Abstract
Bluetongue virus (BTV), the causative agent of bluetongue in ruminants, is an emerging virus in northern Europe. The 2006 outbreak of BTV serotype 8 (BTV-8) in Europe was marked by an unusual teratogenic effect and a high frequency of clinical signs in cattle. Conventional control strategies targeting small ruminants were therefore extended to include cattle. Since cattle were not routinely vaccinated before 2006, the immune responses to BTV have not been studied extensively in this species. With the aims of developing a subunit vaccine against BTV-8 for differentiation between infected and vaccinated animals based on viral protein 7 (VP7) antibody detection and of improving the current understanding of the immunogenicity of BTV proteins in cattle, the immune responses induced by recombinant VP2 (BTV-8) and nonstructural protein 1 (NS1) and NS2 (BTV-2) were studied. Cows were immunized twice (with a 3-week interval) with the experimental vaccine, a commercial inactivated vaccine, or a placebo. The two vaccines induced similar neutralizing antibody responses to BTV-8. Furthermore, the antibody responses detected against VP2, NS1, and NS2 were strongest in the animals immunized with the experimental vaccine, and for the first time, a serotype cross-reactive antibody response to NS2 was shown in cattle vaccinated with the commercial vaccine. The two vaccines evoked measurable T cell responses against NS1, thereby supporting a bovine cross-reactive T cell response. Finally, VP7 seroconversion was observed after vaccination with the commercial vaccine, as in natural infections, but not after vaccination with the experimental vaccine, indicating that the experimental vaccine may allow the differentiation of vaccinated animals from infected animals regardless of BTV serotype. The experimental vaccine will be further evaluated during a virulent challenge in a high-containment facility.
INTRODUCTION
Expanding trade relationships and global climatic changes result in an increasing need for vaccine development to combat vector-borne livestock diseases such as bluetongue (BT), which is spreading into new geographical areas and affecting previously unexposed populations of ruminants (1, 2). The development of vaccines against bluetongue virus (BTV), the causative agent of BT, has a history reaching back to early South African live attenuated vaccines and extending forward to next-generation designs involving the use of more-advanced adjuvants and new vaccine types, such as virus-like particle, subunit, disabled infectious single-cycle, or recombinant vector vaccines (as reviewed by Roy et al. [3]). There is evidence that the use of certain modified live virus vaccines can cause sufficient viremia in vaccinated animals to allow transmission of the vaccine strain to unprotected animals by competent Culicoides midges or to allow reassortment between field and vaccine BTV strains (1, 4–6). Therefore, there is a need for new nonreplicative vaccines that are as efficacious as traditional vaccines. Two other requirements for new-generation vaccine candidates are the abilities to enable differentiation between infected and vaccinated animals (DIVA) and to combat multiple serotypes of BTV with one vaccine. Several experimental DIVA vaccines omitting one or several BTV proteins, such as virus-like particle vaccines (7), capripox, canarypox, or modified vaccinia Ankara virus-based recombinant subunit vaccines (8–12), or DNA vaccines (11, 12), have shown promising results in efficacy studies with sheep or mice, but the diagnostic and immunological importance of antigens excluded in order to fulfill a DIVA characteristic or included in order to protect against multiple BTV serotypes remains to be investigated fully. In order to meet such requirements, it is increasingly evident that knowledge of the roles of individual viral proteins in infection is important but not sufficient; a better understanding of host-pathogen interactions regarding the specific host immune response is needed.
Traditionally, most BT vaccination strategies have targeted sheep, because they generally present with the most severe clinical signs and constitute the largest portions of the ruminant populations in areas in which the disease is endemic (13, 14). Except for mandatory vaccination of all domestic ruminant species in Italy against BTV-2 or BTV-9 in 2002 (15), the commercialization of inactivated vaccines against circulating BTV serotypes in Europe (BTV-1, -2, -4, -8, and -9), beginning in 2005, marked the first time cattle were routinely vaccinated (16), and results showed that immunization of at least 80% of the susceptible ruminant population (including sheep, goats, and cattle) was required to limit the spread of virus (2). As cattle are considered the main amplifying host of BTV, any vaccination campaign that fails to include them may result in the establishment of BTV by allowing a cycle between cattle and the vector (competent Culicoides species) to develop (2). In the case of the 2006 outbreak of BTV-8 in Europe, it appeared essential to vaccinate cattle to limit this possibility and to prevent clinical disease. This had a major indirect impact on trade and the economy within the European Union by causing complications for countries aiming to reclaim their BTV-free status based on serological and clinical proof of freedom from infection (17, 18). The need for DIVA-compliant vaccines is now evident, not only to enable surveillance of outbreaks to prevent serological blindness following vaccination, thereby allowing countries to recover BTV-free status more quickly (3, 19, 20), but also to allow monitoring of vaccine safety and efficacy.
BTV is a double-capsid vector-borne virus with a genome consisting of 10 double-stranded RNA segments encoding 7 structural (viral protein 1 [VP1] to VP7) and 5 nonstructural (nonstructural protein 1 [NS1], NS2, NS3/NS3a, and NS4) proteins (21). To date, 26 serotypes are known (22); they do not confer full cross-protection against each other, although partial cross-protection has been observed (23). VP2 and VP5 are the proteins of the outer capsid. During infection, VP2 plays a significant role in viral penetration of host cells as the primary cell attachment protein of BTV particles (24). It is the most variable protein and is considered the major serotype-defining protein of BTV (19, 25–29). Previous studies have shown that VP2 is required for clinically protective immunological responses in sheep (19, 30) and is essential in inducing the production of neutralizing antibodies (NAs) in mice, sheep, and cattle (19, 26, 31). The protein also is a strong immunogen for cytotoxic T lymphocyte responses in sheep (32), but its role in inducing T cell responses in cattle is unknown.
The importance of inducing T cell responses for clinical and virological protection against BT disease has been shown to be considerable, or even critical, in mice and sheep (33–35). In addition to VP2, proteins such as the nonstructural proteins are suggested to play important roles in inducing cell-mediated immune responses (33, 35, 36). Furthermore, in the serological and cell-mediated immunological assays, NS1 and NS2 have exhibited serotypic cross-reactivity in naturally infected or vaccinated sheep (32, 37) and in experimentally infected rabbits (38), respectively. Although it has been proposed that novel assays detecting antibodies to the nonstructural proteins may allow for DIVA compliance (37, 39), it is evident that the inclusion of proteins that are conserved across serotypes, such as NS1 and NS2, may facilitate the development of effective polyvalent BT vaccines by inducing cross-serotype cell-mediated immune responses (11). The structural protein VP7 is also conserved across BTV serotypes (38) but is considered to induce a serological immune response more than a cell-mediated immune response and is therefore widely used for serological BTV diagnosis (40, 41), including monitoring of all serotypes of BTV infections present in Europe. Therefore, exclusion of this protein from a vaccine may enable DIVA compliance when coupled with well-established diagnostic assays.
In this study, we present an experimental vaccine against BTV-8 consisting of VP2 of BTV-8 and NS1 and NS2 of BTV-2. Besides providing a DIVA characteristic through the absence of VP7, this vaccine was designed to induce serotype-specific neutralizing antibodies and potentially cross-protective cell-mediated immune responses. Theoretically, this formulation can be expanded in the future to target multiple BTV serotypes through the addition of purified recombinant VP2 of other serotypes.
Although much is known about the roles of specific proteins in the pathogenesis of BT disease in sheep, their specific roles in eliciting immune responses are less well studied, especially in cattle. This may prove to be an important piece of the vaccine puzzle, as the clear differences in clinical BT cases among ruminant species suggest that there may be significant divergence in immune responses not only to BTV infections but also to BTV vaccination. Therefore, in addition to evaluation of the VP7-based DIVA characteristics of our experimental vaccine, the aims of this study were to characterize the immunogenicity of our vaccine and to compare it with that of a commercial inactivated vaccine (CV) in cattle.
MATERIALS AND METHODS
Production and purification of recombinant bluetongue proteins.
The VP2-encoding gene of BTV-8 French strain (isolated in 2006) and the NS1-encoding gene of BTV-2 Corsican strain (isolated in 2001) were tagged with 6 His residues at their N-terminal ends, inserted by recombination into individual bacmids, and expressed in a baculovirus expression system (Bac-to-Bac baculovirus expression system; Invitrogen, United Kingdom) after separate infections of Spodoptera frugiperda (Sf9) cells, according to the manufacturer's recommendations. Segment 8 of BTV-2 Corsican strain (isolated in 2001), encoding NS2, was tagged with 6 His residues at the N-terminal end, cloned into a pET28 vector, and expressed in BL21-AI Escherichia coli (Invitrogen, United Kingdom). These recombinant BTV proteins (VP2, NS1, and NS2, respectively) were used to produce the experimental subunit vaccine (SubV) and were used in lymphocyte proliferation assays and enzyme-linked immunosorbent assays (ELISAs). Briefly, 48 to 96 h after infection of Sf9 cells with recombinant baculoviruses expressing either VP2 or NS1, cell suspensions were collected and centrifuged at 300 × g for 10 min. The pellets were frozen at −80°C before purification.
Recombinant BL21-AI E. coli (Invitrogen, United Kingdom) permanently transfected with pET-28b plasmid expressing NS2 was cultured for 5 h in medium containing 0.1% l-arabinose and 1 M isopropyl-β-d-thiogalactopyranoside (IPTG). Bacterial cell suspensions were collected and centrifuged at 500 × g for 10 min, and the pellets were frozen at −80°C before purification.
Briefly, the purification of proteins was performed as follows. Frozen pellets containing VP2, NS1, or NS2 were lysed in sterile water containing 50 mM Na2HPO4, 300 mM NaCl, 5 mM imidazole, and EDTA-free complete protease inhibitor cocktail tablets (Roche Applied Sciences, United Kingdom) (VP2), in the same buffer plus 100 μg/ml lysozyme (Sigma-Aldrich) and 1 U Benzonase nuclease HC (NS2), or in phosphate-buffered saline (PBS) containing 1 mM MgCl2, 20 mM imidazole, 1 U Benzonase nuclease HC, and EDTA-free complete protease inhibitor cocktail tablets (NS1). Proteins were purified from lysed pellets using HisPur cobalt spin plates (Pierce), according to the manufacturer's instructions. Purified proteins were concentrated using Amicon Ultra-15 centrifugal filter units (Millipore, Germany) and were dialyzed in Slide-a-Lyzer 10,000 MWCO dialysis cassettes (Pierce) at 4°C for 40 h in sterile PBS (with three 5-liter buffer changes). VP2 and NS2 were sterilely filtered using 0.45-μm cellulose acetate syringe filters, and all proteins were quantified using the Bradford assay, with readings at 595 nm. NS1, which was not sterilely filtered, was tested at the Swedish Veterinary Institute (Uppsala, Sweden) for the presence of aerobic and anaerobic bacteria and was found to be negative. The presence of each protein was verified using Coomassie blue staining of SDS-PAGE gels and Western blotting with mouse anti-histidine tag monoclonal antibodies (MCA1396; AbD Serotec, United Kingdom). Proteins were stored individually at −80°C until use.
Vaccines.
Each dose of subunit vaccine (SubV) contained 150 μg of each purified recombinant protein (VP2, NS1, and NS2) and 600 μg of AbIsco-300 adjuvant (Isconova AB, Sweden). The final volume was adjusted to 2 ml per dose with sterile PBS. According to the manufacturer, each 1-ml dose of the commercial inactivated vaccine (CV), BTV Pur Alsap 8 (lot L372815; Merial, France), contains at least 7.1 log10 50% cell culture infective doses (CCID50) of BTV-8 before inactivation, as well as aluminum hydroxide and saponin as adjuvant.
Animals, vaccinations, and clinical examinations.
Fifteen healthy, conventionally reared, nonlactating, bovine viral diarrhea virus-free, Swedish red-and-white breed cows (1.5 to 9.5 years of age), from a BTV-free region, were housed in the animal facilities of the Department of Clinical Sciences of the Swedish University of Agricultural Sciences (Uppsala, Sweden). The cows had not been vaccinated against BTV. This experiment was approved by the Ethics Committee of Uppsala (Sweden) (approval no. C153/11).
Groups of five animals were immunized subcutaneously, on the left side of the neck, twice (with a 3-week interval) with either SubV, CV, or 2 ml PBS (control). Clinical examinations and rectal temperature recordings were performed daily before (1 day) and after (3 days) each vaccination, to monitor general and local adverse clinical reactions. Injection site swelling was categorized by size and thickness as none, mild (<3 by 3 cm; flat), moderate (<10 by 10 cm; flat or diffuse), or severe (>10 by 10 cm; raised). Blood samples (25 ml) were obtained from all animals with a BD Vacutainer system (BD Biosciences), in dry heparinized tubes, at day −5 or −4 (0 weeks), day 17 or 18 (3 weeks), day 35 or 36 (6 weeks), and day 59 or 60 (9 weeks).
Bluetongue virus protein-specific lymphocyte proliferation assays.
Peripheral blood mononuclear cells (PBMCs) were obtained from heparinized blood samples from all animals, as described previously (42). Isolated cells were restimulated, in quadruplicate wells of a 96-well plate, with individual proteins and relevant background controls at 0.06 μg of protein per well; Sf9 cell lysate was used as the background control for VP2 and NS1 protein stimulations, and nontransfected BL21-AI E. coli lysate was used as the background control for NS2 protein stimulations. After 5 days of incubation at 37°C in 5% CO2, cell proliferation was quantified using alamarBlue reagent (Invitrogen, United Kingdom), which was added at 20 μl/well for 18 h. Absorbance was measured at 570 nm and 595 nm, and optical density (OD) values were calculated by subtracting values obtained at 595 nm from values obtained at 570 nm for all stimulations (OD = OD570 nm − OD595 nm). Corrected OD (COD) values then were calculated for BTV protein-specific stimulations by subtracting the relevant background control OD values from individual protein OD values (COD = ODprotein − ODrelevant background control).
Bluetongue virus protein-specific ELISAs.
Specific antibodies to BTV-8 VP2 or VP7 were analyzed using commercially available competitive ELISA and double-antigen sandwich ELISA kits (ID Screen bluetongue serotype 8 competition [ID Vet, France] and ID Screen bluetongue early detection [ID Vet] kits, respectively), according to the manufacturer's protocols. Results were expressed as percent inhibition [1 − (ODsample/ODnegative control)] (VP2) or as 100% minus the competition percentage (ODsample/ODpositive) (VP7).
NS1- and NS2-specific antibodies (BTV-2) were analyzed using indirect ELISAs. ELISA plates (Nunc, Denmark) were coated overnight at 4°C and blocked with 2% (wt/vol) bovine serum albumin. Sera were diluted 1:10 with a 60-min incubation (NS1) or 1:25 with a 75-min incubation (NS2) at 37°C on the coated plates and then were incubated for 45 min with horseradish peroxidase-conjugated anti-bovine IgG1 monoclonal antibodies (Svanova Biotech, Sweden). Absorbance values were measured at 450 nm, CODs were calculated, and results were expressed as percentages of positive-control serum CODs.
Gamma interferon (IFN-γ) expression in supernatants of lymphocyte proliferation wells was quantified using a sandwich ELISA kit (ID Screen ruminant interferon gamma kit; ID Vet, France), following the manufacturer's protocol. Results were expressed as COD values.
Serum neutralization assay.
Sera obtained at 6 weeks were analyzed for the presence of specific BTV-8 antibodies by a serum neutralization test, as described previously (43). The range of dilutions was 1:4 to 1:512, and 8,000 Vero cells were added per well, in 100 μl minimal essential medium (Gibco, United Kingdom) supplemented with 1% minimal essential amino acids (Gibco) and 1% HEPES (Gibco). Sera were tested in duplicate, and the neutralizing titer of each serum sample was defined as the highest dilution allowing neutralization of 100 50% tissue culture infective doses (TCID50) of BTV-8.
Statistical analysis.
Analyses among 2 or 3 groups were performed using nonparametric Mann-Whitney and Kruskal-Wallis tests for independent groups, respectively, in the statistical program R (44). Statistical significance was set to a P value of ≤0.05 or ≤0.01 unless otherwise specified. Where applicable, values are provided as the indicated mean ± the standard deviation (SD).
RESULTS
Neutralizing antibodies against BTV-8.
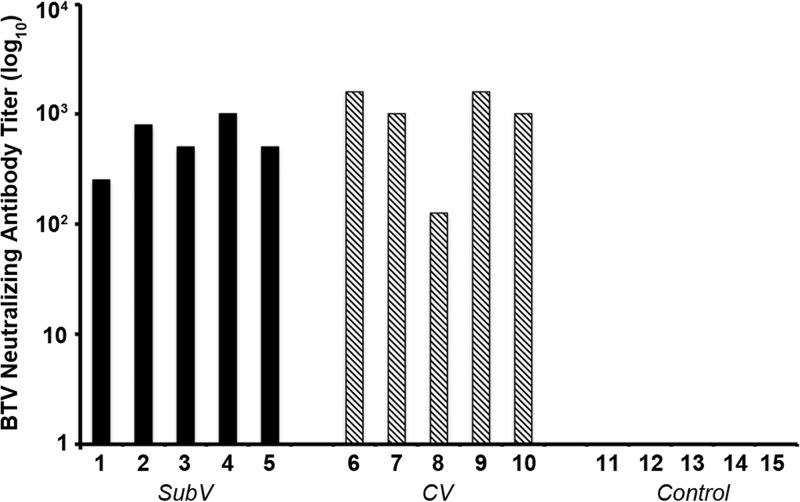
At 6 weeks, high titers of neutralizing antibodies directed against BTV-8 were detected in sera from all animals immunized with either the SubV or the CV but not in the control samples (Fig. 1). The NA levels did not differ significantly between the vaccine groups (P = 0.1666).
Fig 1.
Titers of neutralizing antibodies directed against BTV-8 following vaccination with an experimental subunit vaccine (SubV) (cows 1 to 5), a commercial inactivated vaccine (CV) (cows 6 to 10), or PBS (control; cows 11 to 15). Neutralizing antibodies (NAs) against BTV-8 were titrated in sera obtained 3 weeks after 2 vaccinations given with a 3-week interval. NA titers were calculated using the Reed-Muench method and are expressed as log10 values. No NAs were detected in control animals (titer of 0 ± 0). Each bar represents the average of duplicate determinations.
Kinetics of antibodies against VP2 and VP7 of BTV-8 and NS1 and NS2 of BTV-2.
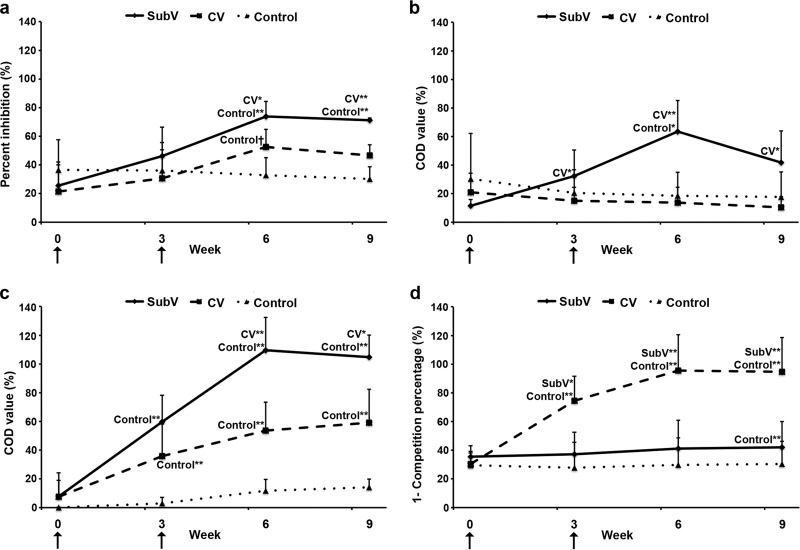
VP2-specific antibodies (BTV-8) were detected by competitive ELISAs in sera of animals in the two vaccine groups (Fig. 2a). Slight antibody responses were observed at 3 weeks and responses were amplified at 6 weeks, while controls remained seronegative (by the manufacturer's definition). Although the VP2-specific antibody levels induced by both vaccines were greater than those observed in the controls at 6 weeks (P ≤ 0.01 and P < 0.06 for SubV and CV, respectively), only animals immunized with the SubV maintained significantly higher levels than the controls at 9 weeks (P ≤ 0.01). Levels of VP2-specific antibodies in SubV-immunized animals were significantly higher than those in CV-immunized animals at both 6 and 9 weeks (P ≤ 0.05 and P ≤ 0.01, respectively).
Fig 2.
Kinetics of protein-specific serum antibodies directed against VP2 of BTV-8, VP7 of BTV, and NS1 and NS2 of BTV-2 in vaccinated cows. Animals (represented by group means, n = 5) were vaccinated twice, with a 3-week interval, with an experimental subunit vaccine (SubV), a commercial inactivated vaccine (CV), or PBS (control) (arrows). Sera were collected at weeks 0, 3, 6, and 9 and tested by competitive ELISA for BTV-8 VP2-specific antibodies (a), indirect ELISA for BTV-2 NS1-specific IgG1 antibodies (b), indirect ELISA for BTV-2 NS2-specific IgG1 antibodies (c), and double-antigen sandwich ELISA for BTV VP7-specific IgM and IgG antibodies (d). Corrected OD (COD) values are presented as percent inhibition (a), percentage of positive reference serum values (b and c), or 100% minus competition percentage (d). Vertical lines, standard deviations. *, P ≤ 0.05, and **, P ≤ 0.01, for comparisons with the indicated groups.
As measured by indirect ELISAs, only animals in the SubV group showed increasing levels of NS1-specific IgG1 antibodies in sera after immunization (Fig. 2b). These levels peaked at 6 weeks (mean, 63.6 ± 21.6%), decreased between 6 and 9 weeks (mean, 41.7 ± 22.3%), and were significantly higher than those in the CV-immunized or control animals at 3, 6, and 9 weeks. Significantly higher levels of NS2-specific IgG1 serum antibodies were detected in the animals in the SubV and CV groups than in the control group at 3, 6, and 9 weeks (Fig. 2c) and in the SubV group than in the CV group at 6 weeks (P ≤ 0.01) and 9 weeks (P ≤ 0.05). VP7-specific IgM and IgG serum antibodies were detected by ELISA only in the CV-immunized animals (Fig. 2d). Positive antibody levels (>50%, as defined by the manufacturer) were detected at 3, 6, and 9 weeks (74.4%, 95.6%, and 94.7%, respectively).
Kinetics of proliferative lymphocyte responses to VP2 of BTV-8 and NS1 and NS2 of BTV-2.
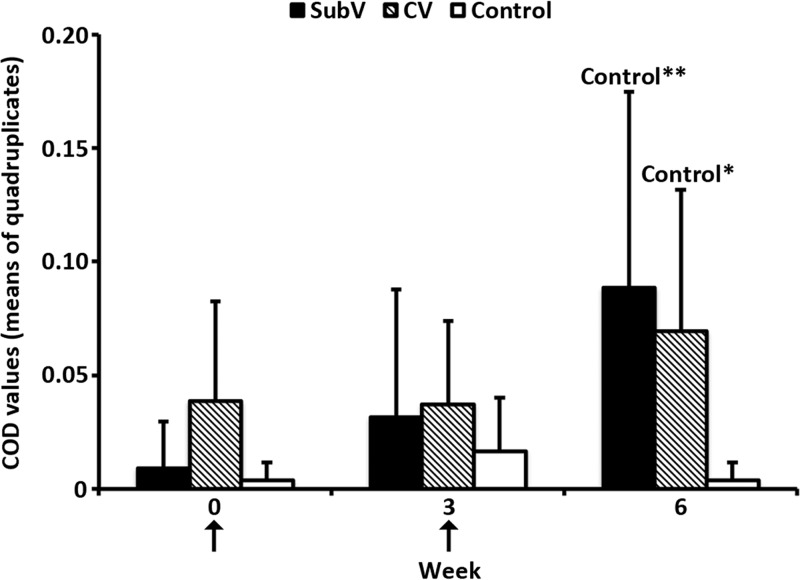
In vitro proliferative lymphocyte responses following restimulation were detected for NS1 but not VP2 or NS2 following immunization with either vaccine. NS1-specific lymphocyte proliferation was detected at 3 weeks in 2/5 animals in each vaccinated group (data not shown) and at 6 weeks in 5/5 (SubV group) and 4/5 (CV group) animals (Fig. 3); these levels were significantly higher than those in the controls (P ≤ 0.01 and P ≤ 0.05, respectively).
Fig 3.
Lymphocyte proliferation in response to BTV-2 NS1-specific restimulation of PBMCs from vaccinated cattle. Animals (represented by group means, n = 5) were immunized twice, with a 3-week interval, with an experimental subunit vaccine (SubV), a commercial inactivated vaccine (CV), or PBS (control) (arrows). Blood was collected at weeks 0, 3, and 6. PBMCs were isolated from all animals and restimulated against NS1 or control antigen. Proliferation is expressed as corrected OD (COD) values (mean of quadruplicate determinations) after 5 days of stimulation and addition of the alamarBlue reagent. Vertical lines, standard deviations. *, P ≤ 0.05, and **, P ≤ 0.01, for comparisons within the indicated groups.
Kinetics of gamma interferon production in response to NS1 of BTV-2.
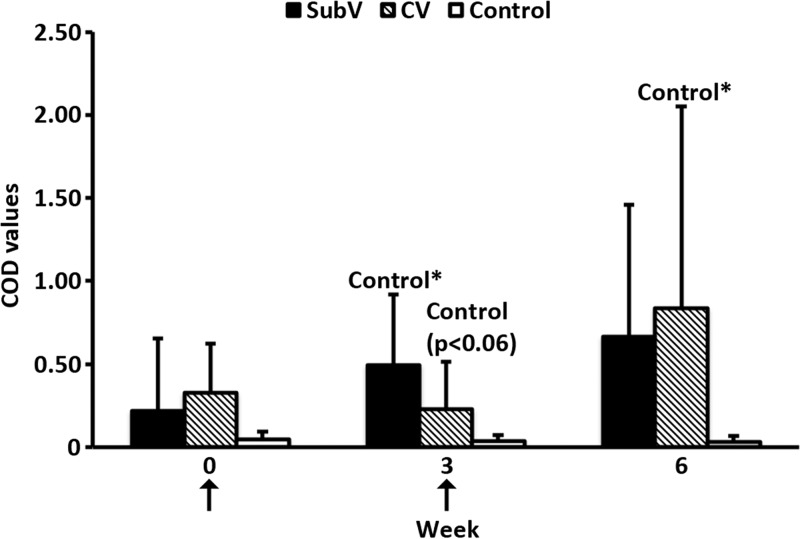
Greater IFN-γ expression in response to NS1-specific stimulation was detected in vaccinated animals than in controls (Fig. 4). Significantly higher mean levels of NS1-specific IFN-γ expression were observed in animals of both vaccine groups at 3 weeks and in the CV-immunized animals at 6 weeks than in controls.
Fig 4.
Kinetics of IFN-γ production induced by BTV protein stimulation of PBMCs from immunized cattle. Animals were immunized twice, with a 3-week interval, with an experimental subunit vaccine (SubV), a commercial inactivated vaccine (CV), or PBS (control) (arrows). Supernatants from PBMCs stimulated with NS1 or control antigen at weeks 0, 3, and 6 were tested by ELISA for the presence of IFN-γ. Data are presented as group averages (n = 5) of corrected OD (COD) values, per the manufacturer's instructions. Vertical lines, standard deviations. *, P ≤ 0.05, for comparisons within the indicated groups.
Vaccine safety.
No severe systemic reactions were observed following vaccination with either vaccine. The animals in the SubV group developed higher rectal temperatures than did those in the other groups at 6 h (SubV group: mean, 38.5°C [range, 38.0 to 39.0°C]; P ≤ 0.05, compared with the control group; CV group: mean, 38.1°C [range, 37.6 to 38.8°C]; control group: mean, 37.9°C [range, 37.7 to 38.5°C]) and 24 h (SubV group: mean, 39.2°C [range, 38.8 to 39.4°C]; P ≤ 0.01, compared with the CV and control groups; CV group: mean, 38.4°C [range, 38.3 to 38.7°C]; P ≤ 0.05, compared with the control group; control group: mean, 38.0°C [range, 37.6 to 38.3°C]) after the first immunization. Mild-to-moderate localized swelling was observed for 48 h following the first immunization in vaccinated animals (SubV group: mild, 2/5 animals; moderate, 3/5 animals; CV group: mild, 2/5 animals), compared with controls. At 24 h after the second vaccination, the SubV-vaccinated animals exhibited higher rectal temperatures (mean, 40.0°C [range, 38.8 to 40.7°C]) and greater swelling than did the CV-immunized animals (mean, 38.2°C [range, 37.7 to 38.6°C]; P ≤ 0.01) and control animals (mean, 38.0°C [range, 37.7 to 38.4°C]; P ≤ 0.01), although mild-to-moderate injection site swelling was observed in animals of both vaccine groups (SubV group: moderate, 4/5 animals; CV group: mild, 4/5 animals; moderate, 1/5 animals) and in 1/5 control animals. No reduction in appetite or change in behavior was observed for any animal throughout the study, and all localized swelling disappeared by 1 week after each vaccination.
DISCUSSION
In this study, we show that a novel subunit BT vaccine safely induced immunological responses in vaccinated cattle comparable to those induced by a commercial inactivated vaccine, including BTV-8 neutralizing antibodies. NS1 and NS2 induced cross-serotype immune responses, suggesting that the experimental vaccine may be adapted to multiple BTV serotypes through the addition of recombinant purified VP2 of different serotypes. Furthermore, although NS2- and VP2-specific cell-mediated immune responses were demonstrated previously in sheep (32, 36), such responses were not observed in this study with cattle. Finally, VP7 seroconversion, as observed after natural infection, occurred following immunization with the commercial vaccine but not the experimental vaccine, which lacked this protein, thus indicating that this component may function as a DIVA marker.
Sera were tested for the presence of neutralizing antibodies to BTV-8 elicited by the two different vaccines. Our results showed the presence of neutralizing antibodies at titers that were similar in the two vaccine groups. Previous studies indicated that the induction of neutralizing antibodies following BTV vaccination is associated with clinical protection, a crucial component of vaccine efficacy (19, 45); therefore, the results suggest that the experimental subunit vaccine may induce a level of protection similar to that observed with the commercial inactivated vaccine. Indeed, the ability of the inactivated vaccine to protect ruminants from disease has been shown through its use in the field (43, 46–49). In addition, it was demonstrated previously that VP2 is a major inducer of neutralizing antibodies following both BTV infection and vaccination (19, 26). Significantly higher levels of VP2-specific antibodies were detected in the sera of SubV-immunized animals than in the sera of CV-immunized or control animals. This may be explained by the quantity of VP2 antigen and its combination with the immunostimulating complex matrix adjuvant used in the experimental vaccine or by changes in protein conformation during the inactivation process for the commercial vaccine.
Only animals in the SubV group demonstrated an increase in NS1 (BTV-2)-specific antibody responses. Although the serotype of NS1 tested in the ELISA differed from that in the commercial vaccine, genetic and serological analyses of the RNA sequences encoding NS1 (segment 5) and of the protein itself show that NS1 is highly conserved across BTV serotypes (38, 50). Furthermore, NS1-specific antibodies were not detected in the sera of sheep immunized with inactivated BT vaccines, in contrast to BTV-infected animals (37, 51). Therefore, as described previously (37), the absence of an antibody response is probably due not to serotype divergence but rather to the small quantity of NS1 present in the CV, in comparison with natural BTV infection or, in the current study, the experimental vaccine. Based on similar observations, NS1 has been proposed as a target for a DIVA test (37). However, since the protein is not removed from the inactivated vaccine by purification, it is probably present in small amounts. Indeed, among cattle that were immunized against BTV-8 several times over several years in the northern part of the vaccination area of Sweden, where the virus itself did not appear to circulate, antibodies against NS1 were detected in 14 of 56 cows with antibodies against VP7 (J. F. Valarcher and L. Renström, unpublished data). If this observation is confirmed, then the potential DIVA characteristics of conventional inactivated vaccines based on NS1 might be impaired, at least at the individual level.
The presence of NS2 antibodies also was evaluated using a serotype in the ELISA that differed from that of the CV. The results showed a clear increase in NS2 (BTV-2)-specific antibodies over time in the sera of animals in both the SubV and CV groups, in contrast to the control group. To the best of our knowledge, this study reports the first time that NS2-specific antibodies have been detected in vaccinated cattle and the first time that NS2-specific antibody cross-reactivity has been demonstrated in vaccinated cattle, although sequence analysis (52) and serological results from experimentally infected rabbits (38) indicate that the protein is highly conserved across BTV serotypes. Interestingly, the observed antibody response to NS2 was the only BTV-specific immune response that was detected in both groups of vaccinated cattle, compared with the control group, after only one immunization. However, the role of NS2-specific antibodies in protection is not known and must be explored further.
In addition to neutralizing antibodies, cell-mediated immune responses are thought to play an important role in clinical and virological protection following BTV vaccination or infection (33–35). To target this type of response, the experimental vaccine in this study contained the AbIsco-300 adjuvant, an immunostimulating complex (ISCOM) matrix that enhances the uptake and activity of antigen-presenting cells and T cells (53). We demonstrated previously that this type of adjuvant elicits antigen-specific antibody and lymphocyte responses with high levels of IFN-γ in cattle (54). In the commercial vaccine, saponins are combined with aluminum hydroxide, also with the aim of inducing humoral and cell-mediated immune responses. In the present study, protein-specific lymphocyte proliferation of PBMCs stimulated with VP2, NS1, and NS2 was determined by subtracting responses to relevant background controls from corresponding protein stimulations, thereby giving low but specific values. The data indicated that NS1 specifically induced a cell-mediated immune response in cattle immunized with either the SubV or the CV in a homologous vaccine-boost strategy. This protein-specific response is also observed as greater levels of IFN-γ expression in the two groups of vaccinated cattle than in the control group. Furthermore, since the inactivated vaccine was serotype 8 and the NS1 test antigen was serotype 2, the results corroborate previous studies of sheep that showed cross-reactive proliferation of NS1-specific T lymphocytes in animals immunized with one serotype of virus (32). Other studies of mice and sheep have shown that the nonstructural proteins NS1, NS2, and NS3 elicit cytotoxic T cell responses to varying degrees (32, 34, 36). However, we did not observe a measurable T cell response to NS2 in vaccinated cattle in this study. Furthermore, although there is evidence of VP2-specific cytotoxic T cell responses in some but not all vaccinated sheep (55), no observable bovine T cell response to VP2 was detected by our assay. These differences in cell-mediated immune responses may be due to differences in vaccine preparations (including adjuvants, amounts of antigen, selection of antigen, types of vectors, and routes of administration) in the studies or to inherent species differences among mice, sheep, and cattle, and they emphasize the importance of understanding protein-specific immune responses on a species level in order to facilitate better vaccine design.
Vaccine safety is a primary concern in vaccine development. Stronger local reactions and hyperthermia were observed in animals vaccinated with the SubV than in those vaccinated with the CV or placebo. We previously correlated such effects with the adjuvant (54), and, although endotoxins were not excluded in the experimental vaccine presented here, protein components underwent dialysis under sterile conditions and were sterilely filtered or demonstrated an absence of bacteria (data not shown) before preparation of the vaccine. Higher rectal temperatures, with mild-to-moderate swelling, were observed in the vaccine groups than in the control group, but no severe clinical signs were observed for any animal. Previous studies of ruminants also demonstrated increases in rectal temperatures following the first and second vaccinations with an inactivated vaccine (48, 56), possibly due to the inclusion of strong adjuvants in the vaccine preparations (56). Therefore, decreasing the quantity of adjuvant may avert the similar local reactions and transient hyperthermia observed in the SubV-immunized animals.
In addition to safety, novel BT vaccines require DIVA compliance. The incursion of multiple serotypes of BTV into Europe from 1998 to 2006 (16) illustrated the need to differentiate between infected and vaccinated animals not only for trade purposes but also for routine surveillance of the movement of BTV serotypes and facilitation of the development of vaccine campaigns designed to prevent the establishment of new BTV serotypes in specific regions. In agreement with previous studies, our data suggest that inactivated vaccines do not induce NS1-specific antibodies or do so only at very low levels following two vaccinations (37), in contrast to natural infection (37, 51). Therefore, NS1 was described as a candidate protein for DIVA assays (37). However, our results indicated that NS1 may be an important inducer of cross-reactive cell-mediated immune responses and consequently might be an important component of a successful polyvalent vaccine, particularly since the importance of T cell responses in combating BTV infections is widely accepted (33, 34). Therefore, the importance of focusing on the use of other proteins to develop DIVA characteristics was clear. In the experimental vaccine presented in this study, NS1 and NS2 were purposely included in the vaccine design with the aim of inducing a cell-mediated immune response able to form the basis of a future polyvalent vaccine against BTV, while VP2 was chosen to generate a protective neutralizing antibody response against BTV-8. VP7 was selected to satisfy DIVA requirements, since this protein is conserved across all BTV serotypes and is detected at an early stage of infection (43). In the 2006 BTV-8 outbreak in Europe, ELISA kits detecting VP7-specific IgG antibodies were used to identify infections, and kits now also identify IgM antibodies as early as 10 days after BTV infection or vaccination (43, 57). Therefore, by including the serotype-determining protein VP2 and excluding VP7, the experimental subunit vaccine is potentially DIVA compliant with the use of two commercially available ELISA kits. High levels of VP2-specific antibodies were detected in the SubV-immunized animals at 6 weeks and VP7-specific antibodies were detected in the CV-immunized animals at just 3 weeks, whereas no other animals developed such antibodies. Although this DIVA characteristic must be tested following BTV infection, it would be highly useful for monitoring BTV and quickly granting BTV-free status to countries affected by outbreaks.
In conclusion, this experimental vaccine against BTV-8 in cattle was shown to induce neutralizing antibodies, specific antibodies to VP2, NS1, and NS2, and cellular immune responses against NS1. Based on these results, the clinical and virological protection induced by this vaccine following an experimental BTV-8 challenge in cattle, as well as its DIVA compliance, will be evaluated next in a Biosafety Level 3 facility available in another country.
ACKNOWLEDGMENTS
This work was supported by the Swedish Research Council Formas (grant 2009-1593) and the Swedish Farmers' Foundation for Agricultural Research (grant H0750358, for the development of immunological assays in cattle).
We thank the staff at Ruminant Medicine and Reproduction, Department of Clinical Sciences, Swedish University of Agricultural Sciences, for maintaining the experimental animals, and we thank J. L. Roque, C. Roy, and associates for supplying the commercial vaccine and hyperimmune sera against BTV-8.
Footnotes
Published ahead of print 29 May 2013
REFERENCES
- 1. Purse BV, Mellor PS, Rogers DJ, Samuel AR, Mertens PPC, Baylis M. 2005. Climate change and the recent emergence of bluetongue in Europe. Nat. Rev. Microbiol. 3:171–181 [DOI] [PubMed] [Google Scholar]
- 2. Caporale V, Giovannini A. 2010. Bluetongue control strategy, including recourse to vaccine: a critical review. Rev. Sci Tech. 29:573–591 [DOI] [PubMed] [Google Scholar]
- 3. Roy P, Boyce M, Noad R. 2009. Prospects for improved bluetongue vaccines. Nat. Rev. Microbiol. 7:120–128 [DOI] [PubMed] [Google Scholar]
- 4. Ferrari G, De Liberato C, Scavia G, Lorenzetti R, Zini M, Farina F, Magliano A, Cardeti G, Scholl F, Guidoni M, Scicluna MT, Amaddeo D, Scaramozzino P, Autorino GL. 2005. Active circulation of bluetongue vaccine virus serotype-2 among unvaccinated cattle in central Italy. Prev. Vet. Med. 68:103–113 [DOI] [PubMed] [Google Scholar]
- 5. Savini G, MacLachlan NJ, Sanchez-Vizcaino Zientara J-MS. 2008. Vaccines against bluetongue in Europe. Comp. Immunol. Microbiol. Infect. Dis. 31:101–120 [DOI] [PubMed] [Google Scholar]
- 6. Veronesi E, Hamblin C, Mellor PS. 2005. Live attenuated bluetongue vaccine viruses in Dorset Poll sheep, before and after passage in vector midges (Diptera: Ceratopogonidae). Vaccine 23:5509–5516 [DOI] [PubMed] [Google Scholar]
- 7. Stewart M, Bhatia Y, Athmaran TN, Noad R, Gastaldi C, Dubois E, Russo P, Thiéry R, Sailleau C, Bréard E, Zientara S, Roy P. 2010. Validation of a novel approach for the rapid production of immunogenic virus-like particles for bluetongue virus. Vaccine 28:3047–3054 [DOI] [PubMed] [Google Scholar]
- 8. Perrin A, Albina E, Bréard E, Sailleau C, Promé S, Grillet C, Kwiatek O, Russo P, Thiéry R, Zientara S, Cêtre-Sossah C. 2007. Recombinant capripoxviruses expressing proteins of bluetongue virus: evaluation of immune responses and protection in small ruminants. Vaccine 25:6774–6783 [DOI] [PubMed] [Google Scholar]
- 9. Boone JD, Balasuriya UB, Karaca K, Audonnet J-C, Yao J, He L, Nordgren R, Monaco F, Savini G, Gardner IA, MacLachlan NJ. 2007. Recombinant canarypox virus vaccine co-expressing genes encoding the VP2 and VP5 outer capsid proteins of bluetongue virus induces high level protection in sheep. Vaccine 25:672–678 [DOI] [PubMed] [Google Scholar]
- 10. Wade-Evans AM, Pullen L, Hamblin C, O'Hara R, Burroughs JN, Mertens PP. 1997. African horsesickness virus VP7 sub-unit vaccine protects mice against a lethal, heterologous serotype challenge. J. Gen. Virol. 78:1611–1616 [DOI] [PubMed] [Google Scholar]
- 11. Calvo-Pinilla E, Navasa N, Anguita J, Ortego J. 2012. Multiserotype protection elicited by a combinatorial prime-boost vaccination strategy against bluetongue virus. PLoS One 7:e34735. 10.1371/journal.pone.0034735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jabbar TK, Calvo-Pinilla E, Mateos F, Gubbins S, Bin-Tarif A, Bachanek-Bankowska K, Alpar O, Ortego J, Takamatsu H-H, Mertens PPC, Castillo-Olivares J. 2013. Protection of IFNAR (−/−) mice against bluetongue virus serotype 8, by heterologous (DNA/rMVA) and homologous (rMVA/rMVA) vaccination, expressing outer-capsid protein VP2. PLoS One 8:e60574. 10.1371/journal.pone.0060574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giovannini A, MacDiarmid S, Calistri P, Conte A, Savini L, Nannini D, Weber S. 2004. The use of risk assessment to decide the control strategy for bluetongue in Italian ruminant populations. Risk Anal. 24:1737–1753 [DOI] [PubMed] [Google Scholar]
- 14. Alpar HO, Bramwell VW, Veronesi E, Darpel KE, Pastoret P-P, Mertens PPC. 2009. Bluetongue virus vaccines past and present, p 397–428 In Mellor PS, Baylis M, Mertens PPC. (ed), Bluetongue. Elsevier, New York, NY [Google Scholar]
- 15. Patta C, Giovannini A, Rolesu S, Nannini D, Savini G, Calistri P, Santucci U, Caporale V. 2004. Bluetongue vaccination in Europe: the Italian experience. Vet. Ital. 40:601–610 [PubMed] [Google Scholar]
- 16. Zientara S, Sánchez-Vizcaíno JM. Control of bluetongue in Europe. Vet. Microbiol. [Epub ahead of print.] 2013 doi: 10.1016/j.vetmic.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 17. MacLachlan NJ, Osburn BI. 2006. Impact of bluetongue virus infection on the international movement and trade of ruminants. J. Am. Vet. Med. Assoc. 228:1346–1349 [DOI] [PubMed] [Google Scholar]
- 18. Hamers C, Galleau S, Chery R, Blanchet M, Besancon L, Cariou C, Werle-Lapostolle B, Hudelet P, Goutebroze S. 2009. Use of inactivated bluetongue virus serotype 8 vaccine against virulent challenge in sheep and cattle. Vet. Rec. 165:369–373 [DOI] [PubMed] [Google Scholar]
- 19. Roy P, Urakawa T, Van Dijk AA, Erasmus BJ. 1990. Recombinant virus vaccine for bluetongue disease in sheep. J. Virol. 64:1998–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bhanuprakash V, Indrani B, Hosamani M, Balamurugan V, Singh R. 2009. Bluetongue vaccines: the past, present and future. Expert Rev. Vaccines 8:191–204 [DOI] [PubMed] [Google Scholar]
- 21. Ratinier M, Caporale M, Golder M, Franzoni G, Allan K, Nunes SF, Armezzani A, Bayoumy A, Rixon F, Shaw A, Palmarini M. 2011. Identification and characterization of a novel non-structural protein of bluetongue virus. PLoS Pathog. 7:e1002477. 10.1371/journal.ppat.1002477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.International Committee on Taxonomy of Viruses 2011. Virus taxonomy: ninth report of the International Committee on Taxonomy of Viruses. Elsevier, Oxford, United Kingdom [Google Scholar]
- 23. Schwartz-Cornil I, Mertens PPC, Contreras V, Hemati B, Pascale F, Bréard E, Mellor PS, MacLachlan NJ, Zientara S. 2008. Bluetongue virus: virology, pathogenesis and immunity. Vet. Res. 39:46. [DOI] [PubMed] [Google Scholar]
- 24. Hassan SS, Roy P. 1999. Expression and functional characterization of bluetongue virus VP2 protein: role in cell entry. J. Virol. 73:9832–9842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maan S, Maan NS, Nomikou K, Veronesi E, Bachanek-Bankowska K, Belaganahalli MN, Attoui H, Mertens PPC. 2011. Complete genome characterisation of a novel 26th bluetongue virus serotype from Kuwait. PLoS One 6:e26147. 10.1371/journal.pone.0026147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huismans H, Erasmus BJ. 1981. Identification of the serotype-specific and group-specific antigens of bluetongue virus. Onderstepoort J. Vet. Res. 48:51–58 [PubMed] [Google Scholar]
- 27. Mertens PPC, Maan NS, Prasad G, Samuel AR, Shaw AE, Potgieter AC, Anthony SJ, Maan S. 2007. Design of primers and use of RT-PCR assays for typing European bluetongue virus isolates: differentiation of field and vaccine strains. J. Gen. Virol. 88:2811–2823 [DOI] [PubMed] [Google Scholar]
- 28. Kahlon J, Sugiyama K, Roy P. 1983. Molecular basis of bluetongue virus neutralization. J. Virol. 48:627–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shaw AE, Ratinier M, Nunes SF, Nomikou K, Caporale M, Golder M, Allan K, Hamers C, Hudelet P, Zientara S, Breard E, Mertens P, Palmarini M. 2013. Reassortment between two serologically unrelated bluetongue virus strains is flexible and can involve any genome segment. J. Virol. 87:543–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stewart M, Dovas CI, Chatzinasiou E, Athmaram TN, Papanastassopoulou M, Papadopoulos O, Roy P. 2012. Protective efficacy of bluetongue virus-like and subvirus-like particles in sheep: presence of the serotype-specific VP2, independent of its geographic lineage, is essential for protection. Vaccine 30:2131–2139 [DOI] [PubMed] [Google Scholar]
- 31. Inumaru S, Roy P. 1987. Production and characterization of the neutralization antigen VP2 of bluetongue virus serotype 10 using a baculovirus expression vector. Virology 157:472–479 [DOI] [PubMed] [Google Scholar]
- 32. Andrew M, Whiteley P, Janardhana V, Lobato Z, Gould A, Coupar B. 1995. Antigen specificity of the ovine cytotoxic T lymphocyte response to bluetongue virus. Vet. Immunol. Immunopathol. 47:311–322 [DOI] [PubMed] [Google Scholar]
- 33. Roy P, Bishop DHL, LeBlois H, Erasmus BJ. 1994. Long-lasting protection of sheep against bluetongue challenge after vaccination with virus-like particles: evidence for homologous and partial heterologous protection. Vaccine 12:805–811 [DOI] [PubMed] [Google Scholar]
- 34. Jones LD, Williams T, Bishop D, Roy P. 1997. Baculovirus-expressed nonstructural protein NS2 of bluetongue virus induces a cytotoxic T-cell response in mice which affords partial protection. Clin. Diagn. Lab. Immunol. 4:297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stott JL, Barber TL, Osburn BI. 1985. Immunologic response of sheep to inactivated and virulent bluetongue virus. Am. J. Vet. Res. 46:1043–1049 [PubMed] [Google Scholar]
- 36. Jones LD, Chuma T, Hails R, Williams T, Roy P. 1996. The non-structural proteins of bluetongue virus are a dominant source of cytotoxic T cell peptide determinants. J. Gen. Virol. 77:997–1003 [DOI] [PubMed] [Google Scholar]
- 37. Anderson J, Mertens PP, Herniman KA. 1993. A competitive ELISA for the detection of anti-tubule antibodies using a monoclonal antibody against bluetongue virus non-structural protein NS1. J. Virol. Methods 43:167–175 [DOI] [PubMed] [Google Scholar]
- 38. Mecham JO, Dean VC, Jochim MM. 1986. Correlation of serotype specificity and protein structure of the five U.S. serotypes of bluetongue virus. J. Gen. Virol. 67:2617–2624 [DOI] [PubMed] [Google Scholar]
- 39. Barros SC, Cruz B, Luís TM, Ramos F, Fagulha T, Duarte M, Henriques M, Fevereiro M. 2009. A DIVA system based on the detection of antibodies to non-structural protein 3 (NS3) of bluetongue virus. Vet. Microbiol. 137:252–259 [DOI] [PubMed] [Google Scholar]
- 40. Hamblin C. 2004. Bluetongue virus antigen and antibody detection, and the application of laboratory diagnostic techniques. Vet. Ital. 40:538–545 [PubMed] [Google Scholar]
- 41. Vandenbussche F, Vanbinst T, Verheyden B, Van Dessel W, Demeestere L, Houdart P, Bertels G, Praet N, Berkvens D, Mintiens K, Goris N, De Clercq K. 2008. Evaluation of antibody-ELISA and real-time RT-PCR for the diagnosis and profiling of bluetongue virus serotype 8 during the epidemic in Belgium in 2006. Vet. Microbiol. 129:15–27 [DOI] [PubMed] [Google Scholar]
- 42. Taylor G, Thomas LH, Wyld SG, Furze J, Sopp P, Howard CJ. 1995. Role of T-lymphocyte subsets in recovery from respiratory syncytial virus infection in calves. J. Virol. 69:6658–6664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bréard E, Belbis G, Hamers C, Moulin V, Lilin T, Moreau F, Millemann Y, Montange C, Sailleau C, Durand B, Desprat A, Viarouge C, Hoffmann B, de Smit H, Goutebroze S, Hudelet P, Zientara S. 2011. Evaluation of humoral response and protective efficacy of two inactivated vaccines against bluetongue virus after vaccination of goats. Vaccine 29:2495–2502 [DOI] [PubMed] [Google Scholar]
- 44.R Development Core Team 2012. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- 45. Huismans H, van Der Walt NT, Cloete M, Erasmus BJ. 1987. Isolation of a capsid protein of bluetongue virus that induces a protective immune response in sheep. Virology 157:172–179 [DOI] [PubMed] [Google Scholar]
- 46. Eschbaumer M, Hoffmann B, König P, Teifke JP, Gethmann JM, Conraths FJ, Probst C, Mettenleiter TC, Beer M. 2009. Efficacy of three inactivated vaccines against bluetongue virus serotype 8 in sheep. Vaccine 27:4169–4175 [DOI] [PubMed] [Google Scholar]
- 47. Bartram DJ, Heasman L, Batten CA, Oura CAL, Plana-Durán J, Yuen HM, Wylie ADM. 2011. Neutralising antibody responses in cattle and sheep following booster vaccination with two commercial inactivated bluetongue virus serotype 8 vaccines. Vet. J. 188:193–196 [DOI] [PubMed] [Google Scholar]
- 48. Gethmann J, Hüttner K, Heyne H, Probst C, Ziller M, Beer M, Hoffmann B, Mettenleiter TC, Conraths FJ. 2009. Comparative safety study of three inactivated BTV-8 vaccines in sheep and cattle under field conditions. Vaccine 27:4118–4126 [DOI] [PubMed] [Google Scholar]
- 49. Wäckerlin R, Eschbaumer M, König P, Hoffmann B, Beer M. 2010. Evaluation of humoral response and protective efficacy of three inactivated vaccines against bluetongue virus serotype 8 one year after vaccination of sheep and cattle. Vaccine 28:4348–4355 [DOI] [PubMed] [Google Scholar]
- 50. Toussaint JF, Sailleau C, Breard E, Zientara S, De Clercq K. 2007. Bluetongue virus detection by two real-time RT-qPCRs targeting two different genomic segments. J. Virol. Methods 140:115–123 [DOI] [PubMed] [Google Scholar]
- 51. Adkison MA, Stott JL, Osburn BI. 1988. Temporal development of bluetongue virus protein-specific antibody in sheep following natural infection. Vet. Microbiol. 16:231–241 [DOI] [PubMed] [Google Scholar]
- 52. Butan C, Tucker P. 2010. Insights into the role of the non-structural protein 2 (NS2) in bluetongue virus morphogenesis. Virus Res. 151:109–117 [DOI] [PubMed] [Google Scholar]
- 53. Morein B, Bengtsson KL. 1999. Immunomodulation by iscoms, immune stimulating complexes. Methods 19:94–102 [DOI] [PubMed] [Google Scholar]
- 54. Hägglund S, Hu K, Vargmar K, Poré L, Olofson Blodörn A-SK, Anderson J, Ahooghalandari P, Pringle J, Taylor G, Valarcher J-F. 2011. Bovine respiratory syncytial virus ISCOMs: immunity, protection and safety in young conventional calves. Vaccine 29:8719–8730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Janardhana V, Andrew ME, Lobato ZI, Coupar BE. 1999. The ovine cytotoxic T lymphocyte responses to bluetongue virus. Res. Vet. Sci. 67:213–221 [DOI] [PubMed] [Google Scholar]
- 56. Pérez de Diego AC, Sánchez-Cordón PJ, de las Heras AI, Sánchez-Vizcaíno JM. 2012. Characterization of the immune response induced by a commercially available inactivated bluetongue virus serotype 1 vaccine in sheep. ScientificWorldJournal 2012:147158. 10.1100/2012/147158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhou E-M, Ridd D, Riva J, Fernando L, Clavijo A. 2001. Development and evaluation of an IgM-capture ELISA for detection of recent infection with bluetongue viruses in cattle. J. Virol. Methods 91:175–182 [DOI] [PubMed] [Google Scholar]