Abstract
Factor H binding protein (fHbp) is a principal antigen in a multicomponent meningococcal vaccine recently licensed in Europe for prevention of serogroup B diseases. The protein recruits the complement downregulator, factor H (fH), to the bacterial surface, which enables the organism to resist complement-mediated bacteriolysis. Binding is specific for human fH. In preclinical studies, mice and rabbits immunized with fHbp vaccines developed serum bactericidal antibody responses, which in humans predict protection against developing meningococcal disease. These studies, however, were in animals whose fH did not bind to the vaccine antigen. Here we review the immunogenicity of fHbp vaccines in human fH transgenic mice. The data suggest that animals with high serum human fH concentrations have impaired protective antibody responses. Further, mutant fHbp vaccines with single amino acid substitutions that decrease fH binding are superior immunogens, possibly by unmasking epitopes in the fH binding site that are important for eliciting serum bactericidal antibody responses. Humans immunized with fHbp vaccines develop serum bactericidal antibody, but achieving broad coverage in infants required incorporation of additional antigens, including outer membrane vesicles, which increased rates of fever and local reactions at the injection site. The experimental results in transgenic mice predict that fHbp immunogenicity can be improved in humans by using mutant fHbp vaccines with decreased fH binding. These results have important public health implications for developing improved fHbp vaccines for control of serogroup B meningococcal disease and for development of vaccines against other microbes that bind host molecules.
VACCINE POTENTIAL OF MENINGOCOCCAL FACTOR H BINDING PROTEIN
Approximately one-third of cases of meningococcal disease in the United States (1), and an even higher proportion in Europe (2, 3), are caused by serogroup B strains. These strains are also responsible for a disproportionate number of cases in infants <1 year old (4) and can cause epidemics, such as the ones that occurred in New Zealand in the 1990s (5) and, more recently, in France (6). The serogroup B polysaccharide consists of α(2→8) N-acetylneuraminic acid, which is an auto-antigen (7). Use of this polysaccharide as a vaccine target therefore raised safety concerns. Alternative approaches for vaccine development against serogroup B strains used detergent-treated outer membrane vesicles (OMV) (8–10), native outer membrane vesicles (NOMV) from mutants with genetically attenuated endotoxin (11–13), or purified noncapsular antigens (reviewed in references 14–17). Specific examples of recombinant protein antigens include NspA (16), Neisseria heparin binding antigen (18) (also referred to as GNA2132 [19]), NadA (20), PorA (21), transferrin binding protein A (22), Opc outer membrane protein (23, 24), and factor H binding protein (fHbp; previously referred to as GNA1870 or LP 2086) (25, 26). One of the most promising protein antigens is fHbp, which is part of a multicomponent meningococcal vaccine recently licensed in Europe for immunization beginning at 2 months of age (27).
fHbp is a surface-exposed lipoprotein expressed by nearly all Neisseria meningitidis strains (28, 29). The protein recruits the complement downregulator, factor H (fH), to the bacterial surface (30), which enables the organism to evade innate immunity (30, 31). The vaccine antigen can be classified into two subfamilies (28) or three variant groups (25) based on cross-reactivity and amino acid sequence similarity. In infants and toddlers, antibodies to fHbp have complement-mediated bactericidal activity only against strains expressing an fHbp from the homologous subfamily or variant group closely matched to that of the vaccine antigen (32–34). In adolescents and adults, serum bactericidal antibody responses to fHbp vaccines appear to be broader than those in infants or toddlers (35, 36). In humans, serum bactericidal activity is the serologic hallmark of protection against developing meningococcal disease (37).
Anti-fHbp antibodies bind to the bacterial surface, activate the classical complement pathway directly, and block binding of fH (38). With less bound fH, the bacteria become more susceptible to anti-fHbp complement-mediated bacteriolysis because there is greater amplification of the alternative complement pathway (39). In many strains, fHbp is relatively sparsely exposed on the bacterial surface (38). Binding of anti-fHbp antibodies to these strains results in insufficient immune complex and, consequently, insufficient Fc density for efficient C1 complex engagement (38). As a result, complement activation via the classical pathway does not proceed to bacteriolysis in the absence of inhibition of fH binding and alternative pathway amplification (39, 40).
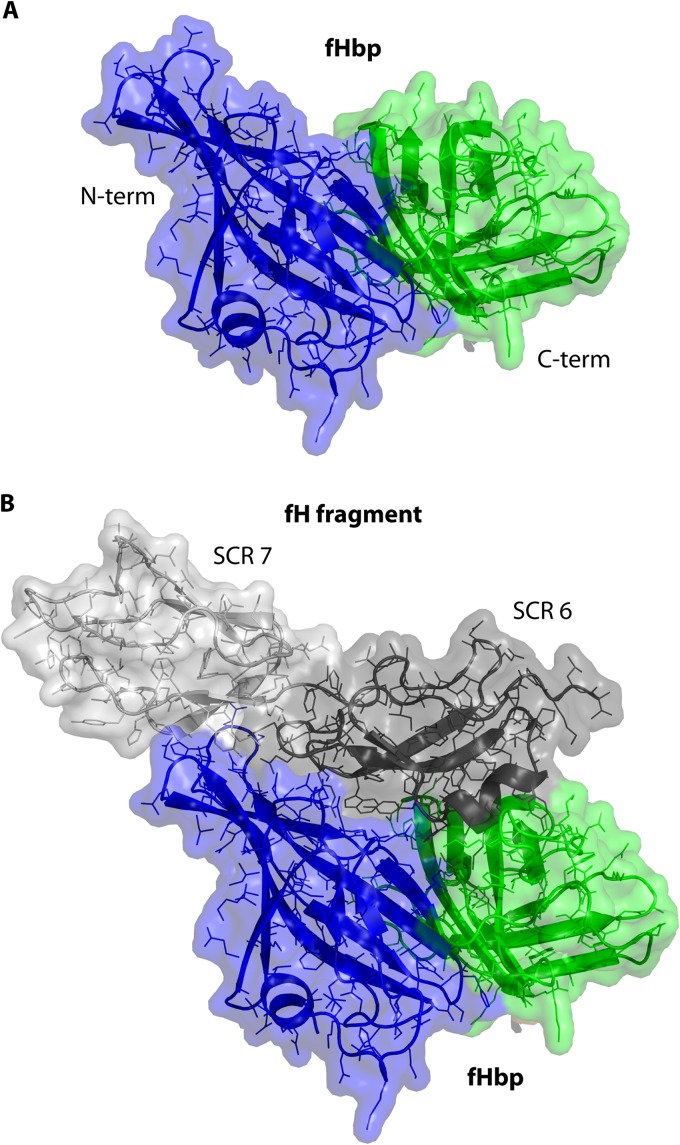
In 2009, we reported that binding of fH to fHbp was specific for human fH (41). Since preclinical fHbp immunogenicity studies had been done in mice and rabbits, the effect of binding of human fH to the vaccine on immunogenicity was not known. In previous studies, most mouse anti-fHbp monoclonal antibodies (MAbs) with bactericidal activity also inhibited binding of fH to fHbp, which suggested that the fHbp epitopes overlapped with the fH binding region in fHbp (42, 43). Conceivably, in immunized humans, fH forms a complex with this region of fHbp and masks important epitopes. A crystal structure of a fragment of fH in complex with fHbp subsequently provided a structural basis for the specificity of binding human fH (44) (Fig. 1).
Fig 1.
Structural models of fHbp. (A) Model of fHbp alone illustrating two domains, N-terminal (blue) and C-terminal (green). Mouse or rabbit fH does not bind to fHbp. In immunized mice or rabbits, epitopes in the fH binding site that are important for eliciting bactericidal antibody are exposed. (B) Model of fHbp in a complex with a fragment of fH (short consensus repeats [SCR] 6 and 7, gray). In humans, the vaccine antigen would be expected to be in a complex with fH and, possibly, mask epitopes in the fH binding site. Models are based on the coordinates of the published crystal structure (44).
The hypothesis that binding of a host molecule to a vaccine antigen impairs immunogenicity (45) can be tested by comparing vaccine immunogenicities in wild-type (WT) mice whose fH does not bind to the vaccine and human fH transgenic mice whose fH binds to the vaccine (46–48). Further evidence is provided by comparative immunogenicity studies in transgenic mice testing mutant fHbp antigens engineered to have decreased fH binding compared with the corresponding antigen that binds fH (46–48).
The purpose of this article is to review data that address whether binding of human fH to fHbp impairs fHbp vaccine immunogenicity and whether mutant fHbp vaccines with decreased fH binding can overcome this impairment. Two published studies reported that mutant fHbp vaccines consisting of either a recombinant protein or native outer membrane vesicles elicited superior serum bactericidal antibody responses in human fH transgenic mice compared with the respective control fHbp vaccines that strongly bind fH (46, 47). A third recently published study suggested that binding of human fH to fHbp did not attenuate immunogenicity (48). In this review, we highlight the differences in methodology and interpretations of these studies to understand better the seemingly disparate observations. As noted above, recombinant fHbp antigens that bind fH are part of vaccines licensed or being developed for prevention of meningococcal disease (33, 34, 49). If fH binding impairs immunogenicity, the effectiveness of these fHbp vaccines in humans can be improved by introduction of amino acid substitutions that decrease or eliminate binding of fH (47, 48, 50). Understanding the effects of these substitutions, therefore, has important scientific, clinical, and public health relevance to the design of optimally immunogenic meningococcal fHbp vaccines.
HUMAN fH TRANSGENIC MICE HAVE LOWER SERUM BACTERICIDAL ANTIBODY RESPONSES TO fHbp VACCINES THAT BIND HUMAN fH THAN DO WILD-TYPE MICE WHOSE fH DOES NOT BIND TO THE VACCINE
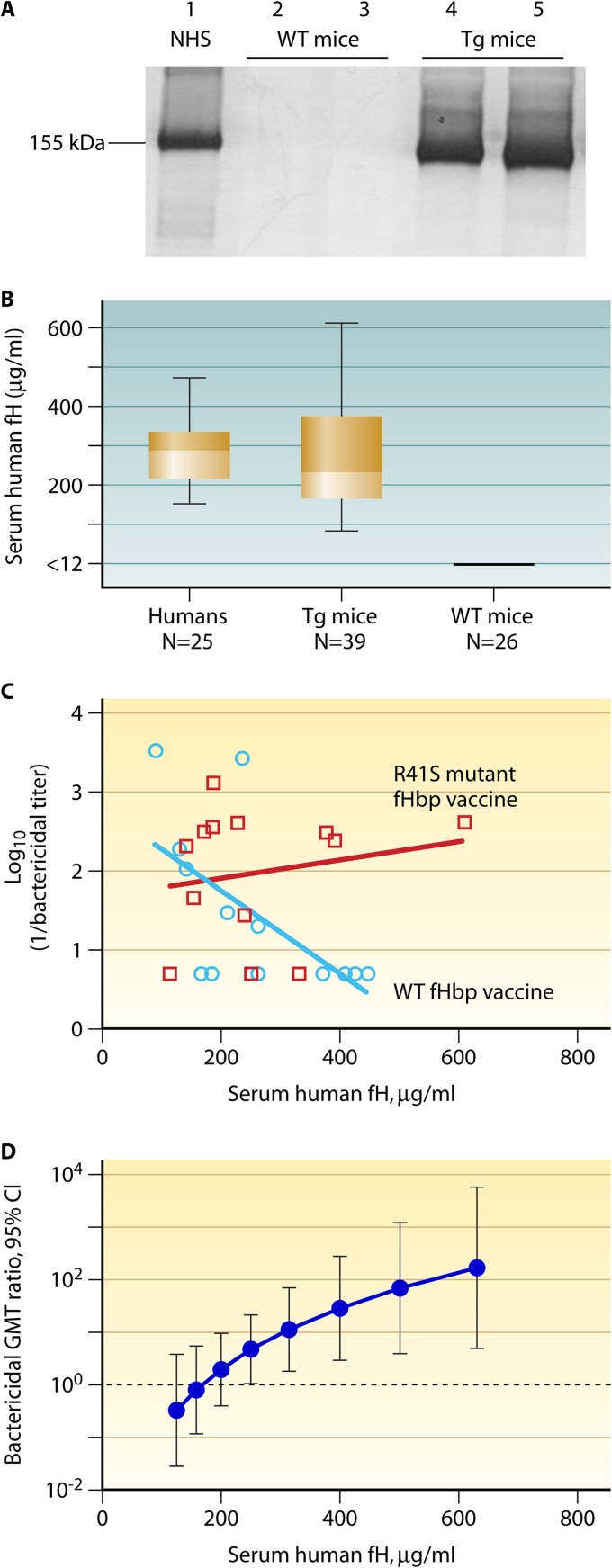
To investigate the effect of fH binding on fHbp immunogenicity, we used a human fH transgenic mouse model that was developed by Peter Rice and colleagues at the University of Massachusetts Medical School (47). These mice were generated by microinjecting BALB/c mouse embryos with a recombinant DNA molecule containing a cytomegalovirus (CMV) enhancer, a chicken β-actin promoter, cDNA encoding the full-length human fH protein, and a rabbit β-globin poly(A) sequence. Sera from mice containing the transgene expressed the full-length human fH protein by Western blotting (Fig. 2A). The CMV enhancer and chicken β-actin promoter were used to achieve high levels of human fH expression to approximate the serum fH concentrations of humans. Using an fHbp capture enzyme-linked immunosorbent assay (ELISA) (47), the median concentration of human fH in the mouse sera was 235 μg/ml, which was similar to that of control human sera assayed in parallel (291 μg/ml; P = 0.22, Mann-Whitney test) (Fig. 2B). However, the range of human fH concentrations in the transgenic mouse sera was greater than the range of fH levels in human sera (P value of 0.008 for differences in the respective variances). Note that in previous studies, the mean fH concentrations in normal human sera or plasma ranged from 210 to 516 μg/ml (51–54), a range which was consistent with the concentrations in the control human sera measured by the fHbp capture ELISA.
Fig 2.
Immunogenicity of mutant and WT fHbp vaccines in human fH transgenic mice in relation to serum human fH concentrations. (A) Detection of human factor H in sera from fH transgenic (Tg) mice by Western blotting. Lane 1, normal human serum (NHS; diluted 1:100); lanes 2 and 3, individual normal (BALB/c) mouse sera (diluted 1:25); lanes 4 and 5, individual transgenic mouse sera (diluted 1:25). Membrane was probed with affinity-purified goat anti-human fH (Complement Technology Inc.) that recognized human, but not mouse, fH. The image is from a replicate experiment, which was performed as part of a previous study (47). (B) Concentrations of human fH in mouse sera measured by a capture ELISA. Positive mice had human fH concentrations of >90 μg/ml, and negative mice had concentrations of <12 μg/ml, which was the lower limit of detection in the assay. For comparison, concentrations of human fH were measured in parallel in stored sera from 25 healthy adult humans. The boxes in the graph extend from the 25th to 75th percentiles. The lines in the middle represent the median values. The whiskers extend from the lowest to the highest value. Data points used to calculate the plots are from Beernink et al. (47). (C) Relationship between serum bactericidal antibody titers measured against serogroup B strain H44/76 and serum human fH concentrations of transgenic mice. The blue circles represent the bactericidal antibody responses of mice immunized with the wild-type control fHbp vaccine that strongly bound fH, measured against serogroup B strain H44/76. The inverse correlation with serum fH concentrations was significant (r = −0.65; P = 0.02; Pearson correlation coefficient between log10 [1/bactericidal titer] and log10 [fH concentration]). The red squares represent the corresponding bactericidal responses of transgenic mice immunized with the R41S mutant fHbp vaccine. The correlation with the serum fH concentrations was not significant (r = +0.18; P = 0.58). The respective r values for the two vaccines were significantly different (P = 0.03). The plots were calculated from previously published data of Beernink et al. (47). (D) Effect of serum human fH concentrations on the ratio of bactericidal antibody responses of transgenic mice immunized with an R41S mutant fHbp vaccine to those of mice immunized with a control fHbp vaccine that strongly bound human fH. The ratios of the geometric mean bactericidal responses of the group immunized with R41S fHbp vaccine to those of the group immunized with control fHbp vaccine were significantly greater than 1 (in favor of the mutant fHbp vaccine) for all human fH concentrations of >250 μg/ml by general linear regression (P < 0.05) and for human fH concentrations of >316 μg/ml (P < 0.01) (47). (Originally published in P. T. Beernink, J. Shaughnessy, E. M. Braga, Q. Liu, P. A. Rice, S. Ram, and D. M. Granoff. 2011. A meningococcal factor H binding protein mutant that eliminates factor H binding enhances protective antibody responses to vaccination. J. Immunol. 186:3606–3614. Copyright © 2011 The American Association of Immunologists, Inc.)
The BALB/c transgenic mice expressed both mouse and human fH (Table 1). The mice appeared to be generally healthy and, when immunized with a control meningococcal serogroup C polysaccharide-protein conjugate vaccine, had serum anti-capsular and anti-carrier protein IgG antibody responses and bactericidal antibody responses similar to those of control BALB/c mice whose sera were negative for human fH (47). In contrast, when the transgenic mice were immunized with an fHbp vaccine that bound fH, the geometric mean serum bactericidal titers in two replicate studies were 4- to 8-fold lower than those of the control mice whose sera were negative for human fH (study 1, P = 0.03; study 2, P = 0.05) (47). Further, in the transgenic mice, there was an inverse correlation between the serum fH levels and the magnitudes of the vaccine response: the higher the serum human fH concentrations, the lower were the serum bactericidal titers to the fHbp vaccine that bound human fH (Pearson correlation coefficient, r = −0.65; P = 0.02) (Fig. 2C, blue line).
Table 1.
Comparison of the methods used to investigate mutant fHbp vaccines in studies using transgenic mice
| Parameter | Value(s) for each mutant fHbp vaccine (reference)a |
||
|---|---|---|---|
| Recombinant fHbp (47) | NOMV (46) | Recombinant fHbp (48) | |
| Vaccine dose (μg of protein) | 20 | 2.5 | 20 |
| Antigen | fHbp R41S-His6 | NOMV expressing fHbp R41S | fHbp E218A/E239A, R41A, and I246A (each with His6) |
| Adjuvant | Aluminum hydroxide | Aluminum hydroxide | Aluminum hydroxide |
| No. of doses of vaccine (route) | 3 (i.p.) | 3 (i.p.) | 3 (i.p.) |
| Bactericidal group B target strain | H44/76 (fHbp ID 1; PorA VR type 7,16) | Cu385 (fHbp ID 1; PorA VR type 19,15) | MC58 (fHbp ID 1; PorA VR type 7,16-2) |
| Mouse genetic background | BALB/c | BALB/c | C57BL/6 |
| Age at immunization (mo) | 1.5 to 2 | 2 to 4 | 3 to 4 |
| Sex | Males and females | Males and females | Not reported |
| Mouse fH | Present | Present | Absent |
| Transgenic fH | Full-length human fH | Full-length human fH | Chimeric mouse-human fHb |
| Enhancer/promoter | CMV/chicken β-actin | CMV/chicken β-actin | None/apoE |
| Serum human or chimeric fH (mean [range]) (μg/ml) | 268 (89 to 610) | 427c (249 to 788) | Not reportedd |
All three studies used WT fHbp ID 1 in variant group 1 for preparing the mutant and control fHbp vaccines. The amino acid residues in the mutants are numbered based on the mature fHbp ID 1 protein sequence (http://pubmlst.org/neisseria/fHbp). i.p., intraperitoneal.
The chimeric fH molecule consisted of human short consensus repeat domains (SCRs) 6 to 8 flanked by the mouse sequences for SCRs 1 to 5 and SCRs 9 to 20.
Only mice that expressed levels of human fH of >240 μg/ml were selected for use in this study.
The serum concentrations of the chimeric fH in the transgenic mouse line were reported in a previous publication to be between 92 and 210 μg/ml (55).
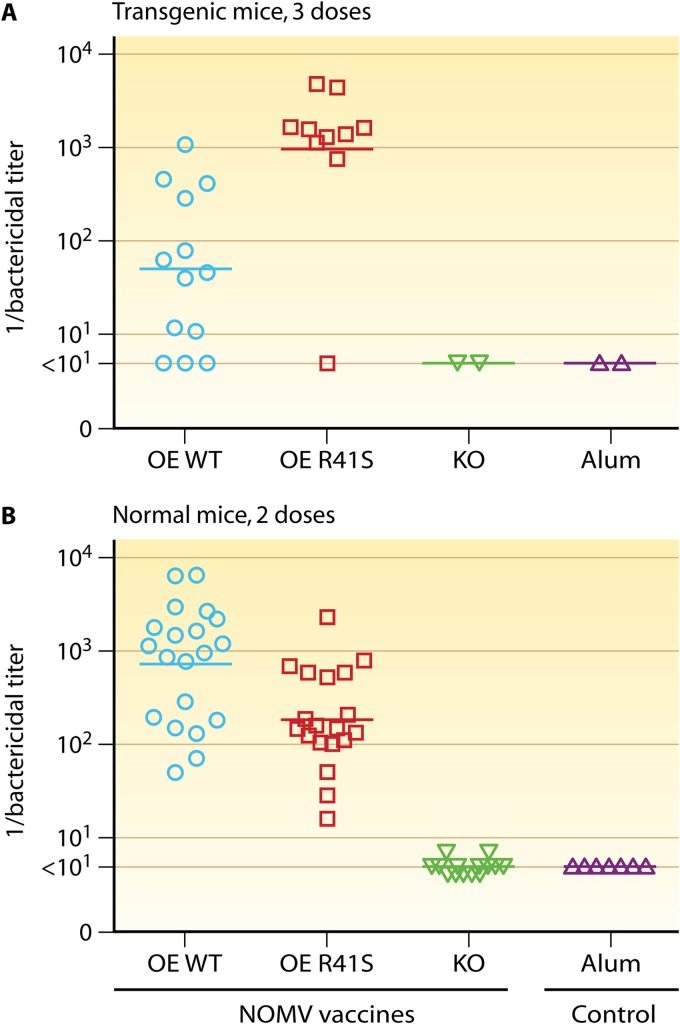
In a subsequent study, we immunized human fH transgenic and wild-type mice with a NOMV vaccine prepared from a mutant meningococcal strain with attenuated endotoxin (LpxL1 knockout) and overexpressed fHbp that bound fH (46). In the normal mice whose fH did not bind to the fHbp vaccine, two doses of the NOMV vaccine with overexpressed WT fHbp elicited higher serum bactericidal anti-fHbp antibody responses than did three doses of the same vaccine given to the human fH transgenic mice whose human fH bound to the vaccine (compare Fig. 3A and B). Thus, in two studies with different fHbp vaccines that strongly bound fH, the human fH transgenic mice had lower serum bactericidal responses than normal mice whose mouse fH did not bind to the vaccines. In contrast, as noted above, the transgenic and normal mice had indistinguishable serum bactericidal antibody responses to a control meningococcal serogroup C conjugate vaccine (47). Therefore, the impairment of the responses of the transgenic mice was specific for the fHbp vaccine that bound human fH. These data supported the hypothesis that binding of a host molecule to a vaccine antigen impaired immunogenicity.
Fig 3.
Serum bactericidal antibody responses of human fH transgenic mice immunized with NOMV vaccines with overexpressed fHbp. Each symbol represents the reciprocal serum titer of an individual mouse; the horizontal lines represent the geometric mean titers. The NOMV vaccines were prepared from mutants of group B strain H44/76 with genetically attenuated endotoxin (LpxL1 knockout) and overexpressed (OE) WT or R41S mutant fHbp ID 1. The test strain, Cu385, has a mismatched PorA variable region type compared to the vaccine strain and expressed fHbp ID 1 that matched the vaccine fHbp antigen (Table 1). OE WT, NOMV vaccine with overexpressed wild-type (WT) fHbp that bound human fH; OE R41S, NOMV vaccine with overexpressed mutant R41S fHbp with lower binding to human fH; KO, control NOMV vaccine from the fHbp knockout; Alum, aluminum hydroxide adjuvant only. (A) Human fH transgenic mice immunized with three injections of vaccine. All mice had serum human fH concentrations of ≥250 μg/ml. The NOMV vaccine with the mutant fHbp elicited 19-fold-higher titers than the NOMV vaccine with WT fHbp that strongly bound human fH (P = 0.001). (B) Normal BALB/c mice whose mouse fH does not bind to fHbp were immunized with two doses of vaccine. The NOMV vaccine with WT fHbp elicited 4-fold-higher titers than the NOMV vaccine with the mutant fHbp (P = 0.003). (Reprinted from reference 46 with permission of the publisher.)
MUTANT fHbp VACCINES WITH DECREASED fH BINDING ARE SUPERIOR IMMUNOGENS IN HUMAN fH TRANSGENIC MICE WITH HIGH SERUM HUMAN fH CONCENTRATIONS
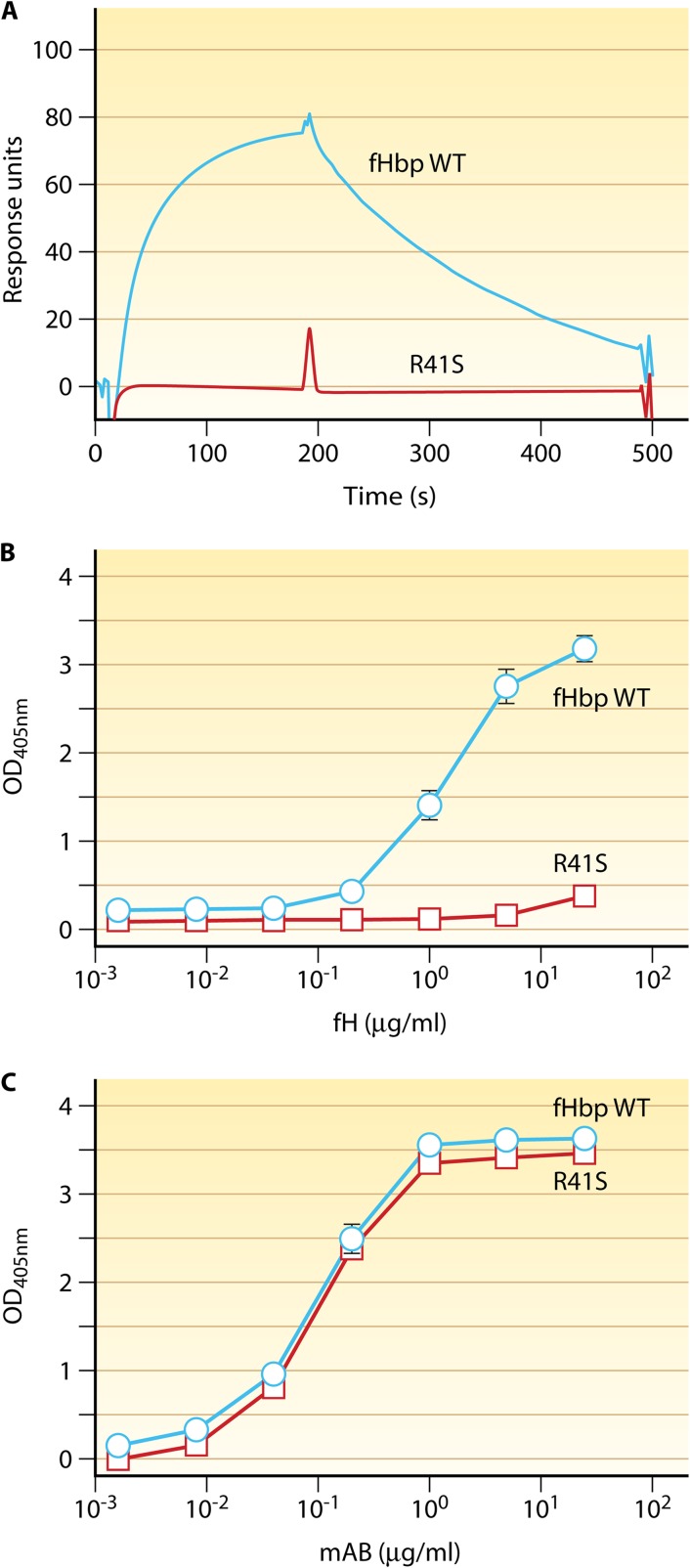
We also immunized human fH transgenic mice with a mutant fHbp vaccine containing a single amino substitution (Arg at residue 41 was replaced by Ser; R41S), which resulted in substantially decreased binding of fH (Fig. 4) (47). In human transgenic mice, the fHbp mutant with decreased fH binding elicited antibodies with greater bactericidal activity than the control fHbp vaccine that bound fH. Overall, the increase in the geometric mean titer (GMT) was only 3-fold. However, in contrast to the results with the fHbp vaccine that bound fH, with the mutant vaccine that did not bind human fH, there was no significant correlation between the serum human fH concentrations and bactericidal responses (Pearson correlation coefficient, r = 0.18; P = 0.57) (Fig. 2C, red line). The test of equality of the two Pearson correlation coefficients showed that the respective correlations between the vaccine groups were significantly different from each other (P = 0.03). Thus, the reason for the relatively small overall increase in the titers elicited by the mutant vaccine likely was the lack of impairment of serum bactericidal antibody responses to the control fHbp vaccine that bound fH when the serum human fH concentrations of the transgenic mice were low (Fig. 2C). Indeed, using general linear regression, we found a statistically significant effect on enhanced immunogenicity of the fHbp, with decreased fH binding only in transgenic mice with serum concentrations of human fH of ≥250 μg/ml (P < 0.05) (Fig. 2D). The model indicated ∼5-fold enhanced immunogenicity of the mutant fHbp vaccine compared with that of the vaccine that bound fH when the serum human fH concentration was ∼250 μg/ml and ∼28-fold enhanced immunogenicity when the serum human fH concentration was ∼400 μg/ml (P < 0.01). We also found that the anti-fHbp antibodies elicited by the mutant fHbp vaccine had greater inhibitory activity for fH binding to fHbp than anti-fHbp antibodies elicited by the control fHbp vaccine that strongly bound fH (47). Thus, unmasking epitopes in the fH binding site of the mutant vaccines appears to affect the anti-fHbp antibody repertoire, and the higher bactericidal activity elicited by the mutant vaccines may have resulted from antibodies with greater fH inhibition, which resulted in less fH bound to the bacteria.
Fig 4.
Replacement of arginine at residue 41 by serine (R41S) decreases binding of fH. (A) By surface plasmon resonance, the fHbp WT (blue line) bound immobilized human fH whereas the R41S mutant (red line) had no detectable binding to fH. Data are for 62.5 nM injection of each fHbp analyte. (B) By ELISA, the fHbp WT (circles with blue line) bound fH, whereas with the R41S substitution (squares with red line), there was no detectable binding of soluble human fH to solid-phase mutant fHbp. OD450, optical density at 450 nm. (C) Binding of anti-fHbp MAb JAR4 to solid-phase wild-type or R41S mutant fHbp indicated that similar amounts of the two proteins were adsorbed to the wells of the microtiter plate and that a conformational epitope in the N-terminal domain was retained by the mutant. (Originally published in P. T. Beernink, J. Shaughnessy, E. M. Braga, Q. Liu, P. A. Rice, S. Ram, and D. M. Granoff. 2011. A meningococcal factor H binding protein mutant that eliminates factor H binding enhances protective antibody responses to vaccination. J. Immunol. 186:3606–3614. Copyright © 2011 The American Association of Immunologists, Inc.)
In the second study with NOMV vaccines, we immunized human fH transgenic mice with a NOMV vaccine with genetically attenuated endotoxin activity and overexpressed WT fHbp, which bound fH strongly, or overexpressed R41S mutant fHbp, which had decreased fH binding (46). The two vaccines had similar levels of fHbp expression. The transgenic mice immunized with the NOMV vaccine with the R41S mutant fHbp had a 19-fold-higher serum bactericidal GMT than the transgenic mice immunized with the control NOMV vaccine with WT fHbp that bound human fH (Fig. 3A). The test strain had a PorA heterologous to that of the vaccine (Table 1), and by antibody depletion studies, the serum bactericidal activity was shown to be directed against fHbp (46). In this second study, we immunized transgenic mice with only serum human fH concentrations of 250 μg/ml or greater. This selection criterion likely contributed to the larger overall increase in immunogenicity of the NOMV vaccine containing the mutant fHbp that did not bind human fH compared to that observed in our first study with the recombinant mutant fHbp vaccine (47).
Our hypothesis is that the enhanced immunogenicity in human fH transgenic mice of mutant fHbp vaccines with decreased fH binding was not a result of increased immunogenicity of the mutant fHbp vaccines per se but from decreased immunogenicity of the fHbp vaccines that bound human fH when serum human fH concentrations were high. Note that in normal BALB/c mice in which mouse fH did not bind to the control or mutant fHbp vaccines, the NOMV-fHbp vaccine with the R41S mutation had decreased serum bactericidal antibody responses compared to those of the control NOMV-fHbp vaccine without the mutation (P = 0.003) (Fig. 3B). Thus, in the absence of human fH, introduction of the R41S amino acid substitution decreased vaccine immunogenicity. This moderate loss of immunogenicity of the mutant fHbp vaccine, which was evident only in normal mice, was more than compensated for in human fH transgenic mice by the greater effect of human fH on decreasing immunogenicity of the NOMV vaccine containing the control fHbp that bound human fH.
Based on protein phylogeny, fHbp variants can be subclassified into three variant groups (25). Interestingly, the R41S substitution, which eliminated fH binding to fHbp in variant group 1, did not affect fH binding to fHbps in variant group 2 (50). In this study, we identified several other amino substitutions in fHbp in variant group 2 that greatly decreased binding of fH (50). Recently, we investigated the immunogenicity of two of these mutant recombinant fHbp vaccines in human fH transgenic mice. Again, we excluded mice with serum human fH concentrations of <250 μg/ml and observed significantly higher serum bactericidal antibody responses to the mutant fHbp vaccines with low binding to human fH than to the control fHbp vaccine that bound fH (P ≤ 0.001) (D. M. Granoff and P. T. Beernink, unpublished data). Thus, in three studies in human fH transgenic mice, we observed higher serum bactericidal antibody responses to mutant fHbp vaccines designed to have decreased fH binding than titers elicited by the respective control fHbp vaccines that strongly bound human fH. In contrast, a recently published study by Johnson and colleagues cast doubt about the superior immunogenicity in transgenic mice of mutant fHbp vaccines with lower fH binding (48). Below we discuss some possible reasons for the discordant results.
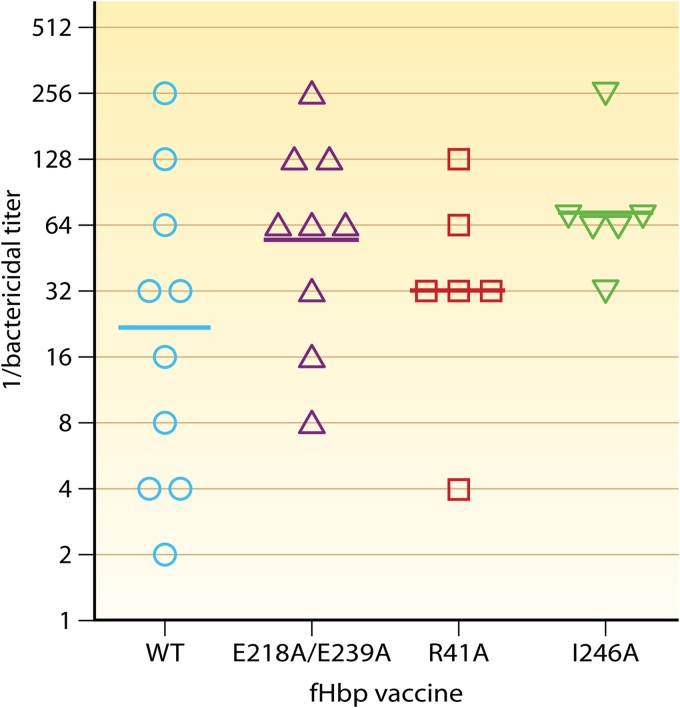
Johnson et al. (48) immunized human fH transgenic mice with three different mutant fHbp vaccines with decreased binding of fH (Fig. 5). The authors reported no significant differences between the serum bactericidal antibody responses of transgenic mice immunized with mutant fHbp vaccines with decreased fH binding and those immunized with a control wild-type fHbp vaccine that strongly bound fH (Fig. 5). The serum bactericidal GMT of mice given the control fHbp vaccine was 1:22, which was lower than that for each of the three fHbp vaccines tested with decreased binding of human fH (1:55, 1:32, and 1:72; GMTs calculated from their data in Fig. 5B of the original paper [48]). Since none of the pairwise comparisons with the control fHbp vaccine that bound fH was statistically significant, the authors concluded that there was no enhancement of immunogenicity by the mutant fHbp vaccines that did not bind human fH.
Fig 5.
Serum bactericidal antibody responses of transgenic mice immunized with mutant recombinant fHbp vaccines that do not bind fH. C57BL/6 mice that lacked endogenous mouse fH and expressed a chimeric mouse-human fH molecule that permitted binding to fHbp were immunized with three doses of a wild-type (WT) fHbp vaccine that bound fH or three mutant vaccines with decreased fH binding (E218A/E239A double mutant, R41A, or I246A). The serum bactericidal GMT of mice given the WT fHbp vaccine was 1:22, which was lower than that for each of the three mutant fHbp vaccines tested (1:55, 1:32, and 1:72); none of the pairwise comparisons with the WT vaccine were significant (P > 0.05). Geometric mean titers (GMT) are shown as horizontal bars as calculated from data in Fig. 5B of the original paper (48). The amino acid residues have been renumbered based on the mature fHbp ID 1 protein sequence (http://pubmlst.org/neisseria/fHbp). (Modified from reference 48 with permission of the publisher.)
Johnson et al. (48) used a previously described transgenic mouse model that was generated in a C57BL/6 background (55) (Table 1). These transgenic mice lacked endogenous mouse fH and expressed a chimeric mouse-human fH molecule that bound to fHbp. Since the short consensus repeat (SCR) domains 6 and 7 of human fH are known to interact with fHbp (44), Johnson et al. employed a chimeric fH molecule that consisted of human SCR domains 6 to 8 flanked by the mouse sequences for SCR domains 1 to 5 and 9 to 20. While the authors proposed that this transgenic model provided a physiologically relevant model to investigate the immunogenicity of WT and mutant fHbp vaccines with decreased binding of human fH, they did not quantify the fH concentrations in the mouse sera. This transgenic mouse line in which fH expression was driven by the apoE promoter (Table 1) originally was reported by Ufret-Vincenty et al. to have serum concentrations of chimeric fH ranging between 92 and 210 μg/ml (55). The published mean serum fH concentrations in humans lie between 210 and 516 μg/ml (47, 51–54). Given our previous report that the superior immunogenicity of mutant fHbp vaccines with low fH binding was observed only in transgenic mice with serum human fH levels of ≥250 μg/ml (Fig. 2D), it is not surprising that Johnson et al. did not observe statistically significant differences in immunogenicity between the control and mutant fHbp vaccines tested in their transgenic mice (48).
Johnson et al. also did not observe a relationship between serum chimeric fH concentrations and serum bactericidal titers in individual transgenic mice (see supplemental Fig. 4 of their publication [48]). The data reported, however, were from transgenic mice immunized with mutant fHbp vaccines in which a lack of correlation was expected, since, in addition to low serum chimeric fH concentrations, the vaccines tested did not bind fH. We, too, did not find a correlation between serum human fH concentrations and serum bactericidal antibody responses of human fH transgenic mice immunized with the R41S mutant fHbp vaccine (Fig. 2C, red regression line). Indeed, these results added credence to the significant inverse correlation we found in transgenic mice immunized with the fHbp vaccine that bound human fH. Thus, in our studies, the impaired serum bactericidal antibody responses of the transgenic mice were specific for the fHbp vaccine that bound human fH when the human fH concentrations were sufficiently high.
We acknowledge that a direct causal link between high serum human fH concentrations and decreased fHbp immunogenicity has not been demonstrated. Apart from the differences in human or chimeric fH concentrations in the two transgenic mouse models, we also cannot exclude the possibility that other differences in study design or reagents might have contributed to the different results reported by Johnson et al. (48) and our group. Most notably, these include different genetic backgrounds of the transgenic mouse strains, use of a mouse line expressing both intact mouse and human fH versus another expressing only chimeric mouse-human factor H, and slight differences in the immunization protocols (Table 1) or the bactericidal assay methods. In addition, potential differences in the affinities of full-length human fH and the chimeric fH for the control fHbp vaccine may possibly have contributed to the differences in immunogenicity. While human fH binds WT fHbp with affinities in the mid-nanomolar range (56, 57), the affinity of the chimeric fH for WT fHbp is not known.
OVERALL CONCLUSIONS
In two published studies, and in a third study not yet published (Granoff and Beernink, unpublished data), we found superior bactericidal antibody responses elicited by mutant fHbp vaccines compared to the respective serum bactericidal responses to control fHbp vaccines that strongly bound human fH. Collectively, the results provide strong evidence that human fH decreases fHbp immunogenicity and that high serum fH concentrations correlate with decreased fHbp immunogenicity. Had we only analyzed the antibody responses of the transgenic mice with serum human fH levels of <250 μg/ml (47), we, too, would not have observed significant differences in immunogenicity between the control and mutant recombinant fHbp vaccines. Thus, the most likely explanation for the lack of superior immunogenicity of the mutant fHbp vaccines tested by Johnson et al. (48) was their use of a transgenic mouse model with serum chimeric fH concentrations that were lower than those expressed in our transgenic model and lower than those found in many healthy humans.
The fHbp antigen in the recently licensed meningococcal serogroup B vaccine is a fusion protein with GNA 2091 (49). The ID 1 fHbp sequence variant used in the fusion protein is known to be a high binder of human fH (56). In human infants and toddlers, the breadth of coverage elicited by the recombinant protein antigens when administered without the OMV component in the multicomponent vaccine (referred to as 3MenB vaccine) was limited (32, 33). One explanation for the limited coverage is an effect of human fH on decreasing fHbp immunogenicity. The addition of OMV (referred to as 4MenB vaccine) improved vaccine coverage (32, 33). In the Findlow et al. study, however, the addition of the OMV was accompanied by higher rates of fever and greater inflammatory reactions at the injection sites than when the recombinant proteins were given without OMV (32). In a subsequent larger study, fever of 39°C or higher occurred in 10% to 15% of infants given the 4MenB vaccine along with routinely recommended vaccines, compared with 3% to 4% of control infants given recommended vaccines only (58). While the clinical importance of these reactions will need to be defined by postlicensure studies in large populations (59), the potential of improving fHbp immunogenicity by using mutant molecules with decreased fH binding may improve vaccine coverage and, possibly, avoid inclusion of the OMV vaccine.
The bivalent fHbp vaccine under development (34) contains recombinant fHbp sequence variants ID 55 (also referred to as B01) in variant group 1 and ID 45 (also referred to as A05) in variant group 3 (34). These two proteins avidly bind human fH (1.6- and 5.2-fold-higher binding than fHbp ID 1, respectively) (56). In toddlers, the bivalent vaccine elicited a limited breadth of bactericidal antibody against four of six primary test strains with fHbp in variant group 1, 2, or 3 (34). Conceivably, using mutant fHbp molecules with decreased fH binding might also improve the breadth of coverage of this vaccine. The prospect of improving vaccine immunogenicity against a potentially fatal disease has important implications for global public health and merits further study, including testing immunogenicity of mutant fHbp vaccines in humans. Indeed, while Johnson et al. did not observe enhanced immunogenicity of mutant fHbp molecules with decreased fH binding, they also concluded that clinical trials were needed to provide definitive evidence of whether the mutants offered superior safety and immunogenicity compared with those of wild-type fHbp vaccines that bind human fH (48).
ACKNOWLEDGMENTS
This work was supported in part by Public Health Service grants R01 AI 046464 and AI 082263 (to D.M.G.), AI 054544 (to S.R.), and AI 070955 and AI 099125 (to P.T.B.) from the National Institute of Allergy and Infectious Diseases, NIH. S.R. was also supported in part by NIH grants AI 032725 and AI 084048, and D.M.G. was supported in part by an endowment from the Clorox Company, Oakland, CA. The laboratory work at Children's Hospital Oakland Research Institute was performed in a facility funded by Research Facilities Improvement Program grant C06 RR 016226 from the National Center for Research Resources, NIH.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Biographies

Dan M. Granoff received his A.B. and M.D. degrees from Johns Hopkins University in Baltimore, MD. He did a pediatric internship at the Children's Hospital of Philadelphia, a pediatric residency at Johns Hopkins Hospital, and a pediatric infectious diseases fellowship at Case Western Reserve University School of Medicine and Cleveland Metropolitan General Hospital in Ohio. He currently holds the Clorox Endowed Chair and is Director of the Center for Immunobiology and Vaccine Development at the Children's Hospital Oakland Research Institute, Oakland, CA. Before moving to Oakland, he was Professor of Pediatrics and Associate Professor of Molecular Microbiology at Washington University School of Medicine, St. Louis, MO, and Director of Pediatric Infectious Diseases at St. Louis Children's Hospital. For more than 10 years, his laboratory has focused on studies of meningococcal factor H binding protein and genetic approaches to developing improved meningococcal outer membrane vesicle vaccines.

Sanjay Ram earned his medical degree at the Seth G. S. Medical College and King Edward VII Memorial Hospital in Mumbai, India. He did a residency in internal medicine at the State University of New York at Buffalo, followed by an infectious diseases fellowship at the Boston University School of Medicine between 1994 and 1998. During his fellowship, Dr. Ram studied complement interactions with Neisseria gonorrhoeae at the Maxwell Finland Laboratory for Infectious Diseases. Subsequently, he has elucidated mechanisms of serum resistance of N. meningitidis. He served as a faculty member at Boston University until 2006 and is now an Associate Professor at the University of Massachusetts Medical School at Worcester, where he continues to study how the pathogenic neisseriae interact with and evade the complement system. He also serves as an infectious diseases consultant at the University of Massachusetts.

Peter T. Beernink is an Associate Scientist at the Children's Hospital Oakland Research Institute in Oakland, CA. He received his A.B. from Cornell University in Ithaca, NY, with a major in biology. He earned his Ph.D. in biology from Boston University in Boston, MA, dissecting the functional role of subunit interactions in the glycolytic enzyme aldolase. Dr. Beernink conducted postdoctoral research at the University of California, Berkeley, where he characterized two allosteric enzymes using crystallographic and other biophysical approaches. He later worked as a biomedical scientist at Lawrence Livermore National Laboratory, where he undertook biochemical studies of human DNA repair proteins. Throughout Dr. Beernink's career, he has been interested in protein structure and macromolecular interactions, and for the past eight years, he has applied these interests to the development of meningococcal protein vaccine antigens, in particular, factor H binding protein.
Footnotes
Published ahead of print 5 June 2013
REFERENCES
- 1. Cohn AC, Macneil JR, Harrison LH, Hatcher C, Theodore J, Schmidt M, Pondo T, Arnold KE, Baumbach J, Bennett N, Craig AS, Farley M, Gershman K, Petit S, Lynfield R, Reingold A, Schaffner W, Shutt KA, Zell ER, Mayer LW, Clark T, Stephens D, Messonnier NE. 2010. Changes in Neisseria meningitidis disease epidemiology in the United States, 1998-2007: implications for prevention of meningococcal disease. Clin. Infect. Dis. 50:184–191 [DOI] [PubMed] [Google Scholar]
- 2. Harrison LH, Trotter CL, Ramsay ME. 2009. Global epidemiology of meningococcal disease. Vaccine 27:B51–B63 [DOI] [PubMed] [Google Scholar]
- 3. Trotter CL, Ramsay ME. 2007. Vaccination against meningococcal disease in Europe: review and recommendations for the use of conjugate vaccines. FEMS Microbiol. Rev. 31:101–107 [DOI] [PubMed] [Google Scholar]
- 4. Shepard CW, Rosenstein NE, Fischer M. 2003. Neonatal meningococcal disease in the United States, 1990 to 1999. Pediatr. Infect. Dis. J. 22:418–422 [DOI] [PubMed] [Google Scholar]
- 5. Baker MG, Martin DR, Kieft CE, Lennon D. 2001. A 10-year serogroup B meningococcal disease epidemic in New Zealand: descriptive epidemiology, 1991-2000. J. Paediatr. Child Health 37:S13–S19 [DOI] [PubMed] [Google Scholar]
- 6. Rouaud P, Perrocheau A, Taha MK, Sesboue C, Forgues AM, Parent Du Chatelet I, Levy-Bruhl D. 2006. Prolonged outbreak of B meningococcal disease in the Seine-Maritime department, France, January 2003 to June 2005. Euro Surveill. 11:178–181 [PubMed] [Google Scholar]
- 7. Finne J, Leinonen M, Makela PH. 1983. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet ii:355–357 [DOI] [PubMed] [Google Scholar]
- 8. Holst J, Feiring B, Naess LM, Norheim G, Kristiansen P, Hoiby EA, Bryn K, Oster P, Costantino P, Taha MK, Alonso JM, Caugant DA, Wedege E, Aaberge IS, Rappuoli R, Rosenqvist E. 2005. The concept of “tailor-made,” protein-based, outer membrane vesicle vaccines against meningococcal disease. Vaccine 23:2202–2205 [DOI] [PubMed] [Google Scholar]
- 9. Holst J, Martin D, Arnold R, Huergo CC, Oster P, O'Hallahan J, Rosenqvist E. 2009. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine 27(Suppl 2):B3–B12 [DOI] [PubMed] [Google Scholar]
- 10. Bjune G, Hoiby EA, Gronnesby JK, Arnesen O, Fredriksen JH, Halstensen A, Holten E, Lindbak AK, Nokleby H, Rosenqvist E, Solberg LK, Closs O, Froholm LO, Lystad A, Bakketeig LS, Hareide B, Halstensen A, Holten E, Eng J. 1991. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet 338:1093–1096 [DOI] [PubMed] [Google Scholar]
- 11. Keiser PB, Biggs-Cicatelli S, Moran EE, Schmiel DH, Pinto VB, Burden RE, Miller LB, Moon JE, Bowden RA, Cummings JF, Zollinger WD. 2011. A phase 1 study of a meningococcal native outer membrane vesicle vaccine made from a group B strain with deleted lpxL1 and synX, over-expressed factor H binding protein, two PorAs and stabilized OpcA expression. Vaccine 29:1413–1420 [DOI] [PubMed] [Google Scholar]
- 12. Koeberling O, Seubert A, Santos G, Colaprico A, Ugozzoli M, Donnelly J, Granoff DM. 2011. Immunogenicity of a meningococcal native outer membrane vesicle vaccine with attenuated endotoxin and over-expressed factor H binding protein in infant rhesus monkeys. Vaccine 29:4728–4734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koeberling O, Seubert A, Granoff DM. 2008. Bactericidal antibody responses elicited by a meningococcal outer membrane vesicle vaccine with overexpressed factor H-binding protein and genetically attenuated endotoxin. J. Infect. Dis. 198:262–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Granoff DM. 2010. Review of meningococcal group B vaccines. Clin. Infect. Dis. 50(Suppl 2):S54–S65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sadarangani M, Pollard AJ. 2010. Serogroup B meningococcal vaccines—an unfinished story. Lancet Infect. Dis. 10:112–124 [DOI] [PubMed] [Google Scholar]
- 16. Martin D, Cadieux N, Hamel J, Brodeur BR. 1997. Highly conserved Neisseria meningitidis surface protein confers protection against experimental infection. J. Exp. Med. 185:1173–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pizza M, Scarlato V, Masignani V, Giuliani MM, Arico B, Comanducci M, Jennings GT, Baldi L, Bartolini E, Capecchi B, Galeotti CL, Luzzi E, Manetti R, Marchetti E, Mora M, Nuti S, Ratti G, Santini L, Savino S, Scarselli M, Storni E, Zuo P, Broeker M, Hundt E, Knapp B, Blair E, Mason T, Tettelin H, Hood DW, Jeffries AC, Saunders NJ, Granoff DM, Venter JC, Moxon ER, Grandi G, Rappuoli R. 2000. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science 287:1816–1820 [DOI] [PubMed] [Google Scholar]
- 18. Serruto D, Spadafina T, Ciucchi L, Lewis LA, Ram S, Tontini M, Santini L, Biolchi A, Seib KL, Giuliani MM, Donnelly JJ, Berti F, Savino S, Scarselli M, Costantino P, Kroll JS, O'Dwyer C, Qiu J, Plaut AG, Moxon R, Rappuoli R, Pizza M, Arico B. 2010. Neisseria meningitidis GNA2132, a heparin-binding protein that induces protective immunity in humans. Proc. Natl. Acad. Sci. U. S. A. 107:3770–3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Welsch JA, Moe GR, Rossi R, Adu-Bobie J, Rappuoli R, Granoff DM. 2003. Antibody to genome-derived neisserial antigen 2132, a Neisseria meningitidis candidate vaccine, confers protection against bacteremia in the absence of complement-mediated bactericidal activity. J. Infect. Dis. 188:1730–1740 [DOI] [PubMed] [Google Scholar]
- 20. Comanducci M, Bambini S, Brunelli B, Adu-Bobie J, Arico B, Capecchi B, Giuliani MM, Masignani V, Santini L, Savino S, Granoff DM, Caugant DA, Pizza M, Rappuoli R, Mora M. 2002. NadA, a novel vaccine candidate of Neisseria meningitidis. J. Exp. Med. 195:1445–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Humphries HE, Williams JN, Blackstone R, Jolley KA, Yuen HM, Christodoulides M, Heckels JE. 2006. Multivalent liposome-based vaccines containing different serosubtypes of PorA protein induce cross-protective bactericidal immune responses against Neisseria meningitidis. Vaccine 24:36–44 [DOI] [PubMed] [Google Scholar]
- 22. West D, Reddin K, Matheson M, Heath R, Funnell S, Hudson M, Robinson A, Gorringe A. 2001. Recombinant Neisseria meningitidis transferrin binding protein A protects against experimental meningococcal infection. Infect. Immun. 69:1561–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carmenate T, Mesa C, Menendez T, Falcon V, Musacchio A. 2001. Recombinant Opc protein from Neisseria meningitidis reconstituted into liposomes elicits opsonic antibodies following immunization. Biotechnol. Appl. Biochem. 34:63–69 [DOI] [PubMed] [Google Scholar]
- 24. Jolley KA, Appleby L, Wright JC, Christodoulides M, Heckels JE. 2001. Immunization with recombinant Opc outer membrane protein from Neisseria meningitidis: influence of sequence variation and levels of expression on the bactericidal immune response against meningococci. Infect. Immun. 69:3809–3816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Masignani V, Comanducci M, Giuliani MM, Bambini S, Adu-Bobie J, Arico B, Brunelli B, Pieri A, Santini L, Savino S, Serruto D, Litt D, Kroll S, Welsch JA, Granoff DM, Rappuoli R, Pizza M. 2003. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J. Exp. Med. 197:789–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fletcher LD, Bernfield L, Barniak V, Farley JE, Howell A, Knauf M, Ooi P, Smith RP, Weise P, Wetherell M, Xie X, Zagursky R, Zhang Y, Zlotnick GW. 2004. Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infect. Immun. 72:2088–2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Granoff DM. 2013. Commentary: European Medicines Agency recommends approval of a broadly protective vaccine against serogroup B meningococcal disease. Pediatr. Infect. Dis. J. 32:372–373 [DOI] [PubMed] [Google Scholar]
- 28. Murphy E, Andrew L, Lee KL, Dilts DA, Nunez L, Fink PS, Ambrose K, Borrow R, Findlow J, Taha MK, Deghmane AE, Kriz P, Musilek M, Kalmusova J, Caugant DA, Alvestad T, Mayer LW, Sacchi CT, Wang X, Martin D, Von Gottberg A, Du Plessis M, Klugman KP, Anderson AS, Jansen KU, Zlotnick GW, Hoiseth SK. 2009. Sequence diversity of the factor H binding protein vaccine candidate in epidemiologically relevant strains of serogroup B Neisseria meningitidis. J. Infect. Dis. 200:379–389 [DOI] [PubMed] [Google Scholar]
- 29. Lucidarme J, Tan L, Exley RM, Findlow J, Borrow R, Tang CM. 2011. Characterization of Neisseria meningitidis isolates that do not express the virulence factor and vaccine antigen factor H binding protein. Clin. Vaccine Immunol. 18:1002–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Madico G, Welsch JA, Lewis LA, Mcnaughton A, Perlman DH, Costello CE, Ngampasutadol J, Vogel U, Granoff DM, Ram S. 2006. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J. Immunol. 177:501–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schneider MC, Exley RM, Chan H, Feavers I, Kang YH, Sim RB, Tang CM. 2006. Functional significance of factor H binding to Neisseria meningitidis. J. Immunol. 176:7566–7575 [DOI] [PubMed] [Google Scholar]
- 32. Findlow J, Borrow R, Snape MD, Dawson T, Holland A, John TM, Evans A, Telford KL, Ypma E, Toneatto D, Oster P, Miller E, Pollard AJ. 2010. Multicenter, open-label, randomized phase II controlled trial of an investigational recombinant meningococcal serogroup B vaccine with and without outer membrane vesicles, administered in infancy. Clin. Infect. Dis. 51:1127–1137 [DOI] [PubMed] [Google Scholar]
- 33. Snape MD, Dawson T, Oster P, Evans A, John TM, Ohene-Kena B, Findlow J, Yu LM, Borrow R, Ypma E, Toneatto D, Pollard AJ. 2010. Immunogenicity of two investigational serogroup B meningococcal vaccines in the first year of life: a randomized comparative trial. Pediatr. Infect. Dis. J. 29:e71–e79 [DOI] [PubMed] [Google Scholar]
- 34. Marshall HS, Richmond PC, Nissen MD, Jiang Q, Anderson AS, Jansen KU, Reynolds G, Ziegler JB, Harris SL, Jones TR, Perez JL. 2012. Safety and immunogenicity of a meningococcal B bivalent rLP2086 vaccine in healthy toddlers aged 18-36 months: a phase 1 randomized-controlled clinical trial. Pediatr. Infect. Dis. J. 31:1061–1068 [DOI] [PubMed] [Google Scholar]
- 35. Richmond PC, Marshall HS, Nissen MD, Jiang Q, Jansen KU, Garces-Sanchez M, Martinon-Torres F, Beeslaar J, Szenborn L, Wysocki J, Eiden J, Harris SL, Jones TR, Perez JL. 2012. Safety, immunogenicity, and tolerability of meningococcal serogroup B bivalent recombinant lipoprotein 2086 vaccine in healthy adolescents: a randomised, single-blind, placebo-controlled, phase 2 trial. Lancet Infect. Dis. 12:597–607 [DOI] [PubMed] [Google Scholar]
- 36. Vu DM, Wong TT, Granoff DM. 2011. Cooperative serum bactericidal activity between human antibodies to meningococcal factor H binding protein and neisserial heparin binding antigen. Vaccine 29:1968–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Borrow R, Andrews N, Goldblatt D, Miller E. 2001. Serological basis for use of meningococcal serogroup C conjugate vaccines in the United Kingdom: reevaluation of correlates of protection. Infect. Immun. 69:1568–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Welsch JA, Ram S, Koeberling O, Granoff DM. 2008. Complement-dependent synergistic bactericidal activity of antibodies against factor H-binding protein, a sparsely distributed meningococcal vaccine antigen. J. Infect. Dis. 197:1053–1061 [DOI] [PubMed] [Google Scholar]
- 39. Giuntini S, Reason DC, Granoff DM. 2012. Combined roles of human IgG subclass, alternative complement pathway activation, and epitope density in the bactericidal activity of antibodies to meningococcal factor H binding protein Infect. Immun. 80:187–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Konar M, Granoff DM, Beernink PT. 28 May 2013. Importance of inhibition of binding of complement factor H for serum bactericidal antibody responses to meningococcal factor H-binding protein vaccines. J. Infect. Dis. 10.1093/infdis.jit239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Granoff DM, Welsch JA, Ram S. 2009. Binding of complement factor H (fH) to Neisseria meningitidis is specific for human fH and inhibits complement activation by rat and rabbit sera. Infect. Immun. 77:764–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Beernink PT, Welsch JA, Bar-Lev M, Koeberling O, Comanducci M, Granoff DM. 2008. Fine antigenic specificity and cooperative bactericidal activity of monoclonal antibodies directed at the meningococcal vaccine candidate, factor H-binding protein. Infect. Immun. 76:4232–4240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Giuntini S, Beernink PT, Reason DC, Granoff DM. 2012. Monoclonal antibodies to meningococcal factor H binding protein with overlapping epitopes and discordant functional activity. PLoS One 7:e34272. 10.1371/journal.pone.0034272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schneider MC, Prosser BE, Caesar JJ, Kugelberg E, Li S, Zhang Q, Quoraishi S, Lovett JE, Deane JE, Sim RB, Roversi P, Johnson S, Tang CM, Lea SM. 2009. Neisseria meningitidis recruits factor H using protein mimicry of host carbohydrates. Nature 458:890–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Meri S, Jordens M, Jarva H. 2008. Microbial complement inhibitors as vaccines. Vaccine 26(Suppl 8):I113–I117 [DOI] [PubMed] [Google Scholar]
- 46. Beernink PT, Shaughnessy J, Pajon R, Braga EM, Ram S, Granoff DM. 2012. The effect of human factor H on immunogenicity of meningococcal native outer membrane vesicle vaccines with over-expressed factor H binding protein. PLoS Pathog. 8:e1002688. 10.1371/journal.ppat.1002688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Beernink PT, Shaughnessy J, Braga EM, Liu Q, Rice PA, Ram S, Granoff DM. 2011. A meningococcal factor H binding protein mutant that eliminates factor H binding enhances protective antibody responses to vaccination. J. Immunol. 186:3606–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Johnson S, Tan L, Van Der Veen S, Caesar J, Goicoechea De Jorge E, Harding RJ, Bai X, Exley RM, Ward PN, Ruivo N, Trivedi K, Cumber E, Jones R, Newham L, Staunton D, Ufret-Vincenty R, Borrow R, Pickering MC, Lea SM, Tang CM. 2012. Design and evaluation of meningococcal vaccines through structure-based modification of host and pathogen molecules. PLoS Pathog. 8:e1002981. 10.1371/journal.ppat.1002981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Giuliani MM, Adu-Bobie J, Comanducci M, Arico B, Savino S, Santini L, Brunelli B, Bambini S, Biolchi A, Capecchi B, Cartocci E, Ciucchi L, Di Marcello F, Ferlicca F, Galli B, Luzzi E, Masignani V, Serruto D, Veggi D, Contorni M, Morandi M, Bartalesi A, Cinotti V, Mannucci D, Titta F, Ovidi E, Welsch JA, Granoff D, Rappuoli R, Pizza M. 2006. A universal vaccine for serogroup B meningococcus. Proc. Natl. Acad. Sci. U. S. A. 103:10834–10839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pajon R, Beernink PT, Granoff DM. 2012. Design of meningococcal factor H binding protein mutant vaccines that do not bind human complement factor H. Infect. Immun. 80:2667–2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hakobyan S, Harris CL, Tortajada A, Goicochea De Jorge E, Garcia-Layana A, Fernandez-Robredo P, Rodriguez De Cordoba S, Morgan BP. 2008. Measurement of factor H variants in plasma using variant-specific monoclonal antibodies: application to assessing risk of age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 49:1983–1990 [DOI] [PubMed] [Google Scholar]
- 52. Ingram G, Hakobyan S, Hirst CL, Harris CL, Pickersgill TP, Cossburn MD, Loveless S, Robertson NP, Morgan BP. 2010. Complement regulator factor H as a serum biomarker of multiple sclerosis disease state. Brain 133:1602–1611 [DOI] [PubMed] [Google Scholar]
- 53. Reynolds R, Hartnett ME, Atkinson JP, Giclas PC, Rosner B, Seddon JM. 2009. Plasma complement components and activation fragments: associations with age-related macular degeneration genotypes and phenotypes. Invest. Ophthalmol. Vis. Sci. 50:5818–5827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Heger A, Kannicht C, Romisch J, Svae TE. 2007. Normal levels of ADAMTS13 and factor H are present in the pharmaceutically licensed plasma for transfusion (Octaplas) and in the universally applicable plasma (Uniplas) in development. Vox Sang. 92:206–212 [DOI] [PubMed] [Google Scholar]
- 55. Ufret-Vincenty RL, Aredo B, Liu X, Mcmahon A, Chen PW, Sun H, Niederkorn JY, Kedzierski W. 2010. Transgenic mice expressing variants of complement factor H develop AMD-like retinal findings. Invest. Ophthalmol. Vis. Sci. 51:5878–5887 [DOI] [PubMed] [Google Scholar]
- 56. Dunphy KY, Beernink PT, Brogioni B, Granoff DM. 2011. Effect of factor H-binding protein sequence variation on factor H binding and survival of Neisseria meningitidis in human blood. Infect. Immun. 79:353–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Seib KL, Serruto D, Oriente F, Delany I, Adu-Bobie J, Veggi D, Arico B, Rappuoli R, Pizza M. 2009. Factor H-binding protein is important for meningococcal survival in human whole blood and serum and in the presence of the antimicrobial peptide LL-37. Infect. Immun. 77:292–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gossger N, Snape MD, Yu LM, Finn A, Bona G, Esposito S, Principi N, Diez-Domingo J, Sokal E, Becker B, Kieninger D, Prymula R, Dull P, Ypma E, Toneatto D, Kimura A, Pollard AJ. 2012. Immunogenicity and tolerability of recombinant serogroup B meningococcal vaccine administered with or without routine infant vaccinations according to different immunization schedules: a randomized controlled trial. JAMA 307:573–582 [DOI] [PubMed] [Google Scholar]
- 59. Cohn AC, Messonnier NE. 2012. Inching toward a serogroup B meningococcal vaccine for infants. JAMA 307:614–615 [DOI] [PubMed] [Google Scholar]