Abstract
Fowl adenoviruses (FAdVs) are a potential alternative to human adenovirus-based vaccine vectors. Our previous studies demonstrated that a 2.4-kb region at the left end of the FAdV-9 genome is nonessential for virus replication and is suitable for the insertion or replacement of transgenes. Our in vivo study showed that the virus FAdV-9Δ4, lacking six open reading frames (ORFs) at the left end of its genome, replicates less efficiently than wild-type FAdV-9 (wtFAdV-9) in chickens that were infected intramuscularly. However, the fecal-oral route is the natural route of FAdV infection, and the oral administration of a vaccine confers some advantages compared to administration through other routes, especially when developing an adenovirus as a vaccine vector. Therefore, we sought to investigate the effects of FAdV-9 in orally inoculated chickens. In the present study, we orally inoculated specific-pathogen-free (SPF) chickens with FAdV-9 and FAdV-9Δ4 and assessed virus shedding, antibody response, and viral genome copy number and cytokine gene expression in tissues. Our data showed that FAdV-9Δ4 replicated less efficiently than did wtFAdV-9, as evidenced by reduced virus shedding in feces, lower viral genome copy number in tissues, and lower antibody response, which are consistent with the results of the intramuscular route of immunization. Furthermore, we found that both wtFAdV-9 and FAdV-9Δ4 upregulated the mRNA expression of alpha interferon (IFN-α), IFN-γ, and interleukin-12 (IL-12). In addition, there was a trend toward downregulation of IL-10 gene expression caused by both viruses. These findings indicate that one or more of the six deleted ORFs contribute to modulating the host response against virus infection as well as virus replication in vivo.
INTRODUCTION
Fowl adenoviruses (FAdVs), of the genus Aviadenovirus and the family Adenoviridae (1), have a worldwide distribution and can be isolated from both sick and healthy birds (2). Infection with pathogenic FAdVs can lead to inclusion body hepatitis (IBH) in broiler chickens, causing very significant losses to the poultry industry worldwide, including in Canada (3, 4). FAdVs are transmitted horizontally and vertically, can cause persistent infections, and are excreted through feces and the respiratory tract (2, 5).
To date, the genomes of four fowl adenoviruses (those of FAdV-1, FAdV-9, FAdV-8, and FAdV-4) have been fully sequenced (6–9), and they are about 10 kb larger than those of mastadenoviruses.
Human adenoviruses (HAdVs) and other mammalian adenoviruses are used both as oncolytic viruses (10–12) and vaccine vectors (13, 14). FAdVs are also suitable vectors; for example, FAdV-1- and FAdV-8-based recombinant viruses have induced protective immune responses against infectious bursal disease virus and infectious bronchitis virus, respectively (15, 16).
The nonpathogenic FAdV-9 has also been developed as a virus vector. We demonstrated that the tandem repeat region 2 (TR-2) at the right end of the genome is dispensable and is suitable for foreign gene insertion (17). More recently, a 2.4-kb region at the left end of the FAdV-9 genome, containing two putative motifs of the packaging signal domain and six open reading frames (ORFs), was shown to be nonessential for virus replication in vitro and in vivo; however, a deletion virus (FAdV-9Δ4) that lacks the six ORFs (0, 1, 1A, 1B, 1C, and 2) replicated less efficiently than the wild-type (unmodified) FAdV-9 (wtFAdV-9) in chickens inoculated intramuscularly, and the antibody (Ab) level was lower in the FAdV-9Δ4-inoculated birds (18). We have also demonstrated that the left end of the FAdV-9 genome is a suitable site for the insertion and replacement of foreign genes (19). Moreover, in chickens immunized with a recombinant virus containing the enhanced green fluorescence protein (EGFP) gene, antibodies were detected against the foreign protein (20). All these studies suggest the importance of the left-end genes of the FAdV-9 genome in virus replication, immune response modulation, and vector design. Moreover, the optimization of delivery routes and regimens is important for overcoming the potential limitations of AdV-based vaccines for both human and animal applications (21).
FAdVs are normally transmitted by the fecal-oral route, so we wanted to learn more about the replication of our vector virus and its effect on the chicken immune system after oral administration. Therefore, the aims of the present work were to study virus replication and host response in chickens that were inoculated orally with an FAdV vector virus (FAdV-9Δ4) and a wild-type virus (wtFAdV-9). Specifically, virus shedding in feces, viral genome copy number in tissues, antibody response, and expression levels in tissues of selected cytokine genes, alpha interferon (IFN-α), IFN-γ, interleukin-10 (IL-10), and IL-12 were determined.
MATERIALS AND METHODS
Viruses and cells.
FAdV-9 (strain A-2A) and FAdV-9Δ4 were propagated and titrated in chicken hepatoma cells (CH-SAH) as described previously (22). The cells were maintained in Dulbecco's modified Eagle's medium and nutrient mixture Ham's F-12 medium (DMEM-F12) supplemented with 10% non-heat-inactivated fetal bovine serum (FBS), 2 mM l-glutamine, 100 U/ml penicillin, and 100 g/ml streptomycin.
Animal experiment.
The experiment was reviewed and approved by the Animal Care Committee of the University of Guelph in accordance with the Guide to the Care and Use of Experimental Animals of the Canadian Council on Animal Care. One hundred thirty-five 1-day-old specific-pathogen-free (SPF) White Leghorn chickens were obtained from the Canadian Food Inspection Agency (CFIA) (Ottawa, Canada) and were housed in the isolation unit of the University of Guelph. At 7 days of age, the chickens were wing tagged and randomly divided into three groups (groups I, II, and III). On day 10, the chickens were inoculated orally with 1.5 × 107 PFU/chick with wtFAdV-9 (group I), FAdV-9Δ4 (group II), or PBS (group III). The chickens were observed daily for clinical signs of infection. To detect virus in the feces, cloacal swabs were collected in 1 ml PBS with antibiotics at 0, 1, 3, 5, 7, 10, 14, 21, and 28 days postinoculation (d.p.i.) and were stored at −80°C until processing. Sample preparation and virus titration were performed as described previously (18). A sample was regarded as negative if it tested negative at least twice and at two different times. Blood samples were collected from all chickens on 0, 7, 14, 21, and 28 d.p.i., and sera were tested for antibodies by enzyme-linked immunosorbent assay (ELISA) using purified FAdV-9 as an antigen and following the method described previously (23). Five chickens from each group were randomly drawn for euthanasia and necropsy on 1, 2, 3, 4, 5, 7, 14, 21, and 28 d.p.i. Liver, cecal tonsil, spleen, and bursa of Fabricius samples were collected and sectioned into two portions: one was placed in a sample bag and stored at −80°C for viral genome copy number determination, as described previously (24), and the other was collected in a 1.5-ml sterile Eppendorf tube containing RNAlater (Invitrogen Canada, Inc., Burlington, Ontario, Canada) and stored at −80°C. The expression levels of the IFN-α, IFN-γ, IL-10, and IL-12 p40 cytokine genes were evaluated by real-time quantitative PCR (RT-qPCR), with β-actin as a reference gene, as described previously (25–27).
Statistical analysis.
Statistical analyses were performed using GraphPad Prism 5.0 software (San Diego, CA). A one-way analysis of variance (ANOVA) was used to determine significant differences between the groups. The critical level for significance was set at a P value of <0.05. The data were expressed as mean ± standard error of the mean (SEM), determined from five individual birds at the designated days.
RESULTS
Throughout the experiment, no clinical signs of infection were seen in any groups of chickens, and there were no pathological lesions at necropsy.
Virus shedding.
Virus titers in cloacal swabs were determined by the plaque assay. No virus was detected in any groups of chickens before inoculation and in the mock-infected group throughout the study. The virus titers in groups inoculated with wtFAdV-9 and FAdV-9Δ4 are shown in Table 1. For FAdV-9Δ4, virus was detected only at days 1 and 7 p.i., and the titers were significantly lower than those of wtFAdV-9-infected chickens. In the wtFAdV-9-infected group, virus was detected with high titers at 1 to 14 d.p.i., but virus was not detected at the later days (21 and 28 d.p.i.). The highest titer appeared at 5 d.p.i with 4.0 ×103 PFU/ml.
Table 1.
Virus titers in the feces of chickens orally inoculated with FAdV-9Δ4 or wtFAdV-9
| Day p.i. | FAdV-9Δ4 |
wtFAdV-9 |
||
|---|---|---|---|---|
| Titer (PFU/ml) | % of chickens shedding the virus | Titer (PFU/ml) | % of chickens shedding the virus | |
| 0 | NDa | 0 | ND | 0 |
| 1 | 1.04 × 102 ± 1.50 × 102 | 88.9 | 8.26 × 102 ± 5.81 × 102 | 100 |
| 3 | ND | 0 | 2.14 × 103 ± 1.46 × 103 | 100 |
| 5 | ND | 0 | 3.97 × 103 ± 4.07 × 103 | 100 |
| 7 | 1.60 × 101 ± 3.51 × 101 | 25 | 4.16 × 102 ± 3.89 × 102 | 100 |
| 10 | ND | 0 | 1.94 × 102 ± 2.65 × 102 | 80 |
| 14 | ND | 0 | 3.21 × 101 ± 3.12 × 101 | 53.3 |
| 21 | ND | 0 | ND | 0 |
| 28 | ND | 0 | ND | 0 |
ND, not detected.
Viral genome copy number in tissues.
Viral genome copy numbers in liver, cecal tonsil, bursa of Fabricius, and spleen samples were determined by quantitative PCR (qPCR), and the results are summarized in Table 2. No viral DNA was detected in the mock-infected chickens. Throughout the study, viral DNA was detected in cecal tonsil and spleen samples from both virus-infected groups from 1 d.p.i. until 21 d.p.i. and also in liver samples collected at 1, 3, 5, and 7 d.p.i. At day 14 p.i., 40% and 60% of the liver samples had detectable virus levels for the FAdV-9Δ4 and wtFAdV-9 groups, respectively. At 21 d.p.i. only 20% of the samples, and only from the wtFAdV-9 group, were positive for virus. Viral DNA was also detected in some samples of the bursa of Fabricius until day 14 p.i.; however, the genome copy numbers were low compared to those in other tissue samples. The viral genome copy number was highest in cecal tonsil samples, and it was higher in the wtFAdV-9 group than in FAdV-9Δ4-infected chickens.
Table 2.
Viral genome copy numbers in tissues of chickens orally inoculated with FAdV-9Δ4 or wtFAdV-9
| Day p.i. | Tissue sample typea | FAdV-9Δ4 |
wtFAdV-9 |
||
|---|---|---|---|---|---|
| Viral genome copy no. (copies/μg total tissue DNA) | % of tissues with virusb | Viral genome copy no. (copies/μg total tissue DNA) | % of tissues with virusb | ||
| 1 | L | 4.27 × 102 ± 1.84 × 101 | 100 | 1.72 × 105 ± 1.76 × 104 | 100 |
| CT | 1.68 × 104 ± 1.58 × 103 | 100 | 1.88 × 106 ± 1.32 × 105 | 100 | |
| B | 1.15 × 102 ± 4.84 × 101 | 100 | 2.17 × 103 ± 1.71 × 102 | 100 | |
| S | 8.77 × 102 ± 6.43 × 101 | 100 | 2.29 × 103 ± 4.62 × 102 | 100 | |
| 3 | L | 2.08 × 101 ± 6.42 × 100 | 100 | 4.12 × 102 ± 1.18 × 101 | 100 |
| CT | 2.98 × 102 ± 1.71 × 101 | 100 | 1.21 × 106 ± 3.08 × 103 | 100 | |
| B | 6.52 × 100 ± 3.76 × 100 | 40 | 9.65 × 100 ± 2.71 × 100 | 60 | |
| S | 3.75 × 102 ± 2.05 × 101 | 100 | 6.03 × 102 ± 2.36 × 101 | 100 | |
| 5 | L | 3.39 × 101 ± 3.88 × 100 | 100 | 8.92 × 102 ± 5.64 × 101 | 100 |
| CT | 2.65 × 103 ± 7.46 × 101 | 100 | 9.80 × 105 ± 9.35 × 102 | 100 | |
| B | 1.86 × 101 ± 2.68 × 100 | 60 | 3.42 × 102 ± 7.14 × 101 | 60 | |
| S | 9.54 × 101 ± 7.1 × 100 | 100 | 1.75 × 102 ± 2.01 × 101 | 100 | |
| 7 | L | 4.55 × 101 ± 4.56 × 100 | 100 | 2.12 × 102 ± 2.46 × 101 | 100 |
| CT | 6.54 × 102 ± 2.79 × 101 | 100 | 7.53 × 103 ± 5.86 × 101 | 100 | |
| B | 8.90 × 100 ± 2.76 × 100 | 40 | 8.28 × 101 ± 2.93 × 100 | 80 | |
| S | 8.15 × 101 ± 3.71 × 100 | 100 | 1.46 × 102 ± 1.71 × 101 | 100 | |
| 14 | L | 1.62 × 101 ± 3.24 × 100 | 40 | 7.64 × 101 ± 2.72 × 100 | 60 |
| CT | 7.30 × 101 ± 5.45 × 100 | 100 | 1.77 × 103 ± 1.63 × 101 | 100 | |
| B | 6.73 × 100 ± 2.26 × 100 | 40 | 3.22 × 101 ± 5.50 × 100 | 40 | |
| S | 2.65 × 101 ± 2.35 × 100 | 100 | 1.27 × 102 ± 5.21 × 101 | 100 | |
| 21 | L | NDc | 0 | 1.56 × 101 ± 4.35 × 100 | 20 |
| CT | 4.35 × 101 ± 1.15 × 100 | 100 | 1.44 × 103 ± 2.93 × 101 | 100 | |
| B | ND | 0 | ND | 0 | |
| S | 1.52 × 101 ± 5.76 × 100 | 100 | 1.15 × 102 ± 2.71 × 101 | 100 | |
L, liver; CT, cecal tonsil; B, bursa of Fabricius; S, spleen.
The percentage of tissues in which the viral genome was detected.
ND, not detected.
Antibody response.
The presence of FAdV-9-specific Ab was determined by ELISA as described previously (23) and is shown in Fig. 1. No antibodies were detected in any groups before inoculation or in the mock-infected group at any time. Over the study, antibody levels increased in both the wtFAdV-9- and FAdV-9Δ4-infected groups from week 1 p.i. until the end of the experiment at week 4 p.i. The antibody response to wtFAdV-9 was significantly higher (P < 0.001) than that to FAdV-9Δ4 throughout the experiment.
Fig 1.
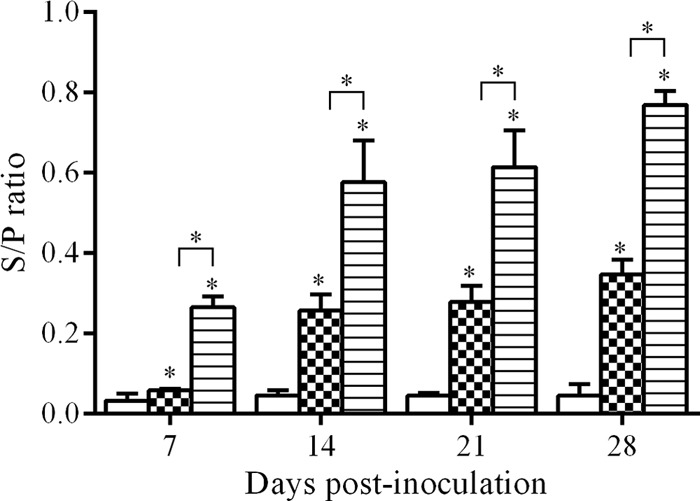
Antibody (IgG) response to FAdV-9 in chickens orally inoculated with FAdV-9Δ4 (checkered bars) or wtFAdV-9 (striped bars) and in mock-inoculated chickens (white bars), as measured by ELISA, shown as S/P (sample-to-positive) ratios. *, statistical significance (P < 0.05) compared to the mock-infected group. Brackets above the bars indicate comparison between wtFAdV-9- and FAdV-9Δ4-infected chickens.
Cytokine gene expression in tissues.
The expression of mRNA of cytokines in the spleen, liver, bursa of Fabricius, and cecal tonsil samples was measured by RT-qPCR.
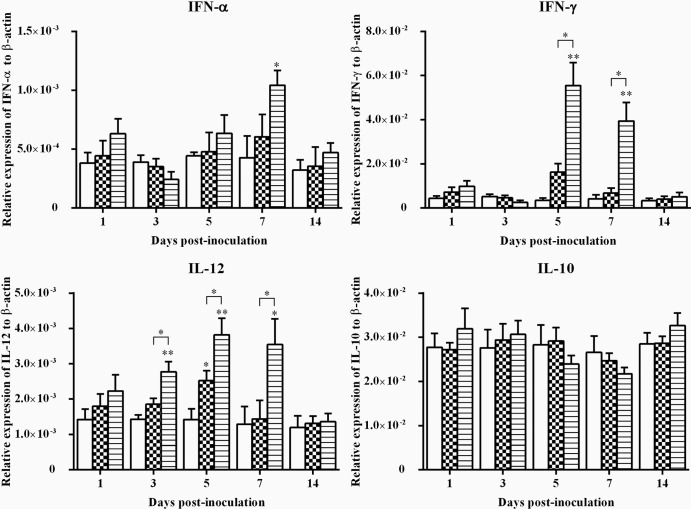
The expression of IFN-α, IFN-γ, IL-10, and IL-12 genes in spleen samples is shown in Fig. 2. There was a statistically significant upregulation (P < 0.05) in the expression of IFN-α in the spleen samples from wtFAdV-9-infected chickens at 7 d.p.i. compared to that in the mock-infected group. In addition, the expression of IFN-γ was significantly upregulated (P < 0.05) at both 5 and 7 d.p.i. upon wtFAdV-9 infection compared to that in both the FAdV-9Δ4-infected and mock-infected groups. IL-12, similar to the pattern of IFN-γ, was also significantly upregulated (P < 0.05) in the spleen samples from wtFAdV-9-infected chickens at 3, 5, and 7 d.p.i. compared to that in the other two groups. Moreover, there was also a significant upregulation (P < 0.05) of the expression of IL-12 in the spleen samples from FAdV-9Δ4-infected chickens. The expression of IL-10 showed some variations, including both upregulation and downregulation, upon wtFAdV-9 or FAdV-9Δ4 infection, although they were not significant (P > 0.05). It should be noted that IL-10 was downregulated, although not significantly, by wtFAdV-9 at 5 and 7 d.p.i., while IFN-γ was significantly upregulated.
Fig 2.
Cytokine mRNA expression in spleen samples from wtFAdV-9 (striped bars), FAdV-9Δ4 (checkered bars), and mock-infected (white bars) chickens. Target and reference gene expression levels were quantified by RT-qPCR, and levels are presented relative to β-actin expression and normalized to a calibrator. Error bars represent standard error of the means. The significance of the regulation level between any two groups was analyzed. *, significant (P < 0.05) upregulation compared to the mock-infected group; **, very significant (0.001 < P < 0.05) upregulation compared to the mock-infected group. Brackets above the bars indicate comparison between wtFAdV-9- and FAdV-9Δ4-infected chickens.
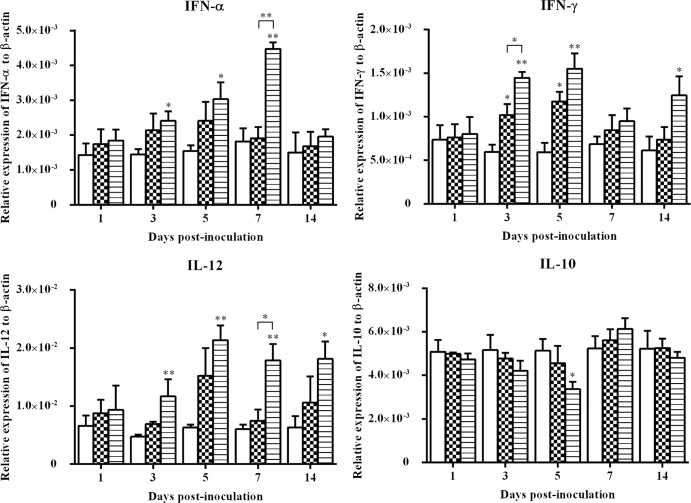
The expression of IFN-α, IFN-γ, IL-10, and IL-12 genes in liver samples is presented in Fig. 3. Similar to the cytokine patterns in spleen samples, upregulation in the expression of IFN-α, IFN-γ, and IL-12 was found in the wtFAdV-9-infected group at certain d.p.i. not seen in the mock-infected group. For example, IFN-α was significantly upregulated (P < 0.05) at 3, 5 and 7 d.p.i., as was the case for IFN-γ at 3, 5, and 14 d.p.i. and for IL-12 at all designated time points except 1 d.p.i. Additionally, compared to FAdV-9Δ4 infection, the wtFAdV-9 caused a greater level (P < 0.05) of induction of IFN-α, IFN-γ, and IL-12 at 7, 3, and 7 d.p.i., respectively. In FAdV-9Δ4 infection, upregulation was noted for only IFN-γ at 3 and 5 d.p.i. (P < 0.05). The expression of IL-10 was downregulated (P < 0.05) in wtFAdV-9-infected chickens at 5 d.p.i, while IFN-γ was significantly upregulated (0.001<P < 0.05) at that time.
Fig 3.
Cytokine mRNA expression in liver samples from wtFAdV-9 (striped bars), FAdV-9Δ4 (checkered bars), and mock-infected (white bars) chickens. Target and reference gene expression levels were quantified by RT-qPCR and are presented relative to β-actin expression and normalized to a calibrator. Error bars represent the standard error of the means. The significance of the regulation level between any two groups was analyzed. *, significant (P < 0.05) up- or downregulation compared to the mock-infected group; **, very significant (0.001 < P < 0.05) up- or downregulation compared to the mock-infected group. Brackets above the bars indicate comparison between wtFAdV-9- and FAdV-9Δ4-infected chickens.
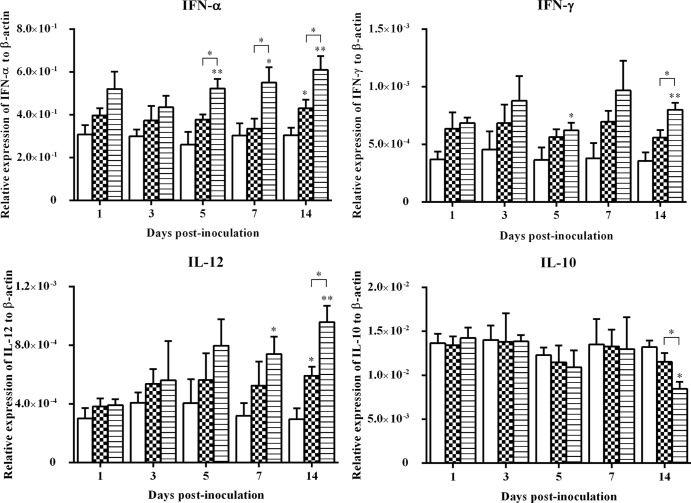
The expression of IFN-α, IFN-γ, IL-10, and IL-12 genes in bursa of Fabricius samples is illustrated in Fig. 4. There was a statistically significant upregulation (P < 0.05) of the expression of IFN-α in bursa samples from wtFAdV-9-infected chickens at 5, 7, and 14 d.p.i. and in bursa samples from FAdV-9Δ4-infected chickens at 14 d.p.i. compared to that in the mock-infected group. For the expression of IFN-γ, significant upregulation (P < 0.05) was found at 5 and 14 d.p.i. in only the wtFAdV-9-infected chickens. IL-12 was upregulated significantly by both wtFAdV-9 at 7 and 14 d.p.i. and by FAdV-9Δ4 at 14 d.p.i. On the other hand, the expression of IL-10 was noted for downregulation at 14 d.p.i., while IFN-γ was significantly upregulated (0.001< P < 0.05) in the wtFAdV-9 group at that time.
Fig 4.
Cytokine mRNA expression in bursa of Fabricius samples from wtFAdV-9 (striped bars), FAdV-9Δ4 (checkered bars), and mock-infected (white bars) chickens. Target and reference gene expression levels were quantified by RT-qPCR and are presented relative to β-actin expression and normalized to a calibrator. Error bars represent the standard error of the means. The significance of the regulation level between any two groups was analyzed. *, significant (P < 0.05) up- or downregulation compared to the mock-infected group; **, very significant (0.001< P < 0.05) up- or downregulation compared to the mock-infected group. Brackets above the bars indicate comparison between wtFAdV-9- and FAdV-9Δ4-infected chickens.
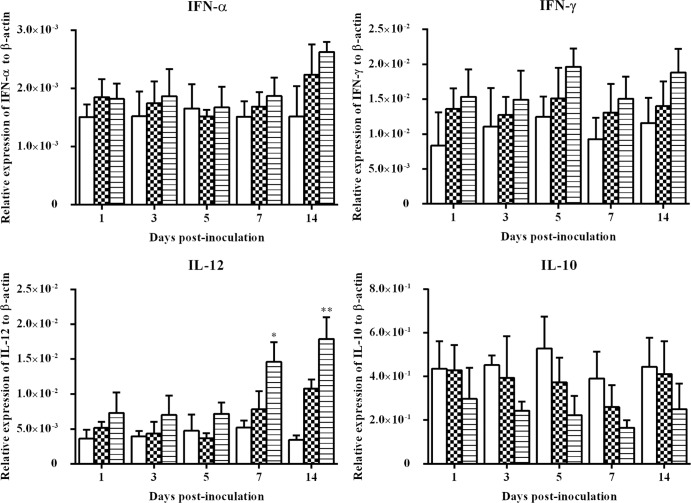
The expression of the IFN-α, IFN-γ, IL-10, and IL-12 genes in cecal tonsil samples is presented in Fig. 5. There was no significant difference (P > 0.05) in the expression of IFN-α, IFN-γ, and IL-10 between any two groups. However, there might be some downregulation of IL-10 expression at 5 and 7 d.p.i. Unlike other cytokines, IL-12 was significantly upregulated (P < 0.05) by wtFAdV-9 infection at 7 and 14 d.p.i. compared to the mock-infected group.
Fig 5.
Cytokine mRNA expression in cecal tonsil samples from wtFAdV-9 (striped bars), FAdV-9Δ4 (checkered bars), and mock-infected (white bars) chickens. Target and reference gene expression levels were quantified by RT-qPCR and are presented relative to β-actin expression and normalized to a calibrator. Error bars represent the standard error of the means. The significance of the regulation level between any two groups was analyzed. *, significant (P < 0.05) up- or downregulation compared to the mock-infected group; **, very significant (0.001< P < 0.05) up- or downregulation compared to the mock-infected group. Brackets above the bars indicate comparison between wtFAdV-9- and FAdV-9Δ4-infected chickens.
DISCUSSION
In the present study, we investigated virus replication and host responses in chickens that were orally inoculated with our adenovirus vector candidate, FAdV-9Δ4, which lacks six ORFs at the left end of the viral genome. FAdV-9Δ4, although it replicated less efficiently in vivo than did wtFAdV-9, induced an antibody response, albeit at a lower level than in wtFAdV-9-inoculated birds. The cytokine gene expression profiles upon virus infection showed that wtFAdV-9 significantly upregulated the mRNA expression of IFN-α, IFN-γ, and IL-12 in all tested tissues except cecal tonsils at least at one tested time point throughout the experiment, while FAdV-9Δ4 did not.
Human adenoviruses, such as HAdV-5, have been extensively investigated for vectored vaccine and gene therapy due to their aptitude for inducing potent innate and adaptive immune responses (10, 13, 28). However, the use of HAdV-based vectors is hampered by the widespread preexisting immunity in humans (13). This initiated interest in the development of nonhuman AdVs, including FAdVs, which are an attractive choice both as vaccine vectors for poultry (15, 16) and as gene therapy vectors. The optimization of delivery routes and application regimens of AdV vectors are also needed to counteract the limitations of HAdV-based vaccines (21). Moreover, oral administration of AdV vectors is better able to avoid systemic neutralizing antibodies than are other routes of administration (29, 30).
Nonpathogenic FAdV-9 is being studied and developed as a vector in our laboratory. Earlier, we employed both oral and intramuscular administration routes to evaluate the Ab response to FAdV-9 (23). However, in that study (23), virus was given through water and feed, which means the amount of virus dose taken up by the chickens was unknown. In more recent studies (18, 20), we evaluated the FAdV-9Δ4 vector virus administered intramuscularly (i.m.), and in the present work, the chickens were inoculated orally. Similar to with i.m. administration, virus was rarely detected (only at days 1 and 7 p.i.) in the feces of the orally inoculated FAdV-9Δ4 group and with titers very significantly lower than those of the wtFAdV-9 group. The route of inoculation did not alter the period of virus shedding for wtFAdV-9-infected chickens. After oral inoculation, virus was detected by both plaque assay and quantitative PCR at 1 d.p.i., which showed the highest viral genome copy number throughout the study. One explanation is that the detected viruses were from the initial inoculum, i.e., the parental viruses. At 3 d.p.i., the viral genome copy number dropped markedly in all tissue samples. A second peak of viral genome copy numbers in the liver and bursa of Fabricius samples from the wtFAdV-9 group occurred at 5 d.p.i., which was well in accord with the highest titer detected at that time point. Similar trends have been seen for FAdV-8 (8). Viral genome copy numbers, indicating the virus load in different tissues, were significantly higher in the wtFAdV-9 group than in the FAdV-9Δ4 group tissue samples except in spleen and were shown to be the highest in the cecal tonsil samples. Similar results have been obtained for both FAdV-8 (8) and FAdV-4 (unpublished data).
The antibody response after oral inoculation in the wtFAdV-9 group was significantly higher (P < 0.001) than in the FAdV-9Δ4 group throughout the study (Fig. 1), which is similar to the i.m. inoculation results of Corredor and Nagy (18). The fact that the mutant virus elicits a less-robust antibody response than the wild-type virus might be advantageous when the same vector virus is considered in a secondary treatment or vaccination. The i.m. inoculation induced a higher Ab level not only for wFAdV-9 but for FAdV-8 (8) and FAdV-4 as well (our unpublished data).
In addition to the Ab response, we investigated the expression of IFN-α, IFN-γ, IL-12, and IL-10 genes at different days after oral inoculation. Type I IFNs are essential for the mediation of potent antiviral responses, and they also upregulate IFN-γ production in natural killer (NK) cells, which induces a T helper 1 (Th1) response that will activate cytotoxic T lymphocytes (CTLs) against virus-infected cells. One of the major roles of IL-10 is to counteract the effects of Th1 responses by inhibiting IFN-γ synthesis (31). Chicken IL-10 also possesses a similar function (32). In the present study, a trend of downregulation of IL-10 gene expression in both wtFAdV-9 and FAdV-9Δ4 groups was found, which was not surprising considering that IFN-γ expression was upregulated by both viruses. Likewise, it was also apparent that IL-10 gene expression, similar to expression of other cytokine genes, was downregulated to a larger extent in wtFAdV-9-infected birds than in FAdV-9Δ4 birds. Previous studies (33, 34) showed that the inflammatory response against an AdV vector in mice was transient and did not extend beyond 24 h, followed by a somewhat resting period of inflammatory gene expression that occurred in the liver samples lasting until 72 h.p.i. At days 4 to 5 p.i. a second dominant peak of inflammatory gene expression appeared in the liver samples, which is consistent with the adaptive immune response (35). The cytokine gene expression in our study was investigated from only day 1 p.i., and thus, the first peak of inflammatory gene expression might have been missed, although this is unlikely. Nevertheless, the second peak beginning about 5 d.p.i. was confirmed.
IFN-α and IFN-γ mRNA expression was upregulated in all tissues, except in the cecal tonsils, of the wtFAdV-9-infected group. The upregulation of these two cytokines was not remarkable soon after infection (day 1 p.i.), but it became statistically significant at days 3, 5, and 7 p.i. These data were similar to the results of our study on FAdV-8 (27). FAdV-9Δ4 infection also upregulated the expression of IFN-α and IFN-γ mostly in the liver and bursa of Fabricius samples. However, the upregulation by FAdV-9Δ4 was less than that by wtFAdV-9 and was statistically significant in the liver samples only at days 3 and 5 p.i. A significant difference was also noted for the expression of IFN-α and IFN-γ between wtFAdV-9 and FAdV-9Δ4 groups, which might be due to the less-efficient replication of FAdV-9Δ4 in inoculated chickens. However, it might also be due to the deleted ORFs (0, 1, 1A, 1B, 1C, and 2) that potentially have roles in modulating the host immune response against FAdV infection, as wtFAdV-9 induced a significantly higher IFN-γ expression than FAdV-9Δ4 at 3 and 5 d.p.i. in spleen samples, where no significant difference was found in terms of the viral genome copy number.
IL-12 is a pleiotropic heterodimeric cytokine comprising two subunits (p35 and p40) and is secreted by monocytes, macrophages, and dendritic cells (36). In mammals, the key role of IL-12 is the initiation and progression of the Th1-type immune response that is typically associated with IFN-γ induction by resting and activated T and NK cells, through inducing the proliferation of the activated T and NK cells (37, 38). Both the p40 and p35 genes of chicken interleukin-12 (chIL-12) are cloned and characterized (39), and similar to the mammalian IL-12, chIL-12 also induces IFN-γ synthesis and proliferative activity of freshly exposed chicken spleen cells. We found that the mRNA expression of IL-12 (p40) was upregulated throughout the study in all selected tissues from both the wtFAdV-9 and FAdV-9Δ4 groups. We found that the mRNA expression of IL-12 (p40) was upregulated in all selected tissues of wtFAdV-9-infected chickens. Moreover, we also noted that on the days when IL-12 was significantly upregulated, IFN-γ was also upregulated, which is in agreement with the results of Degen and coworkers (39).
In conclusion, we investigated virus replication and host responses of orally inoculated chickens with a candidate FAdV vector virus, FAdV-9Δ4. Based on virus shedding and the number of viral genome copies in selected tissues, virus replication in FAdV-9Δ4 was less efficient than that in wtFAdV-9, which was similar to that of intramuscular inoculation. We also demonstrated that both wtFAdV-9 and FAdV-9Δ4 generally upregulated the mRNA expression of IFN-α, IFN-γ, and IL-12 and had a trend of downregulation of IL-10 gene expression in vivo. wtFAdV-9 normally caused a larger extent of regulation than FAdV-9Δ4. Our data suggest that the six deleted ORFs of FAdV-9Δ4 play an important role not only in virus replication in vivo but also in modulating the host response against FAdV infection, the areas we are currently studying.
ACKNOWLEDGMENTS
Li Deng is a recipient of a China Scholarship Council Ph.D. fellowship. This work was supported by the Natural Sciences and Engineering Research Council of Canada, the Canadian Poultry Research Council, and the Ontario Ministry of Agriculture and Food.
We thank Sara Languay and Betty-Anne McBey for their technical assistance and the personnel in the Isolation Unit for their animal care.
Footnotes
Published ahead of print 5 June 2013
REFERENCES
- 1. Harrach B, Benkö M, Both GW, Brown M, Davison AJ, Echavarria M, Hess M, Jones MS, Kajon A, Lehmkuhl HD, Mautner V, Mittal SK, Wadell G. 2012. Family Adenoviridae, p 125–141 In King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ. (ed), Virus taxonomy: classification and nomenclature of viruses: ninth report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, San Diego, CA [Google Scholar]
- 2. Adair BM, Fitzgerald SD. 2008. Group 1 adenovirus infections, p 260–286 In Saif YM, Fadly AM, Glisson JR, McDougald LR, Nolan LK, Swayne DE. (ed), Diseases of poultry, 12th ed Wiley-Blackwell, Ames, IA [Google Scholar]
- 3. Ojkić D, Krell PJ, Tuboly T, Nagy É. 2008. Characterization of fowl adenoviruses isolated in Ontario and Quebec, Canada. Can. J. Vet. Res. 72:236–241 [PMC free article] [PubMed] [Google Scholar]
- 4. Dar A, Gomis S, Shirley I, Mutwiri G, Brownlie R, Potter A, Gerdts V, Tikoo SK. 2012. Pathotypic and molecular characterization of a fowl adenovirus associated with inclusion body hepatitis in Saskatchewan chickens. Avian Dis. 56:73–81 [DOI] [PubMed] [Google Scholar]
- 5. Grgić H, Philippe C, Ojkić D, Nagy É. 2006. Study of vertical transmission of fowl adenoviruses. Can. J. Vet. Res. 70:230–233 [PMC free article] [PubMed] [Google Scholar]
- 6. Chiocca S, Kurzbauer R, Schaffner G, Baker A, Mautner V, Cotten M. 1996. The complete DNA sequence and genomic organization of the avian adenovirus CELO. J. Virol. 70:2939–2949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ojkic D, Nagy É. 2000. The complete nucleotide sequence of fowl adenovirus type 8. J. Gen. Virol. 81:1833–1837 [DOI] [PubMed] [Google Scholar]
- 8. Grgić H, Yang DH, Nagy É. 2011. Pathogenicity and complete genome sequence of a fowl adenovirus serotype 8 isolate. Virus Res. 156:91–97 [DOI] [PubMed] [Google Scholar]
- 9. Griffin BD, Nagy É. 2011. Coding potential and transcript analysis of fowl adenovirus 4: insight into upstream ORFs as common sequence features in adenoviral transcripts. J. Gen. Virol. 92:1260–1272 [DOI] [PubMed] [Google Scholar]
- 10. Cody JJ, Douglas JT. 2009. Armed replicating adenoviruses for cancer virotherapy. Cancer Gene Ther. 16:473–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gallo P, Dharmapuri S, Cipriani B, Monaci P. 2005. Adenovirus as vehicle for anticancer genetic immunotherapy. Gene Ther. 12(Suppl 1):S84–S91. 10.1038/sj.gt.3302619 [DOI] [PubMed] [Google Scholar]
- 12. Shashkova EV, Cherenova LV, Kazansky DB, Doronin K. 2005. Avian adenovirus vector CELO-TK displays anticancer activity in human cancer cells and suppresses established murine melanoma tumors. Cancer Gene Ther. 12:617–626 [DOI] [PubMed] [Google Scholar]
- 13. Lasaro MO, Ertl HC. 2009. New insights on adenovirus as vaccine vectors. Mol. Ther. 17:1333–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sharma A, Tandon M, Ahi YS, Bangari DS, Vemulapalli R, Mittal SK. 2010. Evaluation of cross-reactive cell-mediated immune responses among human, bovine and porcine adenoviruses. Gene Ther. 17:634–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Francois A, Chevalier C, Delmas B, Eterradossi N, Toquin D, Rivallan G, Langlois P. 2004. Avian adenovirus CELO recombinants expressing VP2 of infectious bursal disease virus induce protection against bursal disease in chickens. Vaccine 22:2351–2360 [DOI] [PubMed] [Google Scholar]
- 16. Johnson MA, Pooley C, Ignjatovic J, Tyack SG. 2003. A recombinant fowl adenovirus expressing the S1 gene of infectious bronchitis virus protects against challenge with infectious bronchitis virus. Vaccine 21:2730–2736 [DOI] [PubMed] [Google Scholar]
- 17. Ojkic D, Nagy É. 2001. The long repeat region is dispensable for fowl adenovirus replication in vitro. Virology 283:197–206 [DOI] [PubMed] [Google Scholar]
- 18. Corredor JC, Nagy É. 2010. A region at the left end of the fowl adenovirus 9 genome that is non-essential in vitro has consequences in vivo. J. Gen. Virol. 91:51–58 [DOI] [PubMed] [Google Scholar]
- 19. Corredor JC, Nagy É. 2010. The non-essential left end region of the fowl adenovirus 9 genome is suitable for foreign gene insertion/replacement. Virus Res. 149:167–174 [DOI] [PubMed] [Google Scholar]
- 20. Corredor JC, Nagy É. 2011. Antibody response and virus shedding of chickens inoculated with left end deleted fowl adenovirus 9-based recombinant viruses. Avian Dis. 55:443–446 [DOI] [PubMed] [Google Scholar]
- 21. Thacker EE, Timares L, Matthews QL. 2009. Strategies to overcome host immunity to adenovirus vectors in vaccine development. Expert Rev. Vaccines 8:761–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alexander HS, Huber P, Cao J, Krell PJ, Nagy É. 1998. Growth characteristics of fowl adenovirus type 8 in a chicken hepatoma cell line. J. Virol. Methods 74:9–14 [DOI] [PubMed] [Google Scholar]
- 23. Ojkic D, Nagy É. 2003. Antibody response and virus tissue distribution in chickens inoculated with wild-type and recombinant fowl adenoviruses. Vaccine 22:42–48 [DOI] [PubMed] [Google Scholar]
- 24. Romanova N, Corredor JC, Nagy É. 2009. Detection and quantitation of fowl adenovirus genome by a real-time PCR assay. J. Virol. Methods 159:58–63 [DOI] [PubMed] [Google Scholar]
- 25. Abdul-Careem MF, Hunter BD, Parvizi P, Haghighi HR, Thanthrige-Don N, Sharif S. 2007. Cytokine gene expression patterns associated with immunization against Marek's disease in chickens. Vaccine 25:424–432 [DOI] [PubMed] [Google Scholar]
- 26. Abdul-Careem MF, Hunter BD, Sarson AJ, Mayameei A, Zhou H, Sharif S. 2006. Marek's disease virus-induced transient paralysis is associated with cytokine gene expression in the nervous system. Viral Immunol. 19:167–176 [DOI] [PubMed] [Google Scholar]
- 27. Grgić H, Sharif S, Haghighi HR, Nagy É. 2013. Cytokine patterns associated with serotype 8 fowl adenovirus infection. Viral Immunol. 26:143–149 [DOI] [PubMed] [Google Scholar]
- 28. Yamamoto M, Curiel DT. 2009. Current issues and future directions of oncolytic adenoviruses. Mol. Ther. 18:243–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xiang ZQ, Gao GP, Reyes-Sandoval A, Li Y, Wilson JM, Ertl HC. 2003. Oral vaccination of mice with adenoviral vectors is not impaired by preexisting immunity to the vaccine carrier. J. Virol. 77:10780–10789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tucker SN, Tingley DW, Scallan CD. 2008. Oral adenoviral-based vaccines: historical perspective and future opportunity. Expert Rev. Vaccines 7:25–31 [DOI] [PubMed] [Google Scholar]
- 31. Endharti AT, Rifa'l M, Shi Z, Fukuoka Y, Nakahara Y, Kawamoto Y, Takeda K, Isobe K, Suzuki H. 2005. Cutting edge: CD8+ CD122+ regulatory T cells produce IL-10 to suppress IFN-gamma production and proliferation of CD8+ T cells. J. Immunol. 175:7093–7097 [DOI] [PubMed] [Google Scholar]
- 32. Rothwell L, Young JR, Zoorob R, Whittaker CA, Hesketh P, Archer A, Smith AL, Kaiser P. 2004. Cloning and characterization of chicken IL-10 and its role in the immune response to Eimeria maxima. J. Immunol. 173:2675–2682 [DOI] [PubMed] [Google Scholar]
- 33. Liu Q, Muruve DA. 2003. Molecular basis of the inflammatory response to adenovirus vectors. Gene Ther. 10:935–940 [DOI] [PubMed] [Google Scholar]
- 34. Muruve DA. 2004. The innate immune response to adenovirus vectors. Hum. Gene Ther. 15:1157–1166 [DOI] [PubMed] [Google Scholar]
- 35. Lieber A, He CY, Meuse L, Schowalter D, Kirillova I, Winther B, Kay MA. 1997. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J. Virol. 71:8798–8807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kato T, Hakamada R, Yamane H, Nariuchi H. 1996. Induction of IL-12 p40 messenger RNA expression and IL-12 production of macrophages via CD40-CD40 ligand interaction. J. Immunol. 156:3932–3938 [PubMed] [Google Scholar]
- 37. Cho D, Lee WJ, Halloran PJ, Trinchieri G, Kim YB. 1996. Enhancement of porcine natural killer cell activity by recombinant human and murine IL-12. Cell. Immunol. 172:29–34 [DOI] [PubMed] [Google Scholar]
- 38. Trinchieri G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3:133–146 [DOI] [PubMed] [Google Scholar]
- 39. Degen WG, van Daal N, van Zuilekom HI, Burnside J, Schijns VE. 2004. Identification and molecular cloning of functional chicken IL-12. J. Immunol. 172:4371–4380 [DOI] [PubMed] [Google Scholar]