Abstract
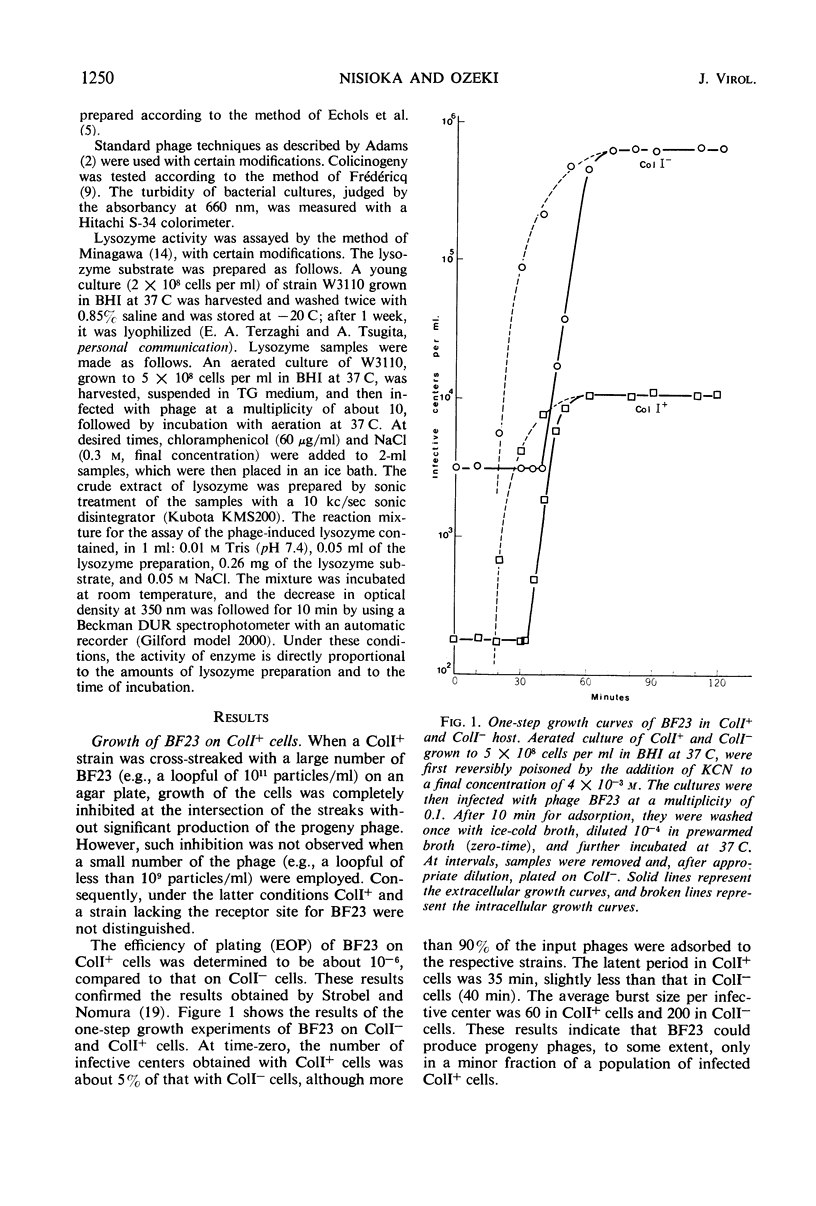
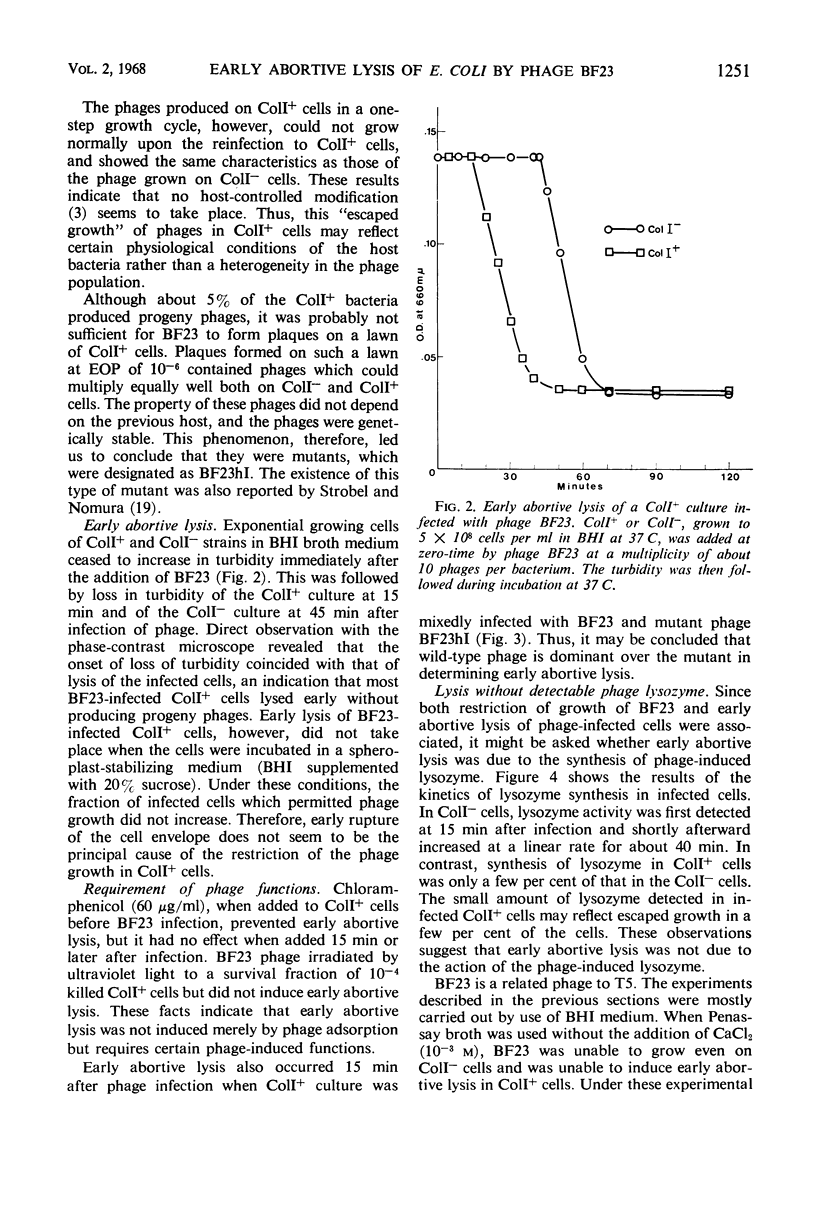
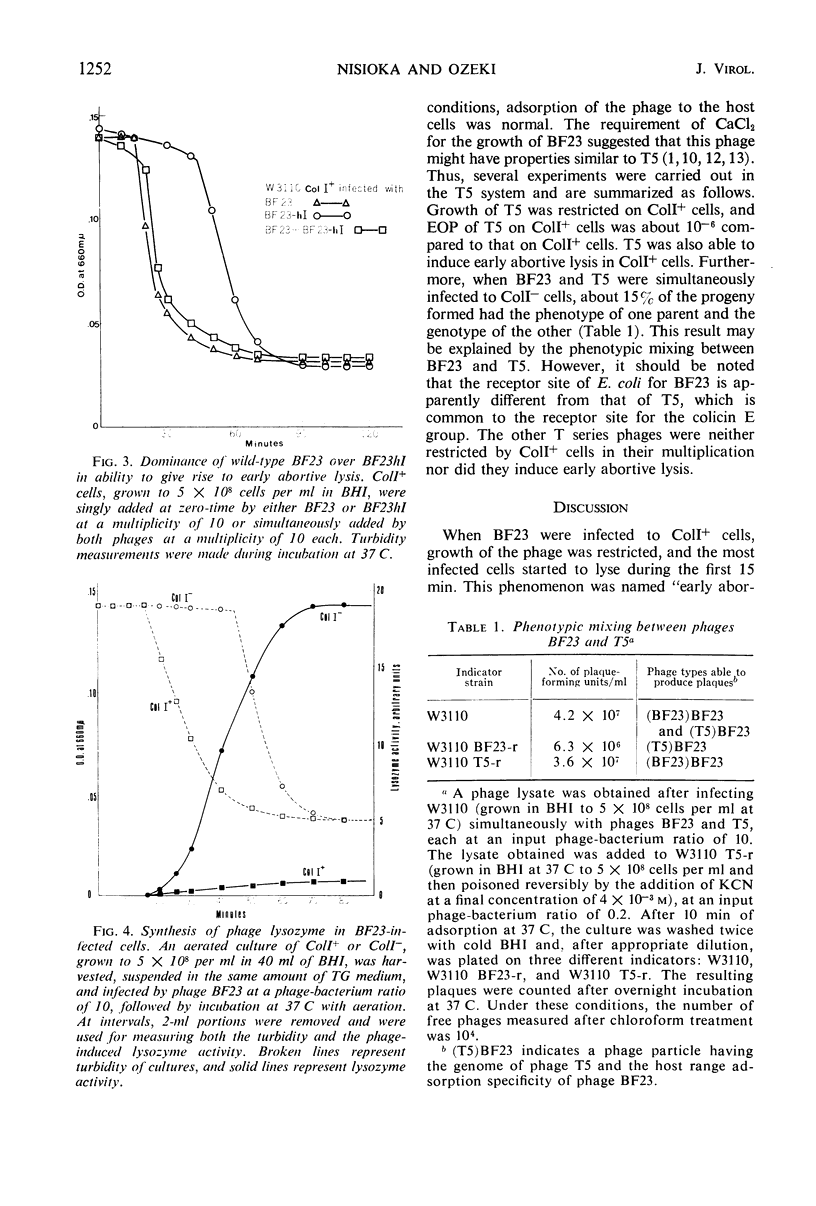
Growth of phage BF23 was restricted in Escherichia coli K-12 strains carrying a colicin I factor (ColIb); most infected cells lysed early without producing progeny phages. Either addition of chloramphenicol before phage infection or ultraviolet irradiation of phage prevented early abortive lysis, an indication that certain phage functions are required for this phenomenon. Very little or no phage-induced lysozyme was synthesized in the infected ColI+ cells. This result suggests that early abortive lysis was not due to the lysozyme action. A small fraction (0.05) of BF23-infected ColI+ cells showed normal phage growth. This “escaped growth” may reflect the physiological state of the host bacteria rather than the heterogeneity of the infecting phage. Host-controlled modification was not observed. A phage mutant, BF23hI, able to grow on ColI+ cells, was isolated and was characterized to be recessive to the wild-type BF23 in its ability to undergo early abortive lysis. Among the T series phages, T5 induced early abortive lysis, and growth of T5 was restricted upon infection to ColI+ cells. These results and the other observations, including the occurrence of phenotypic mixing between BF23 and T5, suggest that these two phages are related to each other even though the receptor sites for BF23 and T5 are apparently different.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARBER W., DUSSOIX D. Host specificity of DNA produced by Escherichia coli. I. Host controlled modification of bacteriophage lambda. J Mol Biol. 1962 Jul;5:18–36. doi: 10.1016/s0022-2836(62)80058-8. [DOI] [PubMed] [Google Scholar]
- Arber W. Host-controlled modification of bacteriophage. Annu Rev Microbiol. 1965;19:365–378. doi: 10.1146/annurev.mi.19.100165.002053. [DOI] [PubMed] [Google Scholar]
- ECHOLS H., GAREN A., GAREN S., TORRIANI A. Genetic control of repression of alkaline phosphatase in E. coli. J Mol Biol. 1961 Aug;3:425–438. doi: 10.1016/s0022-2836(61)80055-7. [DOI] [PubMed] [Google Scholar]
- FATTIG W. D., LANNI F. MAPPING OF TEMPERATURE-SENSITIVE MUTANTS IN BACTERIOPHAGE T5. Genetics. 1965 Jan;51:157–166. doi: 10.1093/genetics/51.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREDERICQ P. Colicins. Annu Rev Microbiol. 1957;11:7–22. doi: 10.1146/annurev.mi.11.100157.000255. [DOI] [PubMed] [Google Scholar]
- FREDERICQ P. Résistance et immunité aux colicines. C R Seances Soc Biol Fil. 1956;150(7):1514–1517. [PubMed] [Google Scholar]
- LANNI Y. T. Invasion by bacteriophage T5. II. Dissociation of calcium-independent and calcium-dependent processes. Virology. 1960 Apr;10:514–529. doi: 10.1016/0042-6822(60)90133-1. [DOI] [PubMed] [Google Scholar]
- LANNI Y. T., MCCORQUODALE D. J., WILSON C. M. MOLECULAR ASPECTS OF DNA TRANSFER FROM PHAGE T5 TO HOST CELLS. II. ORIGIN OF FIRST-STEP-TRANSFER DNA FRAGMENTS. J Mol Biol. 1964 Oct;10:19–27. doi: 10.1016/s0022-2836(64)80024-3. [DOI] [PubMed] [Google Scholar]
- Lanni Y. T., Lanni F., Tevethia M. J. Bacteriophage T5 chromosome fractionation: genetic specificity of a DNA fragment. Science. 1966 Apr 8;152(3719):208–210. doi: 10.1126/science.152.3719.208. [DOI] [PubMed] [Google Scholar]
- MCCORQUODALE D. J., LANNI Y. T. MOLECULAR ASPECTS OF DNA TRANSFER FROM PHAGE T5 TO HOST CELLS. I. CHARACTERIZATION OF FIRST-STEP-TRANSFER MATERIAL. J Mol Biol. 1964 Oct;10:10–18. doi: 10.1016/s0022-2836(64)80023-1. [DOI] [PubMed] [Google Scholar]
- MINAGAWA T. Some characteristics of the internal protein phage T2. Virology. 1961 Apr;13:515–527. doi: 10.1016/0042-6822(61)90283-5. [DOI] [PubMed] [Google Scholar]
- MONK M., CLOWES R. C. THE REGULATION OF COLICIN SYNTHESIS AND COLICIN FACTOR TRANSFER IN ESCHERICHIA COLI K12. J Gen Microbiol. 1964 Sep;36:385–392. doi: 10.1099/00221287-36-3-385. [DOI] [PubMed] [Google Scholar]
- SILVER S. ACRIFLAVINE RESISTANCE: A BACTERIOPHAGE MUTATION AFFECTING THE UPTAKE OF DYE BY THE INFECTED BACTERIAL CELLS. Proc Natl Acad Sci U S A. 1965 Jan;53:24–30. doi: 10.1073/pnas.53.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel M., Nomura M. Restriction of the growth of bacteriophage BF23 by a colicine I (Col I-P9) factor. Virology. 1966 Apr;28(4):763–765. doi: 10.1016/0042-6822(66)90263-7. [DOI] [PubMed] [Google Scholar]
- Tomizawa J., Ogawa T. Inhibition of growth of rII mutants of bacteriophage T4 by immunity substance of bacteriophage lambda. J Mol Biol. 1967 Jan 28;23(2):277–280. doi: 10.1016/s0022-2836(67)80033-0. [DOI] [PubMed] [Google Scholar]



