Abstract
The Deinococcus radiodurans genome encodes five putative quinoproteins. Among these, the Δdr2518 and Δdr1769 mutants became sensitive to gamma radiation. DR2518 with beta propeller repeats in the C-terminal domain was characterized as a radiation-responsive serine/threonine protein kinase in this bacterium. DR1769 contains beta propeller repeats at the N terminus, while its C-terminal domain is a proline-rich disordered structure and constitutes a low-complexity hydrophilic region with aliphatic-proline dipeptide motifs. The Δdr1769 mutant showed nearly a 3-log cycle sensitivity to desiccation at 5% humidity compared to that of the wild type. Interestingly, the gamma radiation and mitomycin C (MMC) resistance in mutant cells also dropped by ∼1-log cycle at 10 kGy and ∼1.5-fold, respectively, compared to those in wild-type cells. But there was no effect of UV (254 nm) exposure up to 800 J · m−2. These cells showed defective DNA double-strand break repair, and the average size of the nucleoid in desiccated wild-type and Δdr1769 cells was reduced by approximately 2-fold compared to that of respective controls. However, the nucleoid in wild-type cells returned to a size almost similar to that of the untreated control, which did not happen in mutant cells, at least up to 24 h postdesiccation. These results suggest that DR1769 plays an important role in desiccation and radiation resistance of D. radiodurans, possibly by protecting genome integrity under extreme conditions.
INTRODUCTION
Desiccation is one of the most hostile conditions that impose physiological constraints, which few organisms can tolerate. Desiccation causes both reversible and irreversible changes in cellular milieus, including protein aggregation, membrane fusion, and damages to DNA, RNA, and proteins (1, 2). In spite of this, there are many organisms that can tolerate prolonged desiccation by entering in the desiccation dormant state and/or protecting cells from the effects of extensive water loss. The roles of disaccharides and certain proteins, like anhydrin and late embryogenesis abundant (LEA)-related proteins, have been reported in the desiccation tolerance of invertebrates (3) and plants (4, 5). The LEA proteins are characterized as having the proline- and glycine-rich low-complexity (LC) regions, which form unstructured conformation under normal physiological conditions. Upon desiccation, these sequences undergo structural transition from an unstructured conformation to a well-defined and functionally viable structure (3, 6). The mechanisms through which these amino acids could contribute to desiccation tolerance are not very clear. However, it has been shown that disordered hydrophilic regions could retain ∼20 times more water than globular proteins of nearly similar sizes, which upon anhydrobiosis leads to the conformational change from a poly-proline type II helix into an α helix (7). An alternative hypothesis also suggests that the side chains of polar amino acids in the LC region could mimic the nature of a water molecule and provide chaperone-like activity in protecting the proteins from aggregation and membrane fusions (8). The LC sequences are common in both eukaryotes (9) and prokaryotes (10).
Deinococcus radiodurans R1 shows superlative potential to survive against the DNA-damaging and mutagenic effect of gamma rays, UVC radiation, and desiccation (11, 12). These phenotypes are attributed to its efficient DNA double-strand break (DSB) repair (13) and strong oxidative stress tolerance mechanisms. Both antioxidant enzymes (14) and nonenzymatic components, like pyrroloquinoline quinine (PQQ) (15, 16), carotenoids (17), and a high ratio of Mn to Fe (18), contribute to the oxidative stress tolerance of this bacterium. The PQQ is characterized as an antioxidant in solution (19) and subsequently as an inducer of eukaryotic-type Ser/Thr protein kinases (STPK) with a role in radiation resistance and DSB repair in D. radiodurans (20) and of STPKs associated with cellular differentiation in the mammalian system (21, 22). The D. radiodurans genome encodes a large number of proteins with an LC region in the primary sequence, and a majority of them are hypothetical proteins (23). The most notable ones suggested for their role in DNA metabolism or protein recycling include DNA polymerase III (DR_2410), MutT/nudix hydrolase (DR_0550), subtilisin family serine protease (DR_1459), a homolog of the LEA (DR_1172), and an uncharacterized open reading frame (ORF) (DR_1769) (10, 24).
Here, we report the detailed characterization of the DR1769 protein for its role in D. radiodurans resistance to radiation and desiccation. The primary sequence of DR1769 is found to have 72-amino-acid-long LC regions at the C terminus, nine PXXP motifs, and 7 beta propeller repeats for PQQ interaction at the N terminus. The Δ1769 mutant showed more than a 3-log cycle loss of wild-type resistance to desiccation and an ∼10-fold decrease in wild-type resistance to gamma radiation. Unlike wild-type cells, more than 80% of mutant cells showed diffused nucleoid after desiccation, and these cells failed to assemble the shattered genome at least up to 48 h of postirradiation recovery. These results suggest that DR1769 has a regulatory role in D. radiodurans resistance to desiccation and gamma radiation, possibly by contributing through DSB repair and protecting the genome under extreme stress conditions.
MATERIALS AND METHODS
Bacterial strains and materials.
The wild-type Deinococcus radiodurans R1 strain (ATCC 13939) was a generous gift from J. Ortner, Germany (25). Wild-type strains and their respective derivatives were grown aerobically in TGY (0.5% Bacto tryptone, 0.3% Bacto yeast extract, 0.1% glucose) broth or on an agar plate at 32°C in the presence of antibiotics, as required. Shuttle expression vector pRADgro and its derivatives were maintained in Escherichia coli strain HB101 in LB broth supplemented with ampicillin (50 μg/ml) and in the presence of chloramphenicol (5 μg/ml), as described earlier (26). Other recombinant techniques were used as described in reference 27.
Construction of expression plasmids.
Genomic DNA of D. radiodurans R1 was prepared as published previously (28). The coding sequences of DR1769 were PCR amplified from genomic DNA of D. radiodurans using gene-specific primers 1769F (5′GCCGGGCCCATGCCTGACCCCGCTGCCCGCCGCT3′) and 1769R (5′CCTATCTAGATCAGCGGCGCACGACGCCCGGGA3′), having the desired restriction sites incorporated at the 5′ end in the respective primers, and gene integrity was confirmed by nucleotide sequencing. The PCR product was ligated at ApaI and XbaI sites in pRADgro (26) to yield pGro1769. The pGro1769 plasmid was transformed into Δdr1769 mutant cells as described earlier (29), and the recombinant clones were scored in the presence of chloramphenicol (5 μg/ml). For overexpression in E. coli and purification of the recombinant DR1769 protein, the coding sequences of the dr1769 gene was PCR amplified using gene-specific primers 1769OF (5′CGGGATCCGTGCTTAAAGACACCATTGA3′) and 1769OR (5′CCGCTGGAGTCAGCCCGGGAATTCCGGCT 3′), cloned in the pET28a vector at BamHI and XhoI sites, to yield pET1769. Similarly, dr2518 was cloned in pET28a+, and the resultant pET2518 plasmid was obtained as described earlier (20). These plasmids were transformed into E. coli BL21(DE3)/pLysS, and inducible expression of recombinant proteins was confirmed on SDS-PAGE.
Protein purification, kinase activity, and PQQ interaction studies.
Transgenic E. coli BL21(DE3)/pLysS cells harboring pET1769 and pET2518 plasmids were induced with 200 μM isopropyl β-d-1-thiogalactopyranoside (IPTG), and recombinant DR1769 and DR2518 proteins were purified under nondenaturing conditions by nickel-nitrilotriacetic acid (Ni-NTA) (Qiagen, Germany) affinity chromatography using the manufacturer protocols and as described in detail in reference 20. Autophosphorylation of DR2518 was checked in the absence and presence of increasing concentrations of DR1769 and 10 nM PQQ in different combinations as described earlier (20). The level of phosphorylation in DR2518 was estimated as the intensity of phosphoprotein bands densitometrically. PQQ interaction with recombinant DR1769 was monitored using a circular dichroism (CD) spectroscopy approach as described earlier (20). In brief, 50 μM purified recombinant DR1769 was incubated with increasing concentrations (10 nM, 50 nM, and 500 nM) of PQQ, and CD spectra were recorded on a CD spectrometer.
Generation of deletion mutants in D. radiodurans.
For generating the deletion mutant of the dr1769 gene in D. radiodurans, pNOK1769 was made in pNOKOUT (30) as described earlier (20). In brief, the 1-kb upstream and 1-kb downstream coding sequences of dr1769 were PCR amplified using gene-specific primers 1769UF (5′ CGGGCCCCATCCCGAGGAATTTGGTGTA3′) and 1769UR (5′ GGAATTCGCGGGCAGCGGGGTCAGGCAT3′) for upstream and 1769DF (5′ GCGGGATCCAGCCGGAATTCCCGGGCGT3′) and 1769DR (5′ GTTGGATCCT TGCCCTGCGCTTCGTCACT3′) for downstream fragments and were cloned at ApaI-EcoRI and BamHI sites of pNOKOUT, respectively, to generate pNOK1769. For generating the pqqE disruption mutation in the Δdr1769 background, the nptII cassette flanking upstream and downstream sequences of pqqE, together with part of the neighboring genes in pNOKpqqE (16), was replaced with the chloramphenicol acetyltransferase (cat) cassette using PstI and BamHI restriction sites to yield pPQCAT (20). The linearized plasmids, like pNOK1769 and pPQCAT, were transformed into D. radiodurans R1 and the Δdr1769 mutant, respectively, and homozygous replacement of wild-type target genes with nptII in the case of the wild type and with pqqE::cat in the case of the dr1769 deletion mutant was confirmed by PCR amplification using sequence-specific primers. Details on the materials used for generating these mutants of D. radiodurans will be provided upon inquiry. The resultant strains were designated the Δdr1769 single mutant and the Δdr1769 pqqE::cat double mutant.
Desiccation, gamma irradiation, and cell survival studies.
For desiccation, the exponentially growing D. radiodurans cells were collected by centrifugation, washed in 4 volumes of 10 mM MgSO4, and resuspended in an equal volume of 10 mM MgSO4. A 100-μl aliquot of this suspension, having nearly 106 CFU each of D. radiodurans R1 and its Δdr1769 mutant, was smeared on different sets of surface-sterilized microscopic slides in triplicate and incubated in desiccators maintained at 5% humidity at 30°C. Relative humidity within the desiccators was continuously monitored online by fitting a hygrometer in the desiccators. One set of both the wild type and mutant was removed at a regular interval (5, 10, 15, and 20 days), and the cells were recovered by soaking with 200 μl TGY under sterilized conditions. The appropriate dilutions of these samples were spread on a TGY agar plate and incubated at 32°C for 72 h, and CFU were counted. The percent cell survival was computed by comparing the number of CFU smeared on the slide at time zero. Untreated controls of both the wild type and mutant were processed identically without desiccation. For radiation treatment, both wild-type and Δdr1769 mutant cells were treated with different doses of UV and gamma radiation as described earlier (26). In brief, the bacteria grown in TGY medium at 32°C were washed and suspended in sterile phosphate-buffered saline (PBS) and treated with different doses of gamma radiation at a dose rate of 3.8 kGy/h (Gamma 5000, 60Co; Board of Radiation and Isotopes Technology, DAE, India). For UVC, the dilutions of these cells were plated and exposed with different doses of UV (254-nm) radiation. The CFU were recorded after 48 h of incubation at 32°C.
Growth recovery and DSB repair studies.
For studying the postdesiccation recovery, D. radiodurans wild-type and Δdr1769 mutant cells were desiccated at ∼5% humidity for 14 days as described above, and cells were suspended in 200 μl fresh TGY. Optical density at 600 nm (OD600) was adjusted to ∼0.2, and 6 replicates of each were aliquoted on a microtiter plate. Growth was measured continuously as OD600 on Bioscreen C, a microplate reader-based absorbance-measuring system (Oy Growth Curves AB Ltd., Helsinki, Finland). Untreated controls of both wild-type D. radiodurans and Δdr1769 mutant cells were processed identically, and OD600 was adjusted to 0.2 and used for continuous monitoring of growth.
The reassembly of the shattered genome was monitored on a pulsed-field gel electrophoresis (PFGE) gel as described earlier (20). In brief, the cells were treated with 6 kGy gamma radiation (3.8 kGy/h) and aliquots were collected at different time intervals during postirradiation recovery (PIR). Cell lysis and NotI restriction digestion were carried out in gel as detailed earlier, and DNA fragments were separated on PFGE under conditions described earlier.
Fluorescence microscopic studies.
Fluorescence microscopy of D. radiodurans cells was carried out on a Zeiss AxioImager (model LSMS10 META; Carl Zeiss) microscope equipped with a 254 Zeiss AxioCam HRm camera as described earlier (31). The D. radiodurans cells desiccated for 14 days at ∼5% humidity were allowed to grow for 24 h, and cells were collected by centrifugation and washed twice with 10 mM MgCl2. Washed cells were stained with 4′,6-diamidino-2-phenylindole (0.5 μg/ml) (DAPI) for the nucleoid and with Nile red (1 μg/ml) for the membrane for 5 min in the dark, 2 μl of cells were spotted on a 0.8% agarose pad, and fluorescence images were taken by exciting at 405 nm for DAPI and at 560 nm for Nile red. Untreated normal-growing cells of both wild-type D. radiodurans and the Δdr1769 mutant were processed similarly and used as controls. Cell and nucleoid sizes were measured using AxioVision 4.8.2 software, and mean values of nucleoid-to-cell diameter were used to perform the statistical t test using Origin Pro 6.1 data analysis software.
Data presented without standard deviations are illustrative of a typical experiment and represent the average from three replicates wherein the variation among replicates was less than 15%. All experiments were repeated at least three times.
RESULTS AND DISCUSSION
DR1769 comprised of beta propeller repeats and low-complexity hydrophilic regions.
The D. radiodurans genome annotated DR1769 as a putative serine/threonine protein kinase-like quinoprotein (32). The levels of dr1769 transcript do not change during postirradiation/desiccation recovery of D. radiodurans (33, 34). The amino acid sequence analysis using SMART software (http://smart.embl-heidelberg.de/) revealed the presence of seven tandem beta propeller repeats in the N-terminal domain and the unstructured LC region at the C-terminal domain of DR1769 (Fig. 1A). Multiple sequence alignments of its N-terminal domain with the conserved amino acid sequences from each class of beta propeller family proteins were carried out using the ClustalX program. It showed that the N-terminal domain of DR1769 had seven WD repeats of approximately 40 amino acids each that start with glycine (G) and end with tryptophan (W) (Fig. 1B). A molecular model generated from DR1769 sequences using the I-TASSER online server showed that the N terminus of this protein produces a typical beta propeller arrangement around the central core through which PQQ interacts in quinoproteins and a disorganized C terminus (Fig. 1C). Recently, another protein, DR2518, having a similar signature of beta propellers, albeit in its C-terminal domain, has been characterized, and its role in radiation resistance of D. radiodurans has been demonstrated (20). The C-terminal of DR1769 contains two hydrophilic LC regions between positions 393 and 465 and positions 548 and 566, forming a stretch of 72 and 18 amino acids, respectively. These LC regions are rich in glycine and proline amino acids. Linear motif analysis of the primary sequence of DR1769 was carried out using Scansite (http://scansite.mit.edu), which showed the presence of a couple of tandem PXXP motifs and also proline-rich motifs (Table 1). Proteins harboring PXXP motifs are generally known for interacting with proteins having SH3 domains, and such proteins' role in signal transduction and in the regulation of polymerization/depolymerization dynamics of the cytoskeleton has been reported (35, 36). It is shown that when an aliphatic residue precedes each proline residue, the characteristic aliphatic-proline (AP) pair is formed, which would establish interaction with the hydrophobic pocket on the SH3 domain (37). The typical SH3 domain binds with the +XXΦPXΦP or ΦPXΦPX+ type of motif, where Φ is a hydrophobic amino acid and + is a basic amino acid. Bacterial proteins having SH3 domains either exactly or closely similar to eukaryotic types have been reported (38). Although DR1769 is found with nine proline-rich motifs arranged in tandem (Table 1), these motifs do not show consensus in the amino acids flanking to proline residues, and the impact of this variation on the function of this protein is not known. However, it may be possible that the small differences in the putative SH3-interacting motif of DR1769 might be helping this protein establish interactions with deinococcal proteins with minor variations in SH3 motifs. Similar observations have been recorded and supported with some examples (39). The functional motif organization in N-terminal and C-terminal domains of DR1769 might predict the regulatory role of this protein in desiccation and radiation resistance of D. radiodurans, possibly by interacting with other deinococcal proteins with putative SH3-type domains. Thus, the role of this protein in extreme phenotypes of this bacterium is being further investigated.
Fig 1.
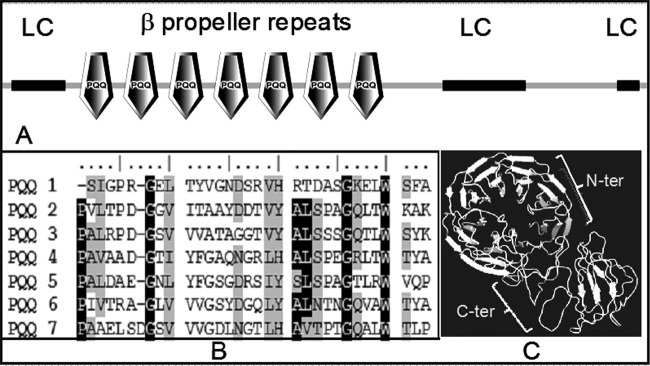
Functional domain analysis of DR1769. (A) Primary sequence of DR1769, searched for the presence of functional motifs showing 7 beta propeller repeats making PQQ-interacting motifs (PQQ) in the N-terminal domain and low complexity (LC) region, largely confined to the C-terminal domain; (B) beta propeller repeats (PQQ1 through PQQ7) showing the typical signature of the WD motif, starting with consensus G and ending with W; (C) modeled structure of DR1769 showing typical arrangement of 7 beta propeller repeats of the N-terminal domain (N-Ter) and disorganized C-terminal domain (C-Ter).
Table 1.
Distribution of PXXP motifs in DR1769, their functional attributes, like matching scores, surface accessibility, and gene card, indicating the similarity of a motif sequence with a similar/closely matching motif in respective candidate proteins
| Site | Matching score | Motif sequence | Surface accessibility score | Gene card |
|---|---|---|---|---|
| P189 | 0.3443 | GAPVFSSPAVAADGT | 0.482 | ABL1 (receptor tyrosine kinase) |
| P462 | 0.4825 | PPVASPAPATARLTP | 0.815 | ABL1 (receptor tyrosine kinase) |
| P12 | 0.4204 | AARRFSLPPFPLAAL | 0.811 | ABL1 (receptor tyrosine kinase) |
| P442 | 0.5069 | QTTPRPQPTPAQPAT | 2.139 | SRC (tyrosine kinase) |
| P438 | 0.6067 | PAPAQTTPRPQPTPA | 3.871 | PLCG1 (phospholipases) |
| P442 | 0.5069 | QTTPRPQPTPAQPAT | 2.139 | SRC (tyrosine kinase) |
| P453 | 0.5072 | QPATPAAPVPPVASP | 0.642 | CRK (adapter protein) |
| P455 | 0.5243 | ATPAAPVPPVASPAP | 0.472 | GRB2 (adapter protein) |
| P15 | 0.6072 | RFSLPPFPLAALALS | 0.399 | HCLS1 (substrate of the antigen receptor-coupled tyrosine kinase) |
The Δdr1769 mutant is sensitive to gamma radiation and hypersensitive to desiccation.
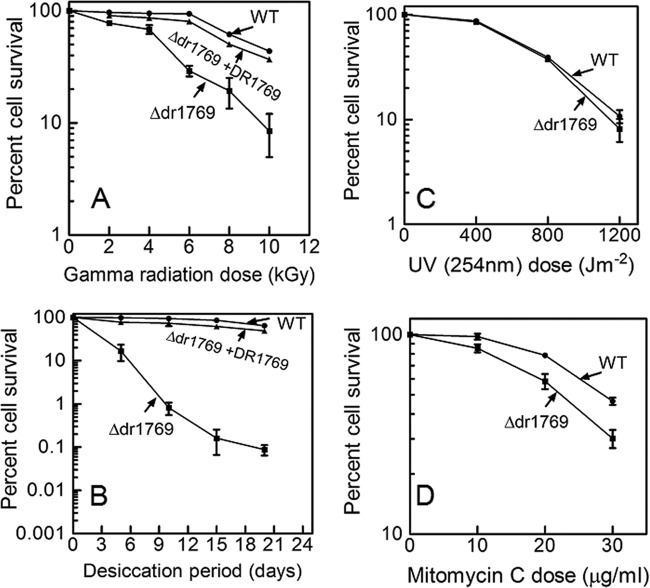
Earlier, it has been shown that D. radiodurans cells devoid of PQQ become sensitive to gamma radiation, and the DSB repair and protein phosphorylation profiles of these cells were significantly affected (16). Five ORFs having putative PQQ-interacting motifs were identified from the genome of D. radiodurans, and these were individually deleted from the genome (20). The Δdr1769 mutant showed nearly a 1.2-log cycle drop in cell survival at 10 kGy (Fig. 2A) and nearly a 3-log cycle loss of resistance to desiccation at 5% humidity (Fig. 2B) for 20 days, compared to those of the wild type. Interestingly, the Δdr1769 mutant responses to UVC radiation of up to 800 J/m2 (Fig. 2C) and hydrogen peroxide were the same as those of the wild type (see Fig. S1 in the supplemental material). These cells' response to mitomycin C (MMC) was marginally different from that of wild-type cells (Fig. 2D). The loss of resistance to desiccation and gamma radiation in the dr1769 mutant was not due to the polar effect of this deletion, as ascertained by in trans expression of a wild-type copy of the DR1769 protein. The mutant cells expressing wild-type DR1769 showed almost complete recovery of gamma radiation as well as desiccation resistance loss in the Δdr1769 mutant (Fig. 2A and B), suggesting that the gamma radiation and desiccation sensitivity in Δdr1769 mutant cells was due to the absence of DR1769 per se. Since DR1769 has 7 beta propeller repeats, making a putative PQQ binding pocket at its N terminus, the possibility of a PQQ-regulating function of this protein was checked by disrupting pqqE (a gene responsible for PQQ synthesis) in the Δdr1769 mutant background. The Δdr1769pqqE::cat double mutant showed increased sensitivity to gamma radiation compared to that of the Δdr1769 single mutant (Fig. 3), but the levels of desiccation resistance in both single and double mutants were the same. It may, however, be noted that the level of double-mutant sensitivity to gamma radiation was almost similar to that of the pqqE::cat single mutant (Fig. 3A compared with Fig. 3A in reference 31), suggesting that the PQQ role(s) in gamma radiation resistance is dominant over DR1769 and both PQQ and DR1769 possibly function through common pathways in desiccation resistance of D. radiodurans. Recently, it has been shown that PQQ contributes to gamma radiation resistance in this bacterium through a radiation- and DNA damage-responsive STPK (20). These results together suggested that DR1769 has a role in desiccation resistance of D. radiodurans.
Fig 2.
DNA damage response of the Δdr1769 mutant. D. radiodurans cells (■), Δdr1769 mutant cells (●), and Δdr1769 cells expressing wild-type DR1769 (▲) were treated with different doses of gamma radiation (A), desiccation (B), UV (254 nm) (C), and mitomycin C (D). Different dilutions of these cells were plated on LB agar plates supplemented with the required antibiotics, numbers of CFU per milliliter were computed, and the percent cell survival was calculated by considering the percentage of CFU of untreated samples as 100%.
Fig 3.
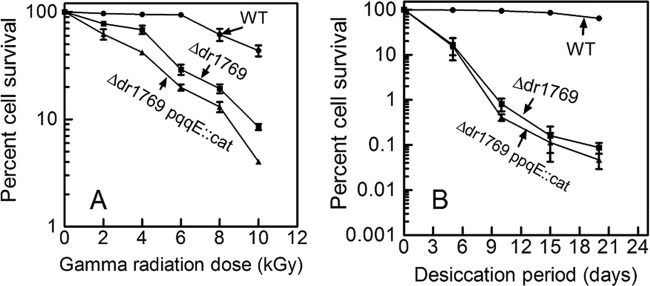
Genetic interaction of pqqE and dr1769 loci in DNA damage response of D. radiodurans. D. radiodurans (■), Δdr1769 mutant (●), and Δdr1769pqqE::cat (▲) cells were treated with different doses of gamma radiation (A) and desiccation (B), the number of CFU per milliliter was computed, and percent cell survival was calculated by considering the percentage of CFU of untreated samples as 100%.
The Δdr1769 mutant showed delayed postdesiccation recovery.
The influence of dr1769 deletion on normal growth of D. radiodurans was determined by comparing mutant growth with that of wild-type cells. We observed that the Δdr1769 mutant grew almost similar to the wild type under normal growth conditions (Fig. 4). However, the growth rates of wild-type and Δdr1769 mutant cells incubated for 20 days at less than 5% humidity recovered differently. The Δdr1769 mutant showed a nearly twice as long lag phase (28 h) as that of the wild type (15 h) (Fig. 4). Earlier, the mutants showing both desiccation and gamma radiation resistance have been characterized in D. radiodurans (40, 41). Since the Δdr1769 mutant grew well under normal growth conditions of D. radiodurans and showed a major defect only during the recovery from desiccation stress, the roles of DR1769 in D. radiodurans resistance to gamma radiation and desiccation are demonstrated.
Fig 4.
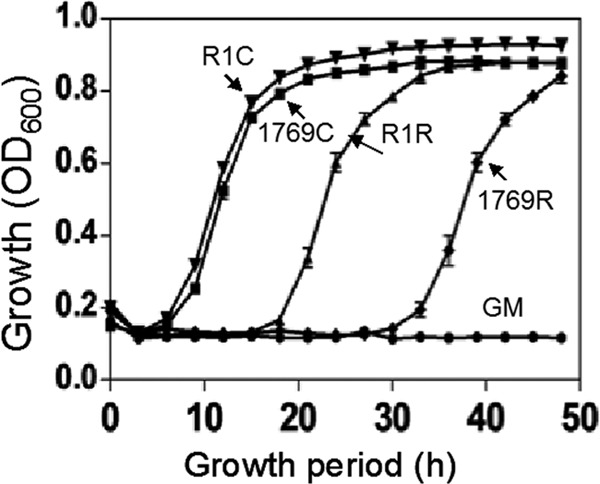
Effect of dr1769 deletion on normal growth and recovery from desiccation. D. radiodurans R1 (▼, ▲) and Δdr1769 mutant (■,⧫) cells were grown in rich medium under normal conditions (▼, ■) and stressed at 5% humidity for 20 h and then allowed to recover (▲, ⧫). Optical density at 600 nm was measured continuously on a microtiter-based density reader. Growth medium (●, GM) was taken as the blank control.
The Δdr1769 mutant showed delayed DSB repair and recovery of desiccated nucleoid.
In order to understand the mechanisms underlying DR1769 roles in desiccation and gamma radiation resistance of D. radiodurans, the kinetics of DSB repair during postirradiation recovery was monitored. D. radiodurans cells exposed to 6 kGy gamma radiation recovered the typical NotI pattern of untreated cells in less than 4 h PIR. On the contrary, the Δdr1769 mutant did not recover the typical NotI profiles of its unirradiated genome up to 48 h PIR (Fig. 5). Since the NotI pattern of the recovered genome of the Δdr1769 mutant was almost similar to the corresponding PFGE bands of the wild type, which did not change beyond 6 h PIR in the mutant, the possibility of DR1769 affecting the slow crossover event of homologous recombination and genome maturation as characterized in the second phase of DSB repair could be argued. Mattimore and Battista have demonstrated that both gamma radiation and desiccation produce nearly similar effects on DNA double-strand break, and the mechanisms contributing to DSB repair could provide resistance to both these DNA-damaging agents (41). Similar results have been reported in the case of the two-component system (TCS) mutant of D. radiodurans that shows sensitivity to both gamma radiation and desiccation (40). Thus, these results suggested that DR1769 contributes to gamma radiation and desiccation resistance possibly by regulating DSB repair.
Fig 5.
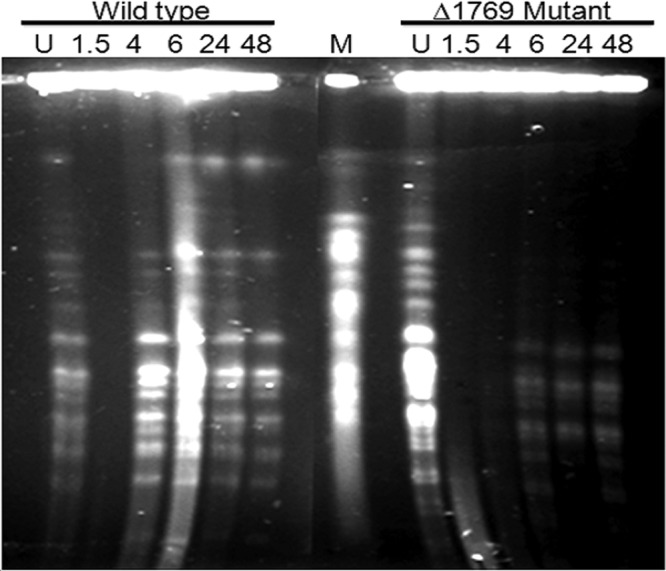
DSB repair kinetics during postirradiation recovery in D. radiodurans. D. radiodurans R1 (wild type) and Δdr1769 mutant (Δdr1769 mutant) cells were treated with 6.5 kGy gamma radiation and allowed to grow at different time periods (1.5, 4, 6, 24, and 48 h), and PFGE was carried out along with unirradiated (U) respective controls.
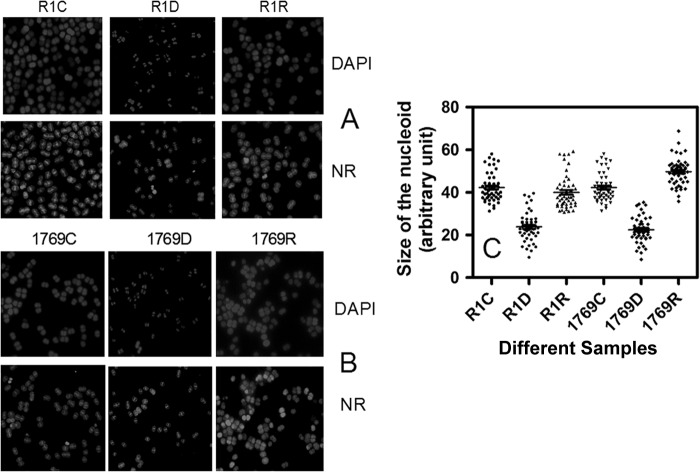
Since the desiccation response of an organism is governed by a number of cellular and molecular changes that can also affect genome structure, the possibility of DR1769 absence affecting the genome compactness of D. radiodurans under desiccation was hypothesized. Both wild-type and mutant cells were grown under identical conditions, and the effect of desiccation on genome compactness was monitored microscopically. The nucleoids in both wild-type (Fig. 6A; see Fig. S2A in the supplemental material for a color picture) and Δdr1769 mutant (Fig. 6B; see Fig. S2B for a color picture) cells desiccated for 20 days at 5% humidity were condensed by ∼2-fold compared to the respective untreated controls. When these cells were allowed to recover in rich medium, the nucleoid in the wild type could return to nearly the same size as that of untreated cells in less than 24 h postdesiccation (Fig. 6C). However, the majority of the mutant cells showed a dispersed nucleoid up to 24 h after desiccation recovery. These results suggested that DR1769 has a role in maintenance of genome integrity possibility by supporting D. radiodurans cells to recover the original genome from desiccation/gamma radiation-mediated DNA double-strand break and nucleoid condensation.
Fig 6.
Effect of dr1769 deletion on nucleoid compactness under desiccation. D. radiodurans R1 (A) and Δdr1769 mutant (B) untreated cells (R1C, 1769C) were incubated at 5% humidity for 24 h (R1D, 1769D) and allowed to recover in rich medium under normal growth conditions (R1R, 1769R). These cells were stained for nucleoid (DAPI) and with Nile red (NR), and images were taken with a fluorescence microscope. (C) The false blue areas of DAPI-stained images were measured using Image J software and plotted in GraphPad Prism software. Data shown here are representative of a reproducible experiment repeated three times independently. For a color version of this figure, you may refer to Fig. S2 in the supplemental material.
Recombinant DR1769 attenuates autokinase activity of DR2518.
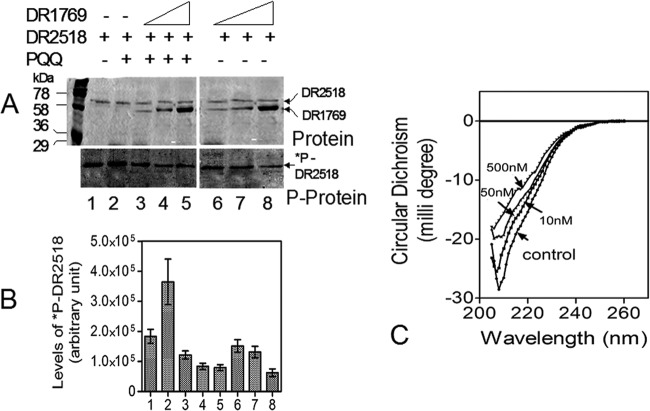
A homology search using the eukaryotic-type consensus SH3 domain in the proteome of D. radiodurans R1 and then multiple sequence alignment of putative hits revealed that there are proteins, including DRA0335 (a putative protein kinase), DR2518 (a kinase characterized for its role in radiation resistance) (20), and drRecA, with SH3-type domains, albeit relatively less conserved compared to those of eukaryotes (see Fig. S3 in the supplemental material). Since DR1769 contains PXXP motifs at the C terminus, which is the signature implicated for interaction with the proteins having the SH3 motif, and DR2518 apparently has the putative SH3 motif, the possibility of DR1769 influencing the autokinase activity of DR2518 was checked. Results showed that the autophosphorylation of DR2518 was reduced by nearly 4-fold in the presence of an ∼5-fold-excess molar ratio of DR1769 to DR2518 (Fig. 7A and B). DR1769 neither showed detectable autokinase activity nor acted as a substrate for DR2518 phosphorylation. However, the incubation of PQQ with purified DR1769 caused a significant change in the secondary structure of the protein (Fig. 7C), and the inhibition of DR2518 autokinase activity by DR1769 was significantly enhanced in the presence of PQQ (Fig. 7B, compare bar 2 with bars 3 to 5 and bar 1 with bars 6 to 8). The molecular basis of DR1769 attenuation of DR2518 activity in the presence/absence of PQQ is not known. However, it appears that DR2518 activity inhibition by DR1769 does not seem to be through the involvement of PQQ, because the effect was seen irrespective of PQQ presence, albeit to a higher magnitude in the presence of PQQ (Fig. 7B, compare lines 3 to 5 with lines 6 to 8). The possibility of DR1769 competing for ATP with DR2518 has also been nearly ruled out, as the former showed neither kinase nor ATPase activity (data not shown). Therefore, these results suggest that PQQ interacts with DR1769, which could attenuate DR2518 autokinase activity possibly through protein-protein interaction in vitro.
Fig 7.
DR1769 effect on DR2518 autophosphorylation and DR1769 interaction with pyrroloquinoline quinone (PQQ). Purified recombinant DR2518 was mixed with increasing molar ratios of 1:1 (lanes 3 and 6), 1:5 (lanes 4 and 7), and 1:10 (lanes 5 and 8) of purified recombinant DR1769 in the presence (lanes 2 to 5) and absence (lanes 1, 6, 7, 8) of 10 nM PQQ and 1μCi [32P]ATP for 1 h. (A) Proteins were separated on an SDS-PAGE gel, the gel was stained with Coomassie blue (Protein) and dried, and autoradiogram (P-protein) was developed. (B) The intensity of phosphoprotein bands of each lane (1 to 8) were computed and plotted. (C) Interaction of PQQ with recombinant purified DR1769 was checked by circular dichroism spectroscopy in the absence (control) and presence of increasing concentrations (10 nM, 50 nM, and 500 nM) of PQQ.
D. radiodurans exhibits extraordinary resistance to several DNA-damaging agents, including radiation and desiccation (42). Since desiccation also produces DSBs and oxidative damage to biomolecules similar to gamma radiation, the molecular basis of desiccation resistance in D. radiodurans had been explained mostly by considering its capabilities of DSB repair and tolerance to oxidative stress. The effect of extreme desiccation on formation of lethal DNA-protein crosslinks has also been reported in a bacterial system (43, 44). However, the depletion of water during desiccation could also destabilize the naive interaction of proteins and eventually their functions, especially those requiring the charge transfer through the formation of salt bridges. The organisms conferring high tolerance to desiccation contain a large number of proteins which have unique structural motifs known for retaining relatively large amounts of water in bound form. One of the features of these proteins is that they have low-complexity regions clustered with positively charged amino acids, like proline and glycine. Calculations made from the hydrodynamic data about the degree of hydration in the AavLEA1 (late embryogenesis abundant protein of Aphelenchus avenae) protein as an example suggested that proteins having such structure can retain nearly 20 times more water in bound form than globular counterparts (7). It has been shown that the proteins containing hydrophilic LC regions protect cells from desiccation effects by inhibiting protein aggregation and membrane fusion during water loss (8). The D. radiodurans genome encodes a large number of such hypothetical proteins with unstructured hydrophilic LC regions (10). Here, we report the characterization of DR1769, a candidate protein having 7 beta propeller repeats with the sensory WD motif at the N terminus and a disorganized hydrophilic LC region along with nine tandem repeats of PXXP motifs at the C terminus, and its role in desiccation and radiation resistance of D. radiodurans. We showed that cells lacking DR1769 become hypersensitive to desiccation and lose the wild-type capability of DSB repair. Both the wild type and the Δdr1769 mutant showed supercondensation of nucleoids upon desiccation. However, the wild type could recover its nucleoid to the size in untreated cells but the mutant's nucleoid continued to be significantly dispersed at least up to 24 h after desiccation recovery. The molecular mechanisms underlying DR1769's roles in DSB repair and nucleoid compaction are not clear. The WD repeat-containing proteins perform a wide range of functions, including regulatory functions in signal transductions, RNA synthesis and processing, genome stabilization, vesicular trafficking, cytoskeletal assembly, cell cycle control, and apoptosis (45, 46). Since DR1769 contains WD repeats and other structural attributes, like C-terminal hydrophilic LC regions and the AP motifs, the role of this protein in protecting the cellular milieu by helping in establishing salt bridges with other proteins during desiccation and by interactions with proteins containing SH3 domain could be suggested. The D. radiodurans genome encodes proteins having putative SH3 motifs (see Fig. S3 in the supplemental material), and the activity of one such protein, DR2518, was inhibited in the presence of purified DR1769 (Fig. 7). DR2518 is an important STPK characterized by its role in radiation resistance, which seems to function through phosphorylation-mediated functional modulation of the DNA metabolic protein (Y. S. Rajpurohit and H. S. Misra, unpublished data). Similarly, we know that RecA is an essential recombinase that is required for maintaining genome integrity in the bacteria. RecA of D. radiodurans is functionally different from other bacteria (47) and is required in the extended synthesis-dependent strand-annealing mechanism of DSB repair in this bacterium (13). Therefore, the possibilities of DR1769 either protecting the SH3-type domain harboring proteins, including RecA and DR2518 in D. radiodurans under desiccation, may be hypothesized. These results suggest that DR1769 contributes in desiccation and gamma radiation resistance of D. radiodurans most likely through the regulation of DSB repair and by protecting nucleoid structure under desiccation.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to S. K. Apte for his critical comments on this work and technical suggestions. We acknowledge the help of Shri A. D. Das in bioinformatic analysis and Shri Vijaya Kumar Charaka and Shri Jaya Kumar for their support in microscopic studies.
Footnotes
Published ahead of print 21 June 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00418-13.
REFERENCES
- 1.Hoekstra AF, Golorina EA, Buitink J. 2001. Mechanisms of plant desiccation tolerance. Trends Plant Sci. 6:431–438 [DOI] [PubMed] [Google Scholar]
- 2.Goyal K, Walton LJ, Tunnacliffe A. 2005. LEA proteins prevent protein aggregation due to water stress. Biochem. J. 388:151–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Browne JA, Dolan KM, Tyson T, Goyal K, Tunnacliffe A, Burnell AM. 2004. Dehydration-specific induction of hydrophilic protein genes in the anhydrobiotic nematode Aphelenchus avenae. Eukaryot. Cell 3:966–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wise MJ. 2003. LEAping to conclusions: a computational reanalysis of late embryogenesis abundant proteins and their possible roles. BMC Bioinformatics 4:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ingram J, Bartels D. 1996. The molecular basis of dehydration tolerance in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47:377–403 [DOI] [PubMed] [Google Scholar]
- 6.Garay-Arroyo A, Colmenero-Flores JM, Garciarrubio A, Covarrubias AA. 2000. Highly hydrophilic proteins in prokaryotes and eukaryotes are common during conditions of water deficit. J. Biol. Chem. 275:5668–5674 [DOI] [PubMed] [Google Scholar]
- 7.Goyal K, Tisi L, Basran A, Browne J, Burnell A, Zurdo J, Tunnacliffe A. 2003. Transition from natively unfolded to folded state induced by desiccation in an anhydrobiotic nematode protein. J. Biol. Chem. 278:12977–12984 [DOI] [PubMed] [Google Scholar]
- 8.Chakrabortee S, Boschetti C, Walton LJ, Sarkar S, Rubinsztein DC, Tunnacliffe A. 2007. Hydrophilic protein associated with desiccation tolerance exhibits broad protein stabilization function. Proc. Natl. Acad. Sci. U. S. A. 104:18073–18078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sim KL, Creamer TP. 2002. Abundance and distributions of eukaryote protein simple sequences. Mol. Cell. Proteomics 1:983–995 [DOI] [PubMed] [Google Scholar]
- 10.Krisko A, Smole Z, Debret G, Nikolic N, Radman M. 2010. Unstructured hydrophilic sequences in prokaryotic proteomes correlate with dehydration tolerance and host association. J. Mol. Biol. 402:775–782 [DOI] [PubMed] [Google Scholar]
- 11.Slade D, Radman M. 2011. Oxidative stress resistance in Deinococcus radiodurans. Microbiol. Mol. Biol. Rev. 75:133–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blasius M, Sommer S, Hubscher U. 2008. Deinococcus radiodurans: what belongs to the survival kit? Crit. Rev. Biochem. Mol. Biol. 43:221–238 [DOI] [PubMed] [Google Scholar]
- 13.Zahradka K, Slade D, Bailone A, Sommer S, Averbeck D, Petranovic M, Lindner AB, Radman M. 2006. Reassembly of shattered chromosomes in Deinococcus radiodurans. Nature 443:569–573 [DOI] [PubMed] [Google Scholar]
- 14.Markillie LM, Varnum SM, Hradecky P, Wong KK. 1999. Targeted mutagenesis by duplication insertion in the radioresistant bacterium Deinococcus radiodurans: radiation sensitivities of catalase (katA) and superoxide dismutase (sodA) mutants. J. Bacteriol. 181:666–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khairnar NP, Misra HS, Apte SK. 2003. Pyrroloquinoline-quinone synthesized in Escherichia coli by pyrroloquinoline-quinone synthase of Deinococcus radiodurans plays a role beyond mineral phosphate solubilization. Biochem. Biophys. Res. Commun. 312:303–308 [DOI] [PubMed] [Google Scholar]
- 16.Rajpurohit YS, Gopalakrishnan R, Misra HS. 2008. Involvement of a protein kinase activity inducer in DNA double-strand break repair and radioresistance of Deinococcus radiodurans. J. Bacteriol. 190:3948–3954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian B, Hua Y. 2010. Carotenoid biosynthesis in extremophilic Deinococcus-Thermus bacteria. Trends Microbiol. 18:512–520 [DOI] [PubMed] [Google Scholar]
- 18.Daly MJ, Gaidamakova EK, Matrosova VY, Vasilenko A, Zhai M, Venkateswaran A, Hess M, Omelchenko MV, Kostandarithes HM, Makarova KS, Wackett LP, Fredrickson JK, Ghosal D. 2004. Accumulation of Mn(II) in Deinococcus radiodurans facilitates gamma-radiation resistance. Science 306:1025–1028 [DOI] [PubMed] [Google Scholar]
- 19.Misra HS, Khairnar NP, Barik A, Indira PI, Mohan H, Apte SK. 2004. Pyrroloquinoline-quinone: a reactive oxygen species scavenger in bacteria. FEBS Lett. 578:26–30 [DOI] [PubMed] [Google Scholar]
- 20.Rajpurohit YS, Misra HS. 2010. Characterization of a DNA damage-inducible membrane protein kinase from Deinococcus radiodurans and its role in bacterial radioresistance and DNA strand break repair. Mol. Microbiol. 77:1470–1482 [DOI] [PubMed] [Google Scholar]
- 21.Rucker R, Chowanadisai W, Nakano M. 2009. Potential physiological importance of pyrroloquinoline quinone. Altern. Med. Rev. 14:268–277 [PubMed] [Google Scholar]
- 22.Misra HS, Rajpurohit YS, Khairnar NP. 2012. Pyrroloquinoline-quinone and its versatile roles in biological processes. J. Biosci. 37:313–325 [DOI] [PubMed] [Google Scholar]
- 23.Makarova KS, Aravind L, Wolf YI, Tatusov RL, Minton KW, Koonin EV, Daly MJ. 2001. Genome of the extremely radiation-resistant bacterium Deinococcus radiodurans viewed from the perspective of comparative genomics. Microbiol. Mol. Biol. Rev. 65:44–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Misra HS, Rajpurohit YS, Kota S. 2013. Physiological and molecular basis of extreme radioresistance in Deinococcus radiodurans. Curr. Sci. 104:194–205 [Google Scholar]
- 25.Schaefer M, Schmitz C, Facius R, Horneck G, Milow B, Funken KH, Ortner J. 2000. Systematic study of parameters influencing the action of Rose Bengal with visible light on bacterial cells: comparison between the biological effect and singlet-oxygen production. Photochem. Photobiol. 71:514–523 [DOI] [PubMed] [Google Scholar]
- 26.Misra HS, Khairnar NP, Kota S, Shrivastava S, Joshi VP, Apte SK. 2006. An exonuclease I-sensitive DNA repair pathway in Deinococcus radiodurans: a major determinant of radiation resistance. Mol. Microbiol. 59:1308–1316 [DOI] [PubMed] [Google Scholar]
- 27.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 28.Battista JR, Park MJ, McLemore AE. 2001. Inactivation of two homologues of proteins presumed to be involved in the desiccation tolerance of plants sensitizes Deinococcus radiodurans R1 to desiccation. Cryobiology 43:133–139 [DOI] [PubMed] [Google Scholar]
- 29.Meima R, Rothfuss HM, Gewin L, Lidstrom ME. 2001. Promoter cloning in the radioresistant bacterium Deinococcus radiodurans. J. Bacteriol. 183:3169–3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kharinar NP, Kamble VA, Misra HS. 2008. RecBC enzyme overproduction affects UV and gamma radiation survival of Deinococcus radiodurans. DNA Repair (Amst.) 7:40–47 [DOI] [PubMed] [Google Scholar]
- 31.Charaka VK, Misra HS. 2012. Functional characterization of chromosome I partitioning system in Deinococcus radiodurans for its role in genome segregation. J. Bacteriol. 194:5739–5748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White O, Eisen JA, Heidelberg JF, Hickey EK, Peterson JD, Dodson RJ, Haft DH, Gwinn ML, Nelson WC, Richardson DL, Moffat KS, Qin H, Jiang L, Pamphile W, Crosby M, Shen M, Vamathevan JJ, Lam P, McDonald L, Utterback T, Zalewski C, Makarova KS, Aravind L, Daly MJ, Minton KW, Fleischmann RD, Ketchum KA, Nelson KE, Salzberg S, Smith HO, Venter JC, Fraser CM. 1999. Genome sequence of the radioresistant bacterium Deinococcus radiodurans R1. Science 286:1571–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Zhou J, Omelchenko MV, Beliaev AS, Venkateswaran A, Stair J, Wu L, Thompson DK, Xu D, Rogozin IB, Gaidamakova EK, Zhai M, Makarova Koonin KS EV, Daly MJ. 2003. Transcriptome dynamics of Deinococcus radiodurans recovering from ionizing radiation. Proc. Natl. Acad. Sci. U. S. A. 100:4191–4196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka A, Earl M, Howell HA, Park MJ, Eisen Peterson SN, Battista JR. 2004. Analysis of Deinococcus radiodurans's transcriptional response to ionizing radiation and desiccation reveals novel proteins that contribute to extreme radioresistance. Genetics 168:21–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li SC, Gish G, Yang D, Coffey AJ, Forman-Kay JD, Ernberg I, Kay LE, Pawson T. 1999. Novel mode of ligand binding by the SH2 domain of the human XLP disease gene product SAP/SH2D1A. Curr. Biol. 9:1355–1362 [DOI] [PubMed] [Google Scholar]
- 36.Mayer BJ. 2001. SH3 domains: complexity in moderation. J. Cell Sci. 114:1253–1263 [DOI] [PubMed] [Google Scholar]
- 37.Kay BK, Williamson MP, Sudol M. 2000. The importance of being proline: the interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J. 14:231–241 [PubMed] [Google Scholar]
- 38.Whisstock JC, Lesk AM. 1999. SH3 domains in prokaryotes. Trends Biochem. Sci. 24:132–133 [DOI] [PubMed] [Google Scholar]
- 39.Kaneko T, Li L, Li SS. 2008. The SH3 domain—a family of versatile peptide- and protein-recognition module. Front. Biosci. 13:4938–4952 [DOI] [PubMed] [Google Scholar]
- 40.Desai SS, Rajpurohit YS, Misra HS, Deobagkar DN. 2011. Characterization of RadS/RadR two-component system role in radiation resistance of Deinococcus radiodurans. Microbiology (SGM) 157:2974–2982 [DOI] [PubMed] [Google Scholar]
- 41.Mattimore V, Battista JR. 1996. Radioresistance of Deinococcus radiodurans: functions necessary to survive ionizing radiation are also necessary to survive prolonged desiccation. J. Bacteriol. 178:633–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Battista JR. 2000. Radiation resistance: the fragments that remain. Curr. Biol. 10:R204–R205 [DOI] [PubMed] [Google Scholar]
- 43.Bieger-Dose A, Dose K, Meffert R, Mehler M, Risi S. 1992. Extreme dryness and DNA-protein cross-links. Adv. Space Res. 12:265–270 [DOI] [PubMed] [Google Scholar]
- 44.Dose K, Gill M. 1995. DNA stability and survival of Bacillus subtilis spores in extreme dryness. Orig. Life Evol. Biosph. 25:277–293 [DOI] [PubMed] [Google Scholar]
- 45.Li D, Roberts R. 2001. WD-repeat proteins: structure characteristics, biological function, and their involvement in human diseases. Cell. Mol. Life Sci. 58:2085–2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith TF, Gaitatzes C, Saxena K, Neer EJ. 1999. The WD repeat: a common architecture for diverse functions. Trends Biochem. Sci. 24:181–185 [DOI] [PubMed] [Google Scholar]
- 47.Kim JI, Sharma AK, Abbott SN, Wood EA, Dwyer DW, Jambura A, Minton KW, Inman RB, Daly MJ, Cox MM. 2002. RecA protein from the extremely radioresistant bacterium Deinococcus radiodurans: expression, purification, and characterization. J. Bacteriol. 184:1649–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.