Abstract
Serological testing to detect toxoplasmosis is of major importance to avoid the possible effects of the disease in newborns. This study assessed anti-Toxoplasma IgG and IgM with the Vidas (bioMérieux), Architect (Abbott), and Liaison (DiaSorin) systems in 631 sera from pregnant women and newborns as well as anti-Toxoplasma IgG avidity with these three systems on 54 sera from pregnant women with positive IgG and IgM. The IgG and IgM results were in agreement in, respectively, 95.2% and 98.3% (Vidas versus Architect) and 96.9% and 95.3% (Vidas versus Liaison) of the samples. Specificities were excellent for all the assays, while Vidas sensitivities ranged (depending on the classification of gray zone results) from 93.8 to 98.4% for IgG (Architect, 84.4 to 93.8%; Liaison, 93.8%) and from 81.8 to 90.9% for IgM (Architect, 63.6%; Liaison, 81.8 to 90.9%). In seroconversion sequences, IgMs were generally detected simultaneously by the three assays, while Architect was the earliest assay to detect IgG. In noninfected children, maternally transmitted IgGs were detected for a longer time with Architect than with the other systems. IgMs were positive in only one infected child with the Vidas and Liaison systems. Significantly more sera were classified in the high-avidity category with Vidas than with Architect. This evaluation shows similar performances for Vidas and more recent systems. The Vidas system adequately detects toxoplasmosis in pregnant women and newborns. This system fits the needs of laboratories working on small routine series for first-line testing as well as expert laboratories, due to a high specificity and a powerful avidity test.
INTRODUCTION
Due to transmission of the apicomplexan protozoan Toxoplasma gondii from a mother with acute infection to her fetus, congenital toxoplasmosis can cause severe diseases or sequelae, mainly concerning the eye (retinochoroiditis) and the brain (1). The severity of congenital toxoplasmosis ranges from asymptomatic or mild infection to severe symptoms (hydrocephalus, microcephaly, or encephalitis) or sequelae (visual impairment, intracranial calcifications, or psychomotor or mental retardation), and even in utero abortion or fetal death (especially when maternal infection occurs early in the pregnancy) (2, 3).
Antibiotic treatment—usually spiramycin, or pyrimethamine associated with a sulfonamide—can be started in several situations: acute infection in a pregnant woman and positive prenatal or postnatal diagnosis of congenital infection. Such treatments have been shown to decrease the risk of infection for the fetus and to avoid critical symptomatology (4, 5). Since this infection is often asymptomatic or with unspecific symptoms in adult patients, serologic tests are useful, especially for the prevention of transmission to the fetus (6). The detection of an acute infection can be made by detecting antibodies in a pregnant woman who was previously seronegative. Presence or absence of immunoglobulin M (IgM) antibodies, titers of IgG antibodies, and antibody kinetics may then help date the infection (7). When a woman presents with anti-T. gondii IgG and IgM at the first serological testing during pregnancy, assessing the avidity of the IgG antibodies may help exclude a recent infection (i.e., an infection in the past 4 months, generally) (7, 8). These serologic tests are also important for the follow-up of newborns from mothers infected during pregnancy, because they allow detection of neosynthesized IgM or IgG antibodies, in addition to highly sensitive, qualitative serologic tests (Western blots) (7, 9–11).
Since the 1940s, serologic tests have evolved from manual to completely automated techniques (12, 13). Evaluations of the Vidas system (bioMérieux) for detection of toxoplasmosis IgG and IgM were first published in the 1990s and involved comparisons with techniques that are, for the most part, no longer commercialized today (14–17). Since then, the system has been poorly evaluated (18) and has been used in studies mainly as a comparator (19–22). Vidas was also the first system allowing the automated assessment of anti-T. gondii IgG avidity (23). Vidas assays are still used today, either as first-line techniques in routine testing for small-volume testing laboratories or as second-line techniques for confirmation and expertise. However, more recently, competitor systems with highly sensitive and specific techniques have emerged (19, 22, 24–26). An updated evaluation of the accuracy and usefulness of the Vidas system compared with more recent techniques is therefore necessary.
The aim of this study was to determine the current performance of the Vidas system for diagnosing toxoplasmosis in pregnant women and newborns. To do this, we compared the results of anti-T. gondii IgG, IgM, and IgG avidity measurements obtained with three automated systems: the Vidas, the Architect (Abbott), and the Liaison (DiaSorin) systems.
MATERIALS AND METHODS
Study design.
Sera were prospectively collected and assessed with the Vidas system as part of the routine work of our clinical laboratory (Parasitology-Mycology Clinical Laboratory, Grenoble University Hospital, Grenoble, France). The analyses with the comparator system Architect were made retrospectively in our laboratory, and those with the comparator system Liaison were made in a private laboratory (Biomnis, Lyon, France).
Patients and sera.
A total of 687 serum samples were included in this study, taken from routinely analyzed, nonimmunocompromised women. Group A contained 500 sera from 433 pregnant women and women of childbearing age whose sera were sent to our laboratory for Toxoplasma serologic tests between September 2011 and January 2012. Group B was constituted of 51 sera from 15 pregnant women presenting with seroconversion during their pregnancy (3 or 4 consecutive serum samples for each patient). Group C comprised 80 serum samples from 19 infants of mothers who seroconverted during their pregnancy (2 to 7 consecutive sera for each patient), 10 of whom developed congenital toxoplasmosis (and were subsequently treated with pyrimethamine-sulfonamide from the first days of life when the diagnosis was prenatal or a few days after diagnosis when it was postnatal) and 9 of whom did not. Diagnosis of congenital toxoplasmosis was made (i) through prenatal amniotic fluid analysis (positive PCR and/or mice inoculation in cases 5, 7, 9, and 10; see Table 7); (ii) on the basis of serological evidence in the first days of life (neosynthesized IgG and/or IgM in newborn serum in cases 1, 4, and 8); or (iii) on the basis of delayed serological evidence (IgM detection at 4 months [case 3] or 5 months [cases 2 and 6]). Group D comprised 54 sera from 53 pregnant women for whom avidity had been routinely assessed during 2010 because of positive IgG and IgM and no evidence of recent or old infection. Sera were stored at −20°C for a few days up to 5 months (group A) or 2 to 5 years (groups B, C, and D). Samples were transported among laboratories at −20°C.
Table 7.
Detailed results of IgG and IgM detection for 10 newborns (group C) with congenital toxoplasmosisa
| Case no. | Age (mo) | Toxo IgG assay results (IU/ml) |
Toxo IgM assay results |
||||
|---|---|---|---|---|---|---|---|
| Vidas | Architect | Liaison | Vidas (index) | Architect (index) | Liaison (AU/ml) | ||
| 1 | 6 | 31 | 32.6 | 35.4 | 0.01 | 0.04 | <3.0 |
| 8 | 16 | 17.7 | 22.1 | 0.03 | 0.04 | <3.0 | |
| 11 | 12 | 14.5 | 17.7 | 0.08 | 0.05 | <3.0 | |
| 21 | 4 | 3.4 | 4.5 | 0.08 | 0.05 | <3.0 | |
| 2 | 0 | 0 | 1 | <3.0 | 0.12 | 0.04 | <3.0 |
| 6 | 50 | 29.6 | 58.3 | 0.07 | 0.03 | <3.0 | |
| 9 | 95 | 38 | 87.5 | 0.09 | 0.04 | <3.0 | |
| 12 | 80 | 37 | 94.2 | 0.17 | 0.07 | 3.8 | |
| 18 | 139 | 75.7 | 126 | 0.17 | 0.07 | 3.2 | |
| 25 | >300 | >200.0 | >400 | 0.2 | 0.13 | 5.1 | |
| 31 | >300 | >200.0 | 334 | 0.24 | 0.09 | 4.3 | |
| 3 | 0 | 184 | 119 | NA | 0.05 | 0.06 | <3.0 |
| 2 | 103 | 52.7 | 101 | 0.03 | 0.04 | <3.0 | |
| 4 | 89 | 29.6 | 81.7 | 0.93 | 0.29 | 33.4 | |
| 8 | 10 | 5.3 | 15.9 | 0.12 | 0.06 | <3.0 | |
| 11 | 5 | 2.7 | 11.7 | 0.09 | 0.04 | <3.0 | |
| 27 | 148 | 14.9 | NA | 0.12 | 0.05 | <3.0 | |
| 4 | 3 | 27 | 22.5 | 41.4 | 0.06 | 0.05 | <3.0 |
| 5 | 19 | 13 | 33.2 | 0.06 | 0.03 | <3.0 | |
| 9 | 20 | 7.5 | 27.2 | 0.12 | 0.04 | <3.0 | |
| 11 | 18 | 8.6 | 33.4 | 0.15 | 0.04 | <3.0 | |
| 13 | 15 | 6.8 | 25.9 | 0.16 | 0.05 | 3.5 | |
| 18 | >300 | >200.0 | NA | 0.33 | 0.19 | 7.6 | |
| 5 | 0 | 102 | 106.7 | 84.3 | 0.41 | 0.1 | <3.0 |
| 2 | 49 | 49 | 50.1 | 0.15 | 0.06 | <3.0 | |
| 11 | 247 | 183 | 274 | 0.1 | 0.05 | <3.0 | |
| 13 | 150 | 126 | 209 | 0.06 | 0.05 | <3.0 | |
| 16 | 157 | 91 | 161 | 0.07 | 0.05 | <3.0 | |
| 6 | 5 | 96 | 40.4 | NA | 0.31 | 0.2 | NA |
| 7 | 32 | 12.9 | 55.1 | 0.11 | 0.05 | 3.0 | |
| 9 | 23 | 6.5 | 37.6 | 0.03 | 0.09 | <3.0 | |
| 14 | 28 | 7.7 | 38.9 | 0.03 | 0.05 | <3.0 | |
| 7 | 1 | 54 | 68.3 | 84.1 | 0.05 | 0.07 | <3.0 |
| 9 | 14 | 6.3 | 16.3 | 0.04 | 0.03 | <3.0 | |
| 12 | 10 | 5.4 | 11.2 | 0.05 | 0.05 | <3.0 | |
| 25 | >300 | 165.3 | >400 | 0.14 | 0.07 | <3.0 | |
| 8 | 5 | 21 | 10.7 | 19.2 | 0.05 | 0.05 | <3.0 |
| 11 | 11 | 4.6 | 9.5 | 0.11 | 0.07 | <3.0 | |
| 15 | 4 | 2.3 | 4.1 | 0.1 | 0.06 | <3.0 | |
| 21 | >300 | >200.0 | >400 | 0.33 | 0.18 | 7.5 | |
| 29 | 235 | 158.9 | 247 | 0.14 | 0.1 | 3.6 | |
| 9 | 10 | >300 | >200.0 | >400 | 0.03 | 0.07 | <3.0 |
| 12 | >300 | >200.0 | 388 | 0.06 | 0.06 | <3.0 | |
| 24 | >300 | >200.0 | >400 | 0.19 | 0.08 | 4.4 | |
| 31 | >300 | >200.0 | >400 | 0.14 | 0.08 | 3.8 | |
| 10 | 8 | 2 | 2.2 | 5.0 | 0.04 | 0.05 | <3.0 |
| 10 | 1 | 1.1 | <3.0 | 0.13 | 0.06 | <3.0 | |
| 17 | >300 | 92.7 | 359 | 0.19 | 0.05 | 3.2 | |
| 24 | 123 | 30.7 | 143 | 0.09 | 0.04 | <3.0 | |
Shaded boxes indicate gray zone results; values in boldface represent positive results. NA, not available.
Serologic tests.
All sera were tested for the detection of anti-T. gondii IgG and IgM antibodies using the Vidas Toxo IgGII and Toxo IgM assays (bioMérieux, Marcy l'Étoile, France) (27, 28), the Architect Toxo IgG and Toxo IgM assays (Abbott Laboratories, Wiesbaden, Germany) (24), and the Liaison Toxo IgG and IgM assays (DiaSorin, Saluggia, Italy) (19).
In addition, group D sera were analyzed with the Vidas Toxo IgG Avidity assay (bioMérieux) (23, 29), the Architect Toxo IgG Avidity assay (Abbott Laboratories) (24, 30), and the Liaison Toxo IgG Avidity II assay (DiaSorin) (19). Cutoff values used to interpret the results were those recommended by the manufacturers (Table 1).
Table 1.
Cutoffs recommended by manufacturers for interpretation of serologic values
| System | Assay (unit)a | Negative/low | Gray zone | Positive/high |
|---|---|---|---|---|
| Vidas | IgG (IU/ml) | <4.0 | 4.0 ≤ x < 8.0 | ≥8.0 |
| IgM (index) | <0.55 | 0.55 ≤ x < 0.65 | ≥0.65 | |
| IgG avidity (index) | <0. 2 | 0.2 ≤ x < 0.3 | ≥0.3 | |
| Architect | IgG (IU/ml) | <1.6 | 1.6 ≤ x < 3.0 | ≥3.0 |
| IgM (index) | <0.50 | 0.50 ≤ x < 0.60 | ≥0.60 | |
| IgG avidity (%) | <50.0 | 50.0 ≤ x < 60.0 | ≥60.0 | |
| Liaison | IgG (UI/ml) | <7.2 | 7.2 ≤ x < 8.8 | ≥8.8 |
| IgM (AU/ml) | <6.0 | 6.0 ≤ x < 8.0 | ≥8.0 | |
| IgG avidity (index) | <0.30 | 0.30 ≤ x < 0.40 | ≥0.40 |
For all three IgG avidity assays, a high avidity value allows exclusion of a primary infection dating back less than 4 months. IU, international unit; AU, arbitrary unit.
Sample characterization.
The presence or absence of anti-T. gondii IgG or IgM was determined by concordance between the three automated systems. To solve discrepancies (i.e., to distinguish a recent from a distant seroconversion or to conclude on the presence or the absence of a congenital transmission), we used results of previous or subsequent sera and global interpretation of the clinical and biological data together with results of our routine homemade IgG and IgM indirect immunofluorescence assays (31) and an IgM immunosorbent agglutination assay (Toxo Isaga IgM; bioMérieux) (32). For 12 sera with undetermined presence or absence of IgG (due to discordant or gray zone results), Sabin-Feldman dye tests were also performed in the Parasitology-Mycology Clinical Laboratory of the Limoges University Hospital, France. This procedure was used to determine whether the results obtained from the compared systems were true or false positives or negatives.
Data analysis.
The sensitivity (Se), specificity (Sp), and positive and negative predictive values (PPV and NPV, respectively) were calculated from group A results as follows: (i) Se = 100 × true positive/(true positive + false negative); (ii) Sp = 100 × true negative/(true negative + false positive); (iii) PPV = 100 × true positive/(true positive + false positive); (iv) NPV = 100 × true negative/(true negative + false negative).
For each of these parameters, the value was calculated in three ways: with gray zone results excluded, with gray zone results included as positive results, and with gray zone results included as negative results.
Cohen's kappa value was calculated to assess the agreement between two compared assays in each group; agreement with kappa values of 0.00 to 0.20 was considered slight, 0.21 to 0.40 fair, 0.41 to 0.60 moderate, 0.61 to 0.80 substantial, and 0.81 to 1.00 almost perfect (33). The Student t test was used to assess differences between Pearson correlation coefficients. The McNemar test was used to compare percentages of high avidity values provided by each assay.
RESULTS
Agreement.
The qualitative IgG and IgM results of the three methods are shown in Tables 2 and 3, respectively. IgG and IgM results from the Vidas and Architect assays were compared for 687 sera, while IgG and IgM results from the Vidas and Liaison assays were compared for 679 and 683 sera, respectively. The general agreement between IgG results from the Vidas assay and from the comparators was almost perfect, reaching 95.2% with the Architect assay and 96.9% with the Liaison assay, as was the general agreement between IgM results, reaching 98.3% with the Architect assay and 95.3% with the Liaison assay. Agreements in each group for both IgG and IgM results are detailed in Table 4.
Table 2.
Comparison of IgG test results obtained by the Vidas system with results obtained by the Architect and the Liaison systems in the four groups of sera included in the study
| System | Result | No. of samples with indicated Vidas result |
|||
|---|---|---|---|---|---|
| Positive | Gray zone | Negative | Total | ||
| Architect | Positive | 192 | 9 | 4 | 205 |
| Gray zone | 5 | 3 | 8 | 16 | |
| Negative | 4 | 3 | 459 | 466 | |
| Total | 201 | 15 | 471 | 687 | |
| Liaison | Positive | 193 | 8 | 4 | 205 |
| Gray zone | 0 | 1 | 1 | 2 | |
| Negative | 2 | 6 | 464 | 472 | |
| Total | 195 | 15 | 469 | 679 | |
Table 3.
Comparison of IgM test results obtained by the Vidas system with results obtained by the Architect and the Liaison systems in the four groups of sera included in the study
| System | Result | No. of samples with indicated Vidas result |
|||
|---|---|---|---|---|---|
| Positive | Gray zone | Negative | Total | ||
| Architect | Positive | 92 | 1 | 0 | 93 |
| Gray zone | 3 | 1 | 0 | 4 | |
| Negative | 6 | 2 | 582 | 590 | |
| Total | 101 | 4 | 582 | 687 | |
| Liaison | Positive | 84 | 1 | 4 | 89 |
| Gray zone | 8 | 1 | 9 | 18 | |
| Negative | 8 | 2 | 566 | 576 | |
| Total | 100 | 4 | 579 | 683 | |
Table 4.
Agreement between the Vidas system and the Architect or the Liaison systema
| Serum group | % agreement (kappa value) between Vidas and Architect or Liaison |
|||||
|---|---|---|---|---|---|---|
| IgG |
IgM |
IgG avidity |
||||
| Architect | Liaison | Architect | Liaison | Architect | Liaison | |
| A | 97.40 (0.89) | 98.60 (0.94) | 99.40 (0.82) | 97.60 (0.59) | ||
| B | 80.39 (0.65) | 83.33 (0.70) | 100.00 (1.00) | 98.00 (0.94) | ||
| C | 87.80 (0.68) | 92.21 (0.80) | 98.78 (NC)c | 97.47 (0.49) | ||
| D | 100.00 (NC) | 100.00 (NC) | 85.19 (0.23) | 68.52 (0.04b) | 82.00 (0.68) | 81.48 (0.66) |
| All groups | 95.20 (0.89) | 96.91 (0.92) | 98.25 (0.93) | 95.31 (0.82) | ||
For all results except the Liaison IgM result for group D (see footnote b), the kappa-related P value was <0.05.
P = 0.53.
NC, the kappa value was not calculable.
Analytical parameters.
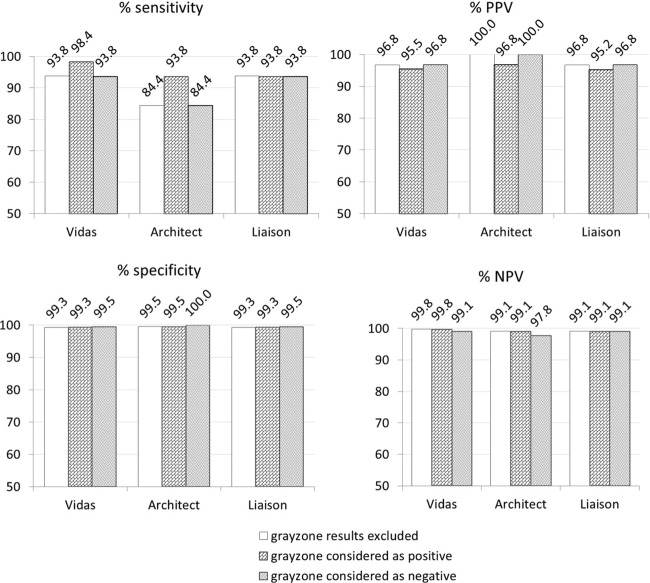
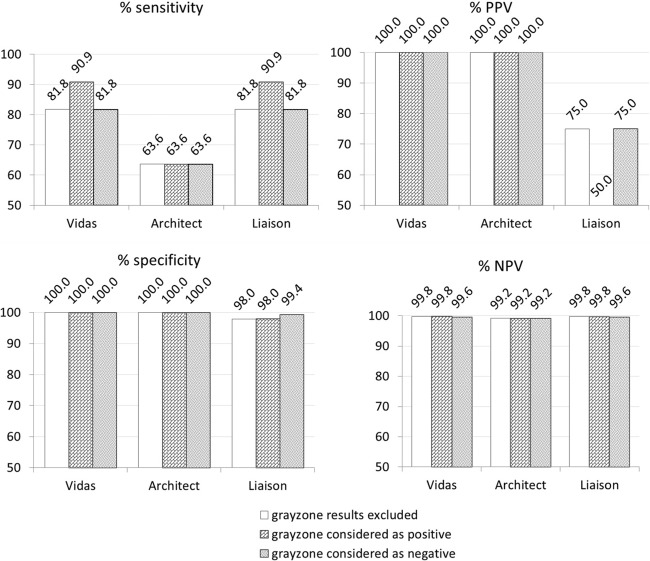
Figures 1 and 2 show the Se, Sp, PPV, and NPV values of each IgG and IgM assay, calculated from group A results. In this group, the prevalence of positive IgG sera was 12.8% (64 of 500 sera were true positives), while the prevalence of positive IgM was 2.2% (11 of 500 sera). In addition, these parameters were calculated in group B for IgM, because of the high prevalence of positive IgM (76.5%) in this group. All parameters reached 100% for each assay, except for the Liaison assay, which had a 97.4% Se and a 91.7% NPV due to a single false-negative result (case 7).
Fig 1.
Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) (%) of the three IgG assays included in this study on 500 samples from pregnant women (group A).
Fig 2.
Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) (%) of the three IgM assays included in this study on 500 samples from pregnant women (group A).
IgG and IgM assay kinetics.
Extensive sequential results of IgG and IgM assays for group B are provided in Table 5. In cases of seroconversion, IgMs were detected at the same time by the three assays, except in case 7, for which the Liaison assay provided the only negative result for the first sample. In four cases, IgGs were detected earlier with the Liaison and Architect assays than with the Vidas assay; notably, in case 11, the Vidas assay provided only gray zone results. In three cases, IgGs were detected earlier with the Architect assay than with the Liaison and Vidas assays. In one case, IgGs were detected earlier with the Liaison and Vidas assays than with the Architect assay.
Table 5.
Antibody kinetics in 15 cases of women with a proven seroconversion (group B)a
| Case no. | Sampling date (day mo yr) | Toxo IgG assays (IU/ml) |
Toxo IgM assays |
||||
|---|---|---|---|---|---|---|---|
| Vidas | Architect | Liaison | Vidas (index) | Architect (index) | Liaison (AU/ml) | ||
| 1 | 27 Mar 2008 | 0 | 0.4 | <3.0 | 0.04 | 0.04 | <3.0 |
| 5 May 2008 | 14 | 19.1 | NA | 2.76 | 3.01 | 57.8 | |
| 19 July 2008 | 99 | 77.5 | 107 | 1.29 | 1.21 | 16.8 | |
| 2 | 20 Sept 2005 | 0 | 0.5 | <3.0 | 0.11 | 0.04 | <3.0 |
| 18 Oct 2005 | 5 | 23 | 26.0 | 8.52 | 15.3 | >160 | |
| 25 Oct 2005 | 22 | 44 | 52.8 | 8.51 | 15.18 | >160 | |
| 3 | 5 Nov 2007 | 0 | 0.9 | <3.0 | 0.02 | 0.05 | <3.0 |
| 14 Mar 2008 | 1 | 1.5 | 6.6 | 1.92 | 2.04 | 47.9 | |
| 29 Mar 2008 | 21 | 34.3 | 51.4 | 2.74 | 3.01 | 69.4 | |
| 4 | 30 June 2007 | 0 | 0.4 | NA | 0.07 | 0.05 | <3.0 |
| 4 Oct 2007 | 4 | 23.5 | 18.3 | 2.87 | 1.93 | 68.3 | |
| 16 Oct 2007 | 22 | 48.9 | 51.3 | 2.62 | 1.73 | 67.1 | |
| 19 Jan 2008 | 73 | 97.8 | 113 | 1.66 | 0.78 | 30.4 | |
| 5 | 5 Feb 2004 | 0 | 1.7 | <3.0 | 0.04 | 0.04 | <3.0 |
| 27 July 2004 | 279 | 186 | 350 | 5.05 | 7.18 | 127 | |
| 10 Aug 2004 | >300 | >200.0 | >400 | 4.64 | 6.27 | 109 | |
| 16 Mar 2005 | 77 | 55.7 | 92.2 | 2.9 | 3.1 | 52.0 | |
| 6 | 16 Nov 2008 | 0 | 0.2 | <3.0 | 0.06 | 0.05 | 4.2 |
| 21 Jan 2009 | 123 | 125 | 158 | 3.74 | 4.63 | 61.8 | |
| 2 Feb 2009 | 132 | 130.8 | 154 | 3.4 | 4.14 | 48.4 | |
| 7 | 27 Nov 2008 | 0 | 0.3 | <3.0 | 0.79 | 1.28 | <3.0 |
| 4 Dec 2008 | 0 | 0.3 | <3.0 | 5.13 | 11.5 | 39.1 | |
| 23 Dec 2008 | 0 | 1.3 | <3.0 | 6.11 | 13.33 | 54.7 | |
| 5 Jan 2009 | 0 | 1.9 | <3.0 | 5.72 | 11.52 | 48.3 | |
| 8 | 30 Nov 2009 | 0 | 0.2 | <3.0 | 0.21 | 0.21 | <3.0 |
| 22 Dec 2009 | 0 | 0.8 | <3.0 | 1.32 | 1.52 | 17.1 | |
| 6 Jan 2010 | 3 | 4 | 8.7 | 1.45 | 1.54 | 24.4 | |
| 8 Apr 2010 | 71 | 56.6 | NA | 0.75 | 0.63 | NA | |
| 9 | 12 May 2010 | 0 | 0.2 | <3.0 | 0.1 | 0.04 | <3.0 |
| 11 June 2010 | 4 | 6.4 | 15.0 | 5.05 | 4.33 | >160 | |
| 16 June 2010 | 11 | 15.5 | 25.6 | 5.12 | 4.16 | >160 | |
| 10 | 14 Jan 2011 | 0 | 0.2 | <3.0 | 0.75 | 0.81 | 10.6 |
| 21 Feb 2011 | 42 | 45.7 | 107 | 3.48 | 5.79 | 72.8 | |
| 29 July 2011 | 73 | 46.8 | 94.6 | 1.09 | 1.05 | 21.8 | |
| 11 | 20 July 2011 | 3 | 7.5 | 9.4 | 5.68 | 5.38 | >160 |
| 22 July 2011 | 5 | 8.3 | 12.3 | 5.82 | 4.87 | >160 | |
| 1 Aug 2011 | 6 | 8 | 19.4 | 5.04 | 3.7 | >160 | |
| 12 | 14 May 2011 | 0 | 0.1 | <3.0 | 0.04 | 0.07 | <3.0 |
| 8 June 2011 | 0 | 1.3 | 5.5 | 6.3 | 14.98 | 65.8 | |
| 22 June 2011 | 30 | 55.9 | 41.7 | 5.77 | 16.21 | 92.3 | |
| 13 | 2 Mar 2010 | 0 | 0.3 | <3.0 | 0.04 | 0.03 | <3.0 |
| 18 Mar 2010 | 8 | 1.2 | 12.9 | 5.88 | 6.39 | 82 | |
| 3 Apr 2010 | 40 | 34.8 | 70.9 | 6.71 | 11.49 | 159 | |
| 25 Sep 2010 | 103 | 70.9 | 108 | 2.23 | 1.79 | 21.5 | |
| 14 | 25 Jan 2011 | 249 | >200.0 | 362 | 2.55 | 1.55 | 31.1 |
| 15 Feb 2011 | 233 | >200.0 | 363 | 1.64 | 1.34 | 27.5 | |
| 10 Mar 2011 | 146 | 194.2 | 362 | 1.71 | 1.2 | 27.2 | |
| 6 Sep 2011 | 80 | 39.9 | 74.4 | 1.3 | 0.63 | 22.5 | |
| 15 | 23 Mar 2011 | 0 | 0 | <3.0 | 0.03 | 0.03 | <3.0 |
| 14 Nov 2011 | 273 | >200.0 | 234 | 4.36 | 5.77 | 98.4 | |
| 1 Dec 2011 | 156 | >200.0 | 292 | 3.6 | 3.5 | 50.9 | |
In some cases, seroconversion was detected by other tests that do not appear in this table. Shaded boxes indicate gray zone results; values in boldface represent positive results. Jan, January; Feb, February; Mar, March; Apr, April; Aug, August; Sep, September; Oct, October; Nov, November; Dec, December; NA, not available.
Extensive sequential results of IgG and IgM assays for children from group C without congenital toxoplasmosis are provided in Table 6. In only one of the nine cases, maternally transmitted IgGs were detected for a longer period of time with the Liaison and Architect assays than with the Vidas assay, while they were detected for a longer period of time with the Architect assay than with the other two assays in three cases. No IgMs were detected by any of the three assays in this subgroup. Extensive sequential results of IgG and IgM assays for infants with congenital toxoplasmosis from group C are provided in Table 7. In case 10, Architect provided a gray zone IgG result for the first sample, which was retested and found to be a true positive with the Sabin-Feldman dye test. In 3 of 10 congenital toxoplasmosis cases, IgMs were detected once in the sequence (in two cases with only the Liaison assay and in one case with both the Liaison and Vidas assays).
Table 6.
Detailed results of IgG and IgM detection for nine newborns (group C) with no congenital toxoplasmosisa
| Case no. | Age (mo) | Toxo IgG assay results (IU/ml) |
Toxo IgM assay results |
||||
|---|---|---|---|---|---|---|---|
| Vidas | Architect | Liaison | Vidas (index) | Architect (index) | Liaison (AU/ml) | ||
| 1 | 0 | >300 | >200.0 | >400 | 0.26 | 0.05 | <3.0 |
| 2 | 133 | 115.5 | 176 | 0.06 | 0.04 | <3.0 | |
| 6 | 17 | 14.4 | 27.8 | 0.03 | 0.04 | <3.0 | |
| 11 | 0 | 1 | <3.0 | 0.08 | 0.05 | <3.0 | |
| 2 | 1 | 112 | 63.5 | 122 | 0.04 | 0.05 | 3.4 |
| 6 | 1 | 2.4 | <3.0 | 0.03 | 0.03 | <3.0 | |
| 13 | 0 | 0.5 | <3.0 | 0.07 | 0.04 | <3.0 | |
| 3 | 1 | 38 | 37.9 | 49.6 | 0.07 | 0.04 | <3.0 |
| 4 | 7 | 11.2 | 18.0 | 0.02 | 0.04 | <3.0 | |
| 6 | 1 | 3 | 4.1 | 0.03 | 0.04 | <3.0 | |
| 8 | 0 | 1 | <3.0 | 0.04 | 0.03 | <3.0 | |
| 4 | 1 | 24 | 18.4 | 43.7 | 0.02 | 0.04 | <3.0 |
| 8 | 0 | 0.9 | <3.0 | 0.01 | 0.04 | <3.0 | |
| 10 | 0 | 0.6 | <3.0 | 0.06 | 0.04 | <3.0 | |
| 5 | 4 | 4 | 3.4 | 13.9 | 0.04 | 0.03 | <3.0 |
| 10 | 0 | 0.2 | <3.0 | 0.03 | 0.03 | <3.0 | |
| 6 | 1 | 58 | 44.6 | 62.5 | 0.01 | 0.03 | <3.0 |
| 3 | 16 | 13 | 20.6 | 0.01 | 0.04 | <3.0 | |
| 5 | 4 | 3.2 | 5.0 | 0.04 | 0.03 | <3.0 | |
| 8 | 0 | 1 | <3.0 | 0.04 | 0.04 | <3.0 | |
| 7 | 4 | 1 | 2.9 | <3.0 | 0.04 | 0.04 | <3.0 |
| 5 | 0 | 3.2 | <3.0 | 0.03 | 0.04 | NA | |
| 8 | 0 | >300 | >200.0 | 399 | 0.31 | 0.05 | <3.0 |
| 2 | 75 | 99.3 | 163 | 0.06 | 0.04 | <3.0 | |
| 4 | 37 | 37.8 | 70.1 | 0.03 | 0.04 | <3.0 | |
| 7 | 9 | 7.8 | 15.1 | 0.05 | 0.04 | <3.0 | |
| 10 | 1 | 1.9 | <3.0 | 0.06 | 0.04 | <3.0 | |
| 9 | 0 | 73 | 46 | 104 | 0.11 | 0.05 | <3.0 |
| 1 | 53 | 22.9 | 57.2 | 0.06 | 0.05 | <3.0 | |
| 3 | 15 | 7.7 | 19.8 | 0.04 | 0.04 | <3.0 | |
| 7 | 2 | 0.9 | NA | 0.02 | 0.03 | NA | |
Shaded boxes indicate gray zone results; values in boldface represent positive results. NA, not available.
IgG avidity assays.
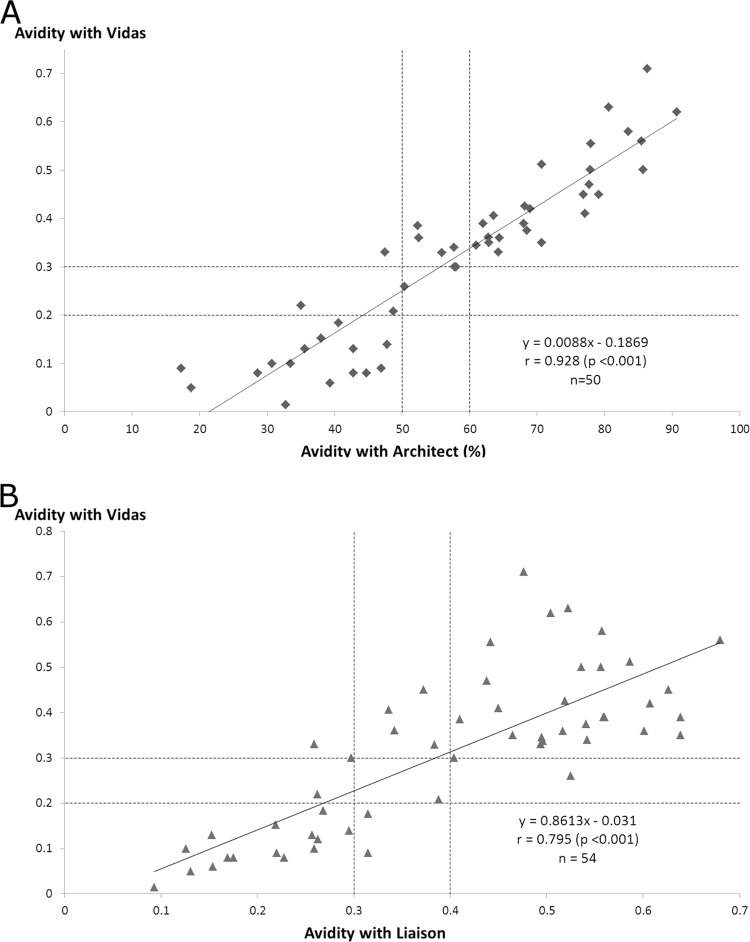
Qualitative IgG avidity results are compared in Table 8. The general agreement between Vidas and the comparators was substantial, reaching 82% with the Architect assay and 81.5% with the Liaison assay (Table 4). Values from the Vidas assay were highly correlated with those from the Architect assay (r = 0.928, P < 0.001), while the correlation was lower with the Liaison assay (r = 0.795, P < 0.001) (Fig. 3). The percentages of high avidity values within the whole group were 63% (34/54), 50% (25/50), and 53.7% (29/54) with the Vidas, Architect, and Liaison assays, respectively. The percentage provided by the Vidas assay was significantly different from that obtained with the Architect assay (P = 0.023) but not from that obtained with the Liaison assay (P = 0.13).
Table 8.
Comparison of IgG avidity test results obtained by the Vidas system with results obtained by the Architect and the Liaison systems in group D
| System | Result | No. of samples with indicated Vidas result |
|||
|---|---|---|---|---|---|
| High | Gray zone | Low | Total | ||
| Architect | High | 25 | 0 | 0 | 25 |
| Gray zone | 6 | 1 | 0 | 7 | |
| Low | 1 | 2 | 15 | 18 | |
| Total | 32 | 3 | 15 | 50 | |
| Liaison | High | 28 | 1 | 0 | 29 |
| Gray zone | 4 | 1 | 2 | 7 | |
| Low | 2 | 1 | 15 | 18 | |
| Total | 34 | 3 | 17 | 54 | |
Fig 3.
Correlation of avidity values obtained from group D sera analyzed with the Vidas and Architect assays (A) and the Vidas and Liaison assays (B); r, correlation coefficient.
DISCUSSION
The aim of this study was to compare the performances of the Vidas Toxo assays with those of more recent, fully automated assays. Our findings show that the performance of the Vidas system in this field is maintained.
The most important characteristic in screening pregnant women is the absence of false-negative IgM results, as this can lead to misdiagnosed acute toxoplasmic infection and to lack of or delay in treatment and clinical/biological follow-up of the fetus and newborn. Se and NPV must therefore be as high as possible. Calculation of the analytical parameters for IgM showed much higher sensitivities for the Vidas and Liaison assays than for the Architect assay. This Se superiority of the Vidas and Liaison assays was increased when gray zone results were interpreted as positive results, allowing better detection of ongoing seroconversions. This is consistent with the analysis of congenital toxoplasmosis (group C) whereby IgMs in one infant were not detected by the Architect assay in case 3 at the time of diagnosis, while they were by the two other systems, Isaga, and Western blotting (neosynthesized IgM). Consequently, the Vidas and Liaison assays showed very good NPV, although the very low prevalence of IgM-positive sera in group A explains the high levels of NPV for all three assays. The high Se of the Vidas IgM assay has been previously shown, although a value higher than with the Liaison assay was found (20). We did not observe this difference in group A, but it is worth noting that IgMs were not detected by the Liaison assay in 14 sera from group D (8 gray zone and 6 negative results; data not shown). Results from group D were not used in the calculation of analytical parameters as positive Vidas IgG and IgM results were major criteria for inclusion in this group. Moreover, the analysis of group B showed a decreased sensitivity for the Liaison assay. However, this was due to a single false-negative serum reflecting a delay in detection of IgM in seroconversion case 7.
Time of detection of IgM in seroconversion cases is also an important criterion, because it is the signal that triggers further serological explorations to confirm or rule out an acute infection. In this respect, Vidas and Architect assays showed similar performances, whereas IgMs were detected later with Liaison in one case, as mentioned above.
All three IgM assays appeared to be very specific, although better Sp values were obtained with the Vidas and Architect assays than with Liaison. The presence of three false positives with the Liaison assay, which was not expected in light of previous data (20), together with the very low prevalence of positive IgM, is the reason for the much lower PPV values calculated for this assay.
However, the Se and Sp values that we found can be discussed, as they depend on how reference results are determined. In this study, we interpreted persistent specific IgM as positive samples, but these persistent IgMs may be considered to be an unnecessary source of additional testing and anxiety for the patient; therefore, these samples could be considered negative. In the current study, all three assays detected IgM in the four cases of acute infection from group A; the differences in IgM Se were observed for the detection of persistent IgM (i.e., past infections) only.
Analytical parameters for IgG showed a higher Se for the Vidas and Liaison assays than for the Architect assay in the general population of pregnant women evaluated in group A. This was true even when gray zone results were interpreted as negative, which is the safest line of conduct in the context of pregnant women screened for acute toxoplasmic infection, since it indicates that the patient should be followed up during pregnancy. In contrast, the Architect assay appeared to be the most able to detect early IgG in pregnant women with an acute infection (group B), while Vidas was the least sensitive assay in this situation, as previously found by Gay-Andrieu et al. (22). In case 11, the Vidas assay retrieved only gray zone values for the last two sera included in the study, but the time period studied is short and this case may reflect atypical kinetics. Indeed, the titers did not rise in these three sera with the Architect system, and the first positive result with Vidas was obtained on serum taken at delivery (44 days later, not included in the study) with a very low IgG titer (10 IU/ml). Case 7 is quite atypical, because IgGs were either not detected or detected at a very low level with the three automated assays. However, this was a proven seroconversion, because specific immunofluorescence assay results went from negative to positive with the third serum. In this context, the gray zone value obtained with the Architect assay is of interest, because it provides an additional indication for seroconversion.
However, concerning antibody kinetics during seroconversion, detection of IgG only formally confirms the diagnosis of seroconversion, which is already highly suspected on the basis of positive or increasing IgM. An interesting kinetics example is shown by case 14: while the Vidas assays showed a decrease from the second and mostly the third serum samples, variations were not shown before the fourth serum by the Architect (mostly because the first two rates exceeded the upper measurement limit) and the Liaison assays. Case 15 shows a similar trend, the decreasing phase being observable only with the Vidas assay. PPV and Sp reached high levels with all three assays, particularly since low prevalence tends to decrease PPV. Very high Sp and PPV (i.e., few false positives) are important during pregnancy, because they give confidence in a positive IgG result, indicating a past immunity requiring no further follow-up. In this study, high NPVs are not very reliable, since the low prevalence of positive IgG increases this parameter.
In line with its better Se in seroconversions, the Architect IgG assay remained positive for sera from newborns with no congenital toxoplasmosis for a longer time period than the two other assays in three of nine cases. However, in these cases, there is a slight advantage for an assay in detecting clearance of IgG as early as possible (i.e., completely negative IgG test; gray zone results are to be interpreted in this situation as positive), since this allows postnatal follow-up to be withdrawn. All three assays detected high-level and persistent IgG in infants presenting with congenital toxoplasmosis (not all the samples were positive, but there were no misdiagnosed cases). Although it was not possible to confirm slight differences in the techniques for postnatal follow-up, there seemed to be a trend for a higher Se of the Architect assay in this population, which is consistent with what was observed in group B and in healthy children from group C.
It should be noted that we took into account only group A sera for calculations in order to be as close to real conditions as possible and because this was the prospective part of the study. However, if groups B and C had been taken into account, the high Se value for the Vidas assay would have been lowered, compared to the Architect and Liaison assays, by the lack of positivity in some seroconversion sera (group B) and in the last serum of serial testing from healthy newborns (group C). Therefore, the present results are overall concordant with previously published data, which characterized the Vidas IgG assay through its high Sp but moderate Se (16, 21).
In this study, avidity values provided by the Vidas assay were highly correlated with those from the Architect and Liaison assays. However, the most important parameter required for an avidity assay is the ability to provide high avidity values for chronic toxoplasmosis. From this point of view, the Vidas assay provided more high values and therefore identified more old infections than the comparators. The difference compared to the Architect assay was significant, but that compared to the Liaison was not, mainly due to one serum that was found to be in the gray zone with Vidas and high with Liaison. This is consistent with recent data that concluded that the Vidas IgG avidity assay is the most accurate assay for detecting infection lasting more than 4 months, compared to other avidity assays commercialized in France, including the Architect assay (34).
The main conclusion to this study is that the Vidas assays are useful for the detection of toxoplasmosis in pregnant women and newborns, as well as for follow-up of newborns with or without congenital transmission of the parasite. This evaluation shows similar performances for Vidas and more recently commercialized systems, notably excellent Sp, and some particularities. The Vidas system provides better Se in screening for both IgG and IgM in pregnant women than the Liaison and Architect assays. The Architect assay and, to a lesser extent, the Liaison assay show a high ability to detect early IgG in seroconversion cases (i.e., a high Se) but have the slight disadvantage of a prolonged detection of passive IgG in healthy newborns. Conversely, in cases of seroconversion, the Vidas system detects IgM as early as the compared assays but is less powerful than the comparators in early detection of newly produced IgG. Consequently, the Vidas system fits the needs of laboratories working on small routine series for first-line testing as well as those of expert laboratories that are often solicited for confirmation, second-line testing due to a high Sp, and a powerful avidity assay.
ACKNOWLEDGMENTS
We thank Sophie Tronchet (Biomnis Laboratory) for performing analyses with the Liaison system.
This study was funded by bioMérieux, Marcy l'Étoile, France. The work was performed at the Laboratory of Parasitology and Mycology, Grenoble University Hospital, Grenoble, France. The Grenoble Parasitology-Mycology Laboratory (J.-B.M., C.D., H.F.H., M.-P.B.-P., and H.P.) has received funding from bioMérieux, Abbott Laboratories, and Roche Diagnostics; M.-L.D. has received funding from bioMérieux.
Footnotes
Published ahead of print 5 June 2013
REFERENCES
- 1. Weiss LM, Dubey JP. 2009. Toxoplasmosis: a history of clinical observations. Int. J. Parasitol. 39:895–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Villena I, Ancelle T, Delmas C, Garcia P, Brezin AP, Thulliez P, Wallon M, King L, Goulet V. 2010. Congenital toxoplasmosis in France in 2007: first results from a national surveillance system. Euro Surveill. 15:pii=19600 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19600 [DOI] [PubMed] [Google Scholar]
- 3. Delair E, Latkany P, Noble AG, Rabiah P, McLeod R, Brézin A. 2011. Clinical manifestations of ocular toxoplasmosis. Ocul. Immunol. Inflamm. 19:91–102 [DOI] [PubMed] [Google Scholar]
- 4. McLeod R, Kieffer F, Sautter M, Hosten T, Pelloux H. 2009. Why prevent, diagnose and treat congenital toxoplasmosis? Mem. Inst. Oswaldo Cruz 104:320–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaye A. 2011. Toxoplasmosis: diagnosis, treatment, and prevention in congenitally exposed infants. J. Pediatr. Health Care 25:355–364 [DOI] [PubMed] [Google Scholar]
- 6. Montoya JG, Remington JS. 2008. Management of Toxoplasma gondii infection during pregnancy. Clin. Infect. Dis. 47:554–566 [DOI] [PubMed] [Google Scholar]
- 7. Robert-Gangneux F, Dardé M-L. 2012. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin. Microbiol. Rev. 25:264–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Remington JS, Thulliez P, Montoya JG. 2004. Recent developments for diagnosis of toxoplasmosis. J. Clin. Microbiol. 42:941–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chumpitazi BF, Boussaid A, Pelloux H, Racinet C, Bost M, Goullier-Fleuret A. 1995. Diagnosis of congenital toxoplasmosis by immunoblotting and relationship with other methods. J. Clin. Microbiol. 33:1479–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pinon JM, Dumon H, Chemla C, Franck J, Petersen E, Lebech M, Zufferey J, Bessieres MH, Marty P, Holliman R, Johnson J, Luyasu V, Lecolier B, Guy E, Joynson DH, Decoster A, Enders G, Pelloux H, Candolfi E. 2001. Strategy for diagnosis of congenital toxoplasmosis: evaluation of methods comparing mothers and newborns and standard methods for postnatal detection of immunoglobulin G, M, and A antibodies. J. Clin. Microbiol. 39:2267–2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tissot Dupont D, Fricker-Hidalgo H, Brenier-Pinchart MP, Bost-Bru C, Ambroise-Thomas P, Pelloux H. 2003. Usefulness of Western blot in serological follow-up of newborns suspected of congenital toxoplasmosis. Eur. J. Clin. Microbiol. Infect. Dis. 22:122–125 [DOI] [PubMed] [Google Scholar]
- 12. Dubey JP. 2008. The history of Toxoplasma gondii—the first 100 years. J. Eukaryot. Microbiol. 55:467–475 [DOI] [PubMed] [Google Scholar]
- 13. Sabin AB, Feldman HA. 1948. Dyes as microchemical indicators of a new immunity phenomenon affecting a protozoon parasite (Toxoplasma). Science 108:660–663 [DOI] [PubMed] [Google Scholar]
- 14. Pelloux H, Ciapa P, Goullier-Fleuret A, Ambroise-Thomas P. 1993. Evaluation of the Vidas system for the serological diagnosis of toxoplasmosis. Ann. Biol. Clin. (Paris) 51:875–878 (In French.) [PubMed] [Google Scholar]
- 15. Candolfi E, Ramirez R, Hadju MP, Shubert C, Remington JS. 1994. The Vitek immunodiagnostic assay for detection of immunoglobulin M Toxoplasma antibodies. Clin. Diagn. Lab. Immunol. 1:401–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hofgärtner WT, Swanzy SR, Bacina RM, Condon J, Gupta M, Matlock PE, Bergeron DL, Plorde JJ, Fritsche TR. 1997. Detection of immunoglobulin G (IgG) and IgM antibodies to Toxoplasma gondii: evaluation of four commercial immunoassay systems. J. Clin. Microbiol. 35:3313–3315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wilson M, Remington JS, Clavet C, Varney G, Press C, Ware D. 1997. Evaluation of six commercial kits for detection of human immunoglobulin M antibodies to Toxoplasma gondii. The FDA Toxoplasmosis Ad Hoc Working Group. J. Clin. Microbiol. 35:3112–3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vlaspolder F, Singer P, Smit A, Diepersloot RJ. 2001. Comparison of immulite with Vidas for detection of infection in a low-prevalence population of pregnant women in The Netherlands. Clin. Diagn. Lab. Immunol. 8:552–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Petersen E, Borobio MV, Guy E, Liesenfeld O, Meroni V, Naessens A, Spranzi E, Thulliez P. 2005. European multicenter study of the Liaison automated diagnostic system for determination of Toxoplasma gondii-specific immunoglobulin G (IgG) and IgM and the IgG avidity index. J. Clin. Microbiol. 43:1570–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Calderaro A, Piccolo G, Peruzzi S, Gorrini C, Chezzi C, Dettori G. 2008. Evaluation of Toxoplasma gondii immunoglobulin G (IgG) and IgM assays incorporating the new Vidia analyzer system. Clin. Vaccine Immunol. 15:1076–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maudry A, Chene G, Chatelain R, Patural H, Bellete B, Tisseur B, Hafid J, Raberin H, Beretta S, Sung RTM, Belot G, Flori P. 2009. Bicentric evaluation of six anti-Toxoplasma immunoglobulin G (IgG) automated immunoassays and comparison to the Toxo II IgG Western blot. Clin. Vaccine Immunol. 16:1322–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gay-Andrieu F, Fricker-Hidalgo H, Sickinger E, Espern A, Brenier-Pinchart M-P, Braun H-B, Pelloux H. 2009. Comparative evaluation of the ARCHITECT Toxo IgG, IgM, and IgG avidity assays for anti-Toxoplasma antibodies detection in pregnant women sera. Diagn. Microbiol. Infect. Dis. 65:279–287 [DOI] [PubMed] [Google Scholar]
- 23. Pelloux H, Brun E, Vernet G, Marcillat S, Jolivet M, Guergour D, Fricker-Hidalgo H, Goullier-Fleuret A, Ambroise-Thomas P. 1998. Determination of anti-Toxoplasma gondii immunoglobulin G avidity: adaptation to the Vidas system (bioMérieux). Diagn. Microbiol. Infect. Dis. 32:69–73 [DOI] [PubMed] [Google Scholar]
- 24. Sickinger E, Gay-Andrieu F, Jonas G, Schultess J, Stieler M, Smith D, Hausmann M, Stricker R, Stricker R, Dhein J, Braun H-B. 2008. Performance characteristics of the new ARCHITECT Toxo IgG and Toxo IgG Avidity assays. Diagn. Microbiol. Infect. Dis. 62:235–244 [DOI] [PubMed] [Google Scholar]
- 25. Prusa A-R, Hayde M, Unterasinger L, Pollak A, Herkner KR, Kasper DC. 2010. Evaluation of the Roche Elecsys Toxo IgG and IgM electrochemiluminescence immunoassay for the detection of gestational Toxoplasma infection. Diagn. Microbiol. Infect. Dis. 68:352–357 [DOI] [PubMed] [Google Scholar]
- 26. Prusa A-R, Hayde M, Pollak A, Herkner KR, Kasper DC. 2012. Evaluation of the Liaison automated testing system for diagnosis of congenital toxoplasmosis. Clin. Vaccine Immunol. 19:1859–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roux-Buisson N, Fricker-Hidalgo H, Foussadier A, Rolland D, Suchel-Jambon A-S, Brenier-Pinchart M-P, Pelloux H. 2005. Comparative analysis of the Vidas Toxo IgG IV assay in the detection of antibodies to Toxoplasma gondii. Diagn. Microbiol. Infect. Dis. 53:79–81 [DOI] [PubMed] [Google Scholar]
- 28. Alvarado-Esquivel C, Sethi S, Janitschke K, Hahn H, Liesenfeld O. 2002. Comparison of two commercially available avidity tests for Toxoplasma-specific IgG antibodies. Arch. Med. Res. 33:520–523 [DOI] [PubMed] [Google Scholar]
- 29. Montoya JG, Liesenfeld O, Kinney S, Press C, Remington JS. 2002. Vidas test for avidity of Toxoplasma-specific immunoglobulin G for confirmatory testing of pregnant women. J. Clin. Microbiol. 40:2504–2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Curdt I, Praast G, Sickinger E, Schultess J, Herold I, Braun HB, Bernhardt S, Maine GT, Smith DD, Hsu S, Christ HM, Pucci D, Hausmann M, Herzogenrath J. 2009. Development of fully automated determination of marker-specific immunoglobulin G (IgG) avidity based on the avidity competition assay format: application for Abbott Architect cytomegalovirus and Toxo IgG Avidity assays. J. Clin. Microbiol. 47:603–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ambroise-Thomas P, Garin JP, Rigaud A. 1966. Improvement of the immunofluorescence technic by the use of counter-dyes. Application to toxoplasmas. Presse Med. 74:2215–2216 (In French.) [PubMed] [Google Scholar]
- 32. Goubet S, Pelloux H, Fricker-Hidalgo H, Goullier-Fleuret A, Ambroise-Thomas P. 1999. Serodiagnosis of toxoplasmosis: comparison of the Elisa Axym (Abbott) kit with the Vidas (bioMérieux) kit, indirect immunofluorescence and Isaga. Ann. Biol. Clin. (Paris) 57:481–484 (In French.) [PubMed] [Google Scholar]
- 33. Landis JR, Koch GG. 1977. The measurement of observer agreement for categorical data. Biometrics 33:159–174 [PubMed] [Google Scholar]
- 34. Murat J-B, L'Ollivier C, Fricker Hidalgo H, Franck J, Pelloux H, Piarroux R. 2012. Evaluation of the new Elecsys® Toxo IgG Avidity assay for toxoplasmosis and new insights into the interpretation of avidity results. Clin. Vaccine Immunol. 19:1838–1843 [DOI] [PMC free article] [PubMed] [Google Scholar]