Abstract
Unisexual reproduction is a novel homothallic sexual cycle recently discovered in both ascomycetous and basidiomycetous pathogenic fungi. It is a form of selfing that induces the yeast-to-hyphal dimorphic transition in isolates of the α mating type of the human fungal pathogen Cryptococcus neoformans. Unisexual reproduction may benefit the pathogen by facilitating sexual reproduction in the absence of the opposite a mating type and by generating infectious propagules called basidiospores. Here, we report an independent potential selective advantage of unisexual reproduction beyond genetic exchange and recombination. We competed a wild-type strain capable of undergoing unisexual reproduction with mutants defective in this developmental pathway and found that unisexual reproduction provides a considerable dispersal advantage through hyphal growth and sporulation. Our results show that unisexual reproduction may serve to facilitate access to both nutrients and potential mating partners and may provide a means to maintain the capacity for dimorphic transitions in the environment.
INTRODUCTION
Sexual reproduction is pervasive and yet has established costs, including the time and energy devoted to locating a mate. A limited availability of mating partners of the opposite sex may further exacerbate this cost. The human fungal pathogen Cryptococcus neoformans illustrates this dilemma because its populations predominantly contain isolates of the α mating type and few, if any, of the a mating type, restricting opportunities for conventional a-α opposite-sex mating (1, 2, 3). C. neoformans may overcome this barrier in part by undergoing unisexual reproduction—an alternative mode of sexual cycle involving cells of only one mating type, most commonly α (4). Unisexual reproduction can confer benefits by generating adaptive genotypic diversity through recombination and by producing spore progeny that are readily dispersed aerially and inhaled as infectious propagules. Here, we report a novel way in which unisexual reproduction benefits C. neoformans, involving hyphal development to promote foraging and increased access to nutrients and facilitating the dispersal of spores.
C. neoformans grows asexually as a budding yeast but differentiates into hyphae upon unisexual reproduction during solo culture of self-fertile isolates on appropriate growth media (4). Unisexual reproduction involves the formation of an extensive monokaryotic mycelium and terminal fruiting structures called basidia wherein meiosis occurs, and long chains of meiotic spores decorate the outer surface of the basidia (5). Components of a pheromone-responsive mitogen-activated protein kinase (MAPK) pathway are responsible for the yeast-to-hypha transition, and the absence of key elements, including the protein kinase Ste7 or the transcription factor Mat2, blocks unisexual reproduction and traps C. neoformans in the yeast form (6, 7, 8). While a functional MAPK pathway is necessary for morphological differentiation, the meiotic machinery, including Dmc1 and Spo11, is essential for culmination of the unisexual cycle, resulting in meiosis and the production of basidiospores (4). In strains lacking Spo11 (Fig. 1), the yeast-to-hypha transition and the formation of basidia proceed normally, but meiosis and sporulation are severely impaired (32).
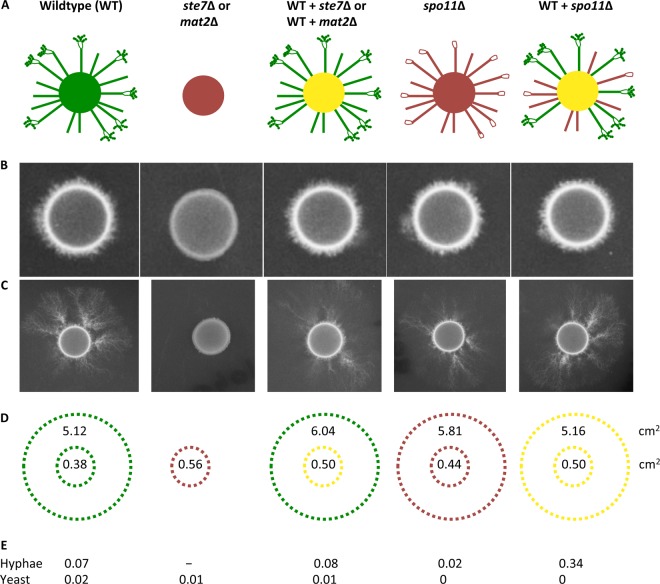
Fig 1.
Unisexual reproduction confers a dispersal advantage. (A to C) Genotypes of the strains used in this study are shown (A), along with their phenotypes for growth on the mating-inducing media MS and V8 agar for 1 week (B) and 3 weeks (C). Green represents the WT, red represents deletion mutants, and yellow represents a mixture of the two. The wild type is capable of unisexual reproduction and differentiates into hyphae, unlike the mat2Δ or ste7Δ mutants, which are incapable of unisexual reproduction. The spo11Δ mutant forms hyphae but produces few viable spores that could be disseminated aerially or via other routes. (D) The surface areas (cm2) covered by yeast cells in the center and the extra surface areas covered by hyphae represent the averages of three replicates. (E) Standard deviations are provided for the respective yeast and hyphal areas. mat2Δ and ste7Δ are represented by one measurement, as they show comparable results. All strains grew to cover the same surface area on YPD (data not shown).
What selective pressures may maintain the ability to undergo the yeast-to-hyphal transition? We tested the hypothesis that morphological differentiation during unisexual reproduction allows this pathogen to disperse and extract nutrients from a larger territory than would be available otherwise. This advantage is independent of genetic exchange or recombination and highlights a possible selective pressure underlying the origin and maintenance of unisexual reproduction with implications for both pathogenic and saprobic microbes.
MATERIALS AND METHODS
Strains.
Strains with deletions of the STE7, MAT2, or SPO11 genes were generated in the XL280 α wild-type (WT) background using overlap PCR products to replace the relevant genes with the nourseothricin (NAT) drug resistance selectable marker. XL946 and XL947 are two independent ste7Δ deletion mutants, XL942 and MF200 are independent mat2Δ deletion mutants, and XL1082 and MF43 are independent spo11Δ deletion mutants. All strains but the wild type are resistant to NAT.
Competition assays.
For each competition assay, 8 × 107 cells/ml each of the WT and a deletion mutant strain were mixed, and the mixture was incubated on yeast extract-peptone-dextrose (YPD), V8 juice agar mixture (4), or MS agar (Sigma catalog no. M5524) at 25°C in the dark. After 2 days on YPD or 1 week on V8 or MS agar, cells from the center of the coincubation culture were sampled. CFU were enumerated on the selective medium YPD-plus-NAT versus YPD to determine the proportion of mutant cells relative to WT cells. We also isolated by micromanipulation ∼50 spores from the hyphae growing at the periphery, and these spores were germinated, scored for NATR or NATS phenotype, and analyzed by PCR for the mat2Δ, ste7Δ, and spo11Δ mutations. Totals of 5,400 spores and 8,640 CFU (160/competition assay) were tested in this fashion.
Growth curve assays.
Strains were grown overnight in liquid YPD medium, and 2 × 107 cells/ml were inoculated in 200 μl in a 96-well plate. The optical density at 600 nm (OD600) was measured every hour over 48 h at room temperature for 6 replicates per strain using a Tecan Sunrise microplate reader.
Pheromone expression.
RNA was isolated from 5 ml of overnight cultures incubated for 24 h at room temperature in YPD or MS agar medium in the dark. Cells were harvested and lyophilized overnight prior to extraction using TRIzol reagent following the manufacturer's instructions (Invitrogen). An amount of 5 μg of total RNA was treated with Turbo DNase (Ambion), and single-stranded cDNA was synthesized using AffinityScript RT-RNase (Stratagene). Quantitative real-time PCR (qRT-PCR) assays were performed in triplicates on an Applied Biosystems 7500 real-time PCR System using Brilliant SYBR green qRT-PCR master mix (Stratagene). A no-template control and melting curves were analyzed to exclude primer artifacts. The expression of the pheromone was normalized relative to that of the endogenous control, GPD1, and the level of expression was determined using the cycle threshold (2−ΔΔCT) approach. The primers used for RT-PCR are listed in Table S3 in the supplemental material. The Student t test was used to establish significant differences in the expression of the pheromone in the mutants compared to its expression in the wild type (significance was determined at a P value of <0.05).
RESULTS AND DISCUSSION
Competition assays were conducted between a wild-type strain and mutants lacking the STE7, MAT2, or SPO11 gene to test whether the wild-type strain, which is capable of unisexual reproduction, produces hyphae that extend onto the medium, covering a wider surface area over time than the ste7 or mat2 deletion mutants, which are incapable of hyphal differentiation via unisexual reproduction (Fig. 1). Over the course of 2 weeks of incubation, the wild type covered >5 times as much surface area of the growth medium as the unisexual defective mutants (t test, P < 0.0001). The spo11 deletion mutants, which have an intact MAPK pathway and are thus able to undergo hyphal differentiation but not meiosis and sporulation (the ultimate hallmarks of sexual reproduction), dispersed as widely as the wild type. However, upon successful meiosis at the tips of hyphae, the wild type gains an additional advantage through spore production, a critical fitness component for filamentous and dimorphic fungi (9, 10).
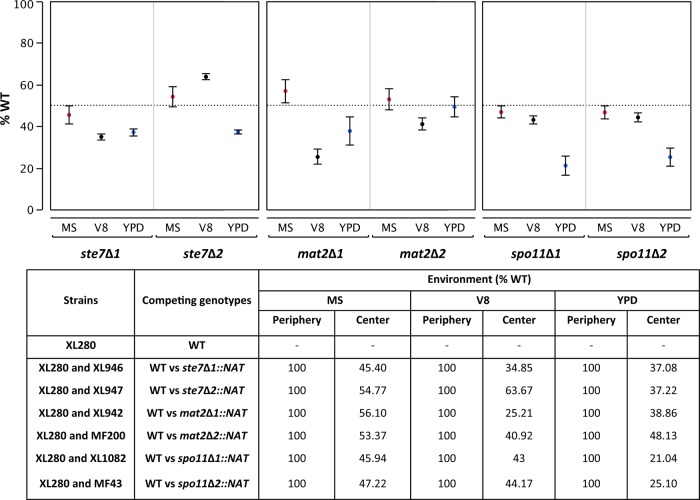
We performed competitive growth assays under several environmental conditions, ranging from (i) nutrient-replete YPD, which supports vegetative growth but not unisex, to (ii) MS or V8 medium, which contain inositol and other factors that induce unisexual reproduction (11, 12). Under MS conditions, the wild type and the mutants were present at equivalent proportions at the center of the coincubation spots, indicating no significant difference in their growth rates; however, the periphery of the spot was overwhelmingly populated by the wild-type spores (50 out of 50 analyzed per competition assay, or 100% WT, NAT-sensitive isolates) produced through unisexual reproduction (Fig. 2; see also Table S1 in the supplemental material). This striking difference observed in spore production at the periphery (with no NAT-resistant mutant isolates recovered) also suggests that minimal or no cell fusion occurs between the two competitors under these experimental conditions. We also randomly sampled CFU from the periphery and found that ste7 or mat2 mutant genotypes were completely absent, whereas spo11 mutants were distributed nonuniformly in the periphery (data not shown). Thus, the wild type, which is proficient in unisexual reproduction, gains an advantage not only by habitat exploration through hyphae but also by dominating the surrounding environment through the production of recombinant spores that can readily be further disseminated aerially. This is notable because C. neoformans can undergo sporadic hyphal development without the production of spores through an unknown pathway that is independent of unisexual reproduction (13). Furthermore, spores disseminated following unisexual reproduction can serve as infectious propagules (14). We also tested an intermediate environment using V8 agar, which supports asexual growth but also induces sexual reproduction. On this medium, the proportion of the wild type varied for different mutants, and the modestly higher proportion of the wild type against some competing genotypes may represent sampling of the spores, as well as yeast cells from the center of the competitive growth spot. The genotype had no effect, in contrast to the environment, which significantly affected the outcome of the competition (see Table S1).
Fig 2.
The competitive fitness of unisexually proficient and deficient strains is environment specific. The results of competition between the wild type and mutant isolates on three media are shown in the top panels. The average proportions (%) of the wild type per environment per mutant genotype from the center of the coincubation spot for three replicates are shown. The whiskers show standard error. The dotted line marks the proportion of the wild type expected under the null hypothesis (50%). We tested two independent deletion mutants each for the STE7, MAT2, and SPO11 genes. We analyzed whether the two independent mutants of the same gene behaved similarly in a given environment using 2-factor analysis of variance (see Table S1 in the supplemental material) to test the effects of the variables and their interaction on the proportion of the wild type. The bottom panel provides the proportions (%) of the wild type when coincubated in competition with the respective deletion mutants on three different media. The binomial two-sample proportion test was used to test for significant deviation from equality (50%) in each competition assay conducted in a given environment (see Table S2 in the supplemental material).
Under the nutrient-replete conditions provided by YPD medium, a modestly decreased proportion of the wild type was observed relative to that of the various mutants (21 to 38% WT). We do not have an explanation for what factors may have caused the wild type to be less abundant than the mutants, but it suggests that under certain conditions, the maintenance of sexual machinery might come with a cost. The trade-off between competitive ability and unisexual reproduction observed in our experiment is reminiscent of the fitness trade-offs observed in Saccharomyces cerevisiae (15) such that mutations that eliminate mating ability were found to confer up to a 2% growth advantage during asexual growth. Moreover, this trade-off between asexual growth rate and mating efficiency was rooted in a cost of gene expression such that the MAPK pathway genes necessary for mating were more prone to loss under natural selection. We conducted growth assays to test whether the low proportion of the wild type on YPD is due to its poor competitive ability being manifested in a lower growth rate than the mutants. Although no significant difference in the doubling time was observed between the strains with mutant genotypes and the wild type in liquid YPD medium (see Fig. S2 in the supplemental material), the relative growth rates may differ on solid versus liquid medium or when in competition.
Importantly, the trade-off observed in our experiment is environment specific and is absent under conditions that induce unisexual reproduction. Similarly, the facultatively sexual fungus Aspergillus nidulans has been found to invest more resources in sexual reproduction in environments in which the fitness is lower (16), suggesting that the level of resources invested in sexual reproduction is in part determined by how well adapted the genotype is to a given environment. In C. neoformans, unisexual reproduction often involves increased pheromone expression. Hence, we hypothesized that the wild type may produce a basal level of the mating pheromone MFα in YPD agar that is sufficient to slow a proportion of the population in the G1 phase of the cell cycle, decreasing growth and resulting in a lower proportion than the mutant genotypes (17, 18). Nutrient deprivation conditions may similarly reduce the growth of both the wild type and the mutant isolates. We tested whether the WT produces more pheromone than mutants when grown in YPD; however, we found no significant difference between the WT and the mutants in pheromone expression levels in YPD (see Fig. S3 in the supplemental material).
Hyphal forms are responsible for host recognition and also allow some pathogenic fungi to invade host tissues and escape immune cell control (19). Although hyphal forms of C. neoformans have not been commonly observed in host tissues (20), our results suggest that differentiation into hyphae upon unisexual reproduction may be advantageous in an environment outside the host, enabling increased access to nutrients, as well as pathogen dispersal. Also, the spores produced at the hyphal tips can be readily disseminated aerially or through other routes (21), further introducing progeny into new and distant environmental niches. Thus, the dispersal advantage of unisexual reproduction is entirely independent of and in addition to the commonly recognized advantages of sexual reproduction, which include genetic exchange, purging of deleterious mutations, and keeping pace with pathogens (22, 23, 24). Furthermore, our experimental paradigm directly tests the outcomes of a scenario where a mutant incapable of unisexual reproduction arises in a wild-type population in nature. The mutant, initially rare, may gain a short-term advantage by trading the unisexual reproduction ability for a yeast mitotic growth advantage, whereas being wild type may come at a modest cost in nutrient-replete environments; however, the wild type could prevail through advantages conferred by hypha and spore production.
This novel role of unisexual reproduction in C. neoformans is similar to the role that pseudohyphal growth may play in Saccharomyces cerevisiae in response to nutrient limitation or mating pheromones (25, 26) and in C. neoformans in response to natural predators (27). Pseudohyphae in S. cerevisiae provide an opportunity to forage for nutrients and locate mating partners (28, 29); in the case of C. neoformans, they enable escape from predators. Likewise, dispersal via unisexual reproduction may also enhance the ability of C. neoformans to locate distant mating partners that may be genetically diverse, thereby introducing useful genetic variation into the population and avoiding the inbreeding depression caused by selfing.
Interestingly, the ascomycete C. albicans was recently found to also be capable of unisexual reproduction (30). C. albicans undergoes a parasexual cycle that may provide an alternative means for chromosome assortment and ploidy reduction other than meiosis (31); however, it may lack the dispersal advantages afforded by the production of spores during unisexual reproduction. Our results demonstrate that the novel unisexual cycle in C. neoformans represents an evolutionarily successful strategy that combines the benefits of sexual reproduction and of dispersal, providing advantages in terms of fecundity, adaptive genetic diversity, and long-term survival.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by NIH/NIAID R37 grant AI39115-15 to J.H.
Footnotes
Published ahead of print 21 June 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/EC.00147-13.
REFERENCES
- 1. Kwon-Chung KJ, Bennett JE. 1978. Distribution of α and a mating types of Cryptococcus neoformans among natural and clinical isolates. Am. J. Epidemiol. 108:337–340 [DOI] [PubMed] [Google Scholar]
- 2. Hull CM, Heitman J. 2002. Genetics of Cryptococcus neoformans. Annu. Rev. Genet. 36:557–615 [DOI] [PubMed] [Google Scholar]
- 3. Lin X, Heitman J. 2006. The biology of the Cryptococcus neoformans species complex. Annu. Rev. Microbiol. 60:69–105 [DOI] [PubMed] [Google Scholar]
- 4. Lin X, Hull CM, Heitman J. 2005. Sexual reproduction between partners of the same mating type in Cryptococcus neoformans. Nature 434:1017–1021 [DOI] [PubMed] [Google Scholar]
- 5. Kozubowski L, Heitman J. 2012. Profiling a killer, the development of Cryptococcus neoformans. FEMS Microbiol. Rev. 36:78–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davidson RC, Nichols CB, Cox GM, Perfect JR, Heitman J. 2003. A MAP kinase cascade composed of cell type specific and non-specific elements controls mating and differentiation of the fungal pathogen Cryptococcus neoformans. Mol. Microbiol. 49:469–485 [DOI] [PubMed] [Google Scholar]
- 7. Lin X, Jackson JC, Feretzaki M, Xue C, Heitman J. 2010. Transcription factors Mat2 and Znf2 operate cellular circuits orchestrating opposite- and same-sex mating in Cryptococcus neoformans. PLoS Genet. 6:e1000953. 10.1371/journal.pgen.1000953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kruzel EK, Giles SS, Hull CM. 2012. Analysis of Cryptococcus neoformans sexual development reveals rewiring of the pheromone-response network by a change in transcription factor identity. Genetics 191:435–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gilchrist MA, Sulsky DL, Pringle A. 2006. Identifying fitness and optimal life-history strategies for an asexual filamentous fungus. Evolution 60:970–979 [PubMed] [Google Scholar]
- 10. Pringle A, Taylor J. 2002. The fitness of filamentous fungi. Trends. Microbiol. 10:474–481 [DOI] [PubMed] [Google Scholar]
- 11. Xue C, Tada Y, Dong X, Heitman J. 2007. The human fungal pathogen Cryptococcus can complete its sexual cycle during a pathogenic association with plants. Cell Host Microbe 1:263–273 [DOI] [PubMed] [Google Scholar]
- 12. Kent CR, Ortiz-Bermudez P, Giles SS, Hull CM. 2008. Formulation of a defined V8 medium to induce sexual development of Cryptococcus neoformans. Appl. Environ. Microbiol. 74:6248–6253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fu J, Morris IR, Wickes BL. 2013. The production of monokaryotic hyphae by Cryptococcus neoformans can be induced by high temperature arrest of the cell cycle and is independent of same-sex mating. PLoS Pathog. 9:e1003335. 10.1371/journal.ppat.1003335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Velagapudi R, Hsueh YP, Geunes-Boyer S, Wright JR, Heitman J. 2009. Spores as infectious propagules of Cryptococcus neoformans. Infect. Immun. 77:4345–4355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lang GI, Murray AW, Botstein D. 2009. The cost of gene-expression underlies a fitness trade-off in yeast. Proc. Natl. Acad. Sci. U. S. A. 106:5755–5760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schoustra S, Rundle HD, Dali R, Kassen R. 2010. Fitness-associated sexual reproduction in a filamentous fungus. Curr. Biol. 20:1350–1355 [DOI] [PubMed] [Google Scholar]
- 17. Courchesne WE, Kunisawa R, Thorner J. 1989. A putative protein kinase overcomes pheromone-induced arrest of cell cycling in Saccharomyces cerevisiae. Cell 22:1107–1119 [DOI] [PubMed] [Google Scholar]
- 18. Bücking-Throm E, Duntze W, Hartwell LH, Manney TR. 1973. Reversible arrest of haploid yeast cells at the initiation of DNA synthesis by a diffusible sex factor. Exp. Cell Res. 76:99–110 [DOI] [PubMed] [Google Scholar]
- 19. Bastidas RJ, Heitman J. 2009. Trimorphic stepping stones pave the way to fungal virulence. Proc. Natl. Acad. Sci. U. S. A. 106:351–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alspaugh JA, Davidson RC, Heitman J. 1999. Morphogenesis of Cryptococcus neoformans, p 217–235 In Ernst JF, Schmidt A. (ed), Dimorphism in human pathogenic and apathogenic yeasts. Karger, Basel, Switzerland [Google Scholar]
- 21. Roper M, Seminara A, Bandi MM, Cobb A, Dillard HR, Pringle A. 2010. Dispersal of fungal spores on a cooperatively generated wind. Proc. Natl. Acad. Sci. U. S. A. 107:17474–17479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heitman J, Sun S, James TY. 2013. Evolution of fungal sexual reproduction. Mycologia 105:1–27 [DOI] [PubMed] [Google Scholar]
- 23. Jokela J, Dybdahl MF, Lively CM. 2009. The maintenance of sex, clonal dynamics, and host-parasite coevolution in a mixed population of sexual and asexual snails. Am. Nat. 174:S43–S53 [DOI] [PubMed] [Google Scholar]
- 24. Morran LT, Schmidt OG, Gelarden IA, Parrish RC, II, Lively CM. 2011. Running with the red queen: host-parasite coevolution selects for biparental sex. Science 333:216–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee SC, Phadke S, Sun S, Heitman J. 2012. Pseudohyphal growth of Cryptococcus neoformans is a reversible dimorphic transition in response to ammonium that requires the Amt1/2 ammonium permeases. Eukaryot. Cell 11:1391–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gimeno CJ, Ljungdahl PO, Styles CA, Fink GR. 1992. Unipolar cell divisions in the yeast Saccharomyces cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell 68:1077–1090 [DOI] [PubMed] [Google Scholar]
- 27. Magditch DA, Liu TB, Xue C, Idnurm A. 2012. DNA mutations mediate microevolution between host-adapted forms of the pathogenic fungus Cryptococcus neoformans. PLoS Pathog. 8:e1002936. 10.1371/journal.ppat.1002936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Erdman S, Snyder M. 2001. A filamentous growth response mediated by the yeast mating pathway. Genetics 159:919–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roberts CJ, Nelson B, Marton MJ, Stoughton R, Meyer MR, Bennett HA, He YD, Dai H, Walker WL, Hughes TR, Tyers M, Boone C, Friend SH. 2000. Signaling and circuitry of multiple MAPK pathways revealed by a matrix of global gene expression profiles. Science 287:873–880 [DOI] [PubMed] [Google Scholar]
- 30. Forche A, Alby K, Schaefer D, Johnson AD, Berman J, Bennett RJ. 2008. The parasexual cycle in Candida albicans provides an alternative pathway to meiosis for the formation of recombinant strains. PLoS Biol. 6:e110. 10.1371/journal.pbio.0060110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alby K, Schaefer D, Bennett RJ. 2009. Heterothallic and homothallic mating in the opportunistic pathogen Candida albicans. Nature 460:890–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Feretzaki M, Heitman J. Genetic circuits that govern bisexual and unisexual reproduction in Cryptococcus neoformans. PLoS Genet., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.