Abstract
This study describes the course of an OXA-48-producing Enterobacteriaceae (OPE) outbreak that started in March 2012 in a neonatal intensive care unit (NICU) in Jerusalem, Israel. During the peak of the outbreak (January to August 2012), there were 49 patients who had proven or suspected acquisition of OPE in the NICU, including 16 with invasive infections, out of a total of 156 patients who were hospitalized during that period. Three children hospitalized in the pediatric ICU were identified as carriers of OPE. Three patients with a previous stay in the affected NICU were identified as OPE carriers upon admission to another hospital. The Ministry of Health was notified and then intervened in July 2012. Intervention included cohorting colonized patients, conducting frequent rectal-culture surveillance, and improving the implementation of infection control practices. As a result, the incidence of OPE acquisition declined to 5 cases in the first 4 months, followed by no new cases in the next 3 months. Thirty-one patient-unique isolates were available for analysis: 29 Klebsiella pneumoniae isolates, all belonging to a single clone (sequence type 39 [ST39]), and 2 isolates from Enterobacter cloacae. All isolates possessed the blaOXA-48 and blaCTX-M-14 genes, which are located on the same plasmid. This plasmid, similar to the global blaOXA-48-harboring vector, has now acquired blaCTX-M-14, leading to resistance to all β-lactam agents.
INTRODUCTION
The OXA-48 carbapenemase was first discovered in various Enterobacteriaceae species isolated in Turkey and other countries in the Middle East (1). Within years, several sporadic cases of OXA-48-producing Enterobacteriaceae (OPE) infections have been reported across Europe. Most of these cases were related to patients with previous exposure to health care facilities in the Middle East and northern Africa (2). In Israel, the first cases of OPE were described in patients coming from the Palestinian Authority (PA) or from neighboring countries (3). As Klebsiella pneumoniae carbapenemase (KPC)-producing Enterobacteriaceae were already endemic to Israel at that time (4), OPE isolates were suspected to be present based on their susceptibility to expanded-spectrum cephalosporins, which is a unique feature of OPE (5). Another unique feature of OPE in Israel and worldwide is the location of blaOXA-48 inside a Tn1999 transposon, harbored by a unique 62-kb IncL/M plasmid that is capable of interspecies self-conjugation (3, 6). In recent years, outbreaks of OPE were reported in several European countries (7–10). These outbreaks were caused mainly by the monoclonal spread of K. pneumoniae strains that coproduce CTX-M-15, conferring resistance also to all cephalosporins. blaCTX-M-15 was located on a separate plasmid in the outbreak isolates and was found inside the same transposon in a single case report (11). All of these outbreaks, as well as other sporadic cases, were reported in adults only. In the present study, we describe the first case of a large-scale OPE outbreak in a neonatal intensive care unit (NICU), the intervention, and its results. The outbreak was propagated by a combination of the monoclonal spread of a single K. pneumoniae strain and horizontal transfer of the modified blaOXA-48-harboring plasmid that cocarries the blaCTX-M-14 gene.
MATERIALS AND METHODS
Setup and design.
Makassed Hospital (MH) is a 250-bed hospital affiliated with the School of Medicine of Al-Quds University and is its main teaching hospital. Located in east Jerusalem, it provides secondary and tertiary medical services for the local Palestinian population and functions as a referral center for patients from the Palestinian Authority and the Gaza Strip. The neonatal intensive care unit (NICU) is a 27-bed unit that cares for premature (including very-low-birth-weight babies) and sick babies who were born there or transferred from other hospitals in the Palestinian territories. It contains intensive care (15 beds) and intermediate care (12 beds) sections.
The intervention team of the Israeli Ministry of Health (MoH) was composed of personnel from the offices of the National Center for Infection Control (NCIC) and the Jerusalem District Health Office (JDHO). Data were collected prospectively from the initiation of intervention (June 2012) and retrospectively from that point back to the time the first cases were identified (March 2012).
General overview of the OPE outbreak and infection control practices at Makassed Hospital.
Prior to the OPE outbreak, infection control practices that included hand hygiene and isolation procedures were implemented in the NICU and throughout the hospital but with suboptimal compliance. Surveillance for carbapenem-resistant Enterobacteriaceae (CRE) was performed on admission in all high-risk patients (defined as patients who either were transferred from another medical center or arrived from the Palestinian territories) and as part of the contact investigation.
The OPE NICU outbreak lasted from March 2012 until the last case of OPE carriage identified in December 2012, resulting in a total of 57 affected patients, including 16 with invasive infections. Aside from this outbreak, the incidence of CRE at MH during this period was low; between July 2012 and April 2013, 5 new cases were identified upon admission, and only 1 case was detected in a hospitalized patient.
Rectal carbapenem-resistant Enterobacteriaceae surveillance cultures and phenotypic characterization of isolates.
In the MH lab, rectal CRE surveillance culture swabs were streaked onto MacConkey agar plates supplemented with either cefotaxime (10 mg/liter) or meropenem (0.5 mg/liter), per the local protocol. Pink colonies that grew around the disks were picked and processed. Bacterial identification was done by manual biochemical tests, and antimicrobial susceptibility testing was done by disk diffusion (12). Complete phenotypic and molecular characterizations were performed by the NCIC lab on available isolates as part of the investigation, but these were not available before the intervention.
In the NCIC lab, rectal CRE surveillance cultures were transported within 4 h to the NCIC lab. Swabs were inoculated into brain heart infusion (BHI) broth and incubated overnight prior to subculture onto MacConkey agar with imipenem (1 mg/liter) (13). Suspicious (pink) colonies were selected for further evaluation. Identification and antimicrobial susceptibility testing were performed by the Vitek2 system (bioMérieux, Marcy-l'Etoile, France). Ertapenem, imipenem, and meropenem MICs were verified by the agar dilution assay (14). Phenotypic characterization included the modified Hodge test (MHT) and synergy testing of ertapenem with and without EDTA (EDTAST) (15).
Molecular characterization of isolates.
Isolates were screened by PCR for the presence of blaKPC (16), blaNDM-1, and blaOXA-48 (5). blaCTX-M, blaTEM, and blaSHV were identified by PCR and sequencing (26, 27). Pulsed-field gel electrophoresis (PFGE) was performed for K. pneumoniae and Enterobacter cloacae isolates as previously described (18). Multilocus sequence typing (MLST) was performed for representative PFGE types of the K. pneumoniae (19) isolates. Since only 29/57 patient-unique isolates were available for molecular and phenotypic analyses (from the latter part of the outbreak), we referred to the isolates as either OPE or CRE, depending on whether full characterization was done or not, respectively.
Plasmid analysis.
Plasmid DNA that was purified from a representative K. pneumoniae isolate and the 2 E. cloacae isolates was transformed into Escherichia coli strain DH10B. Transformants were selected on LB agar supplemented with cefazolin (8 mg/liter) and confirmed by blaOXA-48 PCR. Determinations of plasmid size and restriction fragment length polymorphism (RFLP) were done as previously described (20). The genetic environment of blaOXA-48 was studied by PCR and sequencing (1). The presence of the repA, parA, and traU genes was tested by PCR (6). The genetic environments up- and downstream to blaCTX-M-14 were studied by sequencing of the blaOXA-48/blaCTX-M-14-carrying plasmid, using primers directed to the 5′ and 3′ ends of blaCTX-M-14, respectively. The sequence was analyzed using the ISfinder website (https://www-is.biotoul.fr/) (21).
RESULTS
Epidemiological features of the outbreak.
The first clinical cases of CRE in the NICU were noted in mid-March 2012. Two babies had CRE bacteremia, one with Klebsiella spp. and one with Serratia marcescens, in both cases >72 h from admission. In the second case, the baby was admitted following a short stay in a Palestinian Authority hospital. Subsequently, Klebsiella spp. were isolated in CRE surveillance cultures in 6 babies. In the following 17 weeks until the beginning of the NCIC intervention, 27 new cases were identified (Fig. 1a), with the incidence reaching 9 cases/week. All isolates were identified as either Klebsiella spp. or K. pneumoniae, except for 1 isolate from E. coli and 2 from E. cloacae (Table 1; also see below).
Fig 1.
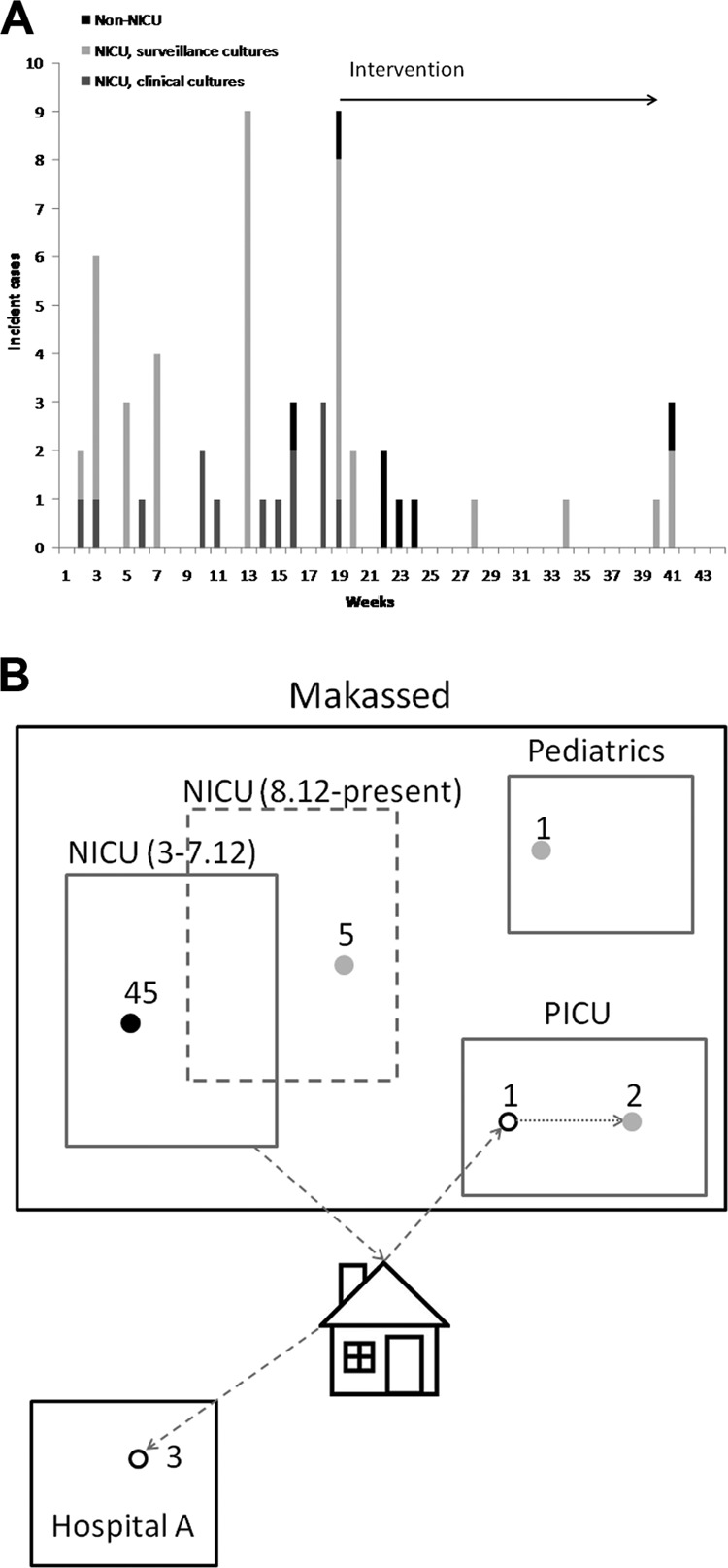
Temporal and spatial analyses of the OXA-48-producing Enterobacteriaceae (OPE) outbreak in the NICU. (A) Incidence of suspected and proven OPE infections (surveillance and clinical cultures). Cases initially identified in the MH NICU (gray bars) and in other MH wards and additional hospitals (black lines) are included. (B) Spatial dissemination of the OPE outbreak outside the NICU. The OPE outbreak was ongoing in the NICU from March 2012 until July 2012 (solid lines), and the NICU was opened to new admissions only after 3 weeks (dashed lines). Filled and open black circles, acquisition of OPE during the initial NICU outbreak (March to July 2012), with the first detection during or following the NICU stay, respectively; gray circles, acquisition of OPE outside the initial NICU outbreak; dashed arrow, home stay prior to readmission; solid arrow, transmission of the outbreak strain.
Table 1.
Microbiological and molecular characteristics of OXA-48-producing Enterobacteriaceae isolates
| Characteristic | Values for: |
|
|---|---|---|
| K. pneumoniae | E. cloacae | |
| No. of isolates | 29 | 2 |
| Sample sourcea | 4 B, 1 S, 24 R | R |
| No. of strainsb (sequence type) | 1 (ST39) | 2 |
| β-lactamase-encoding genes | blaOXA-48, blaCTX-M-14, blaCTX-M-15, blaTEM-1, blaSHV-1 | blaOXA-48, blaCTX-M-14 |
| Carbapenem MIC (mg/liter) (median [range]) | ||
| Ertapenem | 2 (2 to >16) | 3 (2 to 4) |
| Imipenem | 0.5 (0.25 to >8) | 0.75 (0.5 to 1) |
| Meropenem | 0.5 (0.25 to >8) | 0.5 (0.25 to 1) |
| blaOXA-48 plasmid characteristic | ||
| Size (kb) | ∼62 | ∼62 |
| Genes | blaCTX-M-14, repA, parA, traU | blaCTX-M-14, repA, parA, traU |
| Tn1999 type | 1999.2 | 1999.2 |
B, blood; S, sputum; R, rectum.
Typing was done by PFGE and MLST for K. pneumoniae and by PFGE for E. cloacae.
Intervention and outcome.
The NCIC and the JDHO were notified of the outbreak in early July 2012, and an intervention was launched. The measures taken included (i) cohorting colonized patients in a completely separate interim location, with complete separation of staff and equipment from the unaffected babies, (ii) closing the NICU to new admissions (this was done for 3 weeks), (iii) reinforcing infection control practices (including hand hygiene, equipment and environmental cleaning, injection safety, and standard and isolation precautions), (iv) conducting frequent rectal surveillance cultures of all CRE-negative babies in the NICU (at least once a week), (v) conducting CRE surveillance of exposed patients hospitalized in other pediatric wards in the hospital, (vi) conducting CRE surveillance and close monitoring of admissions of exposed infants to other hospitals (via the MoH reporting system), and (vii) submitting all available CRE isolates for molecular studies at the NCIC lab. Implementation of the intervention was overseen in frequent site visits to the hospital undertaken by staff of the NCIC and JDHO. Surveillance of the hospital staff and mothers of the NICU babies was not performed, as colonization among them was not believed to be related to the propagation of the outbreak.
During the first week of the intervention, 8 new cases were identified in the NICU (7 surveillance, 1 clinical). Two additional new carriers were identified in the next month who were all previously exposed (Fig. 1a). The NICU was reopened for new admissions after 3 weeks, with the previously identified carriers cohorted in a separate location. A previously exposed infant readmitted to the pediatric intensive care unit was found to be a carrier of OXA-48-producing K. pneumoniae, and a survey of that unit identified 2 additional carriers, with the infant presumably serving as the index case in that unit. Three previously exposed infants admitted to another hospital were found to be carriers of OPE (Fig. 1b). During the peak of the outbreak (January to August 2012), 49 patients were identified with proven or suspected acquisition of OPE in the NICU out of a total of 156 patients hospitalized in the NICU during that period (31%). Invasive infections (blood and/or cerebrospinal fluid [CSF]) were detected in 16 patients, and 4 of these patients died within 14 days of isolation of the pathogen. One patient carrying the outbreak K. pneumoniae clone who had no known exposure to other carriers or core staff members (i.e., nurses and physicians) was identified in the pediatrics ward. Following reopening of the NICU, 5 additional patients carrying the epidemic K. pneumoniae clone were identified (Fig. 1b). Additional site visits by the MoH teams were conducted, and these patients were cohorted in separate rooms with dedicated nursing staff. Weekly CRE surveillance was continued in the NICU until the present, with no new cases of OPE identified since then.
In addition to the OPE outbreak in the NICU, 5 additional isolates of carbapenem-resistant K. pneumoniae were detected in the pediatrics ward and the pediatric intensive care unit (PICU) of MH: 3 isolates of OPE were detected by surveillance culture upon admission from Gaza and the PA, all presenting a different PFGE pattern than that of the epidemic strain (data not shown), and 1 isolate of KPC-producing K. pneumoniae was detected by surveillance culture upon admission to the PICU of a patient transferred from a hospital in central Israel; an additional isolate was later discovered in another patient in that unit.
Microbiological and molecular features of OXA-48-producing Enterobacteriaceae isolates.
A total of 31 outbreak-related isolates from 29 patients were submitted for analysis to the NCIC laboratory. K. pneumoniae was isolated from all patients, and all isolates belonged to a single PFGE type identified as sequence type 39 (ST39) (Table 1). Two patients also carried OXA-48-producing E. cloacae, belonging to two different PFGE types. All isolates were resistant to ampicillin, cefazolin, piperacillin-tazobactam, ceftriaxone, and ertapenem but had MIC values of <1 mg/liter for imipenem and meropenem (Table 1). The E. coli DH10B transformant isolates from both the K. pneumoniae and E. cloacae donor strains had similar resistance patterns for carbapenems and were also resistant to ampicillin, cefazolin, piperacillin-tazobactam, and ceftriaxone, but not to any other class of antimicrobials. All isolates tested positive by MHT and carried blaOXA-48 and blaCTX-14, and they tested negative by the EDTAST and blaKPC- and blaNDM-based PCR. The K. pneumoniae isolates also carried blaCTX-15, blaTEM-1, and blaSHV-1. The blaOXA-48-harboring plasmids were ∼62 kb in size and carried the repA, parA, and traU genes, as did our previously described blaOXA-48-harboring plasmids (3). Unlike these plasmids, they also carried blaCTX-14 and had an identical restriction fragment length polymorphism pattern that differed from the pattern(s) of these plasmids (data not shown). blaCTX-14 was surrounded by ISEcp1 (GenBank accession no. AJ242809) (Fig. 2). The entire 3.1-kb structure was inserted in position 26950, corresponding to the pOXA-48a plasmid (GenBank accession no. JN626286.1) (6).
Fig 2.
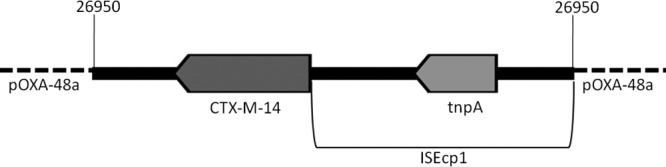
Schematic structure of the blaCTXM-14-containing insert. The orientation of the insert is presented in relation to the pOXA-48a plasmid (GenBank accession no. JN626286.1).
DISCUSSION
In the present study, we describe the first case of OPE in the neonatal and pediatric population. Moreover, this is the first report of an outbreak caused by CRE of any kind in this population. This population has not been spared from nosocomial outbreaks caused by other types of antimicrobial-resistant bacteria, e.g., extended-spectrum β-lactamase-producing Enterobacteriaceae (22), corresponding with the occurrences in other patient populations. It is noteworthy, however, that although sporadic cases of OPE in adults have been described in Israel (3), the first and so far the only OPE outbreak in Israel occurred in the pediatric population. The data regarding the prevalence of OPE in the PA and Gaza are limited, but based on this study and our previous report (3), it appears likely that OPE is endemic to these areas, similar to the situation in other Middle Eastern countries (1, 17).
Due to the fact that several months elapsed between onset of the outbreak and the involvement of the MoH, we were unable to obtain the microbiological data necessary to determine the origin of the outbreak. During the investigation of this outbreak, we identified carriers of additional unrelated OPE who were admitted to other pediatric wards from the PA or Gaza. It is therefore likely that the outbreak strain was introduced in a similar way, following an admission of an unrecognized carrier either directly to the NICU or to one of the other pediatric service facilities. The monoclonal nature of the outbreak and the spread of the outbreak strain to other wards led to the conclusion that although the introduction of the strain via maternal carriage cannot be excluded, this mechanism is unlikely to have played a role in the subsequent propagation of the outbreak.
The epidemic strain presumably spread initially through the NICU, leading to colonization of at least 49 patients with 16 invasive infections. From there, we were able to trace its spread to the PICU following the readmission of a former NICU-exposed baby that led to the transmission of the strain to two additional children. Following intervention, the incidence of new colonization or infection significantly declined. However, despite cohorting the OPE carriers and the allocation of a dedicated nursing staff to care for them, the epidemic strain reappeared in the reopened NICU, presumably due to a single exposed infant who had previously tested negative and then transmitted the strain to 4 additional infants. The strain was also detected in one patient in the pediatrics ward who was not known to be exposed to any of the carriers (Fig. 1b), suggesting transmission via either contaminated equipment or other hospital staff. Surveillance of OPE colonization among hospital staff was not performed, as staff carriage has not been shown to be an important factor in the nosocomial transmission of multidrug-resistant Enterobacteriaceae (23). Indeed, the implementation of proper infection control policies, including hand hygiene and ongoing surveillance of patients at risk, proved to be sufficient to contain the resurgence of the outbreak.
One of the dreaded threats to public health in any local outbreak is the organism's potential to spread via unrecognized carriers to other health care centers (24). The establishment of our nationally coordinated registry of CRE (4) enabled us to alert and communicate with neighboring hospitals, allowing for the detection of carriers upon admission and their prompt isolation, resulting in outbreak containment.
Similar to previous reports from Europe (7–10), the main mechanism of this outbreak was the monoclonal spread of K. pneumoniae. The epidemic clone, ST39, was previously reported as a VIM-producing strain in a nosocomial outbreak in Spain (25) but has never been associated with OPE outbreaks. In addition, we identified two cases of presumed horizontal plasmid transfer in two patients who carried OXA-48-producing E. cloacae in addition to the epidemic strain. The blaOXA-48-harboring plasmid in these isolates was similar in size to the pOXA-48a plasmid and possesses its characteristic genes (6) but differed from it by the presence of blaCTX-M-14 carried along the ISEcp1 transposon. Although additional β-lactamase-encoding genes, including blaCTX-M-15, were also present in the outbreak strain as in previous reports (10), this is only the second report describing the integration of an additional resistance gene into the pOXA-48a plasmid (11). This unique characteristic allowed us to track the route of horizontal plasmid transfer and to differentiate the outbreak strain from nonrelated OPE strains that were introduced to the hospital during the outbreak. The addition of another resistance element to this plasmid is an ominous finding due to the plasmid's demonstrated high degree of transmissibility (1, 3); the added resistance gene in this case renders all recipient strains resistant to cephalosporins, thus limiting therapeutic options even further.
In conclusion, the study of this outbreak teaches us that while standard infection control measures are effective for initial containment, continuous vigilant monitoring is essential in order to detect and respond to a resurgence of the outbreak. It also demonstrates the value of a centralized coordinating body, allowing for the prompt identification of unidentified carriers on admission to other hospitals. In areas where CRE of various types are endemic, a thorough molecular analysis that includes the identification of resistance genes, clones, and plasmids is of paramount importance in an outbreak setting for the accurate assessment of the outbreak's course, containment, and, should it occur, resurgence.
Footnotes
Published ahead of print 26 June 2013
REFERENCES
- 1.Carrër A, Poirel L, Yilmaz M, Akan OA, Feriha C, Cuzon G, Matar G, Honderlick P, Nordmann P. 2010. Spread of OXA-48-encoding plasmid in Turkey and beyond. Antimicrob. Agents Chemother. 54:1369–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. 2012. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin. Microbiol. Rev. 25:682–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adler A, Shklyar M, Schwaber MJ, Navon-Venezia S, Dhaher Y, Edgar R, Solter E, Benenson S, Masarwa S, Carmeli Y. 2011. Introduction of OXA-48-producing Enterobacteriaceae to Israeli hospitals by medical tourism. J. Antimicrob. Chemother. 66:2763–2766 [DOI] [PubMed] [Google Scholar]
- 4.Schwaber MJ, Lev B, Israeli A, Solter E, Smollan G, Rubinovitch B, Shalit I, Carmeli Y, Israel Carbapenem-Resistant Enterobacteriaceae Working Group 2011. Containment of a country-wide outbreak of carbapenem-resistant Klebsiella pneumoniae in Israeli hospitals via a nationally implemented intervention. Clin. Infect. Dis. 52:848–855 [DOI] [PubMed] [Google Scholar]
- 5.Poirel L, Héritier C, Tolün V, Nordmann P. 2004. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 48:15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poirel L, Bonnin RA, Nordmann P. 2012. Genetic features of the widespread plasmid coding for the carbapenemase OXA-48. Antimicrob. Agents Chemother. 56:559–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuzon G, Ouanich J, Gondret R, Naas T, Nordmann P. 2011. Outbreak of OXA-48-positive carbapenem-resistant Klebsiella pneumoniae isolates in France. Antimicrob. Agents Chemother. 55:2420–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paño-Pardo JR, Ruiz-Carrascoso G, Navarro-San Francisco C, Gómez-Gil R, Mora-Rillo M, Romero-Gómez MP, Fernández-Romero N, García-Rodríguez J, Pérez-Blanco V, Moreno-Ramos F, Mingorance J. 2013. Infections caused by OXA-48-producing Klebsiella pneumoniae in a tertiary hospital in Spain in the setting of a prolonged, hospital-wide outbreak. J. Antimicrob. Chemother. 68:89–96 [DOI] [PubMed] [Google Scholar]
- 9.Potron A, Kalpoe J, Poirel L, Nordmann P. 2011. European dissemination of a single OXA-48-producing Klebsiella pneumoniae clone. Clin. Microbiol. Infect. 17:E24–E26. 10.1111/j.1469-0691.2011.03669.x [DOI] [PubMed] [Google Scholar]
- 10.Voulgari E, Zarkotou O, Ranellou K, Karageorgopoulos DE, Vrioni G, Mamali V, Themeli-Digalaki K, Tsakris A. 2013. Outbreak of OXA-48 carbapenemase-producing Klebsiella pneumoniae in Greece involving an ST11 clone. J. Antimicrob. Chemother. 68:84–88 [DOI] [PubMed] [Google Scholar]
- 11.Potron A, Nordmann P, Rondinaud E, Jaureguy F, Poirel L. 2013. A mosaic transposon encoding OXA-48 and CTX-M-15: towards pan-resistance. J. Antimicrob. Chemother. 68:476–477 [DOI] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute 2011. Performance standards for antimicrobial susceptibility testing; 21st informational supplement. Approved standard MS100-S20 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 13.Adler A, Navon-Venezia S, Moran-Gilad J, Marcos E, Schwartz D, Carmeli Y. 2011. Laboratory and clinical evaluation of screening agar plates for detection of carbapenem-resistant Enterobacteriaceae from surveillance rectal swabs. J. Clin. Microbiol. 49:2239–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Approved standard—ninth edition. CLSI document M07-A9 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 15.Giske CG, Gezelius L, Samuelsen Ø, Warner M, Sundsfjord A, Woodford N. 2011. A sensitive and specific phenotypic assay for detection of metallo-β-lactamases and KPC in Klebsiella pneumoniae with the use of meropenem disks supplemented with aminophenylboronic acid, dipicolinic acid and cloxacillin. Clin. Microbiol. Infect. 17:552–556 [DOI] [PubMed] [Google Scholar]
- 16.Schechner V, Straus-Robinson K, Schwartz D, Pfeffer I, Tarabeia J, Moskovich R, Chmelnitsky I, Schwaber MJ, Carmeli Y, Navon-Venezia S. 2009. Evaluation of PCR-based testing for surveillance of KPC-producing carbapenem-resistant members of the Enterobacteriaceae family. J. Clin. Microbiol. 47:3261–3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matar GM, Dandache I, Carrër A, Khairallah MT, Nordmann P, Sabra A, Araj GF. 2010. Spread of OXA-48-mediated resistance to carbapenems in Lebanese Klebsiella pneumoniae and Escherichia coli that produce extended spectrum beta-lactamase. Ann. Trop. Med. Parasitol. 104:271–274 [DOI] [PubMed] [Google Scholar]
- 18.Leavitt A, Carmeli Y, Chmelnitsky I, Goren MG, Ofek I, Navon-Venezia S. 2010. Molecular epidemiology, sequence types, and plasmid analyses of KPC-producing Klebsiella pneumoniae strains in Israel. Antimicrob. Agents Chemother. 54:3002–3006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diancourt L, Passet V, Verhoef J, Grimont PAD, Brisse S. 2005. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J. Clin. Microbiol. 43:4178–4182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goren MG, Carmeli Y, Schwaber MJ, Chmelnitsky I, Schechner V, Navon-Venezia S. 2010. Transfer of carbapenem-resistant plasmid from Klebsiella pneumoniae ST258 to Escherichia coli in patient. Emerg. Infect. Dis. 16:1014–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siguier P, Perochon J, Lestrade L, Mahillon J, Chandler M. 2006. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res. 34:D32–D36. 10.1093/nar/gkj014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paterson DL, Bonomo RA. 2005. Extended-spectrum β-lactamases: a clinical update. Clin. Microbiol. Rev. 18:657–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adler A, Gniadkowski M, Baraniak A, Izdebski R, Fiett J, Salvia A, Samso JV, Lawrence C, Salomon J, Paul M, Schwartzberg Y, Mordechai E, Rossini A, Fierro J, Lammens C, Malhotra-Kumar S, Goossens H, Hryniewicz W, Brun-Buisson YC C. 2013. A multinational study of colonisation with extended-spectrum beta-lactamases (ESBL)-producing Enterobacteriaceae in healthcare personnel and family members of patients admitted to rehabilitation centres. 23rd European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), Berlin, Germany: [DOI] [PubMed] [Google Scholar]
- 24.Lopez JA, Correa A, Navon-Venezia S, Correa AL, Torres JA, Briceño DF, Montealegre MC, Quinn JP, Carmeli Y, Villegas MV. 2011. Intercontinental spread from Israel to Colombia of a KPC-3-producing Klebsiella pneumoniae strain. Clin. Microbiol. Infect. 17:52–56 [DOI] [PubMed] [Google Scholar]
- 25.Valverde A, Ruiz-Garbajosa P, Curiao T, Tato M, Gijón D, Baquero F, Coque TM, Cantón R. 2011. Klebsiella pneumoniae sequence types originally associated with specific ESBLs might act as substrates for recently emerged metallo-beta-lactamases or KPC enzymes. 21st European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), Milan, Italy [Google Scholar]
- 26.Navon-Venezia S, Chmelnitsky I, Leavitt A, Carmeli Y. 2008. Dissemination of the CTX-M-25 family beta-lactamases among Klebsiella pneumoniae, Escherichia coli and Enterobacter cloacae and identification of the novel enzyme CTX-M-41 in Proteus mirabilis in Israel. J. Antimicrob. Chemother. 62:289–295 [DOI] [PubMed] [Google Scholar]
- 27.Woodford N, Fagan EJ, Ellington MJ. 2006. Multiplex PCR for rapid detection of genes encoding CTX-M extended-spectrum (beta)-lactamases. J. Antimicrob. Chemother. 57:154–155 [DOI] [PubMed] [Google Scholar]


