Abstract
To survive in phage-containing environments, bacteria have evolved an array of antiphage systems. Similarly, phages have overcome these hurdles through various means. Here, we investigated how phages are able to circumvent the Lactococcus lactis AbiQ system, a type III toxin-antitoxin with antiviral activities. Lactococcal phage escape mutants were obtained in the laboratory, and their genomes were sequenced. Three unrelated genes of unknown function were mutated in derivatives of three distinct lactococcal siphophages: orf38 of phage P008, m1 of phage bIL170, and e19 of phage c2. One-step growth curve experiments revealed that the phage mutations had a fitness cost while transcriptional analyses showed that AbiQ modified the early-expressed phage mRNA profiles. The L. lactis AbiQ system was also transferred into Escherichia coli MG1655 and tested against several coliphages. While AbiQ was efficient against phages T4 (Myoviridae) and T5 (Siphoviridae), escape mutants of only phage 2 (Myoviridae) could be isolated. Genome sequencing revealed a mutation in gene orf210, a putative DNA polymerase. Taking these observations together, different phage genes or gene products are targeted or involved in the AbiQ phenotype. Moreover, this antiviral system is active against various phage families infecting Gram-positive and Gram-negative bacteria. A model for the mode of action of AbiQ is proposed.
INTRODUCTION
Bacteriophages are ubiquitous in most environments, including foods. Some virulent phages will thrive during food manufacturing processes that rely on rapid bacterial growth or metabolic activities. Lactococcus lactis is a Gram-positive bacterium used in the production of several fermented dairy products. These milk-based cultures can be lysed by a plethora of distinct virulent phages (1), leading to variations in product quality. Numerous antiphage hurdles have been devised over the past decades to cope with this risk (reviewed in references 2 to 5). Yet some phages will persist or emerge in dairy environments (6). L. lactis phages belong to the Caudovirales order (1, 7). Their double-stranded DNA (dsDNA) genomes are within an icosahedral capsid connected to a short (Podoviridae family) or a long noncontractile tail (Siphoviridae). They are also divided into at least 10 genotypes, but three of these, the 936, c2, and P335 groups, contain hundreds of known members and are mostly associated with failed milk fermentations worldwide (1, 8). The complete genome of at least one member of the 10 genotypes is available, with more genomes determined for the most common groups (9). Progress has been made in the structural aspects of the interaction of these phages with their hosts (reviewed in reference 10). However, many phage genes coding for nonstructural proteins have unknown function, and our knowledge of their roles in phage biology is limited.
When infecting bacterial cells, phages may face barriers that will hamper their amplification. These hurdles can prevent the phage adsorption process, inhibit the phage genome ejection into the cell, cut the invading genome, or simply abort another step of the lytic cycle (11). Over 20 lactococcal abortive infection (Abi) systems have been reported (12–15). While some Abi systems stop the replication of several phage genotypes, others inhibit only a few groups (12–15). Of note, not every member of a phage group is sensitive to an Abi system at similar levels (14, 16, 17). Lactococcal Abi systems have been reported to block phage DNA replication, transcription, translation, maturation, and/or lysis, but the mechanistic details are still elusive.
The characterization of Abi-escape phage mutants has led to some mechanistic information. A mutated Orf1 of phage bIL66 (936 group) is no longer able to induce AbiD1 (18–20). The wild-type Orf1 binds to an mRNA secondary structure to activate AbiD1 expression (18). AbiD1 interferes with the phage RuvC-like endonuclease to inhibit replication (20, 21). To resist AbiK, lactococcal phages have evolved mutations in the sak genes, which code for single-strand annealing proteins (17, 22–25). AbiK polymerizes an untemplated DNA molecule via its reverse transcriptase motif to confer phage resistance (26, 27). Phage bIL66M1 (936) needs to acquire a gene (e6) of unknown function from phage bIL170 (936) to avoid abortion by AbiP (28). L. lactis AbiP inhibits the switch-off from early to middle phage gene transcription (29). Lactococcal phages of the 936 group can become insensitive to AbiV due to mutations in the sav gene (30). The interaction between SaV and AbiV leads to a general inhibition of protein synthesis (31). Finally, AbiT-escaping mutants derived from distinct phages (p2, P008, and bIL170/936 group) bypass the antiviral system due to mutations in different genes, indicating a possible phage-dependent activity (32). While two of these phage genes (e14 of bIL170 and orf41 of P008) have no known function, the gene coding for the major capsid protein (orf6 of p2) plays a role in the AbiT phenotype (32), which affects DNA replication and encapsidation (16).
The AbiQ system is active against members of the common 936 and c2 groups (33) as well as rare lactococcal phage groups, with efficiencies of plating (EOPs) of <10−8 for phages Q54 (34), P087 (35), and 949 (9). Recently, it was demonstrated that AbiQ is also a type III toxin-antitoxin (TA) system, with the antitoxin being an RNA molecule (2.8 repeats of 35 nucleotides) and the AbiQ toxin being a protein (172 amino acids, 20.3 kDa) with endoribonuclease activity (36, 37). AbiQ is also related to another Abi, named ToxIN, found in Pectobacterium atrosepticum (37). In this study, we further characterized AbiQ through the analysis of phage escape mutants.
MATERIALS AND METHODS
Bacteria and phage propagation.
Phages and hosts used in this study are listed in Table 1. L. lactis strains were grown in M17 medium supplemented with 0.5% glucose (GM17; Oxoid) at 30°C. When needed, 5 μg/ml of chloramphenicol or erythromycin was added for plasmid maintenance, and 10 mM CaCl2 was added for phage propagation. Escherichia coli strains were grown in LB medium or Trypticase soy broth (TSB) medium and incubated at 37°C with agitation. Chloramphenicol (20 μg/ml) was added to the medium when necessary. For phage amplification, bacteria were grown to an optical density at 600 nm (OD600) of 0.2 prior to the addition of approximately 104 phages per ml and incubated until lysis. When the culture was completely clear, the phage lysate was filtered (0.45-μm-pore-size filter) and stored at 4°C. The efficiency of plating (EOP) was calculated by dividing the phage titer on an AbiQ+ strain by the phage titer on an isogenic AbiQ− strain. To obtain a concentrated phage sample, 1 liter of phage lysate was purified on a discontinuous cesium chloride gradient as described previously (38).
Table 1.
Bacterial strains, plasmids, and phages used in this study
| Bacterial strain, plasmid, or phage | Relevant characteristic(s) | Accession no. | Reference(s) or source |
|---|---|---|---|
| Strains | |||
| Escherichia coli | |||
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15 Tn10 (Tetr)] | Stratagene | |
| MG1655 | F− λ− ilvG- rfb-50 rph-1 | U00096 | 57 |
| DH5-α | supE44 Dlac U169 (f80 lacZDM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | Gibco/BRL | |
| Lactococcus lactis | |||
| IL1403 | Plasmid free; host of P008 and bIL170 | NC_002662 | 58, 59 |
| MG1363 | Plasmid free; host of p2 | NC_009004 | 60, 61 |
| LM0230 | Plasmid free; host of jj50, sk1, 712, and c2 | 62 | |
| Plasmids | |||
| pNZ123 | High-copy-number vector; Cmr, 2.8 kb | 63 | |
| pTRKH2 | High-copy-number vector; Emr, 6.9 kb | 64 | |
| pMIG3 | Medium-copy-number vector, Cmr, 5.5 kb | 65 | |
| pSRQ928 | pNZ123 plus 2.2-kb fragment containing AbiQ | 33 | |
| pSRQ925 | pMIG3 plus 2.2-kb fragment containing AbiQ | 33 | |
| pNZ123-AbiQ | pNZ123 plus AbiQ operon | 36 | |
| pTRKH2-orf38 | pTRKH2 plus orf38 of phage P008 | This study | |
| pTRKH2-orf38M (Q12) | pTRKH2 plus orf38 of phage P008-Q12 | This study | |
| pTRKH2-orf38null | pTRKH2 plus orf38 with two stop codons in positions 5 and 6 of the protein | This study | |
| pTRKH2-orf38fs | pTRKH2 plus orf38 with a reading frame modification in the fifth codon | This study | |
| Phages | |||
| P008 | Siphoviridae, 936 group, propagated on IL1403 | DQ054536 | 46 |
| bIL170 | Siphoviridae, 936 group, propagated on IL1403 | AF009630 | 48 |
| jj50 | Siphoviridae, 936 group, propagated on LM0230 | NC_008371 | 46 |
| sk1 | Siphoviridae, 936 group, propagated on LM0230 | NC_001835 | 66 |
| 712 | Siphoviridae, 936 group, propagated on LM0230 | NC_008370 | 46 |
| p2 | Siphoviridae, 936 group, propagated on MG1363 | GQ979703 | 67 |
| c2 | Siphoviridae, c2 group, propagated on LM0230 | NC_001706 | 49 |
| T1 | Siphoviridae, propagated on MG1655 | NC_005833 | 68 |
| T3 | Podoviridae, propagated on MG1655 | NC_003298 | 69 |
| T4 | Myoviridae, propagated on MG1655 | NC_000866 | 70 |
| T5 | Siphoviridae, propagated on MG1655 | NC_005859 | 71 |
| Lambda vir | Siphoviridae, propagated on MG1655 | NC_001416 | 72 |
| RB69 | Myoviridae, propagated on MG1655 | NC_004928 | 73 |
| HK97 | Siphoviridae, propagated on MG1655 | NC_002167 | 74 |
| Mu | Myoviridae, propagated on MG1655 | NC_000929 | 75 |
| pilHα | Leviviridae, propagated on MG1655 | 76 | |
| 2 | Myoviridae, propagated on MG1655 | KC690136 | 50 |
Phage growth curves were performed at 30°C during 70 min with a starting multiplicity of infection (MOI) of 0.05, as reported elsewhere (39). Growth curves were made at least three times, and all the sampling dilutions were plated in triplicates. The burst size was calculated by dividing the average phage titer after the exponential phase by the average titer before the infected cells began to release new virions. Assays for the efficiency at which the center of infection formed (ECOI) were done in triplicate as described previously (33, 39) with phages P008 and P008-Q12 at an MOI of 0.2 and with the phage-sensitive strain IL1403 (pNZ123) and an AbiQ-containing derivative (pSRQ928). The ECOI was calculated as the number of COIs on the resistant strain divided by number of COIs on the sensitive strain, multiplied by 100.
Phage escape mutants.
Phage plaques from multiple lysates were isolated on a plate containing a lawn of AbiQ+ cells infected with wild-type phages. Each escaping phage plaque was purified three times on a lawn of AbiQ+ cells. Then, phage amplifications were performed in liquid medium until a titer of at least 109 PFU/ml was obtained. To isolate mutants derived from the wild-type AbiQ-sensitive phage P008 (936 group), the strain L. lactis IL1403 containing the AbiQ plasmid pSRQ928 was used. Mutants from wild-type phage bIL170 (936 group) were isolated on L. lactis IL1403 containing the AbiQ plasmid pSRQ925. Wild-type c2 phage was propagated on L. lactis LM0230 harboring pSRQ928 (33). AbiQ-insensitive coliphages were obtained on E. coli MG1655 with the vector pNZ123-AbiQ. To improve phage plaque visualization, 0.5% glycine was added to top agar (40), and/or agarose 0.4% was used to replace agar. The temperature of incubation was also reduced from 30°C to 25°C for L. lactis and from 37°C to 30°C for E. coli.
DNA manipulations and analyses.
Phage DNA was isolated using a Qiagen Maxi Lambda DNA preparation kit with the modifications described previously (41). The genomes of phages P008-Q1, P008-Q12, c2, and c2-Q3 were sequenced at the Plateforme the Séquençage et de Génotypage des Génomes of the Centre Hospitalier Universitaire de Québec-Centre Hospitalier de l'Université Laval, (CHUQ-CHUL). The genomes of phages bIL170, bIL170-Q22, 2, and 2Q4 were sequenced at the Plateforme d'Analyses Génomiques de l'IBIS (Université Laval). Genomes were assembled using the Staden package (http://staden.sourceforge.net/) and edited with Bioedit (http://www.mbio.ncsu.edu/bioedit/bioedit.html). To find open reading frame (ORF) functions, protein sequences were analyzed with BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi), ACLAME (http://aclame.ulb.ac.be/), FASTA (http://www.ebi.ac.uk/Tools/sss/fasta/), the Conserved Domain Database (42), PHYRE 2 (43), the Protein Data Bank (PDB) (44), InterProScan (http://www.ebi.ac.uk/Tools/pfa/iprscan/), and SMART (45) tools. To determine protein properties, ProtParam was used (http://web.expasy.org/protparam/). To evaluate the codon usage of phage orf, the codon usage of L. lactis was used for comparison (http://www.kazusa.or.jp/codon/). For promoters, the consensus −35 box (TTGACA) followed by the −10 box (TATAAT) was visually searched. A stretch of T residues preceded by a hairpin structure (MFOLD, Integrated DNA Technologies, Coralville IA [http://mobyle.pasteur.fr/cgi-bin/portal.py?#forms::mfold]) suggested the presence of rho-independent terminators.
Recombination assay.
A PCR product of the orf38 or a mutated orf38 (orf38M) was cloned in the pTRKH2 shuttle vector using phage P008 and P008-Q12 DNA as templates. These plasmids were subcloned in E. coli XL1-Blue and transformed in L. lactis IL1403 containing pNZ123 or IL1403 containing pSRQ928. All constructs were confirmed by sequencing. The EOPs of phages P008 and P008-Q12 were calculated by dividing the titer of the phage on the tested strain by the titer of the phage on the control strain, phage-sensitive L. lactis IL1403 harboring the empty cloning vectors pNZ123 and pTRKH2. To try to obtain null (two stop codons at positions 5 and 6 of orf38) and frameshift (fs; addition of one nucleotide at the fifth codon) mutations in orf38 (orf38null and orf38fs, respectively), site-directed mutagenesis of the vector pTRKH2-orf38 was used to introduce the targeted modification (36). Primers used were the following: orf38nulA, ATGTACACAGCATAATAAAGAGAGCAAATCATCG; orf38nulB, CGATGATTTGCTCTCTTTATTATGCTGTGTACAT; orf38fsA, ATGTACACAGCAAGAAGAGAGAGAGCAAATCAT; and orf38fsB, ATGATTTGCTCTCTCTCTTCTTGCTGTGTACAT. Resulting plasmids were transformed in E. coli DH5α at first and then in L. lactis IL1403(pSRQ928) and confirmed by sequencing. Ten plaques of phage P008 isolated on L. lactis IL1403 containing pSRQ928 and on L. lactis IL1403(pSRQ928) with pTRKH2-orf38M, pTRKH2-orf38null, or pTRKH2-orf38fs were purified and amplified on IL1403 containing pSRQ928. Then, the orf38 gene of each phage was PCR amplified, and the PCR products were sequenced to identify the mutation.
Detection of ORF38 by mass spectrometry.
L. lactis IL1403 was grown in 10 ml of GM17 at 30°C until an OD600 of 0.5. Then, phage P008 was added at an MOI of 5, and the infection was followed for 15 min. Cells were rapidly pelleted and frozen at −80°C. After cells were thawed on ice, they were resuspended in sample loading buffer and sonicated, and proteins were separated on a 15% SDS-PAGE gel. Protein bands of the size expected for the ORF38 protein (8 kDa) were cut and sent for liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis at the Proteomic platform of the Quebec genomic center (CHUQ-CHUL). The peptide identification results were compared with the phage P008 ORF database, and the identification confidence was determined with Scaffold, version 3, software.
Northern blotting.
A time course infection of L. lactis IL1403 (phage sensitive) or IL1403 containing pSRQ928 (AbiQ+) was performed with phages P008 and P008-Q12 at an MOI of 5 as described previously (36). The following samples were taken: noninfected (NI) and at 2, 10, 20, 30, and 40 min postinfection. Total RNA was purified with TRIzol as described by the manufacturer (Invitrogen), with the addition of a lysozyme pretreatment (60 mg/ml lysozyme for 10 min at 37°C) to increase bacterial lysis. RNA samples were treated with DNase I (Roche) to eliminate residual DNA and protected with RNA inhibitor (Roche), and the RNA concentration was estimated with a NanoDrop 2000 instrument (Thermo-Scientific). Aliquots of 5 μg of RNA were migrated on 1% formaldehyde-agarose denaturing gels and transferred on nylon membranes (38). Northern blot experiments were performed as described previously (34) with 32P-radiolabeled oligonucleotide probes (sense or antisense orientation) to specific genes of phage P008: orf33, orf36, orf37, orf38, orf39, orf40, orf41, orf45, orf52, and orf53 (see supplemental material).
Nucleotide sequence accession number.
The genome of coliphage 2 was deposited in the GenBank database under accession number KC690136.
RESULTS
AbiQ-escaping P008 phage mutants.
In order to identify the target of AbiQ, six wild-type AbiQ-sensitive virulent phages of the 936 group (P008, bIL170, jj50, sk1, 712, and p2) and one phage of the c2 group (c2) were used to infect AbiQ-containing L. lactis cells. All these phages belong to the Siphoviridae family. No phage escape mutants (no plaque) could be observed on plates containing AbiQ-expressing L. lactis cells and high population (>108) of wild-type phages jj50, sk1, 712, and p2. These data suggest that AbiQ strongly inhibits the multiplication of these phages. On the other hand, AbiQ-resistant phages were isolated from phages P008, bIL170, and c2.
The EOP of phage P008 on L. lactis IL1403 carrying AbiQ [IL1403(pNZ123-AbiQ)] is 10−5. Twenty-two P008-derived mutants able to propagate in an AbiQ-containing strain were randomly selected, purified, and characterized (Table 2). The EOPs of these mutants were measured by dividing the titer of each phage lysate on the L. lactis strain IL1403 with AbiQ by the titer of the lysate on the same strain without AbiQ. The EOP of these phage escape mutants was 10−2, an increase of 3 logs compared to the wild-type phage P008. These results also indicated that these phage mutants do not completely escape AbiQ (EOP of 1) but are significantly less affected. The complete genomes (28,538 bp) of two of these AbiQ-escaping mutants (Q1 and Q12) were sequenced, and the sequences were compared to the wild-type genome (GenBank accession number DQ054536) (46). Both AbiQ-escaping phages had only one nucleotide mutation, which was located in the orf38 gene (P008-Q12) or in the ribosome binding site (RBS) located upstream of its start codon (P008-Q1). The genomic region containing orf38 was amplified and sequenced for the other 20 P008-derived AbiQ-escaping mutants, and similarly, all of them had a mutation in the orf38 gene or in its RBS, confirming the importance of this gene for the AbiQ activity. ORF38 has 71 amino acids, a predicted molecular mass of 8.3 kDa, and a pI of 4.5.
Table 2.
Characterization of lactococcal AbiQ escape mutants derived from phages P008, bIL170, and c2
| Phagea | EOPb | Frequencye | Mutated ORF | Amino acid substitution |
|---|---|---|---|---|
| P008 | (1.5 ± 1.3) × 10−5 | |||
| P008-Q1 | (9.0 ± 0.8) × 10−2 | 1/22 | RBS orf38c | |
| P008-Q14 | (8.1 ± 1.1) × 10−2 | 1/22 | RBS orf38d | |
| P008-Q17 | (1.5 ± 0.9) × 10−2 | 1/22 | ORF38 | Met1Leu |
| P008-Q5 | (3.4 ± 0.7) × 10−2 | 1/22 | ORF38 | Thr3Thr |
| P008H2-10 | (1.2 ± 0.4) × 10−2 | 1/22 | ORF38 | Thr3Ile |
| P008H2-7 | (2.6 ± 1.9) × 10−2 | 1/22 | ORF38 | Glu6Gly |
| P008-Q4 | (2.5 ± 0.7) × 10−2 | 1/22 | ORF38 | Ser18Asn |
| P008-Q16 | (3.9 ± 1.3) × 10−2 | 6/22 | ORF38 | Asp23Gly |
| P008-Q19 | (4.0 ± 0.6) × 10−2 | 2/22 | ORF38 | Trp30Stop |
| P008-Q12 | (3.3 ± 1.2) × 10−2 | 6/22 | ORF38 | Pro38Leu |
| P008-Q11 | (4.1 ± 1.3) × 10−2 | 1/22 | ORF38 | Ser49Pro |
| bIL170 | <10−6 | |||
| bIL170-Q2 | (1.7 ± 1.0) × 10−1 | 6/9 | M1 | Met1Phe |
| bIL170-Q22 | (3.2 ± 2.5) × 10−1 | 3/9 | M1 | Gln5Stop |
| c2 | <10−6 | |||
| c2-Q3 | 1 | 5/5 | E19 | Glu17Asp |
Phages in bold indicate those for which the complete genome was sequenced.
The EOPs and standard deviation values were calculated for three assays done in triplicate.
Consensus sequence of the RBS in L. lactis, AGAAAGGAGGT; RBS of orf38 in wild-type phage P008, AGAAAGTCGGT; RBS mutation, AGAAATTCGGT (position 21406).
RBS mutation, AGAAAGTCTGT (position 21403).
Number of times phage was isolated/number of mutants analyzed.
Detailed analyses of these 22 AbiQ-escaping phage mutants revealed 11 distinct mutations, including two (phage P008 mutants Q1 and Q14) in the RBS preceding orf38 (Table 2), suggesting that the level of expression of ORF38 is important to bypass AbiQ. In support of this observation, a mutation in phage escape mutant P008-Q17 led to a substitution of the methionine at position 1 by a leucine (Met1Leu) (Table 2). While the leucine residue could serve as an alternative translational initiation codon, it is used four times less frequently in L. lactis than the traditional methionine codon. Similarly, in phage P008-Q5, the silent mutation Thr3Thr should not change the intrinsic property of the protein, but the mutated codon (ACA) is used half as frequently as the one (ACC) found in the wild-type phage P008. The other mutations led to amino acid changes distributed along the protein and could affect either its conformation or its activity. One mutation also led to a truncated protein (Trp30Stop). Our bioinformatics analyses failed to identify a function. However, this protein is well conserved (>90% identity) in other lactococcal phages of the 936 group. The orf38 is localized in a gene cluster that is expressed early in the phage infection and is likely involved in DNA replication.
AbiQ-escaping bIL170 phage mutants.
Using a similar approach, we isolated AbiQ-escaping mutants derived from the wild-type AbiQ-sensitive virulent lactococcal phage bIL170 (936 group). The genomes of phages bIL170 and P008 share 77.8% identity at nucleotide level, and they both infect L. lactis IL1403 (47). A total of nine AbiQ-escaping bIL170 mutants were characterized. The EOPs of these nine phage mutants were increased from 10−6 (for the wild-type phage) to 10−1 on AbiQ-containing cells (Table 2). The complete genome (31,754 bp) of the phage escape mutant bIL170-Q22 was sequenced and compared to the wild-type genome (GenBank AF009630) (48). Again, a single nucleotide mutation was observed. The mutation was located in the m1 gene and not in the e14 gene (homolog of the P008 orf38 gene). The m1 region was amplified and sequenced for the other escape mutants. Each had a mutation in m1, but only two distinct mutations were observed leading to amino acid changes, Met1Phe and Gln5Stop. In both cases, the mutation probably leads to a defect in M1 protein production. No function could be attributed to M1 (42 amino acids, 4.8 kDa, and pI 4.2) but this protein is well conserved (>90% identity) in lactococcal phages of the 936 group. It shares no similarities with ORF38 of P008. The m1 gene is located in a gene cluster that is starting to be expressed in the middle of the phage lytic cycle.
AbiQ-escaping c2 phage mutants.
We also analyzed AbiQ-escaping mutants from another group of lactococcal phage, namely, c2. Five phage mutants escaping AbiQ (EOP of ∼1) were isolated (Table 2), and the genome (22,172 bp) of one mutant was compared to that of the wild type (GenBank accession number L48605) (49). One nucleotide change was found in the early-expressed e19 phage gene leading to a Glu17Asp substitution in the protein. The same mutation was found in the four other phage mutants. E19 (107 amino acids, 12.5 kDa, and pI 4.6) has no known function but is conserved (gp33; 94% amino acid identity) in the other phage (bIL67) of the c2 group for which the genome is available.
AbiQ-escaping E. coli phage mutants.
We also tested whether AbiQ could be effective against a phage that infects E. coli. We introduced the plasmid pNZ123-AbiQ into E. coli MG1655 and measured the EOPs of 10 coliphages (Table 3). Four phages (T4, Myoviridae family; RB69, Myoviridae; phage 2, Myoviridae; and T5, Siphoviridae) were strongly inhibited by AbiQ, with EOP values reduced by 5 logs, while the six other phages (T1, T3, lambda vir, HK97, Mu, and pilHα) were not affected (Table 3). To our knowledge, this is the first lactococcal Abi system to work against E. coli phages. Moreover, AbiQ could inhibit phages of the Myoviridae family.
Table 3.
Sensitivity of different E. coli phages to AbiQ
| Phage | Family | EOPa |
|---|---|---|
| T1 | Siphoviridae | 1.1 ± 0.1 |
| T3 | Podoviridae | 1.1 ± 0.5 |
| T4 | Myoviridae | (<1.2 ± 0.3) × 10−6 |
| T5 | Siphoviridae | (<5.4 ± 0.5) × 10−7 |
| Lambda vir | Siphoviridae | 1.9 ± 0.1 |
| RB69 | Myoviridae | (<3.5 ± 1.8) × 10−6 |
| HK97 | Siphoviridae | 0.4 ± 0.3 |
| Mu | Myoviriadae | 1.2 ± 0.2 |
| pilHα | Leviviridae | 1.3 ± 0.3 |
| 2 | Myoviridae | (2.0 ± 0.6) × 10−5 |
The EOP and standard deviation values were calculated for three assays done in triplicate.
Despite numerous assays and conditions tested, we could not isolate AbiQ-escaping mutants from T4, T5, and RB69. However, we were able to isolate five AbiQ-resistant mutants derived from coliphage 2, a phage which also infects E. coli O157:H7 strains (50). As the complete genome of the wild-type phage 2 was not available prior to this study, we sequenced it (GenBank accession number KC690136; 136,910 bp), and additional information is available in Table S2 and Fig. S1 in the supplemental material. The genome of one escape mutant (phage 2-Q4) was compared to that of the wild type, and bioinformatics analyses revealed a mutation in a gene coding for a putative protein of 925 amino acids (106.7 kDa). The four other phage mutants had a mutation in the same gene but at different positions (Table 4). The deduced protein, named ORF210, has multiple domains and some similarities with DNA polymerases. It has a putative polynucleotidyl transferase of the RNase H domain localized in the N-terminal part of the protein (amino acids 3 to 217) and a DNA polymerase A palm domain in the C terminus (amino acids 607 to 819). The mutations found in the phage escape mutants 2Q4 (Glu298Gly) and 2Q5 (Met290Val) were close to the polynucleotidyl transferase domain, while the mutations in the phages 2Q1 (Lys331Glu) and 2Q3 (Asn426Lys) were between the two functional domains; the 2Q2 mutation (Val681Ala) is in the putative catalytic site (amino acids 634 to 815).
Table 4.
Phage 2 mutant characterization
| Phagea | EOPb | Frequencyd | ORF210 mutationc |
|---|---|---|---|
| 2 | (2.0 ± 0.6) × 10−5 | ||
| 2Q1 | 0.7 ± 0.1 | 1/5 | Lys331Glu |
| 2Q2 | 0.5 ± 0.1 | 1/5 | Val681Ala |
| 2Q3 | 0.6 ± 0.1 | 1/5 | Asn426Lys |
| 2Q4 | 0.6 ± 0.1 | 1/5 | Glu298Gly |
| 2Q5 | 0.7 ± 0.1 | 1/5 | Met290Val |
Phages in bold are those for which the complete genomes were sequenced.
The EOP and standard deviation values were calculated for three assays done in triplicate.
All mutations occurred in ORF210. Amino acid substitutions are shown.
Number of times phage was isolated/number of mutants analyzed.
No conserved features could be found from these four phage genes (P008, orf38; bIL170, m1; c2, e9; 2, orf210), except that they may be involved in functions related to nucleic acids.
Mutated ORF38 confers insensitivity to AbiQ.
A recombination assay was designed to confirm that a mutated orf38 from the lactococcal phage P008 was responsible for the insensitivity to AbiQ. The plasmid pSRQ928 (AbiQ+) was cotransformed with the plasmid pTRKH2-orf38 wild type or pTRKH2-orf38M (mutated from P008-Q12) in L. lactis IL1403. Of note, orf38 and orf38M are not transcribed when cloned into the pTRKH2 vector (data not shown). The presence of the wild-type orf38 or its mutated version had no effect on the EOPs of either phage in the absence of AbiQ (Table 5). Moreover, the wild-type orf38 gene did not change the phage EOPs in the presence of AbiQ (Table 5). However, the presence of pTRKH2-orf38M increased the EOP of the wild-type phage P008 by 1 log in a strain carrying AbiQ. Ten P008 plaques were purified, and their orf38 genes were PCR amplified and sequenced. The 10 isolated AbiQ-resistant phages had the same mutation in the orf38 as the one found on plasmid pTRKH2-orf38M, strongly suggesting that recombination occurred. A similar experiment was performed with L. lactis cells containing only pSRQ928 (AbiQ+), and only 2 of the 10 phage mutants had acquired this specific mutation. These data confirm that the orf38 is involved in the AbiQ phenotype and that a mutation in this gene allows phages to partially circumvent the antiviral system.
Table 5.
EOPs of phages P008 or P008-Q12 on various L. lactis strains
|
L. lactis IL1403 strain profilea |
EOPb |
|||
|---|---|---|---|---|
| abiQ(pNZ123) | orf38(pTRKH2) | orf38M(pTRKH2) | P008 | P008-Q12 |
| −c,d | − | − | 1.0 ± 0.1 | 1.0 ± 0.1 |
| −d | + | − | 1.0 ± 0.1 | 0.8 ± 0.4 |
| −d | − | + | 0.8 ± 0.4 | 0.5 ± 0.4 |
| +c | − | − | (0.9 ± 0.1) × 10−5 | 0.1 ± 0.1 |
| + | + | − | (1.3 ± 0.3) × 10−5 | 0.2 ± 0.1 |
| + | − | + | (14.9 ± 0.7) × 10−5 | 0.1 ± 0.1 |
The presence (+) and absence (−) of the gene and plasmid are indicated.
The EOP and standard deviation values were calculated for three assays done in triplicate.
The strain also harbors the pTRKH2 empty vector.
The strain also harbors the pNZ123 empty vector.
Orf38 is an essential gene.
While no function could be attributed to ORF38, we tested whether orf38 was an essential gene for P008. Of note, no genetic tool is currently available to generate virulent lactococcal phage mutants. Thus, to construct a null allele of orf38, we first introduced two stop codons at the fifth and sixth positions of orf38 (orf38null) and, in a different construct, a frameshift (fs) in the fifth codon orf38. In both cases, these mutations were generated by site-directed mutagenesis on the pTRKH2-orf38 vector. The vectors were introduced into an L. lactis strain carrying AbiQ. Then, we used the above recombination assay and AbiQ selective pressure to try to generate P008 mutants with a null allele of orf38. Ten plaques of P008 infecting these strains [IL1403(pSRQ928/pTRKH2-orf38null or IL1403(pSRQ928/pTRKH2-orf38fs)] were purified and PCR sequenced in the orf38 gene. No mutant recombined with the plasmid to acquire either of the modifications, strongly suggesting that orf38 is an essential phage gene.
Production of ORF38 during the phage infection.
Since orf38 encodes a well-conserved small protein (8.3 kDa), we tested if this protein was produced during phage infection. An intracellular cell extract from a sample of L. lactis IL1403 infected with P008, 15 min after the beginning of the infection, was migrated on an SDS-PAGE gel, and bands of the expected size for ORF38 were cut and sent for mass spectrometry analysis (LC-MS/MS). The ORF38 protein was detected in the sample with a confidence of 95% (data not shown).
Effects of AbiQ on the growth of P008 and P008-Q12.
To determine the effect of the mutation in orf38 on phage multiplication, we performed growth curve assays of phages P008 and P008-Q12 in the presence or absence of AbiQ. The burst size of P008 in the absence of AbiQ was estimated at 310 ± 67 new phages per infected cell, and its latent period was 39 ± 1 min. We could not estimate those parameters for P008 in AbiQ-containing cells as the phage infection aborted. The burst size of phage P008-Q12 in the absence of AbiQ was 230 ± 47 virions per cells, and its latent period was 43 ± 2 min, suggesting that the mutation in orf38 had a small fitness cost on the escape mutant. The burst size of P008-Q12 was limited to only 9 ± 4 phages per infected AbiQ-containing cell, and the latent period was 47 ± 1 min, confirming that the mutation in orf38 did not confer a complete insensitivity to AbiQ. The efficiency to form the center of infection (ECOI) of both phages was also determined on L. lactis IL1403 strains with or without AbiQ. About half (47.6% ± 5.5%) of P008-Q12-infected AbiQ-containing cells released new virions, which was similar (54.3% ± 12.1%) to the level with P008 infecting the same strain. These results showed that P008-Q12 does not infect an AbiQ-containing strain with maximum efficiency, which is consistent with its EOP of ∼10−2, its low burst size, and its increased latent period. Surprisingly, phage P008 could also replicate in some AbiQ-containing cells.
Effect of AbiQ on the transcription of early- and middle-expressed genes of P008 and P008-Q12.
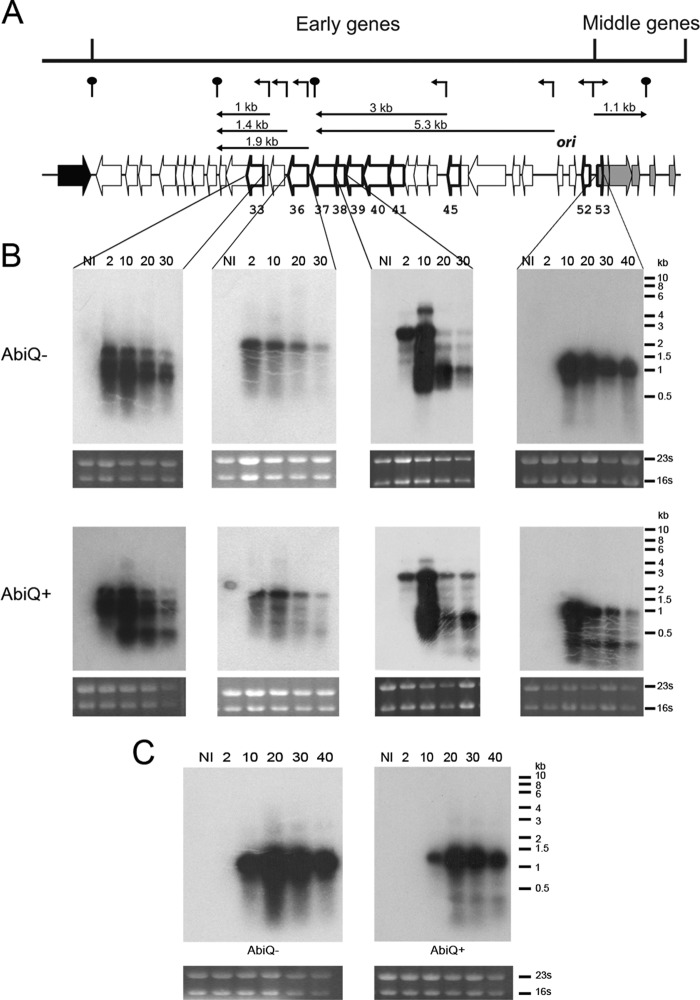
AbiQ is an endoribonuclease that cleaves its RNA antitoxin precursor in vivo to generate small RNAs to neutralize the toxic effect of the protein (36). To test if AbiQ affects phage transcription, a time course of infection of L. lactis IL1403 containing or not AbiQ was performed with phage P008 or P008-Q12. Samples were withdrawn at different times, and the total RNA was extracted and migrated on an agarose gel; Northern blotting carried out using oligonucleotide 32P-labeled probes complementary to 10 phage genes (orf33, orf36, orf37, orf38, orf39, orf40, orf41, orf45, orf52, and orf53). All of these genes are presumably expressed early during the phage infection process, except orf53, which is a so-called middle-expressed gene. Controls with antisense probes did not detect any transcript (data not shown). The detected transcripts obtained by Northern blotting and the bioinformatics analyses were used to generate a transcription map of P008. The results are presented in Fig. 1 and in Fig. S2 in the supplemental material.
Fig 1.
Transcription map of early- and middle-expressed genes from wild-type phage P008 and phage-escaping mutant P008-Q12. (A) Summary of P008 partial transcription map deduced from Northern blotting and bioinformatics analyses. Detected phage P008 genes in the different experiments are represented by arrows with thick lines and numbers (see also Fig. S2 in the supplemental material). The white arrows correspond to early-expressed genes, gray arrows indicate middle-expressed genes, and the black arrow corresponds to a late-expressed gene. Promoters and terminators determined by bioinformatics analyses are above the genome and are represented by broken thin arrows and hairpins, respectively. Transcripts, along with their sizes, detected by Northern blotting and confirmed by bioinformatics analysis are indicated by straight arrows below the promoters-terminators. (B) Transcripts of P008 genes orf33, orf36 orf38, and orf53 detected by Northern blotting during a time course of infection in the absence (−) or in the presence (+) of AbiQ. RNA samples (5 μg) at time NI (noninfected) and at 2, 10, 20, 30, and 40 min were migrated on agarose gels and transferred on nylon membranes. Loading controls of the RNA 23S and 16S migrations are presented below each membrane. Northern blotting was performed with 32P-labeled oligonucleotide probes complementary to the orfs. Ladder size bands are indicated on the right (0.5- to 10-kb ladder; Invitrogen). (C) Transcripts of P008-Q12 gene orf53 detected by Northern blotting during a time course of infection in the absence (−) or in the presence (+) of AbiQ.
Most of the detected P008 transcripts could be assigned to predicted transcripts by bioinformatics. The transcription of the nine early-expressed phage genes was detected 2 min after the beginning of the infection of the sensitive L. lactis cells; it peaked at 10 min and decreased until the end of the sampling period (40 min). The transcription of the middle-expressed gene orf53 started and peaked at 10 min, followed by a decrease until the release of phage particles. The transcription profiles of these P008 genes were similar in the presence of AbiQ. However, with a few probes (orf33, orf38, orf39, orf40, orf41, and orf53), additional small transcripts, smaller than 500 nucleotides (nt) in size, were detected in phage-infected AbiQ-containing cells (Fig. 1; see also Fig. S2 in the supplemental material), suggesting that AbiQ modified the transcription profile of the phage.
Small transcripts were also detected with probes targeting orf38, orf40, and orf53 during the infection with phage P008-Q12, but their concentrations were lower (Fig. 1C; see also Fig. S2). Moreover, P008-Q12 transcription of orf53 differed from transcription of the same gene in P008 wild-type phage in the presence of AbiQ. The transcription started at 10 min but peaked later at 20 min, followed by a decrease over the remaining 20 min (Fig. 1C). This delay may explain the longer latent period of P008-Q12. Taking these results together, AbiQ affects phage transcription profiles.
DISCUSSION
Whole-genome sequencing of wild-type phages and escape mutants is a powerful tool to shed light on the biology of phage resistance mechanisms. Here, we identified four different AbiQ targets/activators in phages infecting Gram-positive L. lactis and Gram-negative E. coli strains. These genes code for proteins with no homology between them, but their genomic context suggests that they are involved with nucleic acids.
The orf38 of L. lactis phage P008 is located in the early-expressed gene cluster involved in phage DNA replication and nucleotide metabolism. This protein is well conserved within 936-like phages, including those tested in this study. As we could not inactivate orf38 and as we could detect ORF38 production during the infection process, these data strongly suggest that ORF38 is essential for phage P008 replication. Interestingly, a homolog of orf38, gene e14 of phage bIL170, is involved in the activity of another lactococcal Abi mechanism, namely, AbiT (32). The AbiT system is made of two genes, which share no similarities with AbiQ, and its molecular mechanism is unknown (32). Unexpectedly, AbiQ-escaping mutants derived from bIL170 were mutated in gene m1 and not in e14. Accordingly, AbiQ-escaping P008 phage mutants are still sensitive to AbiT while AbiT-escaping P008 mutants (mutated in orf41) (32) as well as AbiT-escaping bIL170 mutants (mutated in e14) are also sensitive to AbiQ (data not shown). These data suggest that the genetic context influences Abi's activity.
The m1 gene of phage bIL170 is localized in the middle-expressed gene cluster and appears to have roles in DNA repair and recombination. It is also conserved (>90% amino acid identity) among 936-like phages. A homolog of the m1 gene found in phage bIL166 (936 group), gene orf1, is critical to the activity of the lactococcal AbiD1 system (19, 20). AbiQ and AbiD1 share no similarities other than providing phage resistance. It has been shown that the wild-type Orf1 induces the expression of AbiD1, while a mutated Orf1 does not (18, 21). It was also demonstrated that the C-terminal part of Orf1 is essential for phage bIL66 replication (19). Considering that the mutations in m1 of AbiQ-escaping bIL170 mutants likely resulted in no M1 production, this gene does not seem essential to bIL170 replication. These data illustrate the particularities of each phage, even if they belong to the same genotype (936 group).
Phage escape mutants from the 936 group were obtained only with those (P008 and bIL170) infecting L. lactis strain IL1403. No AbiQ-escaping mutant could be obtained from 936 phages (p2, sk1, jj50, and 712) that infect L. lactis strain MG1363, suggesting that host factors might also be involved. In support of this, the EOP of phage p2 on L. lactis MG1363 was previously (33) shown to be below the detection limit (<10−8) while that of P008 was 10−5 on L. lactis IL1403. Moreover, the significant difference between ECOI values of P008 (54.3%) and p2 (0.8%) could explain why we were able to isolate AbiQ-escaping mutants with P008 but not p2 (33). A summary of phage genes involved in lactococcal Abi phenotypes is given in Table 6.
Table 6.
Summary of the different targets of lactococcal Abi and their links in related 936 phages
| Phage gene homologa |
Timing of expression | Function of gene product | Abi's target/activator(s) | |||
|---|---|---|---|---|---|---|
| P008 | bIL170 | bIL66 | p2 | |||
| orf6-orf7 | l6-l7 | ND | orf6 | Late | Major capsid protein | AbiT |
| orf38b | e14c | e14 | orf33 | Early | Unknown | AbiQ, AbiT |
| orf40 | e12 | e12 | orf35 | Early | SSAP (Sak)e | AbiK |
| orf41 | e11 | orf36 | Early | HNH endonuclease | AbiT | |
| orf53 | m1b | orf1d | orf46 | Middle | AbiD1 activator | AbiD1, AbiQ |
orf genes in bold are experimentally demonstrated to be involved in the Abi phenotype. ND, not determined.
orf gene involved in AbiQ.
orf gene involved in AbiT.
orf gene involved in AbiD1.
SSAP, single-stranded annealing protein.
Analyses of AbiQ-escaping c2 mutants led to the identification of the e19 gene, which is located in the early-expressed gene cluster and is flanked by genes related to DNA replication. Interestingly, the e18 gene of phage c2 has homology with phage P008 orf37, suggesting that e19 (c2) and orf38 (P008) may have analogous functions. Domain prediction (43) suggested that E19 and ORF38 might have DNA/RNA binding activity, but with a low confidence.
While all AbiQ-sensitive wild-type lactococcal phages discussed above belong to the Siphoviridae family, the AbiQ-sensitive coliphage 2 belongs to the Myoviridae family. Therefore, AbiQ has a much broader range than previously reported (33). Nonetheless, a mutation in orf210 of coliphage 2 led to insensitivity to AbiQ. This phage gene likely encodes a DNA polymerase, with similarities to chain A of phage T7 DNA polymerase I (51, 52).
All AbiQ-escaping phage mutants studied here were found to contain a single mutated gene, which is a sharp contrast to ToxIN-escaping phages of Pectobacterium atrosepticum. AbiQ is related to the type III toxin-antitoxin mechanism ToxIN found in P. atrosepticum (36, 37). Pectobacterium phage ϕTE has acquired a pseudo antitoxin (pseudo-ToxI), which is similar to the antitoxin (ToxI) of ToxIN but with fewer repetitions (1.5 repeats in the phage genome compared to 5.5 repeats of 36 bp in the host bacteria) (53). To circumvent the ToxIN system, escaping ϕTE phage mutants either expanded their pseudo-ToxI sequence (from 1.5 to 4.5 to 5.5 repeats) or recombined with the natural ToxI sequence (53). These expanded pseudo-ToxI proteins mimic the natural antitoxin, neutralizing the toxin protein (ToxN) during a phage infection (53). Phage ϕTE belongs to the Myoviridae family and shares similarities to coliphage rv5 as does the coliphage 2 characterized in this study. However, unlike phage ϕTE, coliphage 2 does not appear to encode a pseudo-antiQ (a pseudo-AbiQ antitoxin) in its genome, but both phages encoded the gene ORF210 (ϕTE gp10; 53% amino acid identity). Thus, this study clearly shows that phages can bypass type III toxin-antitoxin systems using at least two different strategies. Interestingly, coliphages T4, Mu, and λvir tested here have also been tested for sensitivity to ToxIN (37). Phages Mu and λvir were found to be insensitive to ToxIN and AbiQ, while T4 is sensitive to AbiQ but not to ToxIN. Hence, these systems share common characteristics but have their own specificities.
We also investigated the impact of one of these AbiQ-escaping mutations on phage fitness. One-step growth curve experiments showed that lactococcal phage P008-Q12 took more time (10%) to complete its lytic cycle and that its burst size was reduced by 26%, indicating that the mutation in orf38 had a fitness cost. Transcriptional analyses supported the above microbiological parameters as gene expression was reduced for phage P008-Q12.
Northern blot experiments coupled with bioinformatic analyses led to a transcriptional map of phage P008 for its early- and middle-expressed genes during infection of L. lactis IL1403. The transcription of early-expressed genes started at 2 min, peaked at 10 min, and decreased until the end of the infection. The transcripts for the middle-expressed gene appeared and peaked at 10 min, followed by a decrease until the end of sampling. This temporal profile was similar to the one reported for the lactococcal phage sk1, another member of the 936 group (54). In the presence of AbiQ, additional small transcripts were observed, likely attributed to the endoribonuclease activity of AbiQ (36), although its specificity still needs to be established.
While the data above failed to provide the exact mode of action of AbiQ, they still offered additional detail. In type III TA systems, the antitoxin molecule forms a pseudoknot structure of three antitoxin repetitions bound to three toxin molecules, leading to a hetero-hexamer triangular structure (55, 56). It has also been demonstrated that the free toxin can cleave, through its endoribonuclease activity, the cognate antitoxins (36, 54) as well as housekeeping bacterial RNA molecules (55, 56), leading to cell death. During the phage infection process, this TA interaction is likely disrupted, leading to cell death and abortion of the phage infection. Since no phage product cleaves the AbiQ antitoxin molecule in vivo (36) and since very distinct phage genes and/or gene products are involved in the AbiQ phenotype, the interaction may be more functional than physical. It is also possible that phage products bind antitoxins, thereby freeing the toxins.
In summary, AbiQ is a powerful resistance mechanism effective against two phage families (Siphoviridae and Myoviridae), including those infecting Gram-positive and Gram-negative bacteria. Its efficiency is likely due to the endoribonuclease activity on phage transcripts. Phages can bypass this system through point mutations in four different phage targets/activators, which demonstrated the complexity of this antiphage system. Finally, a general trend is starting to emerge that several lactococcal Abi systems (AbiD1, AbiK, AbiQ, AbiT, and AbiV) appear to target early-expressed genes as well as proteins involved in activities related to nucleic acids. It is tempting to speculate that the use of multiple antiphage mechanisms in a single strain that are targeting the same phage genomic region may provide the coveted long-term phage resistance to industrially relevant bacteria.
Supplementary Material
ACKNOWLEDGMENTS
We thank Barbara-Ann Conway for editorial assistance.
This work was supported by the Natural Sciences and Engineering Research Council of Canada (Strategic Program) as well as the Ministère du Développement Économique, de l'Innovation et de l'Exportation, Programme de Soutien à la Recherche: Programme de Soutien à des Initiatives Internationales de Recherche et d'Innovation. J.E.S is the recipient of a scholarship from the Fonds Québécois de Recherche sur la Nature et les Technologies. M.B. is the recipient of a scholarship from the Quebec Protein Structure, Function and Engineering Research Network (PROTEO). S.M. holds a Tier 1 Canada Research Chair in Bacteriophages.
Footnotes
Published ahead of print 28 June 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00296-13.
REFERENCES
- 1.Deveau H, Labrie SJ, Chopin MC, Moineau S. 2006. Biodiversity and classification of lactococcal phages. Appl. Environ. Microbiol. 72:4338–4346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garneau JE, Moineau S. 2011. Bacteriophages of lactic acid bacteria and their impact on milk fermentations. Microb. Cell Fact. 10(Suppl 1):S20. 10.1186/1475-2859-10-S1-S20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcó MB, Moineau S, Quiberoni A. 2012. Bacteriophages and dairy fermentations. Bacteriophage 2:149–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samson JE, Moineau S. 2013. Bacteriophages in food fermentations: new frontiers in a continuous arms race. Annu. Rev. Food. Sci. Technol. 4:347–368 [DOI] [PubMed] [Google Scholar]
- 5.Mahony J, Murphy J, van Sinderen D. 2012. Lactococcal 936-type phages and dairy fermentation problems: from detection to evolution and prevention. Front. Microbiol. 3:335. 10.3389/fmicb.2012.00335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moisan M, Moineau S. 2012. Multilocus sequence typing scheme for the characterization of 936-like phages infecting Lactococcus lactis. Appl. Environ. Microbiol. 78:4646–4653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ackermann HW. 1998. Tailed bacteriophages: the order Caudovirales. Adv. Virus Res. 51:135–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moineau S, Lévesque C. 2005. Control of bacteriophages in industrial fermentation, p 286–296 In Kutter E, Sulakvelidze A. (ed), Bacteriophages: biology and applications. CRC Press, Boca Raton, FL [Google Scholar]
- 9.Samson JE, Moineau S. 2010. Characterization of Lactococcus lactis phage 949 and comparison with other lactococcal phages. Appl. Environ. Microbiol. 76:6843–6852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahony J, van Sinderen D. 2012. Structural aspects of the interaction of dairy phages with their host bacteria. Viruses 4:1410–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Labrie SJ, Samson JE, Moineau S. 2010. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 8:317–327 [DOI] [PubMed] [Google Scholar]
- 12.Chopin MC, Chopin A, Bidnenko E. 2005. Phage abortive infection in lactococci: variations on a theme. Curr. Opin. Microbiol. 8:473–479 [DOI] [PubMed] [Google Scholar]
- 13.Durmaz E, Klaenhammer TR. 2007. Abortive phage resistance mechanism AbiZ speeds the lysis clock to cause premature lysis of phage-infected Lactococcus lactis. J. Bacteriol. 189:1417–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haaber J, Moineau S, Fortier LC, Hammer K. 2008. AbiV, a novel antiphage abortive infection mechanism on the chromosome of Lactococcus lactis subsp. cremoris MG1363. Appl. Environ. Microbiol. 74:6528–6537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holubova J, Josephsen J. 2007. Potential of AbiS as defence mechanism determined by conductivity measurement. J. Appl. Microbiol. 103:2382–2391 [DOI] [PubMed] [Google Scholar]
- 16.Bouchard JD, Dion E, Bissonnette F, Moineau S. 2002. Characterization of the two-component abortive phage infection mechanism AbiT from Lactococcus lactis. J. Bacteriol. 184:6325–6332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouchard JD, Moineau S. 2004. Lactococcal phage genes involved in sensitivity to AbiK and their relation to single-strand annealing proteins. J. Bacteriol. 186:3649–3652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bidnenko E, Chopin A, Ehrlich SD, Chopin MC. 2009. Activation of mRNA translation by phage protein and low temperature: the case of Lactococcus lactis abortive infection system AbiD1. BMC Mol. Biol. 10:4. 10.1186/1471-2199-10-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bidnenko E, Chopin MC, Ehrlich SD, Anba J. 2002. Lactococcus lactis AbiD1 abortive infection efficiency is drastically increased by a phage protein. FEMS Microbiol. Lett. 214:283–287 [DOI] [PubMed] [Google Scholar]
- 20.Bidnenko E, Ehrlich D, Chopin MC. 1995. Phage operon involved in sensitivity to the Lactococcus lactis abortive infection mechanism AbiD1. J. Bacteriol. 177:3824–3829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bidnenko E, Ehrlich SD, Chopin MC. 1998. Lactococcus lactis phage operon coding for an endonuclease homologous to RuvC. Mol. Microbiol. 28:823–834 [DOI] [PubMed] [Google Scholar]
- 22.Ploquin M, Bransi A, Paquet ER, Stasiak AZ, Stasiak A, Yu X, Cieslinska AM, Egelman EH, Moineau S, Masson JY. 2008. Functional and structural basis for a bacteriophage homolog of human RAD52. Curr. Biol. 18:1142–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scaltriti E, Launay H, Genois MM, Bron P, Rivetti C, Grolli S, Ploquin M, Campanacci V, Tegoni M, Cambillau C, Moineau S, Masson JY. 2011. Lactococcal phage p2 ORF35-Sak3 is an ATPase involved in DNA recombination and AbiK mechanism. Mol. Microbiol. 80:102–116 [DOI] [PubMed] [Google Scholar]
- 24.Bouchard JD, Moineau S. 2000. Homologous recombination between a lactococcal bacteriophage and the chromosome of its host strain. Virology 270:65–75 [DOI] [PubMed] [Google Scholar]
- 25.Labrie SJ, Moineau S. 2007. Abortive infection mechanisms and prophage sequences significantly influence the genetic make-up of emerging lytic lactococcal phages. J. Bacteriol. 189:1482–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fortier LC, Bouchard JD, Moineau S. 2005. Expression and site-directed mutagenesis of the lactococcal abortive phage infection protein AbiK. J. Bacteriol. 187:3721–3730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang C, Villion M, Semper C, Coros C, Moineau S, Zimmerly S. 2011. A reverse transcriptase-related protein mediates phage resistance and polymerizes untemplated DNA in vitro. Nucleic Acids Res. 39:7620–7629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Domingues S, Chopin A, Ehrlich SD, Chopin MC. 2004. A phage protein confers resistance to the lactococcal abortive infection mechanism AbiP. J. Bacteriol. 186:3278–3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Domingues S, Chopin A, Ehrlich SD, Chopin MC. 2004. The lactococcal abortive phage infection system AbiP prevents both phage DNA replication and temporal transcription switch. J. Bacteriol. 186:713–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haaber J, Rousseau GM, Hammer K, Moineau S. 2009. Identification and characterization of the phage gene sav, involved in sensitivity to the lactococcal abortive infection mechanism AbiV. Appl. Environ. Microbiol. 75:2484–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haaber J, Samson JE, Labrie SJ, Campanacci V, Cambillau C, Moineau S, Hammer K. 2010. Lactococcal abortive infection protein AbiV interacts directly with the phage protein SaV and prevents translation of phage proteins. Appl. Environ. Microbiol. 76:7085–7092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Labrie SJ, Tremblay DM, Moisan M, Villion M, Magadan AH, Campanacci V, Cambillau C, Moineau S. 2012. Involvement of the major capsid protein and two early-expressed phage genes in the activity of the lactococcal abortive infection mechanism AbiT. Appl. Environ. Microbiol. 78:6890–6899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Emond E, Dion E, Walker SA, Vedamuthu ER, Kondo JK, Moineau S. 1998. AbiQ, an abortive infection mechanism from Lactococcus lactis. Appl. Environ. Microbiol. 64:4748–4756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fortier LC, Bransi A, Moineau S. 2006. Genome sequence and global gene expression of Q54, a new phage species linking the 936 and c2 phage species of Lactococcus lactis. J. Bacteriol. 188:6101–6114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villion M, Chopin MC, Deveau H, Ehrlich SD, Moineau S, Chopin A. 2009. P087, a lactococcal phage with a morphogenesis module similar to an Enterococcus faecalis prophage. Virology 388:49–56 [DOI] [PubMed] [Google Scholar]
- 36.Samson JE, Spinelli S, Cambillau C, Moineau S. 2013. Structure and activity of AbiQ, a lactococcal endoribonuclease belonging to the type III toxin-antitoxin system. Mol. Microbiol. 87:756–768 [DOI] [PubMed] [Google Scholar]
- 37.Fineran PC, Blower TR, Foulds IJ, Humphreys DP, Lilley KS, Salmond GP. 2009. The phage abortive infection system, ToxIN, functions as a protein-RNA toxin-antitoxin pair. Proc. Natl. Acad. Sci. U. S. A. 106:894–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sambrook J, Russell DW. 2001. Molecular cloning, a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 39.Moineau S, Durmaz E, Pandian S, Klaenhammer TR. 1993. Differentiation of two abortive mechanisms by using monoclonal antibodies directed toward lactococcal bacteriophage capsid proteins. Appl. Environ. Microbiol. 59:208–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lillehaug D. 1997. An improved plaque assay for poor plaque-producing temperate lactococcal bacteriophages. J. Appl. Microbiol. 83:85–90 [DOI] [PubMed] [Google Scholar]
- 41.Deveau H, van Calsteren MR, Moineau S. 2002. Effect of exopolysaccharides on phage-host interactions in Lactococcus lactis. Appl. Environ. Microbiol. 68:4364–4369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marchler-Bauer A, Zheng C, Chitsaz F, Derbyshire MK, Geer LY, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Lanczycki CJ, Lu F, Lu S, Marchler GH, Song JS, Thanki N, Yamashita RA, Zhang D, Bryant SH. 2013. CDD: conserved domains and protein three-dimensional structure. Nucleic Acids Res. 41:D348–D352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelley LA, Sternberg MJ. 2009. Protein structure prediction on the Web: a case study using the Phyre server. Nat. Protoc. 4:363–371 [DOI] [PubMed] [Google Scholar]
- 44.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. 2000. The Protein Data Bank. Nucleic Acids Res. 28:235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Letunic I, Doerks T, Bork P. 2012. SMART 7: recent updates to the protein domain annotation resource. Nucleic Acids Res. 40:D302–D305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mahony J, Deveau H, Mc Grath S, Ventura M, Canchaya C, Moineau S, Fitzgerald GF, van Sinderen D. 2006. Sequence and comparative genomic analysis of lactococcal bacteriophages jj50, 712 and P008: evolutionary insights into the 936 phage species. FEMS Microbiol. Lett. 261:253–261 [DOI] [PubMed] [Google Scholar]
- 47.Rousseau GM, Moineau S. 2009. Evolution of Lactococcus lactis phages within a cheese factory. Appl. Environ. Microbiol. 75:5336–5344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crutz-Le Coq AM, Cesselin B, Commissaire J, Anba J. 2002. Sequence analysis of the lactococcal bacteriophage bIL170: insights into structural proteins and HNH endonucleases in dairy phages. Microbiology 148:985–1001 [DOI] [PubMed] [Google Scholar]
- 49.Lubbers MW, Waterfield NR, Beresford TP, Le Page RW, Jarvis AW. 1995. Sequencing and analysis of the prolate-headed lactococcal bacteriophage c2 genome and identification of the structural genes. Appl. Environ. Microbiol. 61:4348–4356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahmed R, Bopp C, Borczyk A, Kasatiya S. 1987. Phage-typing scheme for Escherichia coli O157:H7. J. Infect. Dis. 155:806–809 [DOI] [PubMed] [Google Scholar]
- 51.Braithwaite DK, Ito J. 1993. Compilation, alignment, and phylogenetic relationships of DNA polymerases. Nucleic Acids Res. 21:787–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patel PH, Suzuki M, Adman E, Shinkai A, Loeb LA. 2001. Prokaryotic DNA polymerase I: evolution, structure, and “base flipping” mechanism for nucleotide selection. J. Mol. Biol. 308:823–837 [DOI] [PubMed] [Google Scholar]
- 53.Blower TR, Evans TJ, Przybilski R, Fineran PC, Salmond GP. 2012. Viral evasion of a bacterial suicide system by RNA-based molecular mimicry enables infectious altruism. PLoS Genet. 8:e1003023. 10.1371/journal.pgen.1003023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chandry PS, Davidson BE, Hillier AJ. 1994. Temporal transcription map of the Lactococcus lactis bacteriophage sk1. Microbiology 140:2251–2261 [DOI] [PubMed] [Google Scholar]
- 55.Short FL, Pei XY, Blower TR, Ong SL, Fineran PC, Luisi BF, Salmond G. 2013. Selectivity and self-assembly in the control of a bacterial toxin by an antitoxic noncoding RNA pseudoknot. Proc. Natl. Acad. Sci. U. S. A. 110:E241–E249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blower TR, Pei XY, Short FL, Fineran PC, Humphreys DP, Luisi BF, Salmond GP. 2011. A processed noncoding RNA regulates an altruistic bacterial antiviral system. Nat. Struct. Mol. Biol. 18:185–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blattner FR, Plunkett G, Bloch CA, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF, Gregor J, Davis NW, Kirkpatrick HA, Goeden MA, Rose DJ, Mau B, Shao Y. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453–1462 [DOI] [PubMed] [Google Scholar]
- 58.Bolotin A, Wincker P, Mauger S, Jaillon O, Malarme K, Weissenbach J, Ehrlich SD, Sorokin A. 2001. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res. 11:731–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chopin A, Chopin MC, Moillo-Batt A, Langella P. 1984. Two plasmid-determined restriction and modification systems in Streptococcus lactis. Plasmid 11:260–263 [DOI] [PubMed] [Google Scholar]
- 60.Gasson MJ. 1983. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wegmann U, O'Connell-Motherway M, Zomer A, Buist G, Shearman C, Canchaya C, Ventura M, Goesmann A, Gasson MJ, Kuipers OP, van Sinderen D, Kok J. 2007. Complete genome sequence of the prototype lactic acid bacterium Lactococcus lactis subsp. cremoris MG1363. J. Bacteriol. 189:3256–3270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McKay LL, Baldwin KA, Zottola EA. 1972. Loss of lactose metabolism in lactic streptococci. Appl. Microbiol. 23:1090–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Vos WM. 1987. Gene cloning and expression in lactic streptococci. FEMS Microbiol. Lett. 46:281–295 [Google Scholar]
- 64.O'Sullivan DJ, Klaenhammer TR. 1993. High- and low-copy-number Lactococcus shuttle cloning vectors with features for clone screening. Gene 137:227–231 [DOI] [PubMed] [Google Scholar]
- 65.Wells JM, Wilson PW, Le Page RW. 1993. Improved cloning vectors and transformation procedure for Lactococcus lactis. J. Appl. Bacteriol. 74:629–636 [DOI] [PubMed] [Google Scholar]
- 66.Chandry PS, Moore SC, Boyce JD, Davidson BE, Hillier AJ. 1997. Analysis of the DNA sequence, gene expression, origin of replication and modular structure of the Lactococcus lactis lytic bacteriophage sk1. Mol. Microbiol. 26:49–64 [DOI] [PubMed] [Google Scholar]
- 67.Sciara G, Bebeacua C, Bron P, Tremblay D, Ortiz-Lombardia M, Lichiere J, van Heel M, Campanacci V, Moineau S, Cambillau C. 2010. Structure of lactococcal phage p2 baseplate and its mechanism of activation. Proc. Natl. Acad. Sci. U. S. A. 107:6852–6857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roberts MD, Martin NL, Kropinski AM. 2004. The genome and proteome of coliphage T1. Virology 318:245–266 [DOI] [PubMed] [Google Scholar]
- 69.Pajunen MI, Elizondo MR, Skurnik M, Kieleczawa J, Molineux IJ. 2002. Complete nucleotide sequence and likely recombinatorial origin of bacteriophage T3. J. Mol. Biol. 319:1115–1132 [DOI] [PubMed] [Google Scholar]
- 70.Miller ES, Kutter E, Mosig G, Arisaka F, Kunisawa T, Ruger W. 2003. Bacteriophage T4 genome. Microbiol. Mol. Biol. Rev. 67:86–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bradley DE, Kay D. 1960. The fine structure of bacteriophages. J. Med. Microbiol. 23:553–563 [Google Scholar]
- 72.Daniels DL, Schroeder JL, Szybalski W, Sanger F, Coulson AR, Hong GF, Hill DF, Petersen GB, Blattner FR. 1983. Appendix II: complete annotated lambda sequence, p 519–676 In Hendrix RW, Roberts JW, Stahl FW, Weisberg RA. (ed), Lambda II. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 73.Yeh LS, Hsu T, Karam JD. 1998. Divergence of a DNA replication gene cluster in the T4-related bacteriophage RB69. J. Bacteriol. 180:2005–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Juhala RJ, Ford ME, Duda RL, Youlton A, Hatfull GF, Hendrix RW. 2000. Genomic sequences of bacteriophages HK97 and HK022: pervasive genetic mosaicism in the lambdoid bacteriophages. J. Mol. Biol. 299:27–51 [DOI] [PubMed] [Google Scholar]
- 75.Morgan GJ, Hatfull GF, Casjens S, Hendrix RW. 2002. Bacteriophage Mu genome sequence: analysis and comparison with Mu-like prophages in Haemophilus, Neisseria and Deinococcus. J. Mol. Biol. 317:337–359 [DOI] [PubMed] [Google Scholar]
- 76.Coetzee JN, Bradley DE, Fleming J, du Toit L, Hughes VM, Hedges RW. 1985. Phage pilHα: a phage which adsorbs to IncHI and IncHII plasmid-coded pili. J. Gen. Microbiol. 131:1115–1121 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.