Abstract
The ability to identify bacterial pathogens at the subspecies level in clinical diagnostics is currently limited. We investigated whether splitting Escherichia coli species into clonal groups (clonotypes) predicts antimicrobial susceptibility or clinical outcome. A total of 1,679 extraintestinal E. coli isolates (collected from 2010 to 2012) were collected from one German and 5 U.S. clinical microbiology laboratories. Clonotype identity was determined by fumC and fimH (CH) sequencing. The associations of clonotype with antimicrobial susceptibility and clinical variables were evaluated. CH typing divided the isolates into >200 CH clonotypes, with 93% of the isolates belonging to clonotypes with ≥2 isolates. Antimicrobial susceptibility varied substantially among clonotypes but was consistent across different locations. Clonotype-guided antimicrobial selection significantly reduced “drug-bug” mismatch compared to that which occurs with the use of conventional empirical therapy. With trimethoprim-sulfamethoxazole and fluoroquinolones, the drug-bug mismatch was predicted to decrease 62% and 78%, respectively. Recurrent or persistent urinary tract infection and clinical sepsis were significantly correlated with specific clonotypes, especially with CH40-30 (also known as H30), a recently described clonotype within sequence type 131 (ST131). We were able to clonotype directly from patient urine samples within 1 to 3 h of obtaining the specimen. In E. coli, subspecies-level identification by clonotyping can be used to significantly improve empirical predictions of antimicrobial susceptibility and clinical outcomes in a timely manner.
INTRODUCTION
Bacterial species identification is essential for the correct diagnosis of disease and to optimize the empirical choice of antimicrobial treatment before the results of culturing and susceptibility testing are available (up to 2 to 3 days) (1, 2). However, even within a single bacterial species, there is substantial strain-to-strain variation in antimicrobial susceptibilities and virulence (3), and the increasing prevalence of antimicrobial-resistant and multidrug-resistant bacterial pathogens is one of the greatest challenges in clinical medicine today (4, 5). Thus, subspecies-, strain-, or clonal group-level identification might provide significant advantages for the diagnosis of bacterial infections.
Escherichia coli is a leading extraintestinal (found especially in the urine and blood) pathogen in the United States, causing millions of infections and tens of thousands of deaths each year (6). As a clonal species, E. coli contains a limited number of genetically related lineages (i.e., clonotypes) (10). Although several E. coli clonotypes with distinctive antimicrobial susceptibility patterns have been described (11–15), the use of clonotyping as a general predictive marker for antimicrobial susceptibility among unselected extraintestinal clinical E. coli isolates has not been reported. Additionally, the two most-commonly used clonal typing methods for E. coli, multilocus sequence typing (MLST) (24) and pulsed-field gel electrophoresis (PFGE) (25), are poorly suited for associating genetic lineages with susceptibility profiles in clinical practice due to their high costs, slow turnaround times, and/or unsuitably low (for MLST) or variable (for PFGE) levels of discrimination.
We recently reported an E. coli clonal typing method based on variations in internal regions of the genes fumC (a traditional MLST locus encoding fumarase) and fimH (a rapidly evolving locus that encodes the type 1 fimbrial adhesin) (8). The fine resolution provided by CH clonotyping prompted us to correlate CH clonotypes with antimicrobial susceptibility profiles and clinical outcome or presentation, including persistent or recurrent urinary tract infections (UTIs) and sepsis, among >1,600 recent extraintestinal E. coli isolates obtained from multiple clinical microbiology laboratories.
MATERIALS AND METHODS
Isolates and patients.
The primary set of 1,518 recent clinical E. coli isolates consisted of consecutive single-patient human extraintestinal isolates recovered between October 2010 and June 2011 at five clinical microbiology laboratories serving distinct patient populations in Seattle, WA (Group Health Cooperative, Harborview Medical Center, Seattle Children's Hospital, and the University of Washington Medical Center), and Minneapolis, MN (Veterans Affairs Medical Center) (13). For 20% of the isolates (those from Group Health), the supplying clinical laboratory reported that the isolates came almost exclusively from urine specimens, which agrees with the clinical context in that Group Health serves ambulatory patients only. For the rest of the collection, 93% of those isolates were from urine, 2% from blood, and 5% from miscellaneous other sample sites (sputum, wound, abscess, etc.). Another set of E. coli isolates was obtained from the University Hospital in Münster, Germany, and consisted of 161 consecutive isolates from urine samples recovered from July to September 2012.
Among the 1,518 primary set isolates, data regarding the presence of sepsis were available for 1,133 isolates, and data regarding the persistence or recurrence of infection were available for 1,034 urine isolates. The latter were classified as (i) single-episode bacteriuria (no clinical or microbiological evidence of recurrence within 30 days after the index culture) or (ii) recurrent UTI (clinical or microbiological evidence of persistence or recurrence beyond 7 days and within 30 days following the initial resolution of symptoms). Drug-bug mismatch in relation to the initially chosen antimicrobial therapy was analyzed in 676 urine isolates within the primary set (for 10 out of 676 patients, information about recurrence or persistence of infection was not available). The specific regimens used were diverse, and because of small subgroups, a detailed analysis of each regimen in relation to an organism would provide too little value to justify its inclusion in the study. The most relevant consideration analyzed and summarized in the report is whether the particular regimen chosen was active in vitro against each patient's infecting organism. Local institutional review boards approved the study protocol.
Susceptibility testing.
Antimicrobial susceptibility profiles were determined using disk diffusion testing (17). The interpretive criteria were those specified by the Clinical and Laboratory Standards Institute (CLSI) (18).
Clonal typing of E. coli isolates.
Internal (<500 bp) regions of fumC and fimH were amplified by PCR, and the DNA sequences were determined by Sanger sequencing. Each unique combination of fumC and fimH alleles defined a CH clonotype (8). The diversity of the clonotype distribution was evaluated using the Simpson's modified alpha-diversity index (26).
Statistical analysis of antimicrobial susceptibility of major CH clonotypes.
The prevalence of susceptibility to individual agents was calculated for the total primary collection, for individual participating laboratories, and for individual CH clonotypes. Odds ratios (ORs) were calculated for each subgroup (i.e., laboratory or clonotype) relative to the rest of the population. If the OR was >2 or <0.5, its statistical significance was evaluated using Fisher's exact test.
Clonotype-guided algorithm for prediction of antimicrobial resistance.
Each isolate in the primary collection was classified as resistant or susceptible to each of the 11 antimicrobials based on the prevalence of susceptibility to the particular antimicrobial in the rest of isolates belonging to the same clonotype (i.e., for each isolate classification, the susceptibility of the corresponding clonotype was recalculated by excluding the profile of the isolate to be classified). Thus, clonotype-guided antibiotic selection was evaluated only for isolates from clonotypes comprising ≥2 isolates. An isolate was hypothetically “allowed” to be treated with that agent if its clonotype susceptibility to that agent was ≥80%, whereas if its susceptibility was <80%, it was classified as resistant and was “rejected” for treatment with the agent.
Clonotype identification directly in urine specimens.
We used >50 clinical urine specimens (generally, using the boric acid preservative that allows urine to be kept at room temperature) that were submitted to microbiology laboratories for the culture and susceptibility tests. One milliliter of each urine specimen was spun down for 1 min at 10,000 rpm, the sediment resuspended in 100 μl of distilled water, and heated at 98°C for 5 min. This sample was used at a final dilution of 1:10 for either quantitative PCR (qPCR) or pyrosequencing testing. qPCR was performed on LightCycler 2.0 (Hoffmann-La Roche, Inc.) using gene- or single-nucleotide polymorphism (SNP)-based primers specific to the clonotype CH40-30 (also known as H30). The gene-based probes targeted the rMST1 gene region described earlier (7). Pyrosequencing was performed on PyroMark Q24 (Qiagen, Inc.), targeting the short most-clonotype-variable (<60 bp) internal regions of fimH and fumC.
RESULTS
Major clonotypes dominate within E. coli across different clinical laboratories.
A total of 222 distinct CH clonotypes were identified among 1,518 U.S.-based clinical E. coli isolates, comprising 1 to 137 isolates each (Fig. 1). Clonotypes consisting of a single isolate comprised only 7% of all isolates. Nineteen clonotypes that consisted of ≥15 isolates (i.e., ≥1% of the collection) were defined as major (Fig. 1). Clonotype distribution was highly similar across laboratories (Fig. 1), with at least three of the four largest clonotypes overall predominating within each laboratory. The largest clonotype, both overall and at three of the five contributing laboratories, was CH40-30 from sequence type 131 (ST131).
Fig 1.
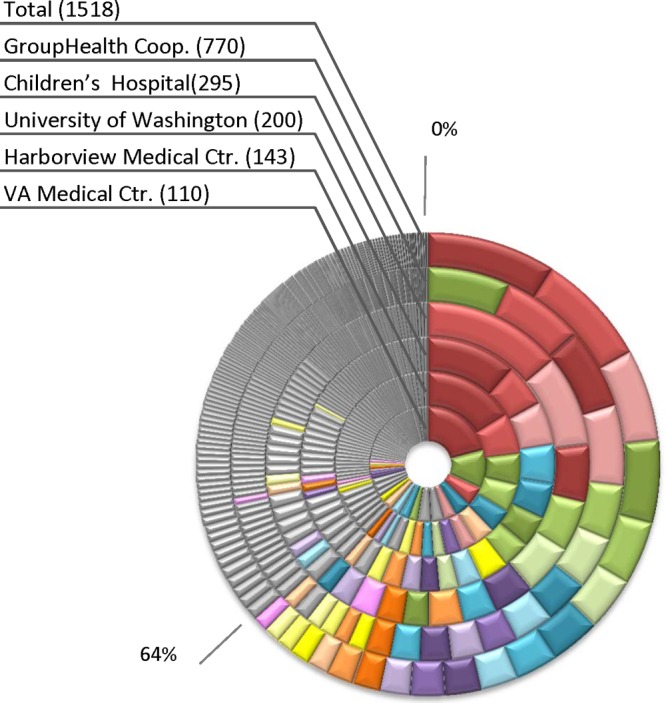
Distribution of isolates by clonotype at different laboratories. The outer ring shows the distribution of clonotypes among isolates from all locations combined (Total), in the order of overall clonotype prevalence. The inner rings show the distribution of clonotypes within individual laboratories, sorted within each ring according to local clonotype prevalence. Clonotypes accounting for >1% of the total collection are color coded consistently across sites, while the others are gray.
Overall, 96 clonotypes were encountered in isolates from at least two different laboratories. In total, the common clonotypes comprised 89.6% of isolates, demonstrating a highly clonal structure of the vast majority of extraintestinal E. coli isolates, found across different geographic areas and patient populations.
Antimicrobial susceptibility profiles of clonotypes are distinct and consistent across different locations.
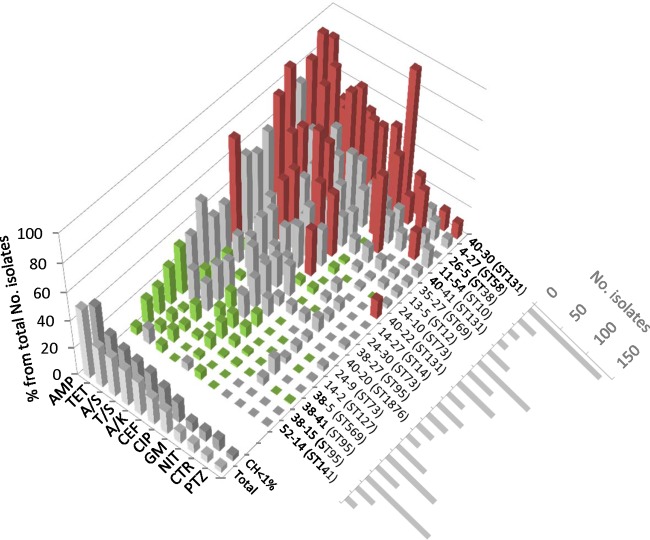
Within each of the 19 major clonotypes, the prevalence of resistance differed by ≥2-fold (higher or lower) (P < 0.05) from that of the rest of population for at least one antimicrobial (Fig. 2). Resistance prevalence within a clonotype did not correlate with the overall population prevalence of the clonotype; for example, among the most-prevalent clonotypes were several of the extensively resistant (e.g., CH40-30 and CH35-27) and the extensively susceptible (e.g., CH38-41 and CH14-2) clonotypes. For a given major clonotype, resistance patterns were highly consistent across laboratories, with only a few statistically significant interlaboratory differences (see Fig. S1A to G in the supplemental material).
Fig 2.
Antimicrobial resistance profiles within the total collection. Cumulative resistance profiles of individual major CH clonotypes (those with ≥1% of isolates) are shown, as well as all other clonotypes combined (37% of isolates), and the total population (Total). The size (number of isolates) of each major clonotype is shown at the lower right. Resistance prevalence values significantly higher (OR ≥ 2) or lower (OR ≤ 0.5) than the mean for the rest of the population at the P < 0.05 level are marked in red or green, respectively.
E. coli clonal typing improves prediction of isolate susceptibility to antibiotics.
Based on the ≥80% susceptibility cutoff triage, CH-based typing allowed for treatment with a particular agent for widely divergent proportions of the isolates (Table 1). Among 5 oral antimicrobials that were most commonly used to treat E. coli infections in the data set, amoxicillin-clavulanate was allowed for 48.1% of isolates, cefazolin for 58%, trimethoprim-sulfamethoxazole for 58%, fluoroquinolones for 79.4%, and nitrofurantoin for 94.1%. In the allowed population, the actual prevalences of resistance to the corresponding antimicrobials ranged from 21.9% (ampicillin) to 3.74% (fluoroquinolones). The relative potential improvement in the prediction of susceptibility to a given agent with the clonotyping-based approach, compared with the total observed susceptibility, ranged from 45.4% (cefazolin) to 78.1% (fluoroquinolones), with an average improvement of 57.4%.
Table 1.
Performance statistics for clonotype-based susceptibility predictions for the primary collection of clinical Escherichia coli isolatesa
| Antibiotics usedb | Observed resistance rate (%)d | Performance of clonotype-based choice of antimicrobial (%)c |
||
|---|---|---|---|---|
| Rejected/resistance | Allowed/resistance | Improvemente | ||
| AMPf | 51.6 | 77.7/60.1 | 22.3/21.9 | 57.5 |
| TETf | 29.5 | 49.4/48.0 | 50.6/11.5 | 61.1 |
| A/Sf | 29.4 | 66.2/37.9 | 33.8/13.0 | 55.9 |
| T/S | 26.9 | 42.0/50.1 | 58.0/10.1 | 62.4 |
| A/K | 25.5 | 51.9/36.5 | 48.1/13.5 | 46.8 |
| CZ | 19.7 | 42.0/32.0 | 58.0/10.7 | 45.4 |
| CIP | 17.1 | 20.6/68.7 | 79.4/3.7 | 78.1 |
| GMf | 8.92 | 17.1/31.4 | 82.9/4.3 | 52.1 |
| NIT | 6.79 | 5.9/27.4 | 94.1/5.5 | 19.2 |
| CTR | 5.38 | 4.3/31.1 | 95.7/4.2 | 21.6 |
| PTZ | 3.96 | 2.1/13.3 | 97.9/3.8 | 5.1 |
A total of 1,518 isolates were typed using a fumC-fimH (CH) scheme and were tested against 11 antimicrobials. Of the isolates, 1,413 out of 1,518 belonged to CH clonotypes that contained >1 isolate (nonsingletons).
AMP, ampicillin; TET, tetracycline; A/S, ampicillin-sulbactam; T/S, trimethoprim-sulfamethoxazole; A/K, amoxicillin-clavulanate; CZ, cefazolin; CIP, ciprofloxacin; GM, gentamicin; NIT, nitrofurantoin; CTR, ceftriaxone; PTZ, piperacillin-tazobactam.
For each isolate, the treatment with an antimicrobial agent was allowed or not based on the prevalence of the susceptibility to this agent in the respective CH clonotype; to avoid bias, each analyzed isolate was excluded from the calculation of prevalence.
Rate (%) of resistant isolates among 1,413 isolates.
Percent improvement toward ideal test (100%) was calculated as (difference between the CH and antibiogram approach)/(difference between the antibiogram approach and 100%) × 100; all improvement rates were statistically significant (P < 0.001, Fisher's exact test), except for with NIT and CTR (P = 0.09) and PTZ (P = 0.43).
Antimicrobials with comparatively limited clinical utility.
Clonotyping predicts antimicrobial susceptibilities across different locations.
The clonotype-guided susceptibility prediction analyses for ciprofloxacin (a fluoroquinolone), which was prescribed in 40% of patients, and trimethoprim-sulfamethoxazole were done separately for the isolates from each clinical laboratory (Table 2), including for a set of 161 E. coli urine isolates from the University Clinics Hospital in Münster, Germany. To avoid potential self-selection bias in the analysis of these smaller groups, the susceptibility classification for the isolates in each subset was done after excluding the susceptibility profiles of that particular subset, i.e., by using the clonotype susceptibility profiles of the remaining isolates from common clonotypes (Table 2).
Table 2.
Performance of clonotype-based susceptibility predictions compared to observed susceptibility among clinical E. coli isolates from different laboratories
| Resistance and improvement rates by antibiotic typeb | Clinical microbiology laboratory and associated performance (%)a |
|||||
|---|---|---|---|---|---|---|
| Group Health Cooperative, Seattle | Children's Hospital, Seattle | University of Washington, Seattle | Harborview Medical Center, Seattle | VA Medical Center, Minneapolis | University of Münster, Münster | |
| T/S | ||||||
| Total resistant | 20.1 | 34.2 | 33.3 | 30.4 | 34.0 | 32.6 |
| Rejected/resistance | 48.8/36.2 | 41.9/58.7 | 43.5/62.6 | 45.6/58.3 | 50.0/51.9 | 56.6/46.6 |
| Allowed/resistance | 51.2/5.0 | 58.1/17.2 | 56.5/11.4 | 54.4/7.4 | 50.0/17.0 | 43.4/14.3 |
| Improvementc | 72.9 | 49.7 | 65.7 | 75.8 | 50.0 | 56.1 |
| CIP | ||||||
| Total resistant | 13.7 | 10.0 | 21.5 | 31.2 | 35.1 | 30.2 |
| Rejected/resistance | 17.5/59.6 | 16.5/54.5 | 26.3/71.2 | 32.8/88.6 | 39.4/72.9 | 33.3/74.4 |
| Allowed/resistance | 82.5/4.1 | 83.5/1.4 | 73.7/3.6 | 67.2/3.6 | 60.6/7.0 | 66.7/8.14 |
| Improvementc | 70.1 | 86.2 | 83.0 | 88.6 | 80.0 | 73.1 |
The set of isolates for which susceptibility was predicted was based on the mean susceptibility of the clonotype to which they belong. This mean susceptibility was calculated in each case for all isolates minus the isolates belonging to the validation set.
T/S, trimethoprim-sulfamethoxazole; CIP, ciprofloxacin.
Percent improvement was calculated as difference between resistance (i.e., potential drug-bug mismatch) in CH-allowed cases and observed resistance based on the actual susceptibility data (divided by the latter, × 100%); all improvements were statistically significant (P < 0.001, Fisher's exact test) unless stated otherwise.
Based on the <80% susceptibility cutoff, CH-based typing rejected treatment with trimethoprim-sulfamethoxazole in 41.9 to 56.6% (mean, 47.7%) of the isolates, depending on the laboratory. Among the remaining allowed isolates, the actual prevalences of trimethoprim-sulfamethoxazole resistance were between 5.0% and 17.2% (mean, 12.1%), which was 2- to 4-fold lower than the overall resistance in the corresponding site. For ciprofloxacin, CH-based typing rejected treatment in 16.5 to 39.4% (mean, 27.6%) of the isolates. In the remaining allowed population, the actual prevalences of resistance to ciprofloxacin ranged from 8.1% to 1.4% (mean, 4.6%), which was 3- to 9-fold lower than the overall resistance prevalence.
Association of UTI persistence or recurrence with clonotypes and drug-bug mismatch.
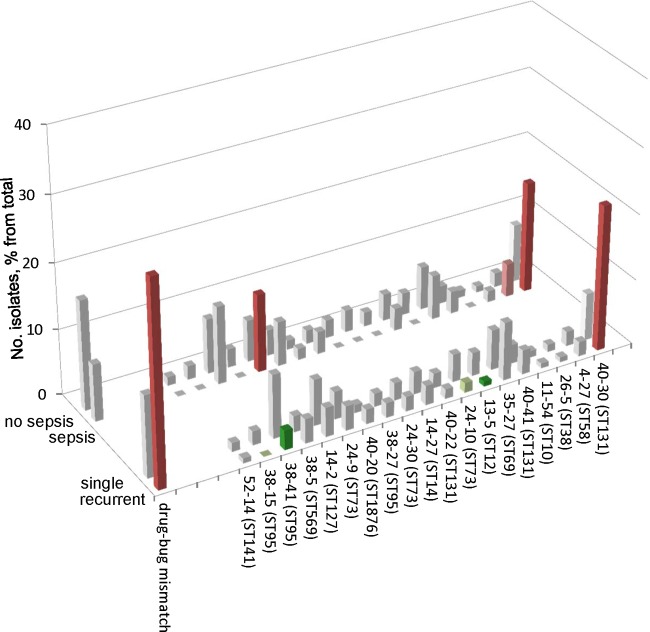
For 1,034 urine isolates, follow-up patient data were available (see Table S1 in the supplemental material). More than 13% (n = 135) of these patients experienced a persistent or recurrent UTI within 30 days. The overall clonal diversity of the isolates associated with persistent or recurrent UTI was significantly lower than that of the remaining isolates (P < 0.001) (see Table S2 in the supplemental material). Thus, a relatively limited subset of CH-based clonotypes of E. coli has an enhanced propensity to cause persistent or recurrent UTIs. Indeed, one clonotype, CH40-30, predominated and was significantly overrepresented among patients with persistent or recurrent UTIs (Fig. 3) (OR, 3.7; P < 0.001). Conversely, certain other clonotypes, including CH38-41 and CH13-5 (P < 0.05), and possibly CH38-15 and CH24-10 (P < 0.10), were negatively associated with persistence or recurrence of infection (Fig. 3).
Fig 3.
Association of recurrence or sepsis with major CH clonotypes. Clonotypes are shown in order of overall resistance prevalence, as in Fig. 2. The drug-bug mismatch bar demonstrates the association of recurrence or sepsis with resistance to the prescribed antimicrobial. Dark red or green, significantly increased or decreased recurrence and/or sepsis in a particular CH clonotype with a P value of <0.05); pale red or green, same as for dark red or green but with a P value of <0.10).
We next assessed the possible effects of drug-bug mismatches on persistence or recurrence of infection. For this, we analyzed actual clinical practices for the subset of urine isolates (n = 666) that were documented to have been treated empirically with only one antimicrobial agent prescribed at or around the day of the visit. The empirically prescribed agents (among the 666 patients with available data) were fluoroquinolones (267 [38%]), trimethoprim-sulfamethoxazole (TMP-SMX) (181 [25%]), first-generation cephalosporins (83 [11%]), third-generation cephalosporins (73 [10%]), nitrofurantoin (45 [6.4%]), penicillins (16 [2.3%]), and second-generation cephalosporins (11 [1.5%]), plus carbapenems, amoxicillin-clavulanate, ampicillin-sulbactam, tetracycline, and gentamicin (each <1%). Of these isolates, 99 (15%) were resistant to the empirically prescribed antimicrobial agent and 27% (27/99) of them were present in persistent or recurrent UTIs (Fig. 3) versus only 10.6% (60/567) of those whose isolate was susceptible to the initially prescribed agent (P < 0.001).
Association of clonotypes with sepsis.
Of the 1,518 primary set isolates, 1,133 were from patients with available clinical diagnostic data, of whom 59 were diagnosed with sepsis (see Table S1 in the supplemental material). The overall clonotype diversity of the 59 sepsis-associated isolates was significantly more limited than that of the 1,074 remaining isolates (P <0.001) (see Table S2 in the supplemental material), indicating that relatively few E. coli CH clonotypes are predisposed to cause bloodstream infections. Among the sepsis-associated isolates, the most prevalent clonotype was CH40-30 (17% of sepsis cases versus 8.9% of patients without sepsis; OR, 2.1) (P = 0.04) (Fig. 3). Other clonotypes associated with sepsis were CH14-2 (13.3% versus 6.33%; OR, 2.3) (P = 0.039) and, potentially, CH4-27 (5.1% versus 2.3%; OR, 2.3) (P = 0.096). In contrast to persistent or recurrent UTIs, sepsis was not significantly associated with resistance to the initially prescribed antimicrobial (Fig. 3).
Clonotype identification directly in urine specimens.
We determined whether clonotype information about E. coli can be determined directly in the patients' urine specimens by using common molecular diagnostics instruments, the LightCycler 2.0 (Roche Diagnostics GmbH) and PyroMark Q24 (Qiagen, Inc.), which utilize quantitative PCR (qPCR) and pyrosequencing approaches, respectively.
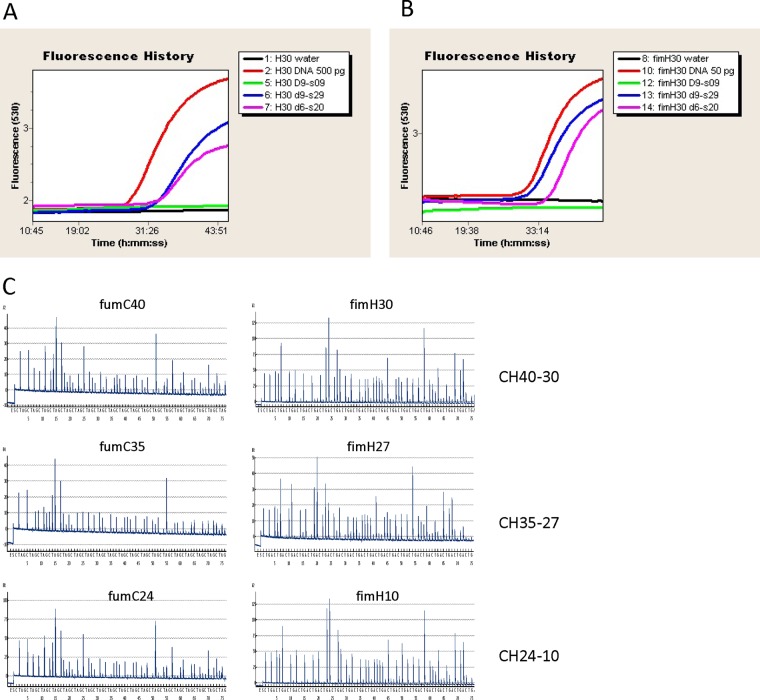
As is typical with traditional molecular diagnostics methods, the qPCR diagnostic tests were based on gene-specific or SNP-specific probes, thus allowing for the detection of one clonotype in a single test run. We designed two probes capable of detecting the predominant CH40-30 clonotype, one based on the rMST1 gene region and another on a canonical SNP in one of the CH clonotyping loci, fimH. The qPCR probes that we designed detected the infecting bacteria directly in the patients' urine specimens (Fig. 4A and B) in all specimens containing the CH40-30 E. coli clonotype with a bacterial load of ≥104 per ml (data not shown). By using qPCR, the clonotype identity was determined within 1 h upon starting processing of a clinical specimen.
Fig 4.
CH clonotyping of E. coli in patients' urine samples. (A) Detection of CH40-30 clones in urine samples using qPCR with RMTS1 gene-specific probe. (B) Detection of CH40-30 clones in urine samples using qPCR with fimH SNP-specific probe. (C) Determination of three different CH clonotypes in urine samples using pyrosequencing of fumC and fimH regions on PyroMark Q24.
Unlike qPCR, pyrosequencing-based tests provide information about the nucleotide sequences of specific gene regions. Thus, this method allows for the detection of many different clonotypes in a single test run, depending on the sequence diversity of the target gene region. We used the test primers against short (<60 bp) highly polymorphic regions within the fumC and fimH loci and, as with qPCR, we were able to determine the clonotype identities of the bacterial isolates directly in patient urine specimens. Illustrative results detecting bacteria identified as belonging to the CH40-30, CH35-27, and CH24-10 clonotypes are presented in Fig. 4C. Different E. coli clonotypes were identified within the clinical specimens that had bacterial loads of ≥103 per ml (data not shown). The pyrosequencing was accomplished generally within 3 h upon the start of processing a clinical specimen.
DISCUSSION
Here, we show that the clonal identities of clinical E. coli isolates, as inferred from two universal loci (fumC and fimH), are linked to distinct antimicrobial susceptibility profiles and clinical manifestations. Clonotyping can be done on the primary specimen well before species-level identification by growth on selective media and susceptibility profile determination, which typically has 2- to 3-day lag time. This suggests the feasibility of using subspecies-level clonotyping to improve patient management, most notably by avoiding the use of ineffective empirical antimicrobials in the clonotypes with a high resistance probability.
While the association of resistance with a few specific clonal groups (e.g., ST131 and ST69) has been established previously, it also is known that resistance genes spread horizontally (as plasmids or mobile chromosomal elements) or emerge by point mutation in different strains or even separate species. We show, however, that despite the highly dynamic nature of these genes, the resistance elements are relatively stably associated with carrier clones across different geographical locales and patient populations, at least over a clinically relevant time frame.
Currently, practitioners are guided in their initial empirical antimicrobial choice by a knowledge of cumulative (historical) susceptibility profiles of relevant isolates from the local area (1). Our findings indicate that a clonotype-guided approach might substantially reduce the likelihood of drug-bug mismatches during the course of initial antimicrobial therapy by providing more specific data about a patient's actual organism.
Historically, in many settings, trimethoprim-sulfamethoxazole and fluoroquinolones (e.g., ciprofloxacin) have been preferred treatment options for UTIs because of their low cost, tolerability, and previously broad efficacy against most isolates of E. coli and other uropathogens (19). However, resistance to these two critical antimicrobials is increasingly prevalent globally among urine isolates. Here, the overall prevalences of resistance to trimethoprim-sulfamethoxazole and fluoroquinolones were as high as 35% and 31%, respectively, among the isolates from some of the participating laboratories. Such high resistance rates increase the likelihood of treatment failure if these drugs are used empirically and may cause practitioners to opt for alternative empirical agents, such as nitrofurantoin for cystitis, or a parenteral agent, such as ceftriaxone, gentamicin, piperacillin-tazobactam, or ertapenem for pyelonephritis or urosepsis (20, 21). However, nitrofurantoin must be taken for 5 days to achieve the same clinical efficacy that is provided by 3 days of trimethoprim-sulfamethoxazole or fluoroquinolones, and it cannot be used in some patient categories, whereas the parenteral agents are more expensive, hazardous, and inconvenient than oral agents.
If clonotyping profiles are made available in a timely fashion as part of clinical laboratory diagnostics, trimethoprim-sulfamethoxazole and/or fluoroquinolones can be used with higher confidence against the majority of clinical E. coli isolates, with projected averages of 3- and 5-fold reductions, respectively, in the likelihood of drug-bug mismatch compared with standard empirical use of the corresponding antimicrobials. Thus, greater certainty from the outset regarding which antimicrobials can and cannot be used reliably for a given patient with suspected E. coli infection might be of great benefit to patients and health care systems alike.
While 50% and 31% of clinical isolates rejected for treatment by trimethoprim-sulfamethoxazole or ciprofloxacin actually are susceptible to these antibiotics, they constitute only 29% and 7.8% of all susceptible isolates (both in rejected and in allowed clonotypes), respectively. Thus, in only a relatively minor proportion of strains, clonotype-guided antimicrobial selection would promote unnecessary use of broader-spectrum antibiotics. Also, antimicrobial therapy would be rejected in 78 and 83% of the resistant isolates overall, respectively, limiting treatment failures due to drug-bug mismatch and possibly reducing the further spread of resistant organisms. Thus, we believe strongly that the proposed method of clonotyping will in fact promote the goals of antimicrobial stewardship, since it responds directly to the call of the Infectious Diseases Society of America (IDSA) for the “development of decision support tools for clinicians, including computer and Internet technology to deliver best practice information at the time the clinical antimicrobial treatment decision is made” (22).
Clonotype-susceptibility association data obtained with one set of isolates might be used to predict susceptibility with reasonable accuracy in a different set of clinical isolates with a distinct geographic origin. Indeed, clonotype-guided antimicrobial choices based on the present U.S. isolates might have decreased drug-bug mismatches 2- to 4-fold among the isolates from Münster, Germany. However, it is also possible that the most accurate predictions might be made by using clonotype-susceptibility association data derived from a comparable geographic area and/or patient population. Since our study was limited to only three urban areas, to further validate this approach, it should be conducted on larger collections that encompass isolates from different geographic regions, e.g., those in the SENTRY and MYSTIC studies (16).
We found that empirical antimicrobial selections that resulted in a drug-bug mismatch were correlated with persistent or recurrent UTIs, as were broadly resistant clonotypes, such as CH40-30, and multidrug-resistant isolates more generally. It seems probable that by reducing the likelihood of a drug-bug mismatch, the clonotyping-guided approach to antimicrobial selection has a strong potential to reduce the likelihood of persistent or recurrent infection. This hypothesis warrants further assessment.
We also documented an association between certain clonotypes and presentation of clinical sepsis. Here again, CH40-30 was the most prominent clonotype, but other smaller clonotypes were also potentially associated with sepsis. Although host factors, such as immunodeficiency, are undoubtedly important in determining whether a patient develops sepsis during the course of an E. coli infection, the observed clonal associations suggest that bacterial traits also contribute significantly. Additional studies are needed to determine whether clonotyping has potential value for the diagnosis, treatment, and/or prevention of sepsis.
The clonotype CH40-30 that our study identified as being strongly associated with recurrent UTI and sepsis, which is the dominant fluoroquinolone-resistant and multidrug-resistant lineage within, appears to have emerged just over a decade ago. Interestingly, the prevalences of E. coli fluoroquinolone and trimethoprim-sulfamethoxazole resistance among CH40-30 isolates have remained unchanged for the last decade (data not shown), indicating the general stability of this clonotype's resistance characteristics over time. The clinical association data presented here indicate that in addition to its antimicrobial resistance determinants, this subclone might also possess important virulence capabilities that make it disproportionately associated with recurrent or persistent UTIs and sepsis.
The significant diagnostic advantage of high-resolution CH typing over traditional MLST is especially evident from the characterization of the CH40-30 clonotype from ST131. The clinical significance and epidemic-like expansion of ST131 have been a focus of a large number of studies over the last decade. However, only recently was the clonal heterogeneity of ST131 discovered. In particular, we have reported that the signature ciprofloxacin resistance of ST131 is due almost exclusively to one of the ST131 subclones, H30, which is defined based on the specific fimH allele it carries and that corresponds here to clonotype CH40-30. In this study, we show for the first time that in addition to its resistance associations, this clonotype is heavily associated with persistent or recurrent UTIs and urosepsis. This is in sharp contrast to other clonotypes of ST131 that combined comprise 35% of the clonal group in our sample and that are not particularly distinct from the rest of the E. coli isolates with respect to either resistance or virulence pattern. Thus, we believe that such novel information about these CH clonotypes is potentially very useful for studying the epidemiology and pathogenic mechanisms underlying UTIs, as well as for diagnostic and therapeutic purposes.
The detection even of just CH40-30 (i.e., the H30 sublineage of ST131) would definitely improve empirical therapy selection by removing >50% of all fluoroquinolone resistance and approximately one-quarter each of third-generation cephalosporin and piperacillin-tazobactam resistance levels. Although this would remove only 17% of trimethoprim-sulfamethoxazole resistance, resistance to this drug is closely associated with another clonotype, CH35-27 (from ST69), such that the added detection of this clonotype (as previously proposed in reference 20) might close this important gap as well. The relative simplicity and rapid nature of the qPCR and pyrosequencing protocols might allow for an easy incorporation into the current clinical microbiology protocols and normal workflow. Depending on the proximity of the laboratory to the point of care (or clinical specimen collection), the clonotype identity can be defined within 1 to 6 h of the patient providing the specimen. Organizing a proper feedback mechanism from the laboratory to the provider will allow the physician to make more-accurate antibiotic selections. This could be done either for the initial prescription, within hours of the patient visit, or on the next day to correct the original prescription if a drug-bug mismatch is predicted (e.g., CH40-30 with fluoroquinolones, or CH35-27 with TMP-SMZ). Even in the latter scenario, clonotyping provides a 24- to 48-h advantage over conventional antibiogram diagnostics, especially for those clonotypes with highly predictable resistance patterns.
The provision of a reliable cost-benefit analysis of predictive diagnostics based on 1- to 4-h clonotyping versus definitive diagnostics based on 2- to 3-day antibiogram testing was outside the scope of this study. However, we believe that the currently estimated reagent cost of a single clonotype-specific qPCR test (≤$2) and of a broad-range clonotype-specific pyrosequencing run (≤$8) are sufficiently low to consider introduction of clonotyping into clinical diagnostics and to encourage its refinement. For other applications, such as less-time-sensitive epidemiologic surveillance, clonotyping costs are likely to compare favorably to currently used alternative methods, such as MLST or PFGE. Even Sanger sequencing-based 2-locus CH typing is obviously cheaper and faster then 5- to 7-locus MLST analysis, with the reagent cost for both being $10 to 15 per locus. No reliable cost estimates are available for PFGE, but PFGE takes 3 to 4 days, plus it is labor-intensive and reliant on subjective interpretation.
While the primary goal of this study was to provide a proof-of-principle that subspecies-level clonotyping of E. coli (and potentially other pathogens) might have predictive diagnostic value, we also demonstrated here the rapidity and low cost (see above) of clonotype determination directly in urine samples. Even considering that a sizable proportion of urine specimens received by laboratories are culture negative or yield contaminated and mixed growth, most culture-positive specimens contain E. coli. Further improvement of clonotyping efficiency could be achieved, for example, by prescreening urine specimens with a dipstick or microscopy to exclude those that are likely to be culture negative, and/or by designing clonotyping primers to work with multiple bacterial species (e.g., Klebsiella pneumoniae also has fumC and fimH loci, but they are not primed by the E. coli-specific primers used here). We are currently optimizing the protocol for practical use to expand its functionality, improve overall performance, and reduce further the cost.
We anticipate that subspecies clonotyping to the level provided by or comparable to that of CH typing might result in a paradigm shift in the management of infections caused by E. coli and other clonal bacterial pathogens. First, it provides prognostic power of antimicrobial resistance. Second, it allows for the genetic typing of clinical isolates to facilitate in-depth epidemiologic analyses of various clonotypes' associations with specific clinical outcomes in relation to treatment regimens, comorbidities, and patient demographics. Third, since clonotyping profiles are based on short DNA sequences, they are discrete and portable, making them suitable for analysis across laboratories, thereby conceivably allowing for the creation of global databases that could be queried by remote users. Based on the present study's results, we cannot comment on the stability of antimicrobial resistance prevalences within individual CH clonotypes over extended time periods, since the study duration was only 3 years. However, over this time interval, there were no evident temporal trends. Additionally, our previously published study regarding clonotype CH40-30 and other clonotypes within ST131 demonstrated the stability of antimicrobial resistance prevalences within those clonotypes over a much greater time interval (i.e., >10 years). Nevertheless, periodic surveys surely are required to update the clonotype versus antibiotic resistance database, and especially to add newly emerged clonotypes. For this, it should be possible to automate the informatics system, allowing for ongoing recalibration of the prediction algorithms, perhaps even at an institution-specific level. This would represent a significant advance over the current practice, which typically is to update the laboratory's cumulative susceptibility report (at the species level) either annually or semiannually. Potentially, the predictive precision of clonotyping for resistance and clinical outcome could be improved by incorporating host factors into the algorithm, including demographic characteristics and comorbidity, as well as previous antibiotic treatment. Recent studies, for example, have demonstrated that electronic health records (EHRs) can be used to identify high-risk patients for targeted relapse prevention strategies directed toward other pathogens (9, 23). Extensive genome-wide analysis of the predominant clonotypes is likely to yield additional and improved genetic markers for molecular diagnostics. Considering the increasing pace of current technological advances, including whole-genome sequencing, subspecies clonotyping of bacterial pathogens might readily be developed as a fast and cost-effective approach in routine clinical diagnostics, rapidly supplying practitioners with potentially critical information about the infecting strain.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ruth Anway, Lucretia Granger, Barbara Grünastel, Sarah Johnson, Elena Kuo, Brett Norquist, and the staff of the Harborview Medical Center Clinical Microbiology Laboratory for their excellent help in collecting isolates and associated clinical data and Steven Moseley for critical revision of the manuscript.
This work was supported by the National Institutes of Health (grant no. ARRA RC4 AI092828) and University of Washington, Department of Microbiology, Sokurenko lab budget no. 75-5533.
Footnotes
Published ahead of print 10 July 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.00984-13.
REFERENCES
- 1.Jenkins SG, Schuetz AN. 2012. Current concepts in laboratory testing to guide antimicrobial therapy. Mayo Clin. Proc. 87:290–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dellit TH, Owens RC, McGowan JE, Jr, Gerding DN, Weinstein RA, Burke JP, Huskins WC, Paterson DL, Fishman NO, Carpenter CF, Brennan PJ, Billeter M, Hooton TM, Infectious Diseases Society of America; Society for Healthcare Epidemiology of America 2007. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin. Infect. Dis. 44:159–177 [DOI] [PubMed] [Google Scholar]
- 3.Rice LB, Bonomo RA. 2007. Mechanisms of resistance to antibacterial agents, p 1114–1145 In Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA. (ed), Manual of clinical microbiology, 9th ed, ASM Press, Washington, DC [Google Scholar]
- 4.Alanis AJ. 2005. Resistance to antibiotics: are we in the post-antibiotic era? Arch. Med. Res. 36:697–705 [DOI] [PubMed] [Google Scholar]
- 5.Spellberg B, Guidos R, Gilbert D, Bradley J, Boucher HW, Scheld WM, Bartlett JG, Edwards J, Jr, Infectious Diseases Society of America 2008. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin. Infect. Dis. 46:155–164 [DOI] [PubMed] [Google Scholar]
- 6.Russo TA, Johnson JR. 2003. Medical and economic impact of extraintestinal infections due to Escherichia coli: focus on an increasingly important endemic problem. Microbes Infect. 5:449–456 [DOI] [PubMed] [Google Scholar]
- 7.Paul S, Linardopoulou EV, Billig M, Tchesnokova V, Price LB, Johnson JR, Chattopadhyay S, Sokurenko EV. 2013. Role of homologous recombination in adaptive diversification of extraintestinal Escherichia coli. J. Bacteriol. 195:231–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weissman SJ, Johnson JR, Tchesnokova V, Billig M, Dykhuizen D, Riddell K, Rogers P, Qin X, Butler-Wu S, Cookson BT, Fang FC, Scholes D, Chattopadhyay S, Sokurenko E. 2012. High-resolution two-locus clonal typing of extraintestinal pathogenic Escherichia coli. Appl. Environ. Microbiol. 78:1353–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hebert C, Du H, Peterson LR, Robicsek A. 2013. Electronic health record-based detection of risk factors for Clostridium difficile infection relapse. Infect. Control Hosp. Epidemiol. 34:407–414 [DOI] [PubMed] [Google Scholar]
- 10.Wirth T, Falush D, Lan R, Colles F, Mensa P, Wieler LH, Karch H, Reeves PR, Maiden MC, Ochman H, Achtman M. 2006. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol. Microbiol. 60:1136–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manges AR, Johnson JR, Foxman B, O'Bryan TT, Fullerton KE, Riley LW. 2001. Widespread distribution of urinary tract infections caused by a multidrug-resistant Escherichia coli clonal group. N. Engl. J. Med. 345:1007–1013 [DOI] [PubMed] [Google Scholar]
- 12.Oteo J, Orden B, Bautista V, Cuevas O, Arroyo M, Martinez-Ruiz R, Perez-Vazquez M, Alcaraz M, Garcia-Cobos S, Campos J. 2009. CTX-M-15-producing urinary Escherichia coli O25b-ST131-phylogroup B2 has acquired resistance to fosfomycin. J. Antimicrob. Chemother. 64:712–717 [DOI] [PubMed] [Google Scholar]
- 13.Johnson JR, Tchesnokova V, Johnston B, Clabots C, Roberts PL, Billig M, Riddell K, Rogers P, Qin X, Butler-Wu S, Price LB, Aziz M, Nicolas-Chanoine MH, Debroy C, Robicsek A, Hansen G, Urban C, Platell J, Trott DJ, Zhanel G, Weissman SJ, Cookson BT, Fang FC, Limaye AP, Scholes D, Chattopadhyay S, Hooper DC, Sokurenko EV. 2013. Abrupt emergence of a single dominant multidrug-resistant strain of Escherichia coli. J. Infect. Dis. 207:919–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nazir H, Cao S, Hasan F, Hughes D. 2011. Can phylogenetic type predict resistance development? J. Antimicrob. Chemother. 66:778–787 [DOI] [PubMed] [Google Scholar]
- 15.Mora A, Blanco M, Lopez C, Mamani R, Blanco JE, Alonso MP, Garcia-Garrote F, Dahbi G, Herrera A, Fernandez A, Fernandez B, Agulla A, Bou G, Blanco J. 2011. Emergence of clonal groups O1:HNM-D-ST59, O15:H1-D-ST393, O20:H34/HNM-D-ST354, O25b:H4-B2-ST131 and ONT:H21,42-B1-ST101 among CTX-M-14-producing Escherichia coli clinical isolates in Galicia, northwest Spain. Int. J. Antimicrob. Agents 37:16–21 [DOI] [PubMed] [Google Scholar]
- 16.Johnson JR, Murray AC, Kuskowski MA, Schubert S, Prère MF, Picard B, Colodner R, Raz R, Trans-Global Initiative for Antimicrobial Resistance Initiative (TIARA) Investigators 2005. Distribution and characteristics of Escherichia coli clonal group A. Emerg. Infect. Dis. 11:141–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauer AW, Kirby WM, Sherris JC, Turck M. 1966. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 45:493–496 [PubMed] [Google Scholar]
- 18.CLSI 2010. Performance standards for antimicrobial susceptibility testing; 20th informational supplement. CLSI M100-S20 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 19.Johnson JR, Stamm WE. 1987. Diagnosis and treatment of acute urinary tract infections. Infect. Dis. Clin. North Am. 1:773–791 [PubMed] [Google Scholar]
- 20.Colgan R, Johnson JR, Kuskowski M, Gupta K. 2008. Risk factors for trimethoprim-sulfamethoxazole resistance in patients with acute uncomplicated cystitis. Antimicrob. Agents Chemother. 52:846–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson JR. 2005. Escherichia coli clonal group A. Clin. Infect. Dis. 41:568. 10.1086/432132 [DOI] [PubMed] [Google Scholar]
- 22.IDSA 2006. Principles and strategies intended to limit the impact of antimicrobial resistance. Infectious Diseases Society of America. http://www.idsociety.org/uploadedFiles/IDSA/Policy_and_Advocacy/Current_Topics_and_Issues/Advancing_Product_Research_and_Development/Antimicrobials/Statements/Principles%20and%20Strategies%20to%20Limit%20the%20Impact%20of%20Antimicrobial%20Resistance.pdf
- 23.Robicsek A, Beaumont JL, Wright MO, Thomson RB, Jr, Kaul KL, Peterson LR. 2011. Electronic prediction rules for methicillin-resistant Staphylococcus aureus colonization. Infect. Control Hosp. Epidemiol. 32:9–19 [DOI] [PubMed] [Google Scholar]
- 24.Maiden MC, Bygraves JA, Feil E, Morelli G, Russell JE, Urwin R, Zhang Q, Zhou J, Zurth K, Caugant DA, Feavers IM, Achtman M, Spratt BG. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. U. S. A. 95:3140–3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfaller MA, Wendt C, Hollis RJ, Wenzel RP, Fritschel SJ, Neubauer JJ, Herwaldt LA. 1996. Comparative evaluation of an automated ribotyping system versus pulsed-field gel electrophoresis for epidemiological typing of clinical isolates of Escherichia coli and Pseudomonas aeruginosa from patients with recurrent gram-negative bacteremia. Diagn. Microbiol. Infect. Dis. 25:1–8 [DOI] [PubMed] [Google Scholar]
- 26.Chattopadhyay S, Feldgarden M, Weissman SJ, Dykhuizen DE, van Belle G, Sokurenko EV. 2007. Haplotype diversity in “source-sink” dynamics of Escherichia coli urovirulence. J. Mol. Evol. 64:204–214 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.