Abstract
Susceptibility breakpoints are crucial for prudent use of antimicrobials. This study has developed the first susceptibility breakpoint (MIC ≤ 0.25 μg/ml) for enrofloxacin against swine Salmonella spp. based on wild-type cutoff (COWT) and pharmacokinetic-pharmacodynamic (PK-PD) cutoff (COPD) values, consequently providing a criterion for susceptibility testing and clinical usage of enrofloxacin.
TEXT
Salmonella spp. are leading zoonosis and food-borne pathogens. Approximately 10 to 20% of all human cases of salmonellosis in the European Union may be the result of contact with pigs and consumption of pig meat (1). Enrofloxacin, an FDA- and China-approved fluoroquinolone member, has been used broadly for treatment of swine disease caused by Gram-positive and -negative bacteria. For guiding susceptibility testing and clinical drug usage, the Clinical and Laboratory Standards Institute (CLSI) has developed a susceptibility breakpoint for enrofloxacin in swine for respiratory disease only (2). Before the present study, no breakpoint for enrofloxacin had been established for swine disease caused by enteric bacteria, such as Salmonella.
In the present study, 214 swine Salmonella isolates were obtained from five representative districts (Henan, Hubei, Zhejiang, Anhui, and Shanghai) in China during the years 2003 to 2010. The MICs of enrofloxacin to these swine Salmonella isolates were determined by agar dilution susceptibility testing according to the CLSI M31-A3 guidance (2). Primary MIC distribution was subjected to statistical goodness-of-fit tests and nonlinear least-squares regressions by following the procedure elaborated in a previous study (3). A wild-type cutoff (COWT) was developed based on the fitted MIC distribution by following CLSI M37-A3 guidance and some previous methods (3–5).
As shown in the primitive enrofloxacin MIC distribution in Fig. 1A, MICs for enrofloxacin against 214 Salmonella isolates were in the range of approximately 0.125 to 8 μg/ml. The percentages at each MIC (0.125, 0.25, 0.5, 1, 2, 4, and 8 μg/ml) were 6.1%, 0.4%, 14.5%, 15%, 43.5%, 5.6%, and 15%, respectively. The standard goodness-of-fit tests demonstrated that primitive MIC distribution did not match a normal distribution, because a bimodal distribution was observed at MICs of 2 μg/ml and 8 μg/ml. To obtain unimodal MIC distribution, the 32 Salmonella isolates with a MIC of 8 μg/ml were consequently removed. The other 182 swine Salmonella isolates were subsequently subjected to nonlinear least-squares regressions and goodness-of-fit tests. The best fit for the unimodal population was found when presumed MIC distribution was defined as being between 0.125 μg/ml and 4.0 μg/ml. In the fitted unimodal MIC distribution (see Fig. 1B), the number of isolates estimated by nonlinear least-squares regression (183 isolates) was closest to the true number of isolates (182 isolates). Of the estimated number of Salmonella strains (183 isolates), more than 95% had enrofloxacin MICs in the range of approximately 0.5 to 2 μg/ml. After NORMINV function and NORMDIST test in Microsoft Excel, the COWT was defined as 2.0 μg/ml. The COWT in our study was slightly higher than susceptibility MIC breakpoints for ciprofloxacin against human Enterobacteriaceae recommended by CLSI M100-S20 (MIC ≤ 1 μg/ml) and the traditional ciprofloxacin breakpoint against Salmonella (MIC ≤ 1 μg/ml) used in the 2002 to 2010 NARMS reports (6, 7). The higher COWT in the present study may due to the different backgrounds of strains isolated from different hosts and different geographical areas.
Fig 1.
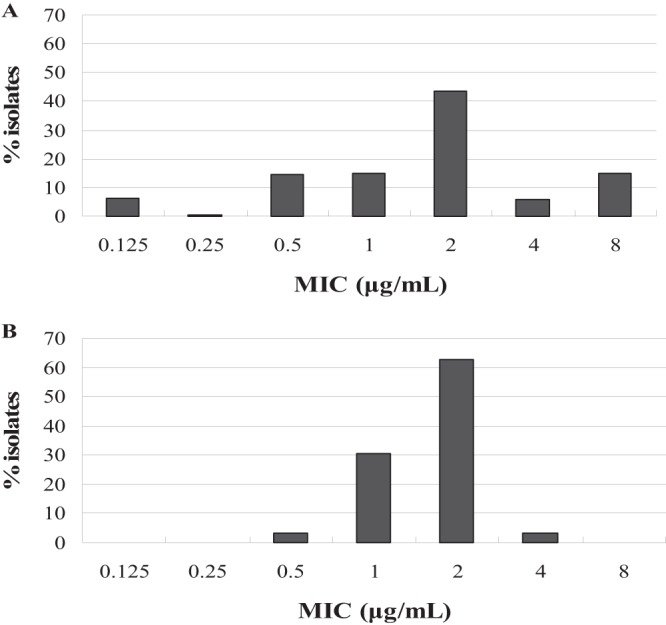
The MIC distribution for enrofloxacin against Salmonella isolates. (A) Primary MIC distribution of 214 Salmonella isolates; (B) fitted MIC distribution of the estimated 183 Salmonella isolates after standard goodness-of-fit tests and nonlinear least-squares regression.
After single-dose intramuscular administration of enrofloxacin (2.5 mg/kg of body weight) to 12 piglets, concentrations of enrofloxacin in plasma were determined by high-performance liquid chromatography (HPLC) according to the method established in our lab (8). The pharmacokinetic (PK) parameters were calculated using 3p97 software. Based on human clinical experience, an area under the concentration-time curve over 24 h in the steady state divided by the MIC (AUC/MIC ratio) value of ≥100 was assumed as pharmacokinetic-pharmacodynamic (PK-PD) target attainment for fluoroquinolones against Gram-negative organisms (9). A 10,000-subject Monte Carlo simulation was constructed using Crystal Ball Professional version 7.2.2 software. The PK-PD susceptibility cutoff (COPD) was defined as the MIC at which the probability of target attainment (PTA) was ≥90% (4).
Our results were in agreement with previous studies which indicated that ciprofloxacin levels (an active metabolite of enrofloxacin) were too low in the plasma of pigs and enrofloxacin could be served as the marker for PK calculation (10, 11). Based on the concentration-time curve of plasma enrofloxacin, a series of pharmacokinetic parameters for the 12 piglets were derived from a one-compartment model (see Table 1). After intramuscular administration of enrofloxacin, the peak drug concentration (Cmax) and AUC were 0.74 ± 0.38 μg/ml and 15.87 ± 3.39 μg · h/ml, respectively. The probability of achieving various AUC/MIC ratios at each MIC value after a 10,000-pig Monte Carlo simulation is presented in Table 2. At a MIC value of ≤0.25 μg/ml, the probabilities of achieving AUC/MIC values of ≥100 were higher than 90%. Therefore, the COPD of enrofloxacin against swine Salmonella was defined as a MIC value of ≤0.25 μg/ml.
Table 1.
Plasma pharmacokinetic parameters after single-dose intramuscular administration of enrofloxacin (2.5 mg/kg of body weight) to piglets (n = 12)
| Pharmacokinetic parametera | Unit | Value ± SD |
|---|---|---|
| A | μg/ml | 2.19 ± 0.18 |
| Ke | 1iter/h | 0.13 ± 0.03 |
| Ka | 1iter/h | 4.97 ± 2.47 |
| Lag time | h | 0.02 ± 0.08 |
| t1/2(Ka) | h | 0.14 ± 0.03 |
| t1/2(Kb) | h | 5.17 ± 1.65 |
| t (peak) | h | 0.74 ± 0.11 |
| Cmax | μg/ml | 0.74 ± 0.38 |
| AUC | (μg/ml) · h | 15.87 ± 3.39 |
| CL/F(s) | ml/kg/h/(μg/ml) | 0.33 ± 0.12 |
| V/F(C) | (ml/kg)/(μg/ml) | 2.30 ± 0.30 |
| Protein binding | % | 41.4–56.6 |
A, zero time intercept for α phase; Ke, elimination rate constant; Ka, absorption rate constant; t1/2(Ka), half-life of absorption; t1/2(Kb), half-life of elimination; t (peak), peak time; Cmax, peak drug concentration; AUC, area under the drug concentration-time curves; CL/F(s), total body clearance; V/F(C), volume of distribution at steady state.
Table 2.
Accumulated probability of attaining the target AUC/MIC ratio at specific MIC breakpoints
| Target AUC/MIC ratio | Accumulated target attainment probability (%) at each MIC (μg/ml) |
||||||
|---|---|---|---|---|---|---|---|
| 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | |
| 50 | 100.00 | 100.00 | 63.90 | 35.82 | 6.47 | 7.58 | 12.85 |
| 60 | 100.00 | 100.00 | 49.13 | 29.86 | 4.94 | 5.70 | 9.42 |
| 70 | 100.00 | 100.00 | 38.89 | 25.43 | 3.96 | 4.67 | 7.19 |
| 80 | 100.00 | 100.00 | 33.46 | 22.08 | 3.26 | 4.05 | 5.47 |
| 90 | 100.00 | 99.99 | 29.22 | 19.51 | 2.80 | 3.48 | 4.42 |
| 100 | 100.00 | 99.94 | 25.47 | 17.11 | 2.40 | 3.10 | 3.64 |
| 110 | 100.00 | 99.74 | 22.33 | 15.21 | 2.08 | 2.70 | 3.01 |
| 125 | 100.00 | 98.43 | 18.97 | 13.03 | 1.79 | 2.40 | 2.17 |
The COPD in our study (MIC ≤ 0.25 μg/ml) was lower than the PK-PD breakpoint for ciprofloxacin recommended by EUCAST (MIC ≤ 0.5 μg/ml) and the susceptibility breakpoint for enrofloxacin against dog Enterobacteriaceae (MIC ≤ 0.5 μg/ml) recommended by CLSI M31-A3 (6, 12). The lower PK-PD breakpoint in our study may be due to the lower dose of drug administration to pigs, because previous studies concluded that the dose of drug administration may affect the PK-PD breakpoint (11, 13, 14). The PK-PD cutoff developed in our study should be more conservative, because it was generated based on the lowest approved dosage regimen for enrofloxacin (15). Based on PK-PD models with the Monte Carlo simulation, a recent study established a ciprofloxacin breakpoint for Gram-negative aerobic bacteria (MIC ≤ 0.125 μg/ml) which was also much lower than the CLSI breakpoint and EUCAST breakpoint (16). Both of the previous studies and our study suggested that CLSI may need to revise the breakpoint for some Gram-negative bacteria (16, 17). Coincidently, a new susceptibility breakpoint for ciprofloxacin against Salmonella (MIC < 0.125 μg/ml) was used in a 2011 NARMS report (7). The discrepancy of susceptibility breakpoint may be due to drug specificity and geographical differences.
Conclusively, our study is unique in the sense that it has established a novel enrofloxacin susceptibility breakpoint against swine Salmonella spp. based on COWT (MIC ≤ 2 μg/ml) and COPD (MIC ≤ 0.25 μg/ml). Since the PK-PD cutoff provided greater value for setting the breakpoint than the wild-type cutoff did (15), the COPD breakpoint (MIC ≤ 0.25 μg/ml) was finally selected as the optimum enrofloxacin susceptibility breakpoint for swine Salmonella. Although further clinical studies are necessary for confirming our findings, our work, to some extent, could provide a criterion for enrofloxacin susceptibility testing and improve prudent use of enrofloxacin for public health.
ACKNOWLEDGMENTS
This work was supported by grants from the National Basic Research Program of China (2013CB127200), National Natural Science Foundation of China (31101856), and the Research Fund for Young Scholars in the Doctoral Program of Higher Education of China (2011014612003).
Footnotes
Published ahead of print 19 June 2013
REFERENCES
- 1.EFSA 19 April 2010, posting date EFSA assesses risk of Salmonella from pig meat. EFSA, Parma, Italy: http://www.efsa.europa.eu/en/press/news/biohaz100419.htm [Google Scholar]
- 2.CLSI 2009. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals; approved standard—third edition. CLSI document M31-A3 Clinical and Laboratory Standard Institute, Wayne, PA [Google Scholar]
- 3.Turnidge J, Kahlmeter G, Kronvall G. 2006. Statistical characterisation of bacterial wild-type MIC value distributions and the determination of epidemiological cut-off values. Clin. Microbiol. Infect. 12:418–425 [DOI] [PubMed] [Google Scholar]
- 4.CLSI 2009. Development of in vitro susceptibility testing criteria and quality control parameters for veterinary antimicrobial agents; approved guideline—third edition, document M37-A3. Clinical and Laboratory Standard Institute, Wayne, PA [Google Scholar]
- 5.Pfaller MA, Diekema DJ, Ghannoum MA, Rex JH, Alexander BD, Andes D, Brown SD, Chaturvedi V, Espinel-Ingroff A, Fowler CL, Johnson EM, Knapp CC, Motyl MR, Ostrosky-Zeichner L, Sheehan DJ, Walsh TJ. 2009. Wild-type MIC distribution and epidemiological cutoff values for Aspergillus fumigatus and three triazoles as determined by the Clinical and Laboratory Standards Institute broth microdilution methods. J. Clin. Microbiol. 47:3142–3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CLSI 2010. Performance standards for antimicrobial susceptibility testing; twentieth informational supplement. CLSI M100-S20, vol 30 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 7.FDA 2010. NARMS retail meat report, 2010. FDA, Washington, DC: http://www.fda.gov/downloads/AnimalVeterinary/SafetyHealth/AntimicrobialResistance/NationalAntimicrobialResistanceMonitoringSystem/UCM293581.pdf [Google Scholar]
- 8.Yu H, Tao Y, Chen D, Pan Y, Liu Z, Wang Y, Huang L, Dai M, Peng D, Wang X, Yuan Z. 2011. Simultaneous determination of fluoroquinolones in foods of animal origin by a high performance liquid chromatography and a liquid chromatography tandem mass spectrometry with accelerated solvent extraction. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 885–886:150–159 [DOI] [PubMed] [Google Scholar]
- 9.Wright DH, Brown GH, Peterson ML, Rotschafer JC. 2000. Application of fluoroquinolone pharmacodynamics. J. Antimicrob. Chemother. 46:669–683 [DOI] [PubMed] [Google Scholar]
- 10.Anadon A, Martinez-Larranaga MR, Diaz MJ, Fernandez-Cruz ML, Martinez MA, Frejo MT, Martinez M, Iturbe J, Tafur M. 1999. Pharmacokinetic variables and tissue residues of enrofloxacin and ciprofloxacin in healthy pigs. Am. J. Vet. Res. 60:1377–1382 [PubMed] [Google Scholar]
- 11.Messenger KM, Papich MG, Blikslager AT. 2012. Distribution of enrofloxacin and its active metabolite, using an in vivo ultrafiltration sampling technique after the injection of enrofloxacin to pigs. J. Vet. Pharmacol. Ther. 35:452–459 [DOI] [PubMed] [Google Scholar]
- 12.EUCAST 2013. Breakpoint tables for interpretation of MICs and zone diameters, version 3.1. http://www.eucast.org/clinical_breakpoints/
- 13.Bimazubute M, Cambier C, Baert K, Vanbelle S, Chiap P, Albert A, Delporte JP, Gustin P. 2009. Penetration of enrofloxacin into the nasal secretions and relationship between nasal secretions and plasma enrofloxacin concentrations after intramuscular administration in healthy pigs. J. Vet. Pharmacol. Therapeut. 33:183–188 [DOI] [PubMed] [Google Scholar]
- 14.Lepe JA, Garcia-Cabrera E, Gil-Navarro MV, Aznar J. 2012. Rifampin breakpoint for Acinetobacter baumannii based on pharmacokinetic-pharmacodynamic models with Monte Carlo simulation. Rev. Esp. Quimioter. 25:134–138 [PubMed] [Google Scholar]
- 15.Turnidge J, Paterson DL. 2007. Setting and revising antibacterial susceptibility breakpoints. Clin. Microbiol. Rev. 20:391–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frei CR, Wiederhold NP, Burgess DS. 2008. Antimicrobial breakpoints for Gram-negative aerobic bacteria based on pharmacokinetic-pharmacodynamic models with Monte Carlo simulation. J. Antimicrob. Chemother. 61:621–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeRyke CA, Kuti JL, Nicolau DP. 2007. Reevaluation of current susceptibility breakpoints for Gram-negative rods based on pharmacodynamic assessment. Diagn. Microbiol. Infect. Dis. 58:337–344 [DOI] [PubMed] [Google Scholar]


