Abstract
Candida albicans forms two types of biofilm in RPMI 1640 medium, depending upon the configuration of the mating type locus. In the prevalent a/α configuration, cells form a biofilm that is impermeable, impenetrable by leukocytes, and fluconazole resistant. It is regulated by the Ras1/cyclic AMP (cAMP) pathway. In the a/a or α/α configuration, white cells form a biofilm that is architecturally similar to an a/α biofilm but, in contrast, is permeable, penetrable, and fluconazole susceptible. It is regulated by the mitogen-activated protein (MAP) kinase pathway. The MTL-homozygous biofilm has been shown to facilitate chemotropism, a step in the mating process. This has led to the hypothesis that specialized MTL-homozygous biofilms facilitate mating. If true, then MTL-homozygous biofilms should have an advantage over MTL-heterozygous biofilms in supporting mating. We have tested this prediction using a complementation strategy and show that minority opaque a/a and α/α cells seeded in MTL-homozygous biofilms mate at frequencies 1 to 2 orders of magnitude higher than in MTL-heterozygous biofilms. No difference in mating frequencies was observed between seeded patches of MTL-heterozygous and MTL-homozygous cells grown on agar at 28°C in air or 20% CO2 and at 37°C. Mating frequencies are negligible in seeded patches of both a/α and a/a cells, in contrast to seeded biofilms. Together, these results support the hypothesis that MTL-homozygous (a/a or α/α) white cells form a specialized “sexual biofilm.”
INTRODUCTION
Candida albicans is the most pervasive fungal pathogen colonizing humans (1). As is the case with bacterial pathogens (2–4), it can colonize host tissues, catheters, and prosthetics by forming multicellular biofilms. However, unlike bacterial pathogens, C. albicans forms two types of biofilms, depending upon the genetic configuration of its mating type locus (MTL) (5). In nature, C. albicans is primarily heterozygous at the MTL locus (a/α) (6–9). To mate, it must first undergo homozygosis to a/a or α/α (10, 11) and then switch from the white to the opaque phenotype (12). This transition also appears to be required for mating of rare haploid cells (13). When in the predominant a/α configuration, cells form robust biofilms on silicone elastomers in RPMI medium (5, 14, 15). Development of these a/α biofilms includes the formation of a basal yeast cell polylayer at the substratum, which forms germ tubes at the distal (top) edge. These germ tubes elongate to form a thick upper layer of vertically oriented hyphae embedded in an extracellular, polymolecular matrix (16, 17). MTL-heterozygous a/α biofilms formed under these conditions have been shown to be impermeable to low- and high-molecular-weight molecules, impenetrable by human polymorphonuclear leukocytes (PMNs), and resistant to fluconazole (5, 18). These traits are consistent with those exhibited by commensal and pathogenic bacterial biofilms (3, 4, 19).
In the predominant a/α configuration, C. albicans cells express the a1-α2 corepressor, which blocks white-opaque switching (7, 12) and mating (10, 11). Upon homozygosis, however, a/a and α/α cells express only a1 or α2, respectively. White-opaque switching and mating are then derepressed because of the absence of the a1-α2 corepressor (7, 12). In the derepressed state, white MTL-homozygous cells must switch to opaque to mate (7, 12, 20). In this derepressed state, white cells, but not opaque cells, form robust biofilms on silicone elastomers in RPMI 1640 medium, which are architecturally similar to a/α biofilms (21–25). They too are composed of a basal yeast cell polylayer and a larger upper region consisting of vertically oriented hyphae embedded in a dense extracellular polymolecular matrix. However, in contrast to a/α biofilms, these MTL-homozygous white cell biofilms do not exhibit the several traits deemed relevant to commensalism and pathogenesis. They are permeable to low- and high-molecular-weight molecules, readily penetrated by phagocytic human white blood cells, and drug susceptible (5, 18, 26). Although they do not exhibit pathogenic traits of a/α biofilms, they have been shown to facilitate chemotropism between the conjugation tubes formed by seeded minority opaque cells, a major step in the mating process (21). The facilitation of mating is consistent with the signal transduction pathway that regulates MTL-homozygous white cell biofilm formation. Whereas MTL-heterozygous biofilm formation is regulated by the Ras1/cyclic AMP (cAMP) pathway (5, 27, 28), MTL-homozygous biofilm formation is regulated by the same pheromones, pheromone receptors, trimeric G protein complex, and mitogen-activated protein (MAP) kinase pathway that regulate the mating response in opaque cells (5, 24–26). The same MAP kinase pathway, however, targets different transcription factors in pheromone-activated white and opaque cells for different phenotypic outcomes, namely, the formation of a white cell biofilm in the former case and the mating response in the latter case. The formation of a white cell biofilm, therefore, is coordinately regulated with the opaque cell mating response by the same signal. This would allow mating-competent opaque cells to signal mating-incompetent white cells to form a sexual biofilm that facilitates mating.
If our general hypothesis is correct, MTL-homozygous biofilms should be far more effective in supporting mating between minority opaque cells than MTL-heterozygous biofilms. Here, using a complementation strategy, we have tested this prediction by performing a comparison of the frequency of mating between minority opaque cells seeded in MTL-heterozygous biofilms and those in MTL-homozygous biofilms. We show that the frequency of complementation in MTL-homozygous biofilms is approximately 10 to >100 times higher than in MTL-heterozygous biofilms, supporting the proposed hypothesis. We further show that there is very little difference in mating frequency between seeded MTL-heterozygous and MTL-homozygous cells grown as patches on nutrient agar under the same conditions as biofilms. These patches consist almost entirely of budding yeast cells. The latter results support the conclusion that the sexual MTL-homozygous biofilm indeed provides a specialized environment that facilitates the mating process.
MATERIALS AND METHODS
Strains.
The a/α strain CAI4 was auxotrophic for URA3 (Δura3/Δura3) (29), the a/a strain GH11 was auxotrophic for URA3 (Δura3/Δura3), and the α/α strain Red3/6 (30) was auxotrophic for ADE2 (Δade2/Δade2). The origins and genetic descriptions of all strains can be found in Table S1 in the supplemental material.
Analysis of mating in biofilms.
All strains used here were auxotrophic and therefore were grown to stationary phase at 28°C in suspension in modified Lee's medium (31) supplemented with adenine (30 μg/ml) and uridine (100 μg/ml). Cells were collected, washed, and resuspended in RPMI 1640 medium (Gibco, Grand Island, NY) supplemented with adenine and uridine. Cells were then mixed in various combinations for a total of 2 × 107 cells in 1 ml of RPMI 1640 medium supplemented with adenine and uridine, and 2 ml of cell suspension was dispersed on silicon elastomer discs (0.1 cm thick, 1 cm in diameter) (Cardiovascular Instrument Corp., Wakefield, MA). The discs were inserted into the wells of a Costar 24-well cluster plate. The elastomer-supported cultures were incubated for 1.5 h at 28°C. The elastomer preparations were then gently rinsed with phosphate buffer solution (PBS) and transferred into wells of a Costar 12-well cluster plate. Each well was filled with 2.5 ml of RPMI medium supplemented with adenine and uridine and placed onto rockers which swayed back and forth gently at a low angle. Incubation was performed under four sets of conditions: 28°C in air, 28°C in 20% CO2, 37°C in air, and 37°C in 20% CO2. After 48 h, cells were removed from the elastomer disc by sonicating the preparations three times for 5 s at 70% amplitude (Vibra Cell Sonic and Materials, Inc.) in 1 ml of Hanks' balanced salt solution (HBSS; Grand Island, NY) without cations and supplemented with 20 mM EDTA. Aliquots were then plated onto agar selection plates that were 10 cm in diameter. Selection agar contained modified Lee's medium lacking adenine and uridine, which allowed assessment of the frequency of complementation (i.e., viable colonies). In select experiments, diluted aliquots were plated onto agar plates 10 cm in diameter containing modified Lee's medium supplemented with adenine and uridine (nonselection plates), to assess the total CFU, and with phloxine B, to score the number of opaque CFU (32). Each experiment included 3 biofilms under each set of conditions. Each biofilm was analyzed on three selection plates containing Lee's medium lacking adenine and uridine, resulting in nine measurements for each condition per experiment. The combined number of plates for four independent experiments was 12. Frequency was computed as the number of complemented CFU per biofilm or, in select experiments, per opaque CFU.
PCR analysis of the MTL configuration.
PCR was used to analyze the heterozygosity of the MTL locus of complemented colonies. PCR for MTLa1 was performed with primer pair MTLa1F (5′-TTGAAGCGTGAGAGGCAGGAG-3′) and MTLa1R (5′-GATTAGGCTGTTTGTTCTTCTCG-3′), and PCR for MTLα2 was performed with primer pair MTLα2F (5′-CATGAATTCACGTCTGGAGGCAC-3′) and MTLα2R (5′-AAGCAGCCAACTCAGGTCAC-3′).
FACS analysis of DNA content.
The DNA content of cells from individual complemented colonies was measured by fluorescence-activated cell sorting (FACS), as described by Paulovich and Hartwell (33), with minor modifications. Cells were harvested from colonies grown for 15 days (i.e., to stationary phase) on selection medium and washed twice in water. The size of colonies analyzed ranged between 5 and 6 mm. Cells were fixed in 1 ml of 95% ethanol overnight at 4°C, washed twice with 50 mM sodium citrate (pH 7.4), and incubated in 50 mM sodium citrate containing 0.25 mg/ml RNase A for 1 h at 50°C. Cells were then treated with proteinase K (20 mg/ml) for 1 h and then stained with propidium iodide (10 μg/ml) in 2 ml of 50 mM sodium citrate. As a control for diploid (2N) and tetraploid (4N) DNA content, strain SC5314 (a/α) was grown to mid-log phase (optical density at 600 nm [OD600] = 0.6) in 10 ml of yeast extract-peptone-dextrose (YPD) medium. An aliquot of 100 μl of stained cells was diluted in 2 ml of 50 mM sodium citrate. FACS was performed on 50,000 cells by using a BD FACSCalibur instrument. The FACScan fluorescence of propidium iodide was excited with a 488-nm argon laser and emitted at 585 nm.
Microscopic analysis of conjugation tubes and fusions.
To image conjugation tubes and shmoos, cells were stained with calcofluor (20). To image fusions in biofilms, we first generated MTL-homozygous a/a and α/α strains that expressed either mCherry or green fluorescent protein (GFP) in the opaque state so that fusants expressed both fluorescent molecules. To accomplish this, the mCherry and GFP genes were individually placed under the regulation of the promoter of the opaque-specific gene OP4 in plasmids pGFP-SAT and pmCherry-SAT, respectively. Plasmid pGFP-SAT was generated by exchanging the SalI-PstI-digested GFP-URA3 fragment in plasmid pGFP70 (34) with the SalI-PstI-digested GFP-caSAT1 fragment from plasmid pNIM1 (35). Plasmid pmCherry-SAT was constructed by substituting mCherry for GFP in plasmid pGFP-SAT. mCherry was obtained by PCR amplification of plasmid pEpGAP-Cherry (36) with primer pair mCherry-1 (5′-GGTTTCAAAAGGTGAAGAAG-3′) and mCherry-2 (5′-ATATGGATCCTTATTTATATAATTCATCCATACC-3′). All sequences were verified. The XbaI-SacI-restricted DNA fragment of each plasmid was then inserted into one of the OP4 alleles of strain GH11 or Red3/6.
MTL-heterozygous or MTL-homozygous biofilms were cast by adding 5% mCherry-expressing α/α opaque cells plus 5% GFP-expressing a/a opaque cells. Biofilms were either fixed in 2% paraformaldehyde and stained with calcofluor (Fluorescent Brightener 28; Sigma) or disrupted in 100 μl of HBSS and stained with calcofluor. Biofilms were imaged by using a Bio-Rad Radiance 2100 MP multiphoton laser scanning confocal microscope equipped with a 20× Plan Fluor water immersion objective and a Nikon TE 2000 microscope. Simultaneous GFP (488-nm excitation/515-nm emission), mCherry (587-nm excitation/610-nm emission), and calcofluor (800-nm excitation/420-nm emission) fluorescent images were acquired as a z-series at 3-μm intervals through areas where fusions occurred in biofilms. Separation of the emissions of the three fluors was achieved by using LASERSHARP 2000 software (Bio-Rad, Hercules, CA) with lambda strobbing to minimize bleedthrough in simultaneous image scan acquisitions. The MicroFire digital camera and software were used for epifluorescence imaging.
Patch analysis.
A total of 2 × 107 cells grown to stationary phase in modified Lee's medium (31) supplemented with uridine and adenine, either unseeded or seeded with opaque cells as in the case of biofilm formation, were suspended in 20 μl of PBS and spotted onto agar containing modified Lee's medium supplemented with adenine and uridine. After 48 h of incubation at 28°C or 37°C in air or 20% CO2, cells from each patch were resuspended in 1 ml of PBS. To assess complementation, 100 μl of undiluted cells was plated onto nutrient agar lacking uridine and adenine. To measure total CFU and opaque CFU, 100 μl of a serial dilution of 10−3, 10−4, and 10−5 cells was spread onto agar containing Lee's medium supplemented with adenine and uridine.
RESULTS
Complementation strategy.
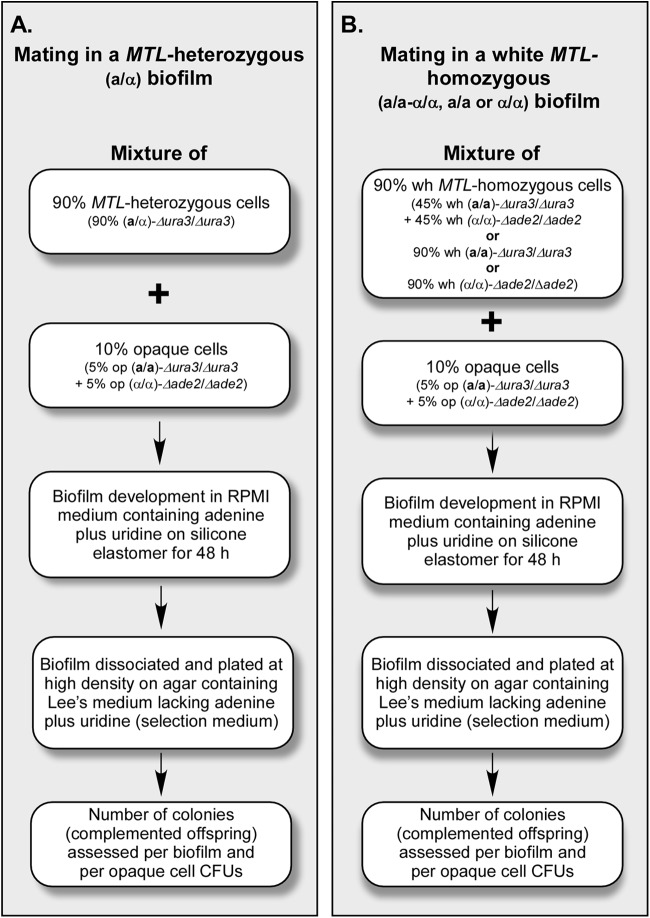
A complementation strategy was used to compare the mating frequencies of seeded minority opaque cells between MTL-heterozygous (Fig. 1A) and MTL-homozygous (Fig. 1B) biofilms. Both majority (90%) MTL-heterozygous and majority (90%) white MTL-homozygous biofilms were cast with minority opaque cells (5% opaque a/a and 5% opaque α/α cells) in a similar fashion. Both majority and minority cells were auxotrophic. The a/α cells were auxotrophic for uridine (Δura3/Δura3), the white and opaque a/a cells were auxotrophic for uridine (Δura3/Δura3), and the white and opaque α/α cells were auxotrophic for adenine (Δade2/Δade2) (see Table S1 in the supplemental material for genotypes and history). There were three types of MTL-homozygous white biofilms cast, white a/a-α/α (half a/a and half α/α white cells), white a/a, and white α/α. Majority cell populations (a/α cells or MTL-homozygous white cells) to which minority opaque cells were added are referred to as “seeded,” and those to which no opaque cells were added are referred to as “unseeded.” For assessing the frequency of complementation (i.e., fusion), biofilms were first developed in RPMI medium (37) supplemented with uridine and adenine (nonselection medium) on silicone elastomer discs for 48 h and then mechanically dissociated, and the same volume of cell suspension was plated at a high density on agar containing modified Lee's medium (31, 38), a defined medium, lacking uridine and adenine (selection medium). On selection plates, only cells that have become prototrophic for both uridine and adenine (i.e., putative fusion products [a/α] of opaque a/a cells [Δura3/Δura3] and opaque α/α cells [Δade2/Δade2]) can form colonies. Cell suspensions were also diluted and plated onto nonselection plates containing phloxine B. The latter platings provided us with the total concentration of CFU per biofilm and the concentration of phloxine B-stained opaque CFU per biofilm. Complementation was tested under four sets of conditions: 28°C in air, 28°C in 20% CO2, 37°C in air, and 37°C in 20% CO2. Under each test condition in each individual experiment, three biofilms were cast, and after 48 h under each set of conditions, biofilms were disrupted, an aliquot of each biofilm suspension was plated onto selection plates, and the plates were scored for the number of complementation events. This resulted in nine measures of complementation per condition per experiment. Four experiments were performed, three of which were performed under all four conditions and one of which was analyzed in air and in 20% CO2 only at 37°C.
Fig 1.
Strategy used to compare the frequencies of complementation events per biofilm between MTL-heterozygous (a/α) (A) and MTL-homozygous (a/a-α/α, a/a, and α/α) (B) biofilms. For opaque-seeded biofilms, either 90% MTL-heterozygous (a/α) cells or 90% MTL-homozygous (50% a/a plus 50% α/α [a/a-α/α], 100% a/a, or 100% α/α) white (wh) cells were mixed with 5% opaque a/a and 5% opaque α/α cells, and the mixture was cast on a silicone elastomer. Controls included unseeded 100% a/α or 100% a/a-α/α cells. After 48 h of development in RPMI medium supplemented with uridine and adenine under the four sets of conditions described, the biofilms were dispersed, and the cells were plated at a high density onto agar containing medium lacking uridine and adenine (selection plates). The frequency of complementation per biofilm was then assessed. Diluted cells were also plated onto agar containing Lee's medium supplemented with uridine and adenine (nonselection plates) plus phloxine B to assess total CFU and to distinguish opaque CFU by phloxine B staining.
Cell composition and architecture of biofilms.
The basic architectures were similar for MTL-heterozygous and MTL-homozygous biofilms seeded or unseeded with minority a/a and α/α opaque cells. After 48 h, all biofilms contained a basal yeast cell polylayer, from which extended vertically oriented hyphae, which have been shown to be embedded in a uniform extracellular matrix (5, 14–16, 39). MTL-heterozygous biofilms, both seeded and unseeded, were on average slightly thicker (10 to 20%) than MTL-homozygous biofilms (see Table S2 in the supplemental material). Seeding caused a 10% increase in the thickness of MTL-homozygous biofilms but not MTL-heterozygous biofilms (see Table S2 in the supplemental material). Because the cells in the upper regions of biofilms were composed of hyphae embedded in a dense matrix, mechanical disruption resulted in multicompartmented hyphal fragments plus matrix, which behaved as single CFU when plated. Opaque cells, however, were individually released when biofilms were disrupted (data not shown). The density of opaque cells in mature biofilms was consistently only slightly higher in MTL-homozygous than in MTL-heterozygous biofilms under the four tested sets of conditions (see Table S4 in the supplemental material). Based on these observations, we have computed the frequencies of complementation as the number of complementation events not only per biofilm but also per opaque CFU. Because the majority of cells in the upper biofilm layers are hyphal, total CFU do not represent total cell compartments and provide inconsistent values. We therefore did not use total CFU to calculate complementation frequencies.
Complementation in unseeded control biofilms.
As was expected, no prototrophic colonies were observed when preparation of disrupted unseeded a/α biofilms were plated onto selection plates (Tables 1 and 2). This was true for all four experiments under all four conditions. However, when preparations of disrupted unseeded a/a-α/α biofilms were plated similarly, low complementation frequencies were observed, ranging on average from 0 to 0.8 per biofilm under the four test conditions (Table 1). This was expected given that opaque cells are formed in white cell populations through spontaneous white-to-opaque switching (32, 40–42). The concentration of spontaneously appearing opaque cells in unseeded a/a-α/α biofilms ranged from 3 × 104 to 1 × 105 per biofilm (see Table S4 in the supplemental material). This suggested that after 48 h of development, no more than 4% of opaque cells in seeded MTL-homozygous biofilms were due to spontaneous switching of majority white cells.
Table 1.
Mean frequencies of complementation events per MTL-heterozygous and MTL-homozygous biofilmsa
| Genotype of majority cells | Addition of 5% op a/a + 5% op α/α | Mean no. of complementation events per biofilm ± SD |
|||
|---|---|---|---|---|---|
| 28°C |
37°C |
||||
| Air | 20% CO2 | Air | 20% CO2 | ||
| a/α | − | 0 | 0 | 0 | 0 |
| + | 2.4 ± 1.4 | 1.3 ± 1.4 | 0 | 0.5 ± 0.6 | |
| a/a-α/α | − | 0 | 0.7 ± 1.2 | 0.8 ± 1.1 | 0.6 ± 1.2 |
| + | 22.6 ± 9.5 | 30.2 ± 12.2 | 34.9 ± 27.3 | 43.2 ± 29.1 | |
| a/a | + | 20.4 ± 12.2 | 21.0 ± 4.7 | 25.4 ± 25.7 | 33.1 ± 23.9 |
| α/α | + | 56.7 ± 68.8 | 102.5 ± 63.6 | 104.7 ± 88.8 | 163.6 ± 139.9 |
The mean frequencies were computed from the average frequencies from four separate experiments. The average frequencies per experiment were then used to compute the mean and standard deviation for each cell mixture and condition. In each experiment, three samples were analyzed from each of three independent biofilms. In one of the four experiments, biofilms were analyzed only at 37°C. op, opaque.
Table 2.
Comparison of MTL-heterozygous and MTL-homozygous complementation frequencies computed per biofilm
| Comparison | Ratio of complementation frequencies |
||||
|---|---|---|---|---|---|
| 28°C |
37°C |
||||
| Air | 20% CO2 | Air | 20% CO2 | ||
| 9× | 23× | >35× | 86× | ||
| 9× | 16× | >25× | 66× | ||
| 24× | 79× | >105× | 328× | ||
Complementation in seeded biofilms.
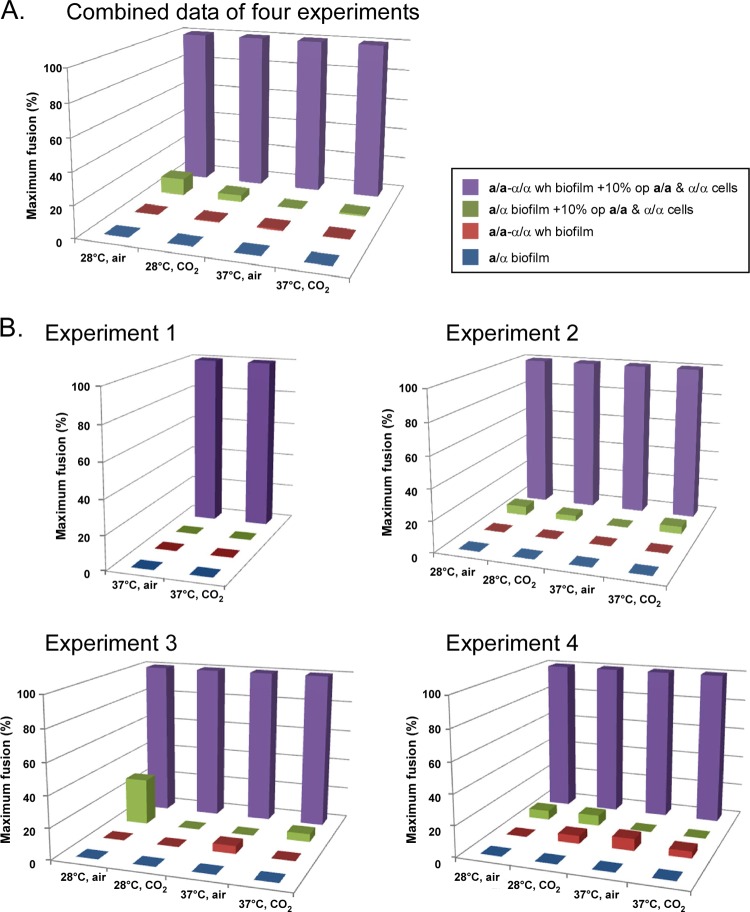
The mean numbers of complementation events per a/α biofilm seeded with a/a and α/α opaque cells under the four sets of conditions were 2.4 ± 1.4, 1.3 ± 1.4, 0, and 0.5 ± 0.6, respectively (Table 1). In contrast, the mean numbers of complementation events per seeded a/a-α/α biofilms were 22.6 ± 9.5, 30.2 ± 12.2, 34.9 ± 27.3, and 43.2 ± 29.1, respectively (Table 1). The numbers of complementation events per seeded a/a-α/α biofilm were therefore 9, 23, >35, and 86 times those of similarly seeded a/α biofilms for the four sets of test conditions (Table 2). The mean number of complementation events per seeded a/a biofilm was similar to that per seeded a/a-α/α biofilm, whereas the mean number of complementation events per seeded α/α biofilm was higher for each tested condition (Table 1). The differences between the complementation frequencies in MTL-heterozygous biofilms and those in MTL-homozygous biofilms are clearly demonstrated by bar graphs in which the average number of complementation events per biofilm was normalized to the highest value attained (100%) under the four sets of conditions (Fig. 2). In Fig. 2A, this has been done for the combined data from experiments 1 to 4, and in Fig. 2B, this has been done for the data for each of the four experiments. In lieu of error bars, one can assess the standard deviations of the data that were normalized in Table 1, and the P values were computed by the Student t test for the combined data from the four experiments between seeded a/a-α/α and a/α biofilms (see Table S3 in the supplemental material). These P values were highly significant for the four sets of test conditions (P values of <0.0001, <0.0001, 0.0003, and <0.0001) (see Table S3 in the supplemental material).
Fig 2.
Histogram comparing complementation frequencies between a/α and a/a-α/α biofilms under the four tested conditions. The pooled data from four experiments (A) and the data for each experiment (B) have been normalized to the highest complementation value (100%) under each test condition. The standard deviations of the mean values of frequencies for the four experiments can be found in Table 1. A table of the P values demonstrating that the differences between the normalized mean frequencies of complementation per a/α biofilm and a/a-α/α biofilm (A) are significant is presented in Table S3 in the supplemental material. This table is in lieu of error bars. A key defining the different MTL genotypes of the biofilms is presented in the box in panel A.
MTL-heterozygous and MTL-homozygous biofilms contained similar concentrations of opaque CFU (see Table S4 in the supplemental material), allowing us to compute and compare the frequencies of complementation events as a function of opaque CFU. Although the differences between a/α and a/a-α/α biofilms were highly significant, the error bars for the bar graphs were relatively high (see standard deviations in Table 1). Thus, additions of error bars to the three-dimensional (3D) plots in Fig. 2 distracted from the main point of the plots, the differences in mean frequencies. The complementation frequencies in MTL-homozygous biofilms, computed as a function of opaque CFU, were again far higher than those in MTL-heterozygous biofilms. At 28°C, the differences between MTL-homozygous (a/a-α/α, a/a, and α/α) and MTL-heterozygous (a/α) biofilms ranged between 50- and 140-fold in air and between 22- and 32-fold in 20% CO2 (see Table S5 in the supplemental material). At 37°C, the differences ranged between 4- and 8-fold in air and 5- and 16-fold in 20% CO2 (see Table S5 in the supplemental material). The differences were again found to be significant by using the Student t test (data not shown). Together, the results demonstrate that even though a/α and MTL-homozygous biofilms are architecturally similar and retain similar numbers of seeded opaque cells at maturity, the frequencies of complementation under all four conditions were far higher in MTL-homozygous biofilms.
Complementation occurs in biofilms and not on selection plates.
We have assumed in the analysis described above that complementation occurs in biofilms, but to assess complementation, cells were plated at a high density onto selection plates (Fig. 1). To verify that the complementation events occurred in biofilms and not in the high-density cell preparations after they were spread onto selection plates, we plated fresh high-density mixtures of a/α cells containing 10% opaque cells (50:50 ratio of a/a and α/α) and a/a-α/α cells containing 10% opaque cells (50:50 ratio of a/a and α/α) directly onto selection plates, at the same high density used to test complementation in biofilms. No complementation occurred on multiple plates in three separate experiments in either seeded a/α or seeded a/a-α/α plates, even after 8 days, demonstrating that in the experimental protocol that we used (Fig. 1), complementation occurred in biofilms.
Complemented strains are a/α and vary in DNA content.
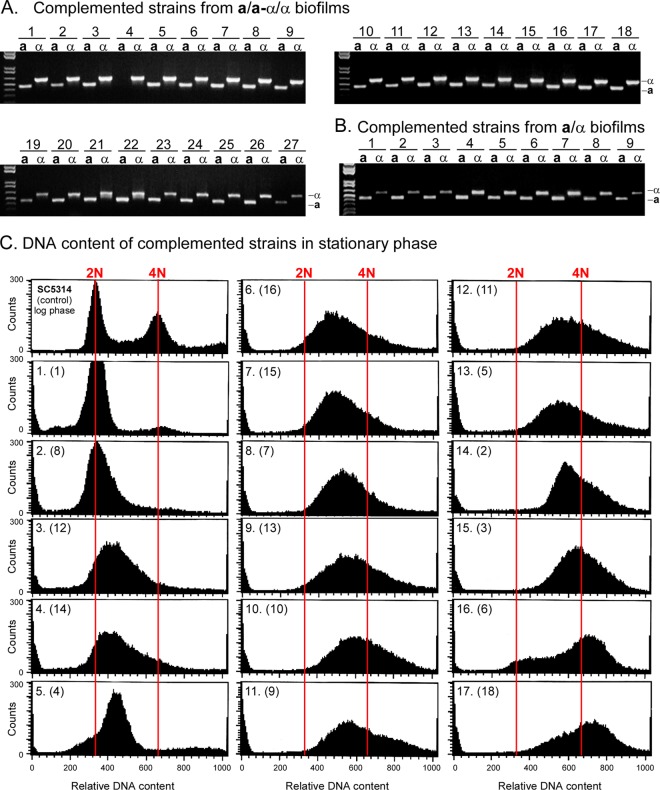
If the complementation of auxotrophic markers in biofilms indeed represents mating fusions, the immediate MTL configuration of the complementation products of opaque a/a (Δura3/Δura3) and α/α (Δade2/Δade2) cells should be a/α. As cells lose chromosomes in the transition to the diploid state (43), they would diversify to a/α, a/a, and α/α. The mating type locus resides on chromosome 5, while the genes for ADE2 and URA3 reside on chromosome 3 (http://www.candidagenome.org/). To test whether the mating type configurations of complemented strains (Δade2/ADE2 and Δura3/URA3) were in fact a/α, we tested cells from colonies that grew on selection plates for the presence of the genes MTLa1, which resides in the a copy of the MTL locus, and MTLα2, which resides in the α copy of the MTL locus (44), by PCR. Of 86 randomly selected complemented clones formed by opaque a/a and α/α cells in mixed white a/a-α/α biofilms, 83 were a/α, 1 was a/a, and 2 were α/α. In Fig. 3A, the MTL genotypes of 27 strains randomly selected from the 86 complemented strains are shown. One of these clones (clone 4) lacked the MTLa1 gene. Of 9 complemented isolates formed by opaque a/a and α/α cells in a/α biofilms, all were MTL heterozygous (Fig. 3B). These results demonstrate that the great majority of complemented strains obtained in either a/α or a/a-α/α biofilms were indeed the result of fusion between opaque a/a and α/α cells.
Fig 3.
The MTL genotype of the complemented strains was predominantly a/α (97%). The DNA contents varied between 2N and >4N. (A) PCR analyses of the MTL genotypes (MTLa1 and MTLα2) of 27 of the 86 complemented offspring tested from seeded MTL-homozygous (a/a-α/α) biofilms. Of the 86 complemented strains, 83 were a/α, 2 were α, and 1 was a. In the limited set shown, all but clone 4 were a/α. (B) PCR analysis of 9 complemented offspring from MTL-heterozygous biofilms. All proved to be a/α. (C) DNA content of complemented strains from MTL-homozygous biofilms analyzed by fluorescence-activated cell sorting (FACS). Cells of complemented strains were grown to stationary phase. As a control, the DNA content of a log-phase culture of strain SC5314 (a/α) was analyzed (top left) to obtain the relative positions of 2N and 4N DNA. The scans are ordered from diploid to tetraploid or greater. The strains analyzed in panel A for MTL configuration are presented in parentheses in panel C.
Since C. albicans is a diploid, fusions should result in tetraploids, which must then undergo chromosome reduction to return to the diploid state (43). To test this prediction, 17 random complemented clones from the set shown in Fig. 3A were grown for 15 days on selection plates to stationary phase so that they would accumulate in G1 (45). They were then analyzed by fluorescence-activated cell sorting (FACS) for DNA content. Strain SC5314 (a/α) collected in the mid-log phase of growth provided measures of cells with DNA contents of 2N and 4N (Fig. 3C, top left), which allowed us to assess the DNA contents of complemented clones through comparisons. The DNA contents of the 17 fusants in stationary phase ranged from 4N to 2N (Fig. 3C), suggesting progressive loss of chromosomes. Surprisingly, even strains which appeared diploid or near diploid by FACS were a/α (clones 1, 8, 12, and 14). This suggests that during chromosome loss, there may be a selective advantage for maintaining the a/α genotype (i.e., heterozygosity of chromosome 5).
Cell biology of complementation.
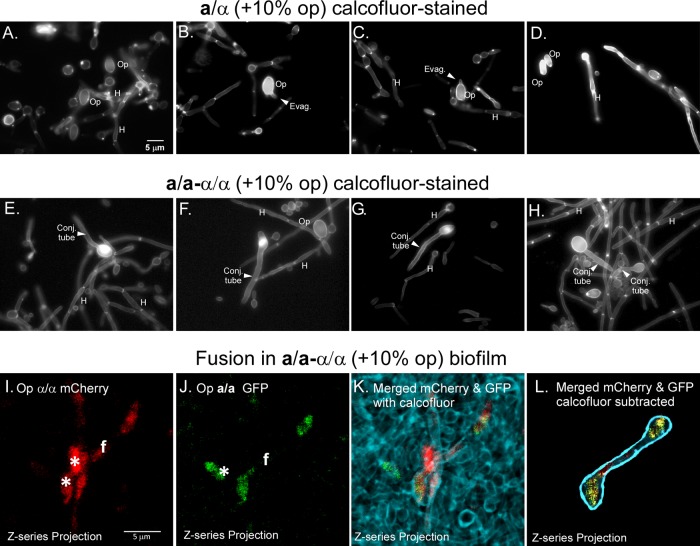
Given that complementation between opaque a/a and opaque α/α cells occurs in MTL-homozygous biofilms at frequencies approximately 1 to 2 orders of magnitude higher than in MTL-heterozygous biofilms, one would expect to observe a higher frequency of conjugation tubes and fusions in the former. Staining of cell preparations with calcofluor can be used to distinguish conjugation tubes from hyphae, given that the latter, but not the former, are compartmentalized by septae that stain intensely with calcofluor (20). In seeded a/α biofilms stained with calcofluor, large oblong opaque cells (Op) were readily identified. A minority of them formed wide evaginations, suggestive of shmoo formation (20) (Fig. 4A to D). Hyphae (H), the predominant phenotype, contained calcofluor-stained septae (Fig. 4A to D). However, long septum-free conjugation tubes were rarely observed in seeded a/α biofilms. In marked contrast, long septum-free conjugation tubes were readily distinguished in seeded white a/a-α/α biofilms (Fig. 4E to H). To visualize fusion in a/a-α/α biofilms, we generated an α/α strain with the fluorescent protein mCherry under the regulation of the OP4 promoter (Δade2/Δade2 OP4/op4::OP4p-mCherry) and an a/a strain with the fluorescent protein GFP also under the regulation of the OP4 promoter (Δura3/Δura3 OP4/op4::OP4p-GFP) (see Table S1 in the supplemental material). In the opaque state, α/α cells would express mCherry, and a/a cells would express GFP, since the OP4 promoter is selectively upregulated in the opaque phase (46). Fusants of opaque mCherry-expressing α/α cells and opaque GFP-expressing a/a cells would be both red (mCherry) and green (GFP), colors which can be separated by using laser scanning confocal microscopy (LSCM) and LASERSHARP 2000 software (Bio-Rad). Superimposing the two colored images would result in yellow when red and green are superimposed. Fusions exhibiting uniform red and green fluorescence were readily observed in white a/a-α/α biofilms seeded with 5% opaque α/α mCherry cells and 5% opaque a/a GFP cells. An example of two opaque cells connected by a conjugation bridge is presented in Fig. 4I to L. By simultaneously staining with calcofluor (Fig. 4K), we confirmed that a bridge existed between two cell bodies and that it contained no septae. In Fig. 4L, calcofluor staining is subtracted, and the fusant outlined is blue. Similar red and green fusants with long conjugation bridges could not be found in seeded a/α biofilms.
Fig 4.
Conjugation tubes and fusions between opaque a/a and opaque α/α cells are readily observed in MTL-homozygous (a/a-α/α) biofilms but not MTL-heterozygous (a/α) biofilms. (A to D) Representative epifluorescence images of calcofluor-stained cells in a/α biofilms that had been seeded with a 10% mixture of opaque a/a and opaque α/α cells. Hyphae (H), which make up the majority of cells, exhibited intensely stained septae, which are absent from conjugation tubes. Opaque cells (Op), which are larger than white cells, stained intensely with calcofluor. Evaginations (Evag) of opaque cells are suggestive of shmoo formation. However, conjugation tubes could be found only rarely in a/α biofilms. (E to H) Conjugation tubes (Conj tube) were readily observed emanating from opaque cells in MTL-homozygous (a/a-α/α) biofilms. These long conjugation tubes could be discriminated from hyphae by the lack of calcofluor-stained septae. (I to L) Example of a fusion between a GFP-expressing opaque a/a cell and mCherry-expressing opaque α/α cells in a white a/a-α/α biofilm. (I) Confocal z-series projection of mCherry staining of a fusant (f). Two individual opaque red α/α cells are in close contact with the fusant. (J) Confocal z-series projection of GFP of the same fusant. One opaque green a/a cell (star) is in close contact with the fusant. (K) Confocal z-series projection of mCherry and that of GFP for a fusant, embedded in calcofluor-stained cells, are imaged simultaneously in a preparation. Merging of red and green produces yellow. (L) The calcofluor-stained nonfusant cells in panels I and J have been subtracted from the images. A drawn-in dark blue outline delimits the edge of the entire fusion profile obtained by calcofluor staining of the same preparation shown in panel K. A z-series of six images was taken at 3-μm intervals for projections. Scale bars, 5 μm.
Complementation in patches.
If the difference in the facilitation of mating between MTL-heterozygous and MTL-homozygous biofilms was specifically a result of the formation of a configuration of the mating type locus and had nothing to do with differences in the specialized biofilm formed by MTL-homozygous cells, the same difference in mating frequencies between seeded MTL-heterozygous and MTL-homozygous biofilms should be observed in seeded patches of cells grown on agar. Alternatively, if differences in complementation frequencies were the result of differences in the alternative biofilms per se, the frequencies should be similar in seeded MTL-heterozygous and MTL-homozygous patches. To distinguish between these alternative possibilities, we seeded majority a/α cells, white a/a-α/α cells, white a/a cells, and white α/α cells with 5% opaque a/a and 5% opaque α/α cells, using the same auxotrophic strains described for biofilms in Fig. 1. The cell mixtures were then spotted onto agar containing Lee's medium supplemented with uridine and adenine (nonselection medium). These agar cultures were then incubated for 48 h under the same four conditions used to analyze biofilms. Cells in patches grown on agar had none of the architecture exhibited by biofilms in RPMI 1640 medium. They consisted almost exclusively of budding cells or disorganized mixtures of yeast cells and hyphae. Cells from patches were then suspended and plated at a high density onto selective medium to assess the frequency of complementation. Cells were also diluted and plated onto nonselection medium containing phloxine B to assess total CFU and opaque CFU. In the case of patches, the frequency of complementation events was computed as a function of CFU, since cells dissociated freely and even hyphae fragmented, probably as a result of the absence of matrix, although they still represented multicompartmented fragments when plated.
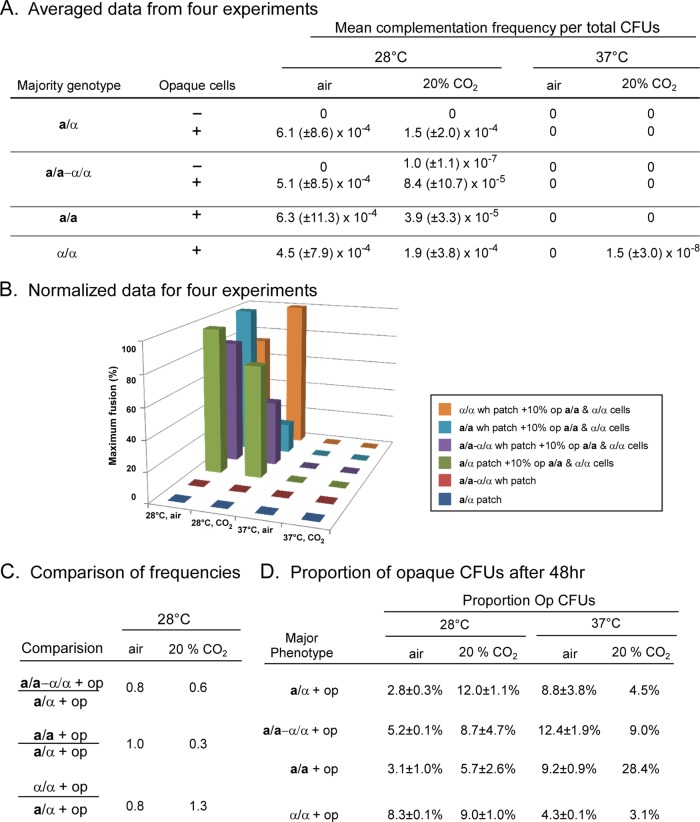
For control patches of unseeded a/α cells grown at 28°C in air or 20% CO2, no prototrophic colonies were obtained on selection plates, as expected (Fig. 5A). For seeded a/α patches, however, the mean frequencies of complementation events per total CFU at 28°C in air and in 20% CO2 were (6.1 ± 8.6) × 10−4 and (1.5 ± 2.0) × 10−4, respectively. In control patches of unseeded a/a-α/α cells, the mean frequency of complementation events per CFU was negligible (Fig. 5A). The mean frequencies of complementation in seeded MTL-homozygous patches (a/a-α/α, a/a, and α/α) grown at 28°C in air or in 20% CO2, however, were similar to those in seeded a/α patches (Fig. 5A). This is evident in bar graphs of normalized data (Fig. 5B). The ratios of complementation frequencies in patches grown at 28°C for seeded a/α versus seeded a/a-α/α, a/a, or α/α patches were 0.8, 1.0, and 0.8, respectively, in air and 0.6, 0.3, and 1.3, respectively, in 20% CO2 (Fig. 5C). In all cases, the differences in mean complementation frequencies between seeded a/α and MTL-homozygous patches proved to be nonsignificant (P value of >0.05) by using the Student t test.
Fig 5.
Frequencies of mating in patches of cells grown on agar containing the same mixtures as biofilms do not exhibit differences between seeded MTL-homozygous and seeded MTL-heterozygous patches. Patches were grown on agar containing nutrient medium supplemented with uridine and adenine for 48 h under the same four sets of conditions as those used for biofilms. (A) Mean frequency of complementation events per patch. The data are averaged from four separate experiments, as was performed for biofilms as described in Fig. 1. Complementation frequencies were computed per CFU. (B) Histogram comparing complementation frequencies in patches for normalized data, as was done in Fig. 2 for biofilms. The key for MTL genotypes is presented in a box. (C) Comparison of complementation frequencies per CFU. (D) Proportion of opaque CFU per patch after 48 h. The data for 37°C and 20% CO2 in panel D were derived from a single CFU obtained in one of four experiments. All data in panels A and D, except for data for 37°C and 20% CO2 in panel D, are presented as means ± standard deviations.
Surprisingly, there was negligible complementation at 37°C in either air or 20% CO2 in both seeded a/α and seeded MTL-homozygous (a/a-α/α, a/a, and α/α) patches (Fig. 5A). This was in direct contrast to seeded biofilms, in which complementation occurred on average at similar frequencies at 28°C and 37°C (Tables 1 and 2). Lack of complementation at 37°C in both a/α and MTL-homozygous patches was not due to a decrease in the concentration of opaque cells (Fig. 5D). There was, however, a difference in the levels of hypha formation between patches that formed at 28°C and patches that formed at 37°C, particularly in a/α patches (see Table S6 in the supplemental material). Cells in unseeded and seeded a/α patches were almost exclusively (>99%) in the yeast form at 28°C but primarily (90%) in the hyphal form at 37°C (see Table S6 in the supplemental material). Thus, one might conclude that hyphae formed in patches inhibited mating, but the results of MTL-homozygous patches did not support that conclusion. Cells in MTL-homozygous patches grown in air at 37°C were primarily (90 to 99%) in the yeast form (see Table S6 in the supplemental material), yet they still did not undergo complementation. Interestingly, at 37°C in air, seeded a/a-α/α and a/a patches contained low levels of shmoos and short conjugation tubes but did not contain fusants (see Table S6 in the supplemental material). Together, the results obtained at 28°C for patches indicate that the difference in mating frequencies between MTL-heterozygous and MTL-homozygous biofilms is not due simply to differences in the MTL configuration but rather to differences in the biofilms formed by cells of the alternative MTL configurations. Moreover, these results demonstrate that MTL-homozygous biofilms provide a specialized environment that facilitates mating at 37°C.
DISCUSSION
We previously demonstrated (5, 15) that when distributed on a silicone elastomer in RPMI 1640 medium, both stationary-phase MTL-heterozygous and stationary-phase white MTL-homozygous yeast cells become adhesive and cohesive, forming a basal polylayer of cells in the budding yeast form. This polylayer then gives rise to an upper layer of vertically oriented hyphae engulfed in an extracellular polymolecular matrix, as originally described by Hawser and Douglas (37). The upper layer accounts for over 80% of the depth of the biofilm. However, we found that even though the architecture appears similar, traits associated with pathogenic bacterial biofilms (2–4) are exhibited by MTL-heterozygous biofilms but not by MTL-homozygous biofilms (5, 15). Whereas a/α biofilms are impermeable to low- and high-molecular-weight molecules, impenetrable by human PMNs, and resistant to fluconazole, a/a and α/α biofilms are permeable, penetrable, and fluconazole susceptible (5, 15). So what role does a MTL-homozygous biofilm play? White a/a and α/α biofilms have been shown to facilitate chemotropism between seeded minority a/a and α/α opaque cells (21). Moreover, in RPMI 1640 medium, formation of an a/α biofilm is regulated by the Ras1/cAMP pathway, whereas a/a and α/α biofilms are regulated by the MAP kinase pathway, the same pathway, including the same pheromone signals, pheromone receptors, trimeric G protein complex, and MAP kinase pathway, that facilitates opaque cell mating (5, 26). Hence, the MTL-homozygous biofilm not only facilitates chemotropism between opaque cells and is regulated by the same signal transduction pathway that regulates the opaque cell mating response but also is coordinated with that response by sharing the pheromone signal released by opaque cells. This has led to the hypothesis that a/α biofilms facilitate commensalism and pathogenesis (i.e., they represent “pathogenic biofilms”) and that pheromone-induced a/a and α/α biofilms facilitate mating (i.e., they represent “sexual biofilms”). If correct, the latter but not the former should facilitate mating when seeded with minority opaque a/a and α/α cells. Here, using a straightforward complementation assay, we present evidence that this is indeed the case. In addition, we show that there is no similar discrimination in mating frequency between seeded patches of MTL-heterozygous and MTL-homozygous cells grown on agar under the same conditions as those for biofilms, demonstrating that the differences in mating frequency are due to differences in the alternative biofilms per se and not simply to the configuration of MTL. It should be noted that this difference holds not only for biofilms formed in RPMI medium at 28°C but also for biofilms formed in Spider medium at 37°C in 20% CO2, even though the cell architecture and deposition of matrix differ dramatically between biofilms formed in the two media, and the frequencies of mating in biofilms formed in Spider medium are lower than the frequencies in biofilms formed in RPMI 1640 medium (K. Daniels, Y.-N. Park, T. Srikantha, and D. R. Soll, submitted for publication).
Biofilm architecture and opaque cell content.
We have found that the general architectures of MTL-heterozygous and MTL-homozygous biofilms were similar under the four sets of conditions. More importantly, there were no major differences between MTL-heterozygous and MTL-homozygous biofilms in the density of seeded opaque cells after 48 h of development under any of the four conditions that would account for the dramatic differences between them in the frequencies of complementation events. In addition, spontaneous switching to the opaque phenotype by majority white cells in MTL-homozygous biofilms accounted for <4% of the opaque cells in seeded MTL-homozygous biofilms after 48 h of development.
Complementation frequencies.
The frequencies of complementation events per biofilm in MTL-homozygous biofilms formed at 28°C, in either air or 20% CO2, were 9- to 79-fold higher than those in MTL-heterozygous biofilms. The frequencies of complementation events per biofilm in MTL-homozygous biofilms at 28°C were consistently higher in 20% CO2 than in air. The frequencies of complementation events per biofilm in MTL-homozygous biofilms were also higher than those in MTL-heterozygous biofilms at 37°C, and again, the frequency in MTL-homozygous biofilms was consistently higher in 20% CO2 than in air. When we computed frequency as a function of opaque cell concentration per biofilm, the frequency in MTL-homozygous biofilms at 28°C was 50- to 140-fold higher than that in MTL-heterozygous biofilms in air and 22- to 32-fold higher in 20% CO2. At 37°C in air and 20% CO2, the frequency of complementation computed as a function of opaque CFU was again far higher in MTL-homozygous biofilms than in MTL-heterozygous biofilms. As we have argued, because the majority of cell compartments in biofilms formed in RPMI 1640 medium are hyphal and embedded in a dense matrix, multicompartmented hyphal fragments of various sizes embedded in matrix gave rise to variable total CFU measured after biofilm disruption. We therefore computed complementation frequencies per biofilm and as a function of total opaque CFU rather than as a function of total CFU. Although the frequencies obtained in biofilms are far lower than those obtained in dense opaque a/a and α/α suspension cultures (47), the latter is the result of collisions resulting from mixing and may be less physiological than a biofilm. Together, our results support the hypothesis that MTL-heterozygous biofilms play a specialized role in pathogenesis (5, 15), whereas MTL-homozygous biofilms play a specialized role in facilitating mating.
Patches are not discriminatory.
If it is the formation of an MTL-homozygous biofilm with a specialized matrix that facilitates mating, then we would not expect to observe differences in mating frequencies in patches of a/α versus a/a-α/α, a/a, or α/α cells grown on agar under the same four sets of conditions in which we tested biofilms, since patches lack matrix and biofilm architecture. Both seeded MTL-heterozygous and seeded MTL-homozygous biofilms formed in RPMI 1640 medium were highly organized, containing a basal yeast cell polylayer and an upper region of vertically oriented hyphae embedded in an extracellular matrix. In the case of MTL-heterozygous biofilms, it is the specialized matrix formed by genes regulated by the transcription factor Bcrl that confers the specialized attributes of impermeability, impenetrability, and drug resistance (15). It has been hypothesized that a factor other than Bcrl regulates the genes that form the specialized matrix in MTL-homozygous biofilms that facilitate mating. In marked contrast, patches formed by MTL-heterozygous and MTL-homozygous cells at 28°C on agar are not architecturally complex. At 28°C, they consist almost entirely of tightly packed, noncohesive yeast cells with little space between them for a complex matrix. At 28°C, seeded minority opaque cells underwent complementation in MTL-heterozygous and MTL-homozygous patches at similar frequencies in both air and 20% CO2. Therefore, while the frequency of mating was far higher in MTL-homozygous biofilms than in MTL-heterozygous biofilms, there was no such discrimination in patches, supporting the conclusion that it is the architecture and composition of the matrix of MTL-homozygous biofilms per se and not simply the configuration of the mating type locus that facilitate mating.
Surprisingly, at 37°C, we observed virtually no conjugation between seeded opaque cells in either MTL-heterozygous or MTL-homozygous patches. In four independent experiments, each in air and in 20% CO2, only one complementation event was observed in a single patch of seeded α/α cells in 20% CO2. Seeded MTL-homozygous patches contained concentrations of opaque cells at 37°C that were similar to those at 28°C, so it was not a lack of opaque cells that was responsible for this result. In marked contrast, there was little difference in the low frequencies of complementation events between 28°C and 37°C for seeded a/α biofilms and little difference in the elevated frequencies for seeded MTL-homozygous (a/a-αα, a/a, or α/α) biofilms between 28°C and 37°C. Because seeded a/α patches consisted of over 90% hyphae, one might argue that it was this characteristic (i.e., hypha formation in patches) that inhibited complementation. However, this explanation is insufficient given that seeded a/a-α/α, a/a, and α/α patches at 37°C contained on average only 10% hyphae in air and 50% hyphae in 20% CO2 but still did not support complementation. The dramatic reduction in complementation at 37°C in patches but not in biofilms supports the hypothesis that MTL-homozygous biofilms create a specialized environment that facilitates mating at physiological temperatures.
Conclusion.
The results presented here demonstrate that MTL-homozygous but not MTL-heterozygous biofilms, formed on silicone elastomers in RPMI 1640 medium, facilitate mating in air or in 20% CO2 and at 28°C or 37°C. These results in turn support the hypothesis that C. albicans can form two types of biofilms, depending in the configuration of the MTL locus. In the a/α configuration, C. albicans cells form a biofilm that is impermeable, impenetrable by leukocytes, and drug resistant, characteristics that facilitate commensalism and pathogenesis (a “pathogenic” biofilm) but not mating. In the a/a or α/α configuration, C. albicans cells expressing the white phenotype form a biofilm that facilitates mating (a “sexual” biofilm), which does not exhibit the characteristics that facilitate commensalism and pathogenesis. The basis for discrimination appears to be in the formation of alternative matrices, the matrix of a/α biofilms regulated by the transcription factor Bcr1 (15, 17, 48), and the matrix of a/a and α/α biofilms by an as-yet-unidentified transcription factor (15). Given that this appears to be the first identified case in a microbial pathogen of alternative biofilms, one pathogenic and the other sexual, one wonders if such separation of function has evolved in other microbial pathogens but has gone unnoticed.
Supplementary Material
ACKNOWLEDGMENTS
This research was funded by the Developmental Studies Hybridoma Bank (DSHB), a NIH National Resource.
We are indebted to Sandy Beck for assembling the manuscript.
Footnotes
Published ahead of print 14 June 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/EC.00112-13.
REFERENCES
- 1. Odds FC. 1998. Should resistance to azole antifungals in vitro be interpreted as predicting clinical non-response? Drug Resist. Updat. 1:11–15 [DOI] [PubMed] [Google Scholar]
- 2. Hoiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. 2010. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 35:322–332 [DOI] [PubMed] [Google Scholar]
- 3. Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322 [DOI] [PubMed] [Google Scholar]
- 4. Hall-Stoodley L, Costerton JW, Stoodley P. 2004. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2:95–108 [DOI] [PubMed] [Google Scholar]
- 5. Yi S, Sahni N, Daniels KJ, Lu KL, Srikantha T, Huang G, Garnaas AM, Soll DR. 2011. Alternative mating type configurations (a/alpha versus a/a or alpha/alpha) of Candida albicans result in alternative biofilms regulated by different pathways. PLoS Biol. 9:e1001117. 10.1371/journal.pbio.1001117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Legrand M, Lephart P, Forche A, Mueller FM, Walsh T, Magee PT, Magee BB. 2004. Homozygosity at the MTL locus in clinical strains of Candida albicans: karyotypic rearrangements and tetraploid formation. Mol. Microbiol. 52:1451–1462 [DOI] [PubMed] [Google Scholar]
- 7. Lockhart SR, Pujol C, Daniels KJ, Miller MG, Johnson AD, Pfaller MA, Soll DR. 2002. In Candida albicans, white-opaque switchers are homozygous for mating type. Genetics 162:737–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Odds FC, Hanson MF, Davidson AD, Jacobsen MD, Wright P, Whyte JA, Gow NA, Jones BL. 2007. One year prospective survey of Candida bloodstream infections in Scotland. J. Med. Microbiol. 56:1066–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tavanti A, Davidson AD, Johnson EM, Maiden MC, Shaw DJ, Gow NA, Odds FC. 2005. Multilocus sequence typing for differentiation of strains of Candida tropicalis. J. Clin. Microbiol. 43:5593–5600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hull CM, Raisner RM, Johnson AD. 2000. Evidence for mating of the “asexual” yeast Candida albicans in a mammalian host. Science 289:307–310 [DOI] [PubMed] [Google Scholar]
- 11. Magee BB, Magee PT. 2000. Induction of mating in Candida albicans by construction of MTLa and MTLalpha strains. Science 289:310–313 [DOI] [PubMed] [Google Scholar]
- 12. Miller MG, Johnson AD. 2002. White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell 110:293–302 [DOI] [PubMed] [Google Scholar]
- 13. Hickman MA, Zeng G, Forche A, Hirakawa MP, Abbey D, Harrison BD, Wang YM, Su CH, Bennett RJ, Wang Y, Berman J. 2013. The ‘obligate diploid’ Candida albicans forms mating-competent haploids. Nature 494:55–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baillie GS, Douglas LJ. 1998. Effect of growth rate on resistance of Candida albicans biofilms to antifungal agents. Antimicrob. Agents Chemother. 42:1900–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Srikantha T, Daniels KJ, Pujol C, Kim E, Soll DR. 2013. Identification of genes upregulated by the transcription factor Bcr1 that are involved in impermeability, impenetrability, and drug resistance of Candida albicans a/alpha biofilms. Eukaryot. Cell 12:875–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Douglas LJ. 2003. Candida biofilms and their role in infection. Trends Microbiol. 11:30–36 [DOI] [PubMed] [Google Scholar]
- 17. Finkel JS, Mitchell AP. 2011. Genetic control of Candida albicans biofilm development. Nat. Rev. Microbiol. 9:109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Srikantha T, Daniels KJ, Pujol C, Sahni N, Yi S, Soll DR. 2012. Nonsex genes in the mating type locus of Candida albicans play roles in a/alpha biofilm formation, including impermeability and fluconazole resistance. PLoS Pathog. 8:e1002476. 10.1371/journal.ppat.1002476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mittelman MW. 1998. Structure and functional characteristics of bacterial biofilms in fluid processing operations. J. Dairy Sci. 81:2760–2764 [DOI] [PubMed] [Google Scholar]
- 20. Lockhart SR, Zhao R, Daniels KJ, Soll DR. 2003. Alpha-pheromone-induced “shmooing” and gene regulation require white-opaque switching during Candida albicans mating. Eukaryot. Cell 2:847–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Daniels KJ, Srikantha T, Lockhart SR, Pujol C, Soll DR. 2006. Opaque cells signal white cells to form biofilms in Candida albicans. EMBO J. 25:2240–2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sahni N, Yi S, Daniels KJ, Huang G, Srikantha T, Soll DR. 2010. Tec1 mediates the pheromone response of the white phenotype of Candida albicans: insights into the evolution of new signal transduction pathways. PLoS Biol. 8:e1000363. 10.1371/journal.pbio.1000363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sahni N, Yi S, Pujol C, Soll DR. 2009. The white cell response to pheromone is a general characteristic of Candida albicans strains. Eukaryot. Cell 8:251–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yi S, Sahni N, Daniels KJ, Pujol C, Srikantha T, Soll DR. 2008. The same receptor, G protein, and mitogen-activated protein kinase pathway activate different downstream regulators in the alternative white and opaque pheromone responses of Candida albicans. Mol. Biol. Cell 19:957–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yi S, Sahni N, Pujol C, Daniels KJ, Srikantha T, Ma N, Soll DR. 2009. A Candida albicans-specific region of the alpha-pheromone receptor plays a selective role in the white cell pheromone response. Mol. Microbiol. 71:925–947 [DOI] [PubMed] [Google Scholar]
- 26. Yi S, Sahni N, Daniels KJ, Lu KL, Huang G, Srikantha T, Soll DR. 2011. Self-induction of a/a or alpha/alpha biofilms in Candida albicans is a pheromone-based paracrine system requiring switching. Eukaryot. Cell 10:753–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nobile CJ, Mitchell AP. 2005. Regulation of cell-surface genes and biofilm formation by the C. albicans transcription factor Bcr1p. Curr. Biol. 15:1150–1155 [DOI] [PubMed] [Google Scholar]
- 28. Nobile CJ, Nett JE, Hernday AD, Homann OR, Deneault JS, Nantel A, Andes DR, Johnson AD, Mitchell AP. 2009. Biofilm matrix regulation by Candida albicans Zap1. PLoS Biol. 7:e1000133. 10.1371/journal.pbio.1000133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fonzi WA, Irwin MY. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Srikantha T, Morrow B, Schroppel K, Soll DR. 1995. The frequency of integrative transformation at phase-specific genes of Candida albicans correlates with their transcriptional state. Mol. Gen. Genet. 246:342–352 [DOI] [PubMed] [Google Scholar]
- 31. Bedell GW, Soll DR. 1979. Effects of low concentrations of zinc on the growth and dimorphism of Candida albicans: evidence for zinc-resistant and -sensitive pathways for mycelium formation. Infect. Immun. 26:348–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Anderson JM, Soll DR. 1987. Unique phenotype of opaque cells in the white-opaque transition of Candida albicans. J. Bacteriol. 169:5579–5588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Paulovich AG, Hartwell LH. 1995. A checkpoint regulates the rate of progression through S phase in S. cerevisiae in response to DNA damage. Cell 82:841–847 [DOI] [PubMed] [Google Scholar]
- 34. Park YN, Strauss A, Morschhauser J. 2004. The white-phase-specific gene WH11 is not required for white-opaque switching in Candida albicans. Mol. Genet. Genomics 272:88–97 [DOI] [PubMed] [Google Scholar]
- 35. Park YN, Morschhauser J. 2005. Tetracycline-inducible gene expression and gene deletion in Candida albicans. Eukaryot. Cell 4:1328–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Keppler-Ross S, Noffz C, Dean N. 2008. A new purple fluorescent color marker for genetic studies in Saccharomyces cerevisiae and Candida albicans. Genetics 179:705–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hawser SP, Douglas LJ. 1995. Resistance of Candida albicans biofilms to antifungal agents in vitro. Antimicrob. Agents Chemother. 39:2128–2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee KL, Buckley HR, Campbell CC. 1975. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida albicans. Sabouraudia 13:148–153 [DOI] [PubMed] [Google Scholar]
- 39. Hawser SP, Douglas LJ. 1994. Biofilm formation by Candida species on the surface of catheter materials in vitro. Infect. Immun. 62:915–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bergen MS, Voss E, Soll DR. 1990. Switching at the cellular level in the white-opaque transition of Candida albicans. J. Gen. Microbiol. 136:1925–1936 [DOI] [PubMed] [Google Scholar]
- 41. Rikkerink EH, Magee BB, Magee PT. 1988. Opaque-white phenotype transition: a programmed morphological transition in Candida albicans. J. Bacteriol. 170:895–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Slutsky B, Staebell M, Anderson J, Risen L, Pfaller M, Soll DR. 1987. “White-opaque transition”: a second high-frequency switching system in Candida albicans. J. Bacteriol. 169:189–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bennett RJ, Johnson AD. 2003. Completion of a parasexual cycle in Candida albicans by induced chromosome loss in tetraploid strains. EMBO J. 22:2505–2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hull CM, Johnson AD. 1999. Identification of a mating type-like locus in the asexual pathogenic yeast Candida albicans. Science 285:1271–1275 [DOI] [PubMed] [Google Scholar]
- 45. Reed SI. 1980. The selection of S. cerevisiae mutants defective in the start event of cell division. Genetics 95:561–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Morrow B, Srikantha T, Anderson J, Soll DR. 1993. Coordinate regulation of two opaque-phase-specific genes during white-opaque switching in Candida albicans. Infect. Immun. 61:1823–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lachke SA, Lockhart SR, Daniels KJ, Soll DR. 2003. Skin facilitates Candida albicans mating. Infect. Immun. 71:4970–4976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Taff HT, Nett JE, Zarnowski R, Ross KM, Sanchez H, Cain MT, Hamaker J, Mitchell AP, Andes DR. 2012. A Candida biofilm-induced pathway for matrix glucan delivery: implications for drug resistance. PLoS Pathog. 8:e1002848. 10.1371/journal.ppat.1002848 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.