Abstract
CD40 and CD40 ligand (CD40L) have costimulatory effects as part of a complex series of events in host immunity. In this study, the expression of CD40 and CD40L on peripheral blood mononuclear cells (PBMCs) isolated from cattle with Johne's disease were measured on freshly isolated PBMCs and on cells cultured for 8, 24, and 72 h in the presence or absence of live Mycobacterium avium subsp. paratuberculosis and exogenous gamma interferon, interleukin 10, and transforming growth factor β. Results demonstrated greater CD40 and CD40L expression on fresh PBMCs obtained from animals in the clinical stage of disease (symptomatic) than those from healthy control animals or cows in the subclinical stage of disease (asymptomatic). A similar expression profile with greater magnitude was noted for cultured PBMCs, with increased CD40 expression after 8 and 24 h of culture and increased CD40L expression between 24 and 72 h on PBMCs obtained from clinically infected animals. The addition of live M. avium subsp. paratuberculosis to cell cultures resulted in downregulation of CD40L expression in naturally infected cows, regardless of the disease stage. In contrast, the addition of live M. avium subsp. paratuberculosis to cultures resulted in upregulation of CD40 expression on cells obtained from clinically infected animals, while a decrease in expression was noted for healthy and subclinically infected cows. No effects of exogenous cytokines on CD40 or CD40L expression were observed. These results clearly point for the first time to a disparity in the expression of these costimulatory molecules on immune cells from cattle in different stages of Johne's disease and suggest further investigation into their roles in paratuberculosis pathogenesis.
INTRODUCTION
Mycobacterium avium subsp. paratuberculosis, the causative agent of Johne's disease, generally colonizes in the lower part of the small intestine (1). However, little is known about the mechanism of uptake of Mycobacterium avium subsp. paratuberculosis into the antigen-presenting cells present there. It has been demonstrated experimentally that, within a few hours after M. avium subsp. paratuberculosis ingestion, the bacteria translocate across the M cells lining Peyer's patches and can be detected in subepithelial macrophages (1). Since M. avium subsp. paratuberculosis can reside within phagosomes of macrophages and even replicate, it has been hypothesized that mycobacteria use a mechanism of selective entry into macrophages to create an environment that does not trigger macrophage defense mechanisms. It was noted, for example, that uptake of mycobacteria via mannose receptor-facilitated phagocytosis did not elicit macrophage activation (e.g., phagosome maturation) (2). After uptake, the susceptibility and resistance to M. avium subsp. paratuberculosis infection and disease progression represent a struggle between the bacteria and host immunity. The host immune system responds to M. avium subsp. paratuberculosis infection by recruiting more macrophages to the site of infection, as the number of macrophages present in the ileum of naturally infected cows was reported to be higher than that in noninfected control animals (3). As in other mycobacterial diseases, it has been suggested that the host immune system responds to M. avium subsp. paratuberculosis infection by recruiting and activating lymphocytes such as γδ T cells, CD4+ T cells, and cytolytic CD8+ T cells at the site of infection (4). Infiltration of infected tissues with lymphocytes and macrophages leads to thickening of the intestine, and the mucosal surface becomes corrugated in appearance over time, leading to malabsorption of nutrients and the extreme weight loss that is associated with clinical disease (3–5). Lesions in paratuberculosis infections are due mainly to this coordinated influx of macrophages and lymphocytes to the site of infection (3, 5). Therefore, the immune response that develops following initial exposure to M. avium subsp. paratuberculosis controls but does not eradicate the pathogen, leading to persistence of the bacterial load and continuous activation of the immune response.
The immunopathogenesis that occurs with M. avium subsp. paratuberculosis infection of macrophages may result in subjugation of the immune response against this intracellular infection and disruption of the host's efforts to contain the disease (6). The induction of gamma interferon (IFN-γ) that is usually present in the asymptomatic stage of the disease represents activation of the host cell-mediated immune response, but this response begins to deviate with progressive increases in anti-inflammatory responses to M. avium subsp. paratuberculosis, as represented by increased levels of interleukin 10 (IL-10) and transforming growth factor β (TGF-β) (6, 7). These cytokines are known to have inhibitory roles in the destruction of intracellular M. avium subsp. paratuberculosis, perhaps through their suppressive effects on IFN-γ production (6, 8).
CD40 and CD40 ligand (CD40L) are costimulators expressed by antigen-presenting cells and T cells, respectively (9). Their presence is necessary for generation of humoral immune responses, as well as priming and activation of antigen-specific T cells toward either Th1 or Th2 immunity (9). The absence or impairment of this interaction leads to the induction of tolerance (9). Although little is known about B cell contributions to immunity during M. avium subsp. paratuberculosis infections, it has been shown that B cells are highly activated in the early stages of infection, expressing CD5, a marker of antigen recognition (10, 11). B cells also may become activated upon linkage of CD40 on the cell surface with CD40L present on activated T cells. To our knowledge, the involvement of CD40-CD40L interactions in the immune responses of cattle during different stages of M. avium subsp. paratuberculosis infection has not been addressed prior to this report. However, it was previously demonstrated that the addition of live M. avium subsp. paratuberculosis to CD40L-treated monocyte-derived macrophages resulted in decreased expression of the inducible nitric oxide (NO) synthase (iNOS) and IL-12p40 genes, suggesting that M. avium subsp. paratuberculosis infection may subvert the ability of macrophages to interact with T cells (12). The assimilation of accumulated data regarding inflammatory processes at the site of M. avium subsp. paratuberculosis infection suggests a gap in our understanding of the initial trigger and sustainment of inflammatory responses in the immunopathogenesis of M. avium subsp. paratuberculosis infection. The current study proposed that the CD40-CD40L interaction might be, through its well-studied function in immune response activation, a key regulator of paratuberculosis pathophysiology. Therefore, the expression of these costimulatory molecules on peripheral blood mononuclear cells (PBMCs) obtained from healthy and naturally infected cows was assessed. Further assessment of CD40 and CD40L expression on PBMCs was performed after in vitro M. avium subsp. paratuberculosis infection and stimulation of cells with key regulatory cytokines (IFN-γ, IL-10, and TGF-β).
MATERIALS AND METHODS
Animals.
Holstein dairy cows (ranging in age from 3 to 6 years) used in this study were placed in three groups, consisting of five noninfected healthy cows, five cows naturally infected with M. avium subsp. paratuberculosis but without symptoms (i.e., subclinical stage), and four cows with the clinical form of Johne's disease. Infection was monitored bacteriologically for the fecal shedding of M. avium subsp. paratuberculosis using standard culture methods with Herrold's egg yolk agar containing mycobactin J, amphotericin, nalidixic acid, and vancomycin (Becton, Dickinson, Sparks, MD), as previously described (13). Animals shedding more than 100 CFU of M. avium subsp. paratuberculosis per gram of feces, with weight loss and intermittent diarrhea, were considered to be in the clinical stage of the disease. Subclinically infected cows were shedding less than 10 CFU/g of feces. All animals were housed in Association for Assessment and Accreditation of Laboratory Animal Care-accredited facilities, and all animal-related procedures were approved by the IACUC of the National Animal Disease Center (Ames, IA). Cows with disease were housed on-site separate from healthy control cows, to prevent cross-contamination between groups.
Blood collection, culture conditions, and sample collection.
Peripheral blood mononuclear cells (PBMCs) were isolated from the buffy coat fractions of peripheral blood collected from the jugular vein in 2× acid-citrate-dextrose solution (1:10). These cells were resuspended to 2 × 106 cells/ml in complete medium consisting of RPMI 1640 medium with 2 mM l-glutamine and 25 mM HEPES (Gibco, Grand Island, NY) supplemented with 10% fetal calf serum (Gibco), 100 μg/ml streptomycin sulfate (Gibco), and 0.25 μg/ml amphotericin B (Gibco). CD40 and CD40L expression was evaluated on freshly isolated PBMCs (day 0) as well as on PBMCs cultured in 24-well flat-bottomed plates (Nunc Technologies, Rochester, NY) at 2 × 106 cells/ml. The use of unfractionated PBMCs in culture allowed cell-cell contact to occur between macrophage, T cell, and B cell populations, thus promoting contact between cells for antigen presentation and cytokine secretion. Cultures were maintained for 7 days at 39°C in a humidified atmosphere with 5% CO2, to allow development of monocyte-derived macrophages before ex vivo infection with live M. avium subsp. paratuberculosis (strain 19698; NADC). On day 7, adherent cells were quantitated in replicate wells, and then unfractionated PBMC cultures were infected with live M. avium subsp. paratuberculosis at a multiplicity of infection (MOI) of 10 bacteria per counted adherent cell. Control wells containing unfractionated PBMCs but not M. avium subsp. paratuberculosis were maintained and harvested at this time point for comparison. Experiments were also designed to evaluate the effects of exogenous cytokines such as IFN-γ, IL-10, and TGF-β on CD40 and CD40L expression. Cytokines were added to the PBMC cultures 18 h before ex vivo infection with live M. avium subsp. paratuberculosis. The doses of exogenous cytokines added to the wells and the culture conditions (including the period of infection and the MOI) used in this study were determined previously in our laboratory and were correlated with several experimental parameters, such as expression of surface activation and identification molecules (determined by flow cytometric analyses), cytokine secretion, nitric oxide production, and phagocytosis and killing of M. avium subsp. paratuberculosis by adherent cells in culture (6, 14, 15). Therefore, cells were stimulated with either 100 ng/ml of bovine IFN-γ (generously donated by Novartis Animal Health, Basel, Switzerland), 100 ng/ml of human IL-10 (Peprotech, Rocky Hill, NJ), or 10 ng/ml of human TGF-β (Peprotech) or were left without stimulation. Cells were collected for flow cytometric analysis at 8, 24, and 72 h after ex vivo infection with live M. avium subsp. paratuberculosis for cells that were stimulated or not stimulated with exogenous cytokines.
Bacteria.
Middlebrook 7H9 broth (pH 6.0; BD) supplemented with oleic acid-albumin-dextrose complex (BD) was used to grow M. avium subsp. paratuberculosis strain 19698 (NADC). The bacteria were harvested, washed three times with phosphate-buffered saline (PBS) (0.15 M, pH 7.4), and resuspended in PBS to a final concentration of 109 CFU/ml, as determined spectrophotometrically by absorbance at 540 nm (absorbance at 540 nm = 1.0). Bacterial stocks were then frozen in PBS at −80°C until they were used in experiments. Prior to in vitro infection, frozen bacterial stocks were thawed and clumps were dispersed by brief sonication at 25 W for 40 s with a Tekmar sonic disruptor (Lorton, VA). The frozen bacterial stocks were monitored for viable counts by culturing of serial 10-fold dilutions on Herrold's egg yolk medium. Viable counts in stocks were reduced to approximately 1 × 108 CFU/ml after thawing and sonication.
CD40 and CD40L expression on PBMCs.
For analysis of the expression of total CD40 and CD40L on cells, a two-color flow cytometric protocol was used. Briefly, 2 × 105 cells were incubated with a primary antibody specific for bovine CD40 (1 μg/ml; generously donated by Mark Estes), followed by incubation with peridinin-chlorophyll-protein complex-conjugated rat anti-mouse IgG1 (diluted 1:25; Becton, Dickinson, San Jose, CA). To detect bovine CD40L, cells were stained with an anti-mouse CD40L antibody (MR1, diluted 1:10; BD) that was demonstrated to cross-react with bovine CD40L. Additional wells were set up for analyses of CD4, CD8, γδ T cells, B cells, monocytes, and the CD5 marker, as previously described (15). All wells received 10 μg/ml of DAPI (4′,6-diamidino-2-phenylindole) (Sigma) to differentiate live cells from dead cells and to allow gating on viable cells. Cells were then washed and resuspended in 200 μl of BD FACS Lyse (BD) for immediate flow cytometric analysis. Samples were evaluated using 30,000 events per sample, using a FACScan flow cytometer (CellQuest software; Becton, Dickinson). Analysis was conducted by gating on mononuclear cells, based on forward and side scatter characteristics (FlowJo; Tree Star, Inc., San Carlos, CA).
Statistical analysis.
Data were analyzed using the PROC Mixed procedure of SAS (SAS Institute, Cary, NC). Values were reported as least-squares mean values ± the standard errors of the mean (SEM). When significant (P < 0.05) effects due to infection, in vitro treatment, or time were detected, a means comparison was conducted using the Tukey-Kramer post hoc test.
RESULTS
Effects of cow infection status on CD40 and CD40L expression on fresh PBMCs.
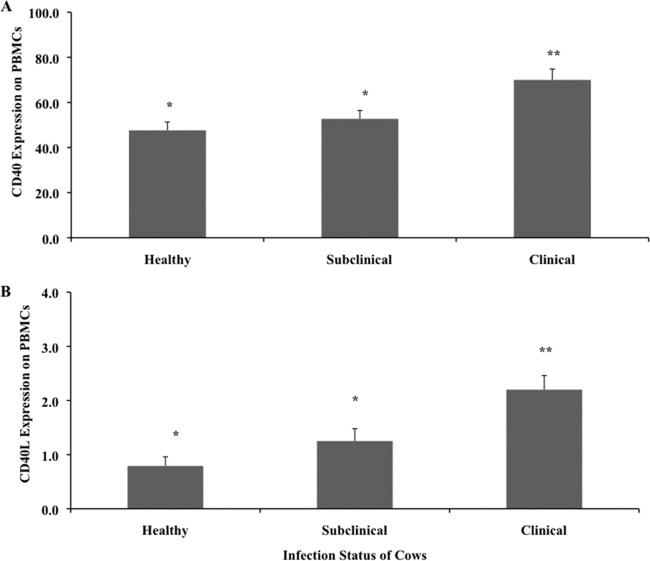
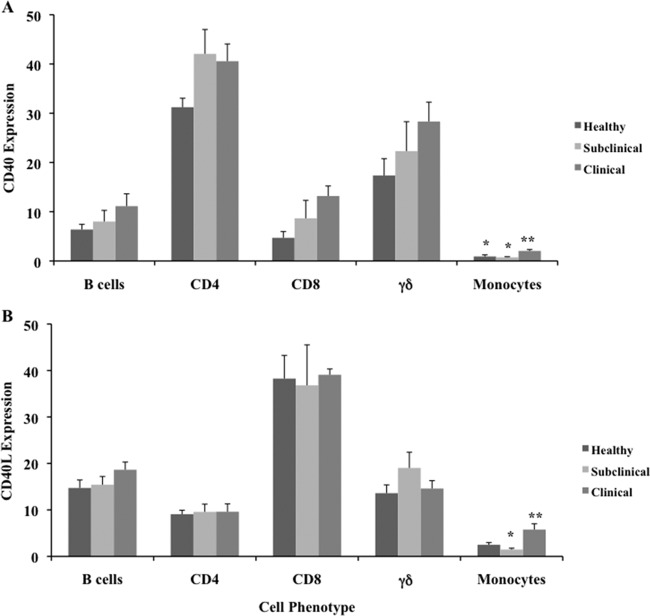
Cows that were naturally infected with M. avium subsp. paratuberculosis and were at the clinical stage of the disease had greater (P < 0.05) expression of CD40 on freshly isolated PBMCs than did healthy or subclinically infected cows (Fig. 1A). Subclinical infection with M. avium subsp. paratuberculosis did not affect the expression of CD40 on fresh PBMCs, as values were similar to those for healthy control cows. In the present study, CD40 expression was detected on 47.7 and 54.3% of the PBMCs obtained from healthy and subclinically infected animals, respectively, whereas nearly 70% of the PBMCs isolated from clinically infected cows expressed this molecule (Fig. 1A). In contrast, CD40L expression on freshly isolated PBMCs was below 2.5% in all treatment groups (Fig. 1B). Although cows in the clinical stage of disease did have significantly (P < 0.05) higher expression of CD40L than did the other treatment groups, there was a trend toward higher CD40L expression in subclinically infected cows than in healthy controls (Fig. 1B). Upon further assessment of CD40 expression on subpopulations of fresh PBMCs, greater expression (P < 0.05) was observed on monocytes, with strong trends (P < 0.10) for greater expression on CD8+ and γδ T cells isolated from clinically infected cows versus noninfected control cows (Fig. 2A). In addition, CD40 expression was higher on CD4+ T cells in both groups of naturally infected cows than in healthy animals. There was little discrimination in CD40L expression on cell subsets in freshly isolated PBMCs; however, an increase in CD40L expression was noted on monocytes from clinically infected cows (Fig. 2B).
Fig 1.
Expression of CD40 (A) and CD40L (B) on freshly isolated PBMCs obtained from healthy control cows and cows naturally infected with M. avium subsp. paratuberculosis in the subclinical and clinical stages of infection. Data are presented as percentage of cells (mean ± SEM). Significant differences between treatment groups are designated by different numbers of asterisks (P < 0.05).
Fig 2.
Expression of CD40 (A) and CD40L (B) on subpopulations of cells within freshly isolated PBMCs obtained from healthy control cows and cows naturally infected with M. avium subsp. paratuberculosis in the subclinical and clinical stages of infection. Data are presented as percentage of cells (mean ± SEM). Significant differences between treatment groups are designated by different numbers of asterisks (P < 0.05).
Effects of cow infection status and in vitro infection with live M. avium subsp. paratuberculosis on CD40 expression on cultured PBMCs.
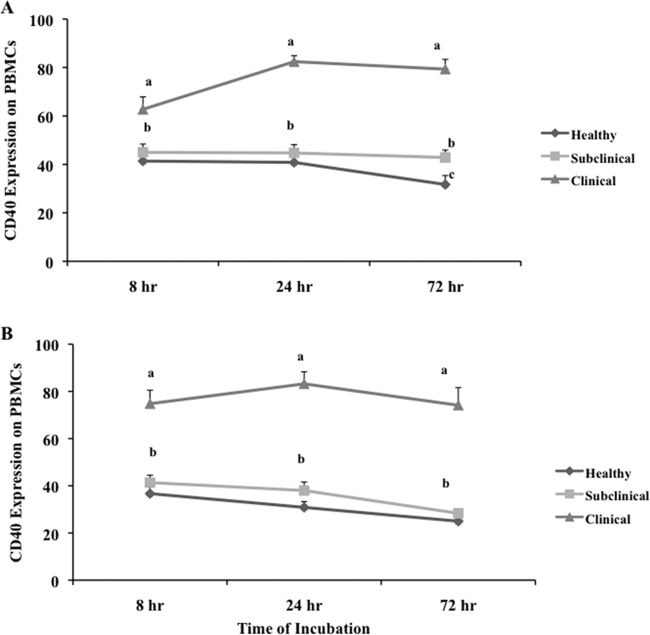
The pattern of CD40 expression on unfractionated PBMCs after culture was similar to that of freshly isolated PBMCs, with higher (P < 0.05) levels of expression on cells isolated from clinically infected cows than from healthy controls or subclinically infected animals at all time points (Fig. 3A). Culture of cells resulted in a 14% increase (P < 0.05) in CD40 expression between 8 and 24 h for clinically infected cows, with no further increases at 72 h of culture. In contrast, a 28.9% decrease in CD40 expression was observed between 24 and 72 h of culture for cells isolated from healthy controls (Fig. 3A). Similar levels of CD40 expression were noted for cells from subclinically infected cows regardless of incubation period, with levels consistently lower (P < 0.05) than those of clinically infected cows at all time points and higher (P < 0.05) than those of control cows at 72 h (Fig. 3A).
Fig 3.
Expression of CD40 on PBMCs isolated from healthy control cows and cows naturally infected with M. avium subsp. paratuberculosis in the subclinical and clinical stages of infection. Unfractionated PBMCs were cultured in complete medium for 7 days and then were incubated for an additional 8, 24, and 72 h in complete medium (A) or complete medium with live M. avium subsp. paratuberculosis (B) at a 10:1 MOI. Data are presented as percentage of cells (mean ± SEM). Significant differences between treatment groups within a time point are designated by different letters (P < 0.05). Significant differences due to the time of incubation are discussed in the text.
The addition of live M. avium subsp. paratuberculosis to PBMCs isolated from healthy control cows resulted in reductions in CD40 expression at all time points, in comparison with cultures without M. avium subsp. paratuberculosis (Fig. 3B). A similar but more significant decrease (26.7%) in CD40 expression was observed between 8 and 72 h with the addition of M. avium subsp. paratuberculosis to cells from healthy cows. CD40 expression on cells from subclinically infected animals also decreased (P < 0.05) over time, an effect that was not observed in replicate cultures without M. avium subsp. paratuberculosis. Therefore, the steady-state CD40 expression observed on cells obtained from subclinically infected animals in the corresponding noninfected cultures appeared to be disrupted in some manner by the addition of M. avium subsp. paratuberculosis. Cultured cells from the clinically infected group showed a significant boost in CD40 expression at 8 h (from 65.6% to 71.7%), compared with the noninfected cultures, but no further increases in expression were detected by 24 h of culture with M. avium subsp. paratuberculosis. The impact of adding live M. avium subsp. paratuberculosis to cultured cells resulted in further stratification of CD40 expression between the clinically infected cows and the healthy or subclinically infected cows at all time points.
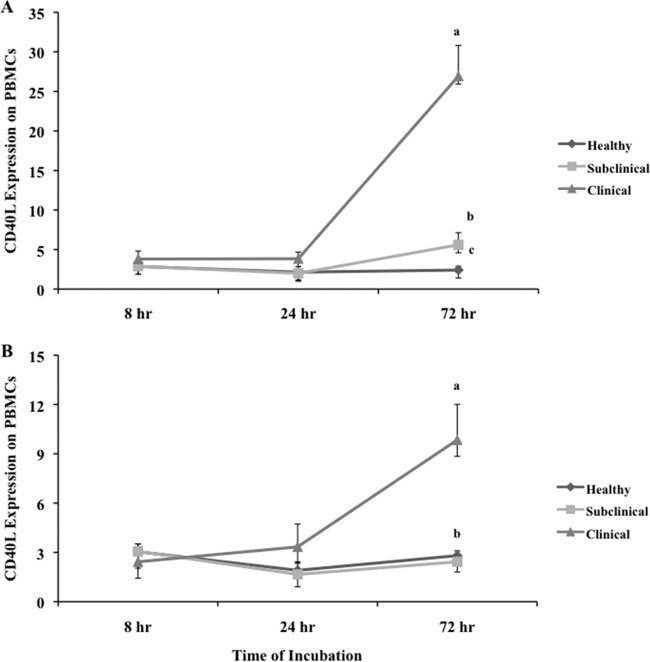
Effects of cow infection status and in vitro infection with live M. avium subsp. paratuberculosis on CD40L expression on cultured PBMCs.
CD40L expression on cells from healthy animals increased after 8 h of incubation, compared with freshly isolated PBMCs (from 0.8% to 2.62%), but remained constant at all successive time points (Fig. 4A). Culture also produced an increase in CD40L expression on PBMCs isolated from subclinically infected cows within 8 h (Fig. 4A), compared with fresh PBMCs. In addition, CD40L expression on cells from subclinically infected cows increased (P < 0.05) 2-fold between 8 and 72 h of culture. Even more dramatic increases (i.e., 10-fold) in CD40L expression were noted for cells obtained from clinically infected animals, with more than 25% of cultured cells expressing CD40L at 72 h of culture, and animals identified in the clinical stage of the disease showed the highest CD40L expression at all time points (Fig. 4B). Cells obtained from subclinically infected animals and cultured for 72 h had significantly (P < 0.05) higher CD40L expression than did cells from healthy animals at the same time point.
Fig 4.
Expression of CD40L on PBMCs isolated from healthy control cows and cows naturally infected with M. avium subsp. paratuberculosis in the subclinical and clinical stages of infection. Unfractionated PBMCs were cultured in complete medium for 7 days and then were incubated for an additional 8, 24, and 72 h in complete medium (A) or complete medium with live M. avium subsp. paratuberculosis (B) at a 10:1 MOI. Data are presented as percentage of cells (mean ± SEM). Significant differences between treatment groups within a time point are designated by different letters (P < 0.05). Significant differences due to the time of incubation are discussed in the text.
Although CD40L expression on PBMCs was relatively low regardless of animal group, the addition of live M. avium subsp. paratuberculosis to cell cultures resulted in further attenuation of expression (Fig. 4B). At 72 h of culture, the level of CD40L expression was decreased on cells obtained from both subclinically and clinically infected cows with the addition of live M. avium subsp. paratuberculosis, while the expression level on cells from healthy animals was not significantly changed by ex vivo infection. At either 8 or 24 h of incubation, the pattern of CD40L expression for all experimental groups did not change due to the presence of M. avium subsp. paratuberculosis in cultures.
Effects of addition of exogenous IFN-γ, IL-10, and TGF-β on CD40 and CD40L expression on cultured PBMCs.
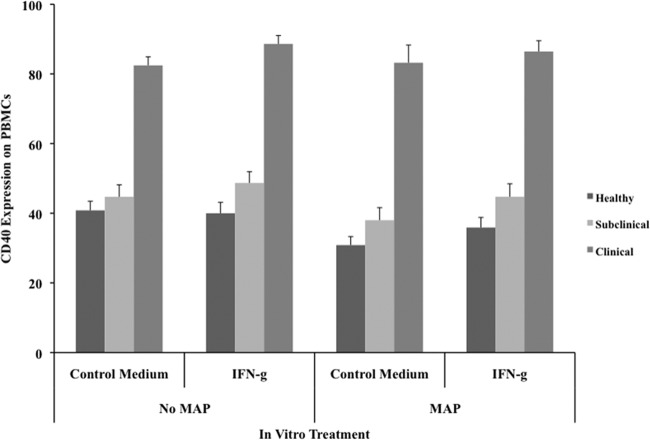
Stimulation of cultured PBMCs with cytokines did not result in any significant changes in either CD40 or CD40L expression (data not shown). There was only a moderate trend for increased expression of CD40 on cells from naturally infected cattle with the addition of IFN-γ to cultures, as shown after 24 h of incubation (Fig. 5). The addition of IFN-γ further modulated CD40 levels by alleviating some of the reduced expression noted on cells from healthy or subclinically infected cows after incubation with live M. avium subsp. paratuberculosis. Interestingly, there was also a trend for IFN-γ-mediated downregulation of CD40L expression on cells from clinically infected cows after 72 h of culture, regardless of the presence of M. avium subsp. paratuberculosis (data not shown).
Fig 5.
Effects of exogenous IFN-γ on CD40 expression on PBMCs isolated from healthy control cows and cows naturally infected with M. avium subsp. paratuberculosis in the subclinical and clinical stages of infection. Unfractionated PBMCs were cultured in complete medium for 7 days and then were incubated for an additional 24 h in complete medium or complete medium with live M. avium subsp. paratuberculosis (MAP) at a 10:1 MOI. IFN-γ was added to the PBMC cultures 18 h before infection with live M. avium subsp. paratuberculosis. Data are presented as percentage of cells (mean ± SEM).
Coexpression of the CD5 activation marker on CD40+ B cells.
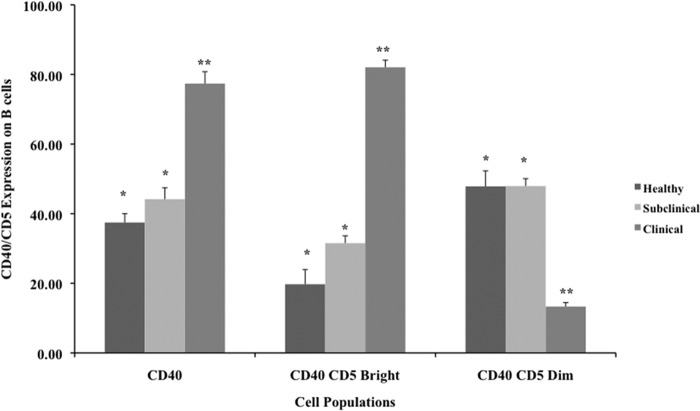
Cells isolated from cows in the clinical stage of infection had a distinct CD5bright subpopulation that was highly (P < 0.01) expressed on CD40+ B cells after 72 h of culture (Fig. 6). Conversely, a CD5dim subpopulation was predominant in the CD40+ B cell population for both healthy and subclinically infected cows.
Fig 6.
Expression of the CD5 marker on CD40+ B cells in PBMC cultures after 72 h of incubation. PBMCs were isolated from healthy control cows and cows naturally infected with M. avium subsp. paratuberculosis in the subclinical and clinical stages of infection. Data are presented as percentage of cells (mean ± SEM). Significant differences between treatment groups are designated by different numbers of asterisks (P < 0.05).
DISCUSSION
Infection with M. avium subsp. paratuberculosis results in a complex process of immunopathogenesis that is mediated by multiple inflammatory pathways. The results presented in this study suggest the importance of CD40-CD40L interactions in M. avium subsp. paratuberculosis infections. Animals in the clinical stage of Johne's disease displayed prominent expression of these costimulatory molecules on freshly isolated or cultured PBMCs, which distinguished them from healthy control and subclinically infected cows. It also was clear that cultured PBMCs obtained from animals in the subclinical stage of the disease had higher CD40L expression than did healthy animals, but levels were still much lower than the expression levels detected on cultured PBMCs from clinically infected animals. CD40 expression levels on cultured cells obtained from different experimental groups were variable. While cells obtained from subclinically infected animals exhibited consistent expression of CD40 at different incubation time points tested, a decrease in CD40 expression on cultured PBMCs obtained from healthy animals was noted after 72 h and an increase in expression was detected after 24 h of incubation for cells obtained from clinically infected animals. In addition, in vitro infection with live M. avium subsp. paratuberculosis resulted in different levels of expression of these molecules at different stages of Johne's disease. For example, a decrease in CD40 expression after ex vivo infection was noted for cells obtained from subclinically infected or healthy animals, while an increase in expression was noted for cells obtained from clinically infected animals.
Interactions between CD40 and CD40L represent a major costimulatory cycle that augments immune responses and promotes gastrointestinal inflammation (16, 17). CD40 and CD40L are overexpressed in forms of inflammatory bowel disease (IBD) such as Crohn's disease and ulcerative colitis (16). Similar to the pathogenesis of Johne's disease in cattle, Crohn's disease in humans is marked by chronic enteric granulomatous inflammation that disrupts nutrient absorption and fluid retention, leading to diarrhea and continuous weight loss (18). The persistence of inflammation in such chronic human enteric diseases was suggested to be a consequence of CD40-CD40L interactions (16). This may be due to the fact that, once CD40 expression is induced, the expression persists for a protracted period of time not only on immune cells but also on nonimmune cells such as fibroblasts, endothelial cells, and mesenchymal cells in IBD-affected mucosa (19). The interaction of recruited CD40L+ T cells with CD40 leads to the production of chemokines, cytokines, adhesion molecules, inflammatory mediators, and nitric oxide (NO) by these cells (16, 20–23). In addition, CD40 ligation with antigen-presenting cells rescues these cells from apoptosis in IBD lesions (16). Therefore, the increased expression of CD40L and CD40 in animals with clinical Johne's disease might be employed as a mechanism of macrophage survival in the granulomatous lesions in tissues, despite the fact that they contain large numbers of bacilli. In IBD, high IFN-γ production has been cited as one likely mechanism responsible for increased expression of CD40 by these cells in the mucosae of IBD patients (16). Increased NO and IFN-γ production has been observed in naturally infected cows in the subclinical stage of the disease, characterizing strong cell-mediated immune responses (14). These changes may be a portent of the slight increases in CD40 and CD40L expression noted in subclinically infected animals, followed by the dramatic increases noted in clinically infected cows.
CD40-CD40L interactions tend to enhance IFN-γ production and upregulate production of NO and other proinflammatory mediators (23–25), which is in contrast to the downregulation of proinflammatory molecules that is observed as animals progress from the subclinical stage to the clinical stage of the disease (6, 7, 14). The subclinically and clinically infected cows used in the current study demonstrated a disparity in antigen-specific IFN-γ responses, with moderate to high IFN-γ levels being noted for subclinically infected cows and moderate to low IFN-γ levels for clinically infected cows, although there was tremendous upregulation of CD40-CD40L expression in the clinically affected animals.
One likely explanation for this finding might be the substantial increase in B cells that is associated with the clinical stage of Johne's disease (26). Therefore, the high degree of CD40 expression detected in this stage of the disease might be associated primarily with B cells and not other antigen-presenting cells. It is well documented that engagement of CD40 and CD40L is necessary for IFN-γ production and thus Th1 development only if the molecules are expressed on appropriate cells (23–25). B cell activation through CD40-CD40L interactions induces negative regulation of immune responses through the production of IL-10, which is known to suppress cell-mediated immune responses (27). Therefore, while CD40-CD40L engagement between macrophages and T cells may result in a positive feedback loop involving IFN-γ and IL-12, the presentation of CD40 on B cells to T cells or other cells expressing CD40L may produce a switch to Th2-mediated responses such as antibody and IL-10 production.
It was previously demonstrated that a high percentage of B cells express CD5 molecules on their surfaces during M. avium subsp. paratuberculosis infection, particularly cells from cows in the clinical stage of disease (10, 28). Further, prolonged CD40 stimulation markedly increased the number of mouse spleen CD1d+ CD5+ B cells, as well as their expression of IL-10 following mitogen stimulation (29, 30). It also is well documented that CD40L interactions with B cells signal proliferation, immunoglobulin switching, and antibody secretion and result in prolonged survival of B cells at the different stages of their lives (31). Such events appear to fit the outcome presented in the clinical stage of Johne's disease. Thus, the upregulated expression of CD40 and CD40L may be synonymous with upregulation of anti-inflammatory cytokine expression or suppression of proinflammatory cytokine production.
Expression of CD5 molecules on conventional B cells is CD40 dependent and becomes upregulated after B cell receptor engagement (32, 33). CD5 acts as an activation marker for B cells and helps B cells to survive (32). CD5 also is considered an inhibitor of B cell receptor signaling and leads to induction of B cell tolerance in vivo, thus controlling overactivation of B cells (33). Hence, it can be hypothesized that, in the clinical stage of Johne's disease, upregulation of CD5 expression on B cells may occur through CD40-CD40L stimulation, labeling these B cells as active cells. As a consequence, high CD5 expression may invoke a negative feedback mechanism for these B cells, resulting in downregulated responses to mycobacterial antigens that, in the later stage of the disease, become widely distributed in the host. In the present study, CD40 expression was upregulated on PBMCs obtained from cows in the clinical stage of disease after in vitro infection with live M. avium subsp. paratuberculosis, whereas an inhibitory effect on CD40 expression was noted for cells obtained from healthy or subclinically infected animals. These results suggest that regulatory feedback mechanisms affected by B cells may differ during the different stages of Johne's disease.
The inhibitory or stimulatory effects of ex vivo infection were not reversed or enhanced by fortification of cell cultures with different cytokines except for a slight reversal in cell cultures supplemented with exogenous IFN-γ. Mimicking the cytokine paradigm of increased IL-10 and TGF-β levels observed during the clinical stage of Johne's disease theoretically should have blocked CD40 engagement of T cells, with reductions in IL-12 and IFN-γ production by T cells in the intestinal lamina propria (34). The addition of IL-10 and TGF-β to cell cultures in the present study did not affect either CD40 or CD40L expression on PBMCs. Perhaps the absence of major effects due to exogenous cytokine treatment suggests that cytokine concentrations, times of addition to cultures, and incubation periods need to be manipulated in order to magnify the effects these cytokines might have on CD40 and CD40L expression. The current study demonstrated that ex vivo infection of cells obtained from naturally infected animals with live M. avium subsp. paratuberculosis resulted in significant decreases in CD40L expression. Ex vivo infection of macrophages with Mycobacterium tuberculosis resulted in the production of soluble factors such as TGF-β and prostaglandin E2, which reduced CD40L expression on T cells (35). In the present study, ex vivo M. avium subsp. paratuberculosis infection induced TGF-β and IL-10 production in cell cultures, with higher levels of secretion being observed for cows in the clinical stage of disease (data not shown). This suggests that clinically infected cows shifted their immune responses to Th2-mediated responses associated with the production of anti-inflammatory cytokines, key mediators of immunological tolerance (7, 8).
In summary, little information is available on key mechanisms of the immunopathogenesis of M. avium subsp. paratuberculosis infection in cattle. It is clear that shifts in the interactions between macrophages, T cells, and B cells are integral to the progression from the subclinical asymptomatic stage of infection to the more advanced stage of clinical disease. Data from the present study implicate two key costimulatory factors, CD40 and CD40L, on these immune cell populations in the disruption of host immunity, leading to the pathogenesis of clinical disease. Further assessment of cell activation markers on key populations of immune cells during M. avium subsp. paratuberculosis infection will improve our understanding of this unique and challenging disease.
ACKNOWLEDGMENTS
We thank Trudy Tatum for her technical assistance and Donnie Anderson for his excellent animal care.
Footnotes
Published ahead of print 12 June 2013
REFERENCES
- 1. Momotani E, Whipple DL, Thiermann AB, Cheville NF. 1988. Role of M cells and macrophages in the entrance of Mycobacterium paratuberculosis into domes of ileal Peyer's patches in calves. Vet. Pathol. 25: 131– 137 [DOI] [PubMed] [Google Scholar]
- 2. Astarie-Dequeker C, N′Diaye EN, Le Cabec V, Rittig MG, Prandi J, Maridonneau-Parini I. 1999. The mannose receptor mediates uptake of pathogenic and nonpathogenic mycobacteria and bypasses bactericidal responses in human macrophages. Infect. Immun. 67: 469– 477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee H, Stabel JR, Kehrli ME., Jr 2001. Cytokine gene expression in ileal tissues of cattle infected with Mycobacterium paratuberculosis. Vet. Immunol. Immunopathol. 82: 73– 85 [DOI] [PubMed] [Google Scholar]
- 4. Coussens PM. 2001. Mycobacterium paratuberculosis and the bovine immune system. Anim. Health Res. Rev. 2: 141– 161 [PubMed] [Google Scholar]
- 5. Cheville NF, Hostetter J, Thomsen BV, Simutis F, Vanloubbeeck Y, Steadham E. 2001. Intracellular trafficking of Mycobacterium avium ss. paratuberculosis in macrophages. Dtsch. Tierarztl. Wochenschr. 108: 236– 243 [PubMed] [Google Scholar]
- 6. Khalifeh MS, Stabel JR. 2004. Effects of gamma interferon, interleukin-10, and transforming growth factor beta on the survival of Mycobacterium avium subsp. paratuberculosis in monocyte-derived macrophages from naturally infected cattle. Infect. Immun. 72: 1974– 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khalifeh MS, Stabel JR. 2004. Upregulation of transforming growth factor-beta and interleukin-10 in cows with clinical Johne's disease. Vet. Immunol. Immunopathol. 99: 39– 46 [DOI] [PubMed] [Google Scholar]
- 8. Othieno C, Hirsch CS, Hamilton BD, Wilkinson K, Ellner JJ, Toossi Z. 1999. Interaction of Mycobacterium tuberculosis-induced transforming growth factor B1 and interleukin-10. Infect. Immun. 67: 5730– 5735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Kooten C, Banchereau J. 1997. Functional role of CD40 and its ligand. Int. Arch. Allergy Immunol. 113: 393– 399 [DOI] [PubMed] [Google Scholar]
- 10. Stabel JR, Khalifeh MS. 2008. Differential expression of CD5 on B lymphocytes in cattle infected with Mycobacterium avium subsp. paratuberculosis. Vet. Immunol. Immunopathol. 126: 211– 219 [DOI] [PubMed] [Google Scholar]
- 11. Stabel JR, Bannantine JP, Eda S, Robbe-Austerman S. 2011. Induction of B cell responses upon experimental infection of neonatal calves with Mycobacterium avium subsp. paratuberculosis. Clin. Vaccine Immunol. 18: 1139– 1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sommer S, Purdrith CB, Colvin CJ, Coussens PM. 2009. Mycobacterium avium subspecies paratuberculosis suppresses expression of IL-12p40 and iNOS genes induced by signaling through CD40 in bovine monocyte-derived macrophages. Vet. Immunol. Immunopathol. 128: 44– 52 [DOI] [PubMed] [Google Scholar]
- 13. Stabel JR. 1997. An improved method for cultivation of Mycobacterium paratuberculosis from bovine fecal samples and comparison to three other methods. J. Vet. Diagn. Invest. 9: 375– 380 [DOI] [PubMed] [Google Scholar]
- 14. Khalifeh MS, Al-Majali AM, Stabel JR. 2009. Role of nitric oxide production in dairy cows naturally infected with Mycobacterium avium subsp. paratuberculosis. Vet. Immunol. Immunopathol. 131: 97– 104 [DOI] [PubMed] [Google Scholar]
- 15. Stabel JR, Waters WR, Bannantine JP, Lyashchenko K. 2011. Mediation of host immune responses after immunization of neonatal calves with a heat-killed Mycobacterium avium subsp. paratuberculosis vaccine. Clin. Vaccine Immunol. 18: 2079– 2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Danese S, Sans M, Fiocchi C. 2004. The CD40/CD40L costimulatory pathway in inflammatory bowel disease. Gut 53: 1035– 1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xu J, Treem WR, Roman C, Anderson V, Rubenstein R, Schwarz SM. 2011. Ileal immune dysregulation in necrotizing enterocolitis: role of CD40/CD40L in the pathogenesis of disease. J. Pediatr. Gastroenterol. Nutr. 52: 140– 146 [DOI] [PubMed] [Google Scholar]
- 18. Momotani E, Romona NM, Yoshihara K, Momtani Y, Hori M, Ozaki H, Eda S, Ikegami M. 2012. Molecular pathogenesis of bovine paratuberculosis and human inflammatory bowel diseases. Vet. Immunol. Immunopathol. 148: 55– 68 [DOI] [PubMed] [Google Scholar]
- 19. Schonbeck U, Libby P. 2001. The CD40/CD154 receptor/ligand dyad. Cell. Mol. Life Sci. 58: 4– 43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yellin MJ, Brett J, Baum D, Matsushima A, Szabolcs M, Stern D, Chess L. 1995. Functional interactions of T cells with endothelial cells: the role of CD40L-CD40-mediated signals. J. Exp. Med. 182: 1857– 1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yellin MJ, Winikoff S, Fortune SM, Baum D, Crow MK, Lederman S, Chess L. 1995. Ligation of CD40 on fibroblasts induces CD54 (ICAM-1) and CD106 (VCAM-1) up-regulation and IL-6 production and proliferation. J. Leukoc. Biol. 58: 209– 216 [DOI] [PubMed] [Google Scholar]
- 22. Thienel U, Loike J, Yellin MJ. 1999. CD154 (CD40L) induces human endothelial cell chemokine production and migration of leukocyte subsets. Cell. Immunol. 198: 87– 95 [DOI] [PubMed] [Google Scholar]
- 23. Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. 1996. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J. Exp. Med. 184: 747– 752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McDyer JF, Goletz TJ, Thomas E, June CH, Seder RA. 1998. CD40 ligand/CD40 stimulation regulates the production of IFN-gamma from human peripheral blood mononuclear cells in an IL-12- and/or CD28-dependent manner. J. Immunol. 160: 1701– 1707 [PubMed] [Google Scholar]
- 25. Tian L, Noelle RJ, Lawrence DA. 1995. Activated T cells enhance nitric oxide production by murine splenic macrophages through gp39 and LFA-1. Eur. J. Immunol. 25: 306– 309 [DOI] [PubMed] [Google Scholar]
- 26. Waters WR, Stabel JR, Sacco RE, Harp JA, Pesch BA, Wannemuehler MJ. 1999. Antigen-specific B-cell unresponsiveness induced by chronic Mycobacterium avium subsp. paratuberculosis infection of cattle. Infect. Immun. 67: 1593– 1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Duddy ME, Alter A, Bar-Or A. 2004. Distinct profiles of human B cell effector cytokines: a role in immune regulation? J. Immunol. 172: 3422– 3427 [DOI] [PubMed] [Google Scholar]
- 28. Stabel JR, Robbe-Austerman S. 2011. Early immune markers associated with Mycobacterium avium subsp. paratuberculosis infection in a neonatal calf model. Clin. Vaccine Immunol. 18: 393– 405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. 2009. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J. Immunol. 182: 7459– 7472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. 2008. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 28: 639– 650 [DOI] [PubMed] [Google Scholar]
- 31. Rush JS, Hodgkin PD. 2001. B cells activated via CD40 and IL-4 undergo a division burst but require continued stimulation to maintain division, survival and differentiation. Eur. J. Immunol. 31: 1150– 1159 [DOI] [PubMed] [Google Scholar]
- 32. Gagro A, McCloskey N, Challa A, Holder M, Grafton G, Pound JD, Gordon J. 2000. CD5-positive and CD5-negative human B cells converge to an indistinguishable population on signalling through B-cell receptors and CD40. Immunology 101: 201– 209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gary-Gouy H, Harriague J, Bismuth G, Platzer C, Schmitt C, Dalloul AH. 2002. Human CD5 promotes B-cell survival through stimulation of autocrine IL-10 production. Blood 100: 4537– 4543 [DOI] [PubMed] [Google Scholar]
- 34. Kelsall BL, Stuber E, Neurath M, Strober W. 1996. Interleukin-12 production by dendritic cells: the role of CD40-CD40L interactions in Th1 T-cell responses. Ann. N. Y. Acad. Sci. 795: 116– 126 [DOI] [PubMed] [Google Scholar]
- 35. Samten B, Thomas EK, Gong J, Barnes PF. 2000. Depressed CD40 ligand expression contributes to reduced gamma interferon production in human tuberculosis. Infect. Immun. 68: 3002– 3006 [DOI] [PMC free article] [PubMed] [Google Scholar]