Abstract
Trypanosomes compartmentalize many metabolic enzymes in glycosomes, peroxisome-related microbodies that are essential to parasite survival. While it is understood that these dynamic organelles undergo profound changes in protein composition throughout life cycle differentiation, the adaptations that occur in response to changes in environmental conditions are less appreciated. We have adopted a fluorescent-organelle reporter system in procyclic Trypanosoma brucei by expressing a fluorescent protein (FP) fused to a glycosomal targeting sequence (peroxisome-targeting sequence 2 [PTS2]). In these cell lines, PTS2-FP is localized within import-competent glycosomes, and organelle composition can be analyzed by microscopy and flow cytometry. Using this reporter system, we have characterized parasite populations that differ in their glycosome composition. In glucose-rich medium, two parasite populations are observed; one population harbors glycosomes bearing the full repertoire of glycosome proteins, while the other parasite population contains glycosomes that lack the usual glycosome-resident proteins but do contain the glycosome membrane protein TbPEX11. Interestingly, these cells lack TbPEX13, a protein essential for the import of proteins into the glycosome. This bimodal distribution is lost in low-glucose medium. Furthermore, we have demonstrated that changes in environmental conditions trigger changes in glycosome protein composition. These findings demonstrate a level of procyclic glycosome diversity heretofore unappreciated and offer a system by which glycosome dynamics can be studied in live cells. This work adds to our growing understanding of how the regulation of glycosome composition relates to environmental sensing.
INTRODUCTION
Trypanosoma brucei, the causative agent of human African trypanosomiasis, has a complex life cycle, with developmental stages in the bloodstream of the mammalian host and the tsetse fly vector. Each host provides a distinct environment in which the parasites must survive. Bloodstream-form (BSF) parasites are bathed in glucose and generate ATP exclusively by glycolysis. While in the tsetse fly, the procyclic-form (PF) parasites experience a drop in glucose levels with a concomitant increase in the availability of amino acids (namely, proline). Under these conditions, the parasite adapts its metabolism, generating ATP from both glycolysis and amino acid metabolism (1).
In trypanosomes, many of the enzymes involved in glycolysis are contained within membrane-bounded organelles called glycosomes (reviewed in references 2, 3, and 4). Similarities between the metabolic activities and the matrix protein import machineries of glycosomes and peroxisomes indicate an evolutionary relationship between the two organelles. In contrast to peroxisomes, however, glycosomes are essential, making mechanisms of glycosome biogenesis and maintenance attractive drug targets.
Glycosome dynamics are governed by a number of processes, including organelle biogenesis, protein import, and changes in protein composition. In trypanosomes, a number of proteins involved in import have been characterized, and recent studies have begun to identify processes involved in glycosome turnover and remodeling (5–7). However, very little is known about organelle biogenesis.
Proper regulation of glycosome number and composition is essential to T. brucei. Silencing of trypanosome PEX proteins involved in glycosome matrix protein import (TbPEX5, -7, -10, -6, -12, and -14) causes the mislocalization of glycosome proteins to the cytosol and compromised growth (8–12). Reduction in the expression of PEX genes involved in glycosome biogenesis (TbPEX11and TbPEX19) through RNA interference (13, 14) results in parasites harboring fewer, larger glycosomes, while overexpression of TbPEX11 results in cells with many smaller glycosomes (14). As observed after silencing of other PEX genes, reduction in the levels of TbPEX11 and TbPEX19 also resulted in growth arrest.
T. brucei glycosomes are extensively remodeled during differentiation between BSF and PF parasites. In a recent study, 159 proteins from the glycosomes of BSFs and PFs were identified by proteomics (15). Of these proteins, approximately 35% were found in both stages of the life cycle. These constitutively expressed proteins included enzymes involved in glycolysis, purine salvage, pyrimidine biosynthesis, phospholipid degradation, and glycerol-ether lipid biosynthesis. Forty-two percent of the proteins were PF specific, and these included proteins involved in the pentose phosphate pathway and the Calvin-Benson cycle and oxygen radical- and peroxide-detoxifying enzymes.
While it is understood that the metabolic repertoire of the glycosome is variable, the details of glycosome biogenesis and changes in protein composition are unknown. Recent work suggests that glycosomal proteins can be turned over during the differentiation process in a pathway that is mechanistically analogous to autophagy (16). Glycosomes colocalize with lysosomes during differentiation when there is a change in glycosome protein expression. Autophagy of glycosomes may also play a role in the parasites' response to the environment, as this pathway appears to be induced under starvation conditions when parasites are moved from nutrient-rich culture to phosphate-buffered saline (PBS) (16, 17). PBS-induced autophagy is not restricted to T. brucei but has also been reported in the related kinetoplastid Leishmania major (18). In contrast to our knowledge of the changes that occur during the differentiation process, less is known about how the protein composition of PF glycosomes changes in response to different environmental conditions.
In yeast and mammalian cells, peroxisomes can proliferate through the growth and division of existing organelles as well as through de novo synthesis from the endoplasmic reticulum (ER). Aside from the observations that the silencing and overexpression of TbPEX11 lead to defects in glycosome number and morphology, nothing is known about how glycosomes proliferate through the growth and division of existing organelles, and to our knowledge, a de novo pathway of glycosome biogenesis in T. brucei has not been examined. Searches of the trypanosome genome have failed to identify any homologs of genes involved in de novo biogenesis; however, this is not surprising as these proteins are not well conserved and trypanosome sequences are highly divergent from those of higher eukaryotes.
To study glycosome dynamics, we have adopted a fluorescent-organelle reporter system used to study peroxisome biogenesis in yeasts and mammals for use in procyclic-form T. brucei. In this system, mature glycosomes import glycosomally targeted fluorescent protein (FP), while immature organelles do not. Using flow cytometry and electron microscopy (EM), we have identified and characterized two parasite populations that differ in their glycosome composition. One population contains “mature” glycosomes harboring the expected repertoire of glycosomal proteins, including TbPEX13, a protein essential for import of proteins into glycosomes, while the other population contains glycosomes, as demonstrated by EM, but expresses few or no glycosome proteins, including TbPEX13. The relative proportions of each parasite population varied under different medium conditions. In the presence of glucose, two distinct populations were present, i.e., one “dim” population harboring immature glycosomes and one “bright” population harboring mature glycosomes, with very few cells of intermediate fluorescence observed. Interestingly, in low-glucose medium this bimodal population structure was lost, and a range of cells with different fluorescence intensities was observed. When parasites were moved from low-glucose medium into medium containing 5 mM glucose, cells of intermediate fluorescence were lost within 24 h. In addition to changes in steady-state glycosome composition under different medium conditions, changes in glycosome expression as monitored by peroxisome targeting sequence 2 (PTS2)-FP expression were observed in live cells during changes in environmental conditions. This change was initiated within 3 h of cell passage from log-phase culture to fresh medium and was complete within 24 h.
The results presented here demonstrate a level of glycosome diversity previously unrecognized. Furthermore, this is the first demonstration that PF parasites harbor immature glycosomes and provides the first suggestion that T. brucei glycosomes may proliferate via a de novo pathway. The glycosome reporter system utilized in these studies provides a rapid, high-throughput, real-time protocol to monitor processes such as autophagy, which likely regulate glycosome remodeling, in live cells.
MATERIALS AND METHODS
Reagents.
All reagents were purchased from Fisher Scientific unless specified.
Growth and transfection of parasites.
T. brucei 29-13 procyclic-form parasites, which express T7 RNA polymerase as well as the tetracycline repressor, were maintained in SDM-79 as described previously (19). PF-PTS2-FP (previously named pXS2-Aldo-PTS-eYFP [20]) and PF-FP were maintained in SDM-79 containing G418 (15 μg/ml), hygromycin (50 μg/ml), and blasticidin (15 μg/ml). Clonal cell lines were obtained by limiting dilution (∼0.33 parasite/well) into 96-well plates.
Generation of fluorescent reporter strains.
To generate PF-FP parasites, the open reading frame of green fluorescent protein (GFP) was cloned using the HindIII site into the pXS2 expression vector (21) (a kind gift provided by J. Bangs, University of Wisconsin) in which the neomycin resistance gene was replaced with the blasticidin resistance gene (pXS2bla). This plasmid integrates into the tubulin locus, and constitutive expression is driven by the procyclic acidic repetitive protein (PARP) promoter. Orientation was confirmed by sequencing. PF parasites were stably transfected as described previously (22) with 10 μg of the MluI-linearized pXS2-FP construct and selected by supplementing the growth medium with 15 μg/ml blasticidin.
Live-cell microscopy.
For analysis of fluorescence in live cells, parasites were collected by centrifugation (800 × g, 10 min) and washed once with PBS. Cell pellets were then resuspended in Vectashield mounting medium (Vector Laboratories, Burlingame, CA) and visualized on a Zeiss Axiovert 200 M using AxioVision software version 4.6.3 for image analysis.
Transmission electron microscopy (TEM).
For ultrastructural analysis, PF parasites were fixed in 2% paraformaldehyde–2.5% glutaraldehyde in 100 mM phosphate buffer for 1 h at room temperature. Samples were washed in phosphate buffer and postfixed in 1% osmium tetroxide (Polysciences Inc., Warrington, PA) for 1 h. Samples were then rinsed extensively in distilled water (dH2O) prior to en bloc staining with 1% aqueous uranyl acetate (Ted Pella Inc., Redding, CA) for 1 h. Following several rinses in dH2O, samples were dehydrated in a graded series of ethanol solutions and embedded in Eponate 12 resin (Ted Pella Inc.). Ultrathin sections of 90 nm were obtained with a Leica Ultracut UCT ultramicrotome (Leica Microsystems Inc., Bannockburn, IL), stained with uranyl acetate and lead citrate, and viewed on a JEOL 1200 EX transmission electron microscope (JEOL USA Inc., Peabody, MA) at the Molecular Microbiology Imaging Facility, Washington University School of Medicine, St. Louis, MO.
Glycosome measurements.
ImageJ software (http://rsb.info.nih.gov/ij/) was used to analyze electron microscopy images of both bright and dim parasite populations. The area of cell visible and individual glycosomes were measured for each image. To calculate glycosome density, the total number of glycosomes was divided by the area of cell visible. This value was then converted to glycosomes/100 μm2. To calculate the percentage of cell area occupied by glycosomes, the area of all glycosomes was summed and divided by the area of cell visible, for each image analyzed. These values were averaged for each population. From the dim population, 13 images, containing 24 cells and 87 glycosomes, were analyzed. From the bright population, 13 images, 26 cells, and 119 glycosomes were analyzed.
Cytometric analysis, cell sorting, and Western blot analysis.
Enhanced yellow fluorescent protein (YFP) fluorescence in live cells was monitored using either a BD FACScan flow cytometer, Accuri C6, or BD Influx cell sorter. Fluorescence emission at 530/540 nm (fluorescein isothiocyanate [FITC] channel) was collected after excitation with a 488-nm laser, and data were analyzed using FlowJo software (TreeStar Inc., Ashland, OR). Cells were sorted directly into SDM-79 using the BD Influx with a 100-μm tip at a sheath pressure of 12 lb/in2 and a drop frequency of 28.7 kHz, and samples were processed for EM and Western analysis immediately. Cell viability after sorting was confirmed by microscopy and estimated (by counting of live cells) to be >90%. For Western blotting, cell lysates (5× 106 to 107 cells) were resolved by 12% SDS-PAGE and transferred to Protran nitrocellulose. Blots were processed as described in reference 23 and probed with antibodies to T. brucei hexokinase (TbHK) (1:10,000), phosphofructokinase (PFK) (1:10,000), fructose 1,6-bisphosphatase (FBPase) (1:10,000), triosephosphate isomerase (TIM) (1:10,000), glyceraldehyde 6-phosphate dehydrogenase (G6PDH) (1:10,000), glycerol kinase (GK) (1:10,000), and PEX13 (1:10,000), which were provided by Paul Michels (de Duve Institute and Université Catholique de Louvain, Brussels, Belgium) (9). Rabbit antiglycosome antibodies (1:1,000) 2841D were provided by Marilyn Parsons (24) (SBRI). TbPEX11 antibodies (1:4,000) were provided by Christine Clayton (14) (Universität Heidelberg, Heidelberg, Germany). Mouse anti-GFP antibodies (1:1,000) (Molecular Probes, Eugene, OR) were used to detect enhanced YFP (eYFP).
Analysis of environmentally dependent changes in glycosome composition.
Cells cultured for extended periods of time are diminished in their ability to respond to environmental changes. Therefore, immediately after transformants emerge from drug selection, stable cell lines are stored in freezing medium (24 mM KCl, 0.03 mM CaCl2, 2 mM K2HPO4, 5 mM HEPES, 0.4 mM EDTA, and 1 mM MgCl2 in 50% glycerol) in liquid nitrogen (LN2). Before use, cells were thawed and seeded at a density of 1 × 105/ml. When cells reached log phase (∼6 × 106/ml), they were passed into fresh medium to a final concentration of 1 × 105/ml or 5 × 105/ml and analyzed by flow cytometry over 24 h. Prior to each remodeling assay, cells were seeded to a density of 1 × 105/ml and grown to log phase. After transformation, cells were passaged no more than twice after, which they were decontaminated and discarded and new cells were transformed.
RESULTS
Cytometric analysis reveals two cell populations that differ in their relative fluorescence.
We have previously generated a fluorescent glycosomal reporter system for use in live cells by fusing the peroxisome-targeting sequence 2 (PTS2) of aldolase, which targets proteins to the glycosome (25), to the open reading frame of eYFP (FP). In this cell line, PF-PTS2-FP (previously named 29-13-pXS2-Aldo-PTS-eYFP), constitutively expressed PTS2-FP is targeted to glycosomes as indicated by fluorescence microscopy (Fig. 1A) (20).
Fig 1.
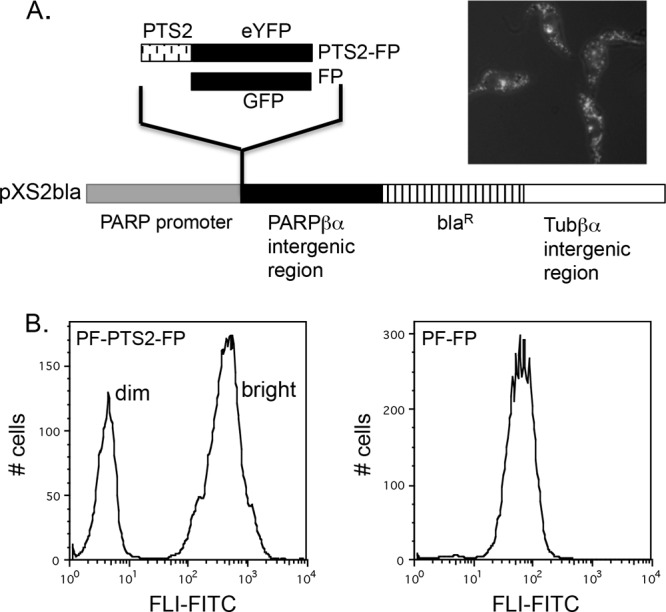
Heterogeneous distribution of PF-PTS2-FP. (A) PTS2-eYFP and GFP were cloned into the expression vector pXS2bla, a derivative of pXS2 (2) in which the neomycin resistance gene has been replaced with the gene encoding blasticidin resistance. The expression plasmid (image modified from reference 2) contains the procyclin promoter (gray box), the PARPβα intergenic region (black box), the Tubβα intergenic region (white box), and the blasticidin resistance gene (hatched box). Fluorescent PF-PTS2-FP cells were incubated with Lysotracker and imaged by fluorescence microscopy. (B) Histograms of PF-PTS2-FP and PF-FP cultures. Fluorescence at 530/540 nm (FL1-FITC) was measured. A total of 10,000 events of each population were analyzed.
Flow cytometry of PF-PTS2-FP cultures revealed two distinct populations (one “bright” and one “dim”) that differed by almost two orders of magnitude in relative fluorescence (Fig. 1B). This was in contrast to PF-FP cells expressing GFP lacking a PTS2 signal sequence (PF-FP) (Fig. 1B), which yielded a single peak. Bright and dim cell populations in the PF-PTS2-FP cultures excluded propidium iodide equally, suggesting that viability was not compromised in the cells with reduced fluorescence (data not shown). Four independent clonal cell lines obtained by two separate transformations were analyzed, and all contained both bright and dim populations, further suggesting that the differences in expression were not due to unexpected integration events or artifacts introduced via selection.
Cells of different fluorescence intensities differ in glycosome protein composition but have similar glycosome morphologies.
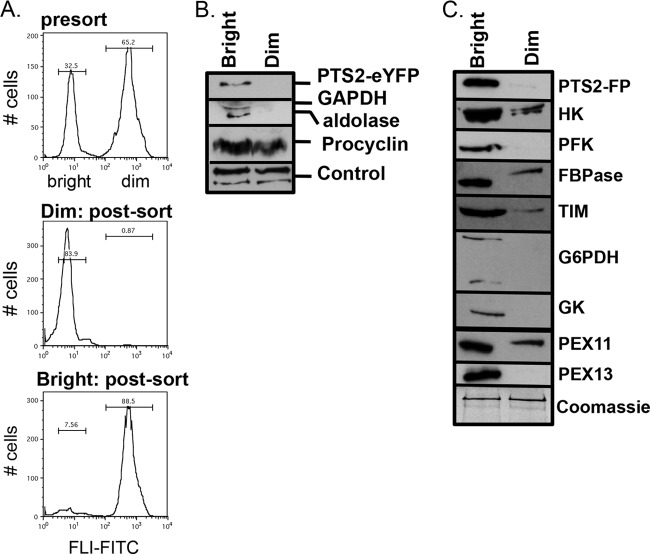
To explore the absence of PTS2-FP in the dim cells and directly assess the expression of other glycosomal proteins, both bright and dim populations were analyzed by Western blotting. Cells were sorted according to their relative fluorescence intensities, and sorting efficiencies were confirmed by cytometric analysis of the sorted populations (Fig. 2A). Lysates from equal cell equivalents (5 × 105) of each population were then analyzed by Western blotting using antibodies that recognize eYFP and antisera (2841D) (24) that recognize the two glycosomal matrix proteins aldolase and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Notably, the cells from the dim population lacked detectable levels of PTS2-FP, aldolase, and GAPDH but expressed equivalent levels of the surface protein procyclin (Fig. 2B). Further Western analysis was used to assess the expression of other glycosomal proteins in bright and dim cells (Fig. 2C). Dim cells expressed low or undetectable levels of TbHK, FPBase, TIM, G6PDH, and glycerol kinase. Interestingly, TbPEX11, an integral glycosome protein, was detected in dim cells, while a protein essential to glycosome protein import, TbPEX13, was not detectable. These findings suggest that dim cells harbor glycosomes but that they are not competent to import matrix proteins.
Fig 2.
Western blot analysis of dim and bright cells. PT-PTS2-FP cultures at 107/ml were analyzed by flow cytometry and sorted into two populations based on their fluorescence in the FITC channel (530/540 nm). (A) Histograms of initial presort population (top panel) and postsort analysis of 10,000 events from the dim population (middle panel) and bright population (bottom panel) sorts. (B) A total of 5 × 105 cell equivalents of each population were resolved by SDS-PAGE and analyzed by Western blotting. Procyclin and a cross-reactive protein (control) were used as loading controls. (C) Western analysis of lysates probed with antibodies against glycosome resident proteins. Coomassie blue-stained protein is shown as a loading control. HK, hexokinase; PFK, phosphofructokinase; FBPase, fructose 1,6-bisphosphatase; TIM, triosephosphate isomerase; GK, glycerol kinase.
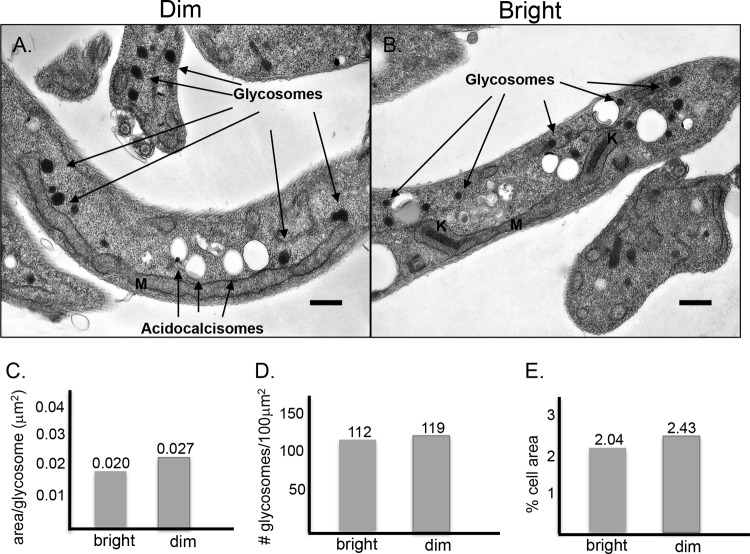
We next used transmission electron microscopy to confirm the presence of glycosomes in dim cells as well as to analyze the morphology of these organelles in the different cell populations (dim [Fig. 3A] and bright [Fig. 3B]). In both bright and dim cells, electron-dense glycosomes were observed. There was no dramatic difference in the size, number, or location of glycosomes in each of the populations. Bright cells had an average of 112 glycosomes/100 μm2 cell area (Fig. 3D), with an average area of 0.02 μm2 (Fig. 3C) and making up 2% of the cell area (Fig. 3E). Dim cells had an average glycosome of 119 cells/100 μm2 (Fig. 3D), with an average area per glycosome of 0.027 μm2 (Fig. 3C) and comprising 2.43% of the area of the cell (Fig. 3E).
Fig 3.
Transmission electron microscopy. Fluorescence-activated cell sorting (FACS) was used to sort cultures based on their fluorescence intensities. (A and B) Dim (A) and bright (B) cells were fixed and processed for TEM as described in Materials and Methods. Arrows indicate glycosomes and acidocalcisomes. Mitochondria (M) and kinetoplasts (K) are indicated. Scale bar = 0.5 μm. The average area/glycosome (C), number of glycosomes/cell area (D), and percent cell area comprised of glycosomes were calculated as described in Materials and Methods.
Cells of intermediate fluorescence are observed in low-glucose medium.
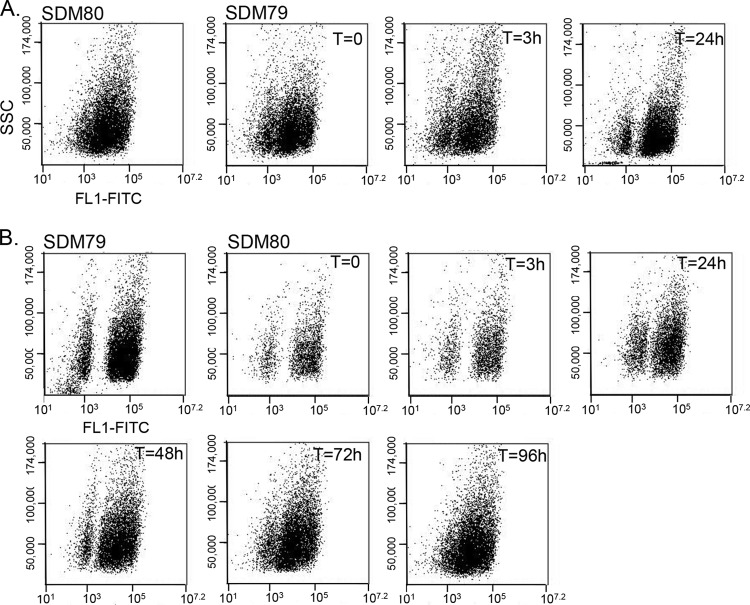
Dim cells have glycosomes, as demonstrated by EM. However, the lack of TbPEX13 suggested that these glycosomes were not able to import glycosomal proteins. It is known that the mislocalization of glycosomal proteins is lethal when cells are grown in standard SMD-79 with glucose. We therefore hypothesized that cells harboring a large number of immature glycosomes must repress glycosome protein expression under standard culturing conditions. In the absence of glucose, the mislocalization of glycosome proteins is less detrimental to the parasites, and we reasoned that under these conditions, cells of intermediate fluorescence would be detected. To test this, we grew cells in low-glucose medium (SDM-80), which was used previously to study how PF metabolism changes in response to extracellular levels of glucose and proline (26), and monitored fluorescence by flow cytometry (Fig. 4). Indeed, when cultures were grown under low-glucose conditions, cells of intermediate fluorescence were observed, resulting in a single broad peak consisting of cells exhibiting a continuous range of fluorescence intensities. Furthermore, addition of glucose (5 mM) to SDM-80 restored the bimodal population distribution, indicating that glucose is the medium component responsible for these differences in glycosome composition (Fig. 4).
Fig 4.
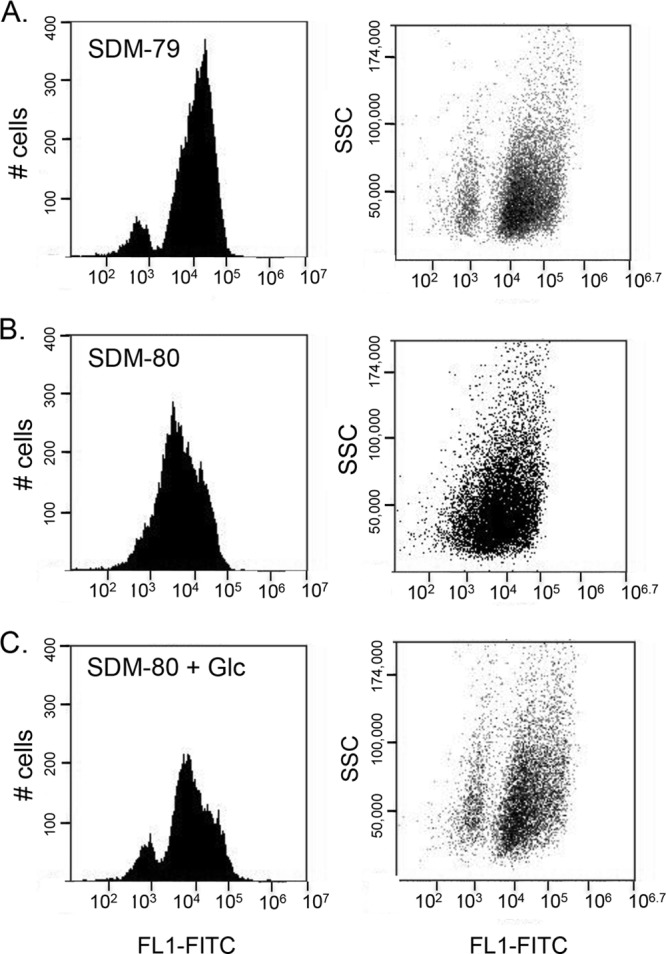
Glycosome composition is regulated by extracellular glucose levels. Cells were grown in SDM-79 (A), SDM-80 (B), and SDM-80 plus glucose (C) and analyzed by flow cytometry. Side scatter (SSC) and fluorescence at 530/540 nm (FL1-FITC) of 10,000 events were plotted.
We next measured the rate at which this intermediate population changed in response to different medium conditions. Cells grown in SDM-80 were passed into SDM-79 and analyzed by flow cytometry. A decrease in the number of intermediate cells was observed at 1 h, and by 24 h two distinct populations were observed. In contrast, when cells were moved from SDM-79 into SDM-80, it took longer for the intermediate population to appear (Fig. 5). By day 5, a continuous range of cells with various levels of fluorescence intensities was observed. Because cells harboring immature glycosomes are so well resolved in SDM-79, we have chosen to use these conditions to study changes in glycosome composition that occur in response to changing environmental conditions.
Fig 5.
Kinetics of medium-dependent glycosome remodeling. (A) Cells grown in SDM-80 were passed into SDM-79 and analyzed by flow cytometry. (B) Cells grown in SDM-79 were passed into SDM-80. Side scatter (SSC) and fluorescence at 530/540 nm (FL1-FITC) of 10,000 events were plotted.
Changes in medium conditions trigger changes in glycosome protein expression.
In yeast and mammalian cells, peroxisome turnover is triggered by changes in medium conditions (27). To test the effect that environmental changes have on glycosome composition, we passed cells from a log-phase culture into fresh SDM-79 at a concentration of 105/ml and measured bright and dim populations by flow cytometry. When cells were passed from log-phase culture to fresh medium at a concentration of 1 × 105/ml, the number of cells falling within the left gate (which includes dim cells exhibiting background fluorescence as well as cells of intermediate fluorescence) increased 3-fold, with 30.1% dim cells present at 3 h postpassage (Fig. 6B) as opposed to 10.8% of cells in the left gate in log-phase culture (Fig. 6A). By 24 h, the original population distribution was restored (Fig. 6C). When cells were subjected to a less dramatic change in cell number and medium conditions by passing them to a final concentration of 5 × 105/ml, there was no change in population distribution (Fig. 6D and E).
Fig 6.
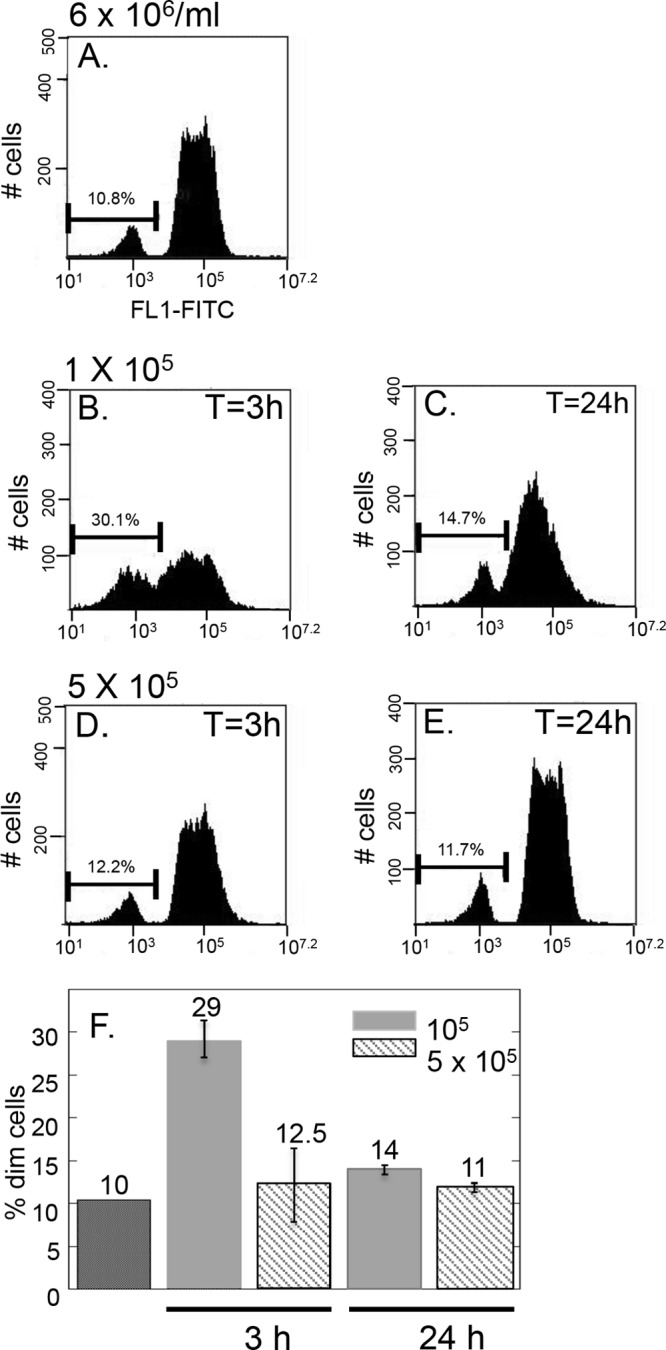
Passage into fresh medium triggers changes in glycosome composition. Cells were passed from log-phase culture (A) to 1 × 105/ml (B and C) or 5 × 105/ml (D and E) and analyzed by flow cytometry at 3 h (B and D) and 24 h (C and E). All cells were passaged and grown in SDM-79. F. Experiments were performed in triplicate and averages and standard deviations of percent dim cells calculated.
DISCUSSION
We have generated a fluorescent-glycosome reporter system in T. brucei that allows us to quickly visualize glycosomes in large numbers of live cells under a variety of different conditions. Using this system, we have identified two populations of PF parasites in culture that harbor different glycosomes. This work reveals that the PF glycosome composition varies in an environmentally dependent manner (Fig. 7).
Fig 7.
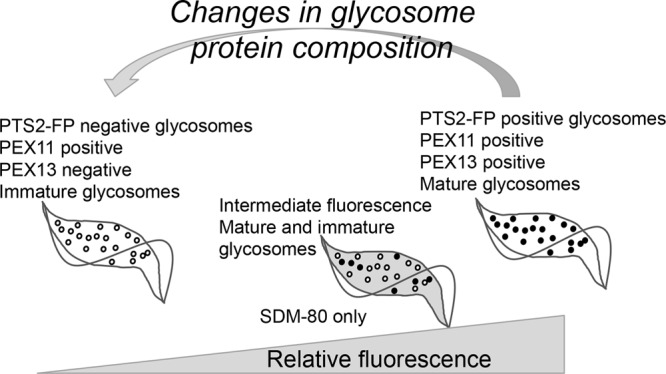
Proposed model for glycosome dynamics in T. brucei. In SDM-79, two parasite populations, one bright and one dim, are present. Dim cells harbor a majority of immature glycosomes that are import incompetent and repress glycosome protein expression. Bright cells harbor enough mature glycosomes to support glycosome protein import. In low-glucose (SDM-80) medium, cells of intermediate fluorescence are observed, presumably because under these conditions any potential cytosolic localization of glycosome proteins is tolerated. Dilution of log-phase cultures into fresh medium triggers changes in glycosome composition, resulting in a transient increase in dim parasites.
Dim cells express very low or undetectable levels of a number of glycosome matrix proteins. TEM showed that dim cells do harbor glycosomes, a finding that is consistent with the expression of the integral glycosome membrane protein, TbPEX11, in these cells. These results, along with the absence of TbPEX13, a protein involved in glycosome protein import, suggested that dim parasites harbor immature glycosomes that are unable to import glycosome proteins. We propose that because these organelles are import incompetent and because mislocalization of glycosomal proteins is lethal under most conditions (12, 28), the expression of glycosomal proteins must be repressed until enough mature organelles are present to correctly localize glycosomal matrix protein. We hypothesize that this tight regulation of glycosome protein is responsible for the bimodal population distribution observed in standard SDM-79 medium. Consistent with this hypothesis is the observation that when cells are grown under low-glucose conditions in which the mislocalization of glycosome proteins is tolerated, cells with intermediate fluorescence intensities are observed.
Several lines of evidence argue against the supposition that these observations are a consequence of misregulated expression of recombinant fluorescent protein in a transgenic system. First, it should be noted that the expression of cytosolic GFP from the same expression vector (pXS2 derivative) yielded a single homogenous population, indicating that the bimodal population is likely not a consequence of unidentified regulation signals in the expression constructs or integration into unanticipated sites in the genome. Furthermore, the dim cells lack not only the PTS2-eYFP reporter but also aldolase and GAPDH as well as a number of other glycosome proteins. It is unlikely that transformation and expression artifacts would affect expression of proteins other than PTS2-FP.
De novo biogenesis of peroxisomes has been demonstrated in yeast and mammalian cells (29, 30). In this process, the sequential maturation of peroxisomes is observed, whereby preperoxisomal vesicles bud from the ER. In Saccharomyces cerevisiae, the peroxin PEX3 was fused to yellow fluorescent protein and found to localize to immature preperoxisomal vesicles which bud from the ER. The protein first localized to the ER with another peroxin, PEX19. PEX3-PEX19-containing foci were then found to bud off from the ER, and newly formed peroxisomes capable of importing matrix proteins developed into mature glycosomes or fused with existing organelles (31). Another example of the temporal regulation of peroxisome protein import is seen in the yeast Hansenula polymorpha. In this model system, peroxisomes first grow by the incorporation of lipids and proteins. After reaching a threshold size, the organelles give rise to new ones through fission. The progenitor organelle is metabolically active but no longer capable of importing new protein (32, 33). To our knowledge, the presence of a de novo biogenesis process or sequential glycosome maturation in T. brucei has never been examined, and to date, database analysis has failed to reveal a PEX3 homolog.
We found that changes in glycosome composition occur rapidly in response to changes in environmental conditions. Passage of cells from a log-phase culture to fresh medium triggered a transient increase in the percentage of “dim” cells that fell within the left gate. This change was initiated within 3 h, and the original population distribution was restored within 24 h. We find that the manner in which the cells are cultured dramatically affects their ability to respond to changes in the environment. In some cases, this increase in the “dim” population approached 100%, while cells cultured for long periods of time become insensitive to changes in the environment (data not shown). To control for this, cells used in remodeling assays are thawed from stabilates and seeded at 1 ×105/ml and grown to a density of 6 × 106/ml before being passed into fresh medium. Cells used in the assays are not passed more than twice. We have found that these conditions provide the most reproducible, although not always the most dramatic, results. Changes in cellular metabolism as well as changes in medium composition that occur during growth in culture likely contribute to the variability of the cells' response to changes in environment. We are currently in the process of identifying and characterizing the effects of different variables on changes in glycosome composition, as we believe this is important to understanding the biological relevance of such responses.
The time frame of these changes in glycosome composition is consistent with the process of autophagy. To date, we have not been able to demonstrate any effect of autophagy inhibitors such as 3-methyladenine and wortmannin on remodeling. However, it is difficult to determine if these compounds work to inhibit autophagy under our experimental conditions. We are in the process of assessing the efficacy of such inhibitors in blocking autophagy under our conditions. Furthermore, we are using TEM to assess the extent to which autophagic structures are formed during this remodeling process.
Dilution of cells from a log-phase culture into fresh medium changes the carbon source availability and triggers a change in glycosome composition. Peroxisome remodeling in response to environmental conditions is documented in a number of organisms. For example, in S. cerevisiae, changes in peroxisome composition are observed when cells are moved from an oleic acid-based medium to one in which glucose is included as a carbon source (34). Under these conditions, the peroxisome matrix protein Fox3p is degraded. In methanol medium, the yeast Pichia pastoris has large, clustered peroxisomes, whereas small, diffused organelles are present when it is grown in oleic acid (27). In another yeast, Hansenula polymorpha, pexophagy is induced when cells are moved from methanol to glucose medium.
We observed a change in glycosome composition when cells were passed back to 1 × 105/ml but not when they were passed to 5 × 105/ml. At this point, we do not know if this change in glycosome composition is the result of changes in cell density or medium composition, as the cells were not washed between passages. It is possible that the residual medium carried with cells through passage affected the cellular response. Separating these two variable has been difficult because washing the cells before diluting them to 1 ×105/ml greatly reduces cell viability.
In T. brucei, it is possible that the two parasite populations have different metabolic capacities. Parasites containing dim glycosomes lacking aldolase and GAPDH may rely heavily on amino acid metabolism and therefore do not require the large number of glycolytic enzymes normally housed in the glycosome.
Additionally, de novo-synthesized glycosomes may be populated primarily with proteins carried from the ER, which is likely the source of origin. The lack of PEX13 may block the import of metabolically important proteins and serve as a means to regulate maturity. Further work on the protein composition and metabolic status of each population will be required to address this model.
We do not know if glycosomes of BSF parasites exhibit the type of plasticity observed in PF parasites. We predict that the obligate dependence on glucose metabolism may prevent or repress dramatic changes in glycosome matrix protein composition.
ACKNOWLEDGMENTS
We acknowledge Wandy Beatty at the Washington University School of Medicine Medical Microbiology Imaging Facility (St. Louis, MO) for the TEM images.
This work was supported in part by NIH grant 2R15A1075326 to J.C.M.
Footnotes
Published ahead of print 24 May 2013
REFERENCES
- 1.Bringaud F, Riviere L, Coustou V. 2006. Energy metabolism of trypanosomatids: adaptation to available carbon sources. Mol. Biochem. Parasitol. 149:1–9 [DOI] [PubMed] [Google Scholar]
- 2.Moyersoen J, Choe J, Fan E, Hol WG, Michels PA. 2004. Biogenesis of peroxisomes and glycosomes: trypanosomatid glycosome assembly is a promising new drug target. FEMS Microbiol. Rev. 28:603–643 [DOI] [PubMed] [Google Scholar]
- 3.Parsons M. 2004. Glycosomes: parasites and the divergence of peroxisomal purpose. Mol. Microbiol. 53:717–724 [DOI] [PubMed] [Google Scholar]
- 4.Parsons M, Furuya T, Pal S, Kessler P. 2001. Biogenesis and function of peroxisomes and glycosomes. Mol. Biochem. Parasitol. 115:19–28 [DOI] [PubMed] [Google Scholar]
- 5.Galland N, Michels PA. 2010. Comparison of the peroxisomal matrix protein import system of different organisms. Exploration of possibilities for developing inhibitors of the import system of trypanosomatids for anti-parasite chemotherapy. Eur. J. Cell Biol. 89:621–637 [DOI] [PubMed] [Google Scholar]
- 6.Brennand A, Gualdron-Lopez M, Coppens I, Rigden DJ, Ginger ML, Michels PA. 2011. Autophagy in parasitic protists: unique features and drug targets. Mol. Biochem. Parasitol. 177:83–99 [DOI] [PubMed] [Google Scholar]
- 7.Duszenko M, Ginger ML, Brennand A, Gualdron-Lopez M, Colombo MI, Coombs GH, Coppens I, Jayabalasingham B, Langsley G, de Castro SL, Menna-Barreto R, Mottram JC, Navarro M, Rigden DJ, Romano PS, Stoka V, Turk B, Michels PA. 2011. Autophagy in protists. Autophagy 7:127–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galland N, Demeure F, Hannaert V, Verplaetse E, Vertommen D, Van der Smissen P, Courtoy PJ, Michels PA. 2007. Characterization of the role of the receptors PEX5 and PEX7 in the import of proteins into glycosomes of Trypanosoma brucei. Biochim. Biophys. Acta 1773:521–535 [DOI] [PubMed] [Google Scholar]
- 9.Verplaetse E, Rigden DJ, Michels PA. 2009. Identification, characterization and essentiality of the unusual peroxin 13 from Trypanosoma brucei. Biochim. Biophys. Acta 1793:516–527 [DOI] [PubMed] [Google Scholar]
- 10.Krazy H, Michels PA. 2006. Identification and characterization of three peroxins—PEX6, PEX10 and PEX12—involved in glycosome biogenesis in Trypanosoma brucei. Biochim. Biophys. Acta 1763:6–17 [DOI] [PubMed] [Google Scholar]
- 11.Saveria T, Kessler P, Jensen BC, Parsons M. 2007. Characterization of glycosomal RING finger proteins of trypanosomatids. Exp. Parasitol. 116:14–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kessler PS, Parsons M. 2005. Probing the role of compartmentation of glycolysis in procyclic form Trypanosoma brucei: RNA interference studies of PEX14, hexokinase, and phosphofructokinase. J. Biol. Chem. 280:9030–9036 [DOI] [PubMed] [Google Scholar]
- 13.Banerjee SK, Kessler PS, Saveria T, Parsons M. 2005. Identification of trypanosomatid PEX19: functional characterization reveals impact on cell growth and glycosome size and number. Mol. Biochem. Parasitol. 142:47–55 [DOI] [PubMed] [Google Scholar]
- 14.Lorenz P, Maier AG, Baumgart E, Erdmann R, Clayton C. 1998. Elongation and clustering of glycosomes in Trypanosoma brucei overexpressing the glycosomal Pex11p. EMBO J. 17:3542–3555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colasante C, Ellis M, Ruppert T, Voncken F. 2006. Comparative proteomics of glycosomes from bloodstream form and procyclic culture form Trypanosoma brucei brucei. Proteomics 6:3275–3293 [DOI] [PubMed] [Google Scholar]
- 16.Herman M, Perez-Morga D, Schtickzelle N, Michels PA. 2008. Turnover of glycosomes during life-cycle differentiation of Trypanosoma brucei. Autophagy 4:294–308 [DOI] [PubMed] [Google Scholar]
- 17.Li FJ, Shen Q, Wang C, Sun Y, Yuan AY, He CY. 2012. A role of autophagy in Trypanosoma brucei cell death. Cell. Microbiol. 14:1242–1256 [DOI] [PubMed] [Google Scholar]
- 18.Besteiro S, Williams RA, Morrison LS, Coombs GH, Mottram JC. 2006. Endosome sorting and autophagy are essential for differentiation and virulence of Leishmania major. J. Biol. Chem. 281:11384–11396 [DOI] [PubMed] [Google Scholar]
- 19.Wang Z, Morris JC, Drew ME, Englund PT. 2000. Inhibition of Trypanosoma brucei gene expression by RNA interference using an integratable vector with opposing T7 promoters. J. Biol. Chem. 275:40174–40179 [DOI] [PubMed] [Google Scholar]
- 20.Dodson HC, Morris MT, Morris JC. 2011. Glycerol 3-phosphate alters Trypanosoma brucei hexokinase activity in response to environmental change. J. Biol. Chem. 286:33150–33157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bangs JD, Brouch EM, Ransom DM, Roggy JL. 1996. A soluble secretory reporter system in Trypanosoma brucei. Studies on endoplasmic reticulum targeting. J. Biol. Chem. 271:18387–18393 [DOI] [PubMed] [Google Scholar]
- 22.Beverley SM, Clayton CE. 1993. Transfection of Leishmania and Trypanosoma brucei by electroporation. Methods Mol. Biol. 21:333–348 [DOI] [PubMed] [Google Scholar]
- 23.Morris MT, DeBruin C, Yang Z, Chambers JW, Smith KS, Morris JC. 2006. Activity of a second Trypanosoma brucei hexokinase is controlled by an 18-amino-acid C-terminal tail. Eukaryot. Cell 5:2014–2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parker HL, Hill T, Alexander K, Murphy NB, Fish WR, Parsons M. 1995. Three genes and two isozymes: gene conversion and the compartmentalization and expression of the phosphoglycerate kinases of Trypanosoma (Nannomonas) congolense. Mol. Biochem. Parasitol. 69:269–279 [DOI] [PubMed] [Google Scholar]
- 25.Blattner J, Dorsam H, Clatyon CE. 1995. Function of N-terminal import signals in trypanosome microbodies. FEBS Lett. 360:310–314 [DOI] [PubMed] [Google Scholar]
- 26.Lamour N, Riviere L, Coustou V, Coombs GH, Barrett MP, Bringaud F. 2005. Proline metabolism in procyclic Trypanosoma brucei is down-regulated in the presence of glucose. J. Biol. Chem. 280:11902–11910 [DOI] [PubMed] [Google Scholar]
- 27.Till A, Lakhani R, Burnett SF, Subramani S. 2012. Pexophagy: the selective degradation of peroxisomes. Int. J. Cell Biol. 2012:512721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furuya T, Kessler P, Jardim A, Schnaufer A, Crudder C, Parsons M. 2002. Glucose is toxic to glycosome-deficient trypanosomes. Proc. Natl. Acad. Sci. U. S. A. 99:14177–14182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolf J, Schliebs W, Erdmann R. 2010. Peroxisomes as dynamic organelles: peroxisomal matrix protein import. FEBS J. 277:3268–3278 [DOI] [PubMed] [Google Scholar]
- 30.Nagotu S, Veenhuis M, van der Klei IJ. 2010. Divide et impera: the dictum of peroxisomes. Traffic 11:175–184 [DOI] [PubMed] [Google Scholar]
- 31.Tabak HF, van der Zand A, Braakman I. 2008. Peroxisomes: minted by the ER. Curr. Opin. Cell Biol. 20:393–400 [DOI] [PubMed] [Google Scholar]
- 32.Veenhuis M, Sulter G, van der Klei I, Harder W. 1989. Evidence for functional heterogeneity among microbodies in yeasts. Arch. Microbiol. 151:105–110 [DOI] [PubMed] [Google Scholar]
- 33.Waterham HR, Keizer-Gunnink I, Goodman JM, Harder W, Veenhuis M. 1992. Development of multipurpose peroxisomes in Candida boidinii grown in oleic acid-methanol limited continuous cultures. J. Bacteriol. 174:4057–4063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hutchins MU, Veenhuis M, Klionsky DJ. 1999. Peroxisome degradation in Saccharomyces cerevisiae is dependent on machinery of macroautophagy and the Cvt pathway. J. Cell Sci. 112:4079–4087 [DOI] [PubMed] [Google Scholar]