Abstract
Molecular diagnosis of malaria offers many potential advantages over microscopy, including identification of malaria to the species level in an era with few experienced microscopists. We developed high-throughput multiplex 5′ nuclease quantitative PCR (qPCR) assays, with the potential to support large studies, to specifically identify Plasmodium falciparum, P. vivax, P. ovale, P. malariae, and P. knowlesi. We compared qPCR to microscopy and confirmed discordant results with an alternative target PCR assay. The assays specifically detected 1 to 6 parasites/μl of blood. The clinical sensitivities (95% confidence intervals [CIs]) of the 4-plex assay to detect microscopically confirmed malaria were 95.8% (88.3 to 99.1%) for P. falciparum, 89.5% (75.2 to 97.1%) for P. vivax, 94.1% (71.3 to 99.9%) for P. ovale, and 100% (66.4 to 100%) for P. malariae. The specificities (95% CIs) were 98.6% (92.4 to 100%) for P. falciparum, 99% (84.8 to 100%) for P. vivax, 98.4% (94.4 to 99.8%) for P. ovale, and 99.3% (95.9 to 100%) for P. malariae. The clinical specificity for samples without malaria was 100%. The clinical sensitivity of the 5-plex assay for confirmed P. knowlesi malaria was 100% (95% CI, 69.2 to 100%), and the clinical specificity was 100% (95% CI, 87.2 to 100%). Coded retesting and testing with an alternative target PCR assay showed improved sensitivity and specificity of multiplex qPCR versus microscopy. Additionally, 91.7% (11/12) of the samples with uncertain species by microscopy were identified to the species level identically by both our multiplex qPCR assay and the alternative target PCR assay, including 9 P. falciparum infections. Multiplex qPCR can rapidly and simultaneously identify all 5 Plasmodium species known to cause malaria in humans, and it offers an alternative or adjunct to microscopy for clinical diagnosis as well as a needed high-throughput tool for research.
INTRODUCTION
Malaria is an important cause of morbidity and mortality worldwide, with up to half of the world's population at risk (1). More than 1 million deaths occur each year, of which 90% are in sub-Saharan Africa (1); however, many cases go unreported. Commercial rapid diagnostic tests (RDTs) for malaria yield only qualitative results and vary widely in diagnostic sensitivity and specificity (2). Microscopy has been the historic diagnostic standard but depends on the skill of the microscopist. Further, Bayesian latent-class models suggest that microscopy can be insensitive (with likely sensitivity ranges of 50 to 90%) for low-level parasitemia (3). Under optimal conditions, the analytical sensitivity of thick-film microscopy is 10 to 30 parasites per μl of blood (4). Moreover, the differentiation of species can be difficult even for the most experienced microscopists. Simian Plasmodium knowlesi was found to cause potentially fatal infections in humans only after molecular tools enabled its distinction from the morphological mimics Plasmodium falciparum and Plasmodium malariae (5, 6). Finally, large epidemiologic and clinical studies require not only detection of low-level parasitemia and accurate identification to the species level but also high throughput to be practical. Therefore, we designed high-throughput multiplex 5′ nuclease quantitative PCR (qPCR) assays to simultaneously identify, quantitate, and distinguish the 5 species of Plasmodium now recognized to cause human malaria.
MATERIALS AND METHODS
Cohort of patients with clinical malaria and control subjects.
The study cohort included patients with clinical malaria after travel to or residence in regions in which malaria is endemic (most frequently sub-Saharan Africa or Southeast Asia), from whom blood samples were submitted between 2004 and 2012 for clinical diagnosis of malaria by thin- and thick-blood-smear microscopy at the Johns Hopkins University and Duke University clinical microbiology laboratories or a university-based reference laboratory. To assess clinical sensitivity, discarded deidentified EDTA-anticoagulated blood samples from the aforementioned cohort that had been archived and stored at −20 to −80°C were used. To assess clinical specificity, discarded deidentified EDTA-anticoagulated blood samples from patients who presented to Johns Hopkins Hospital with no known bloodstream infection or with microscopy-confirmed babesiosis (a clinical mimic of malaria) were used.
Ethics.
The study was reviewed by the ethics committee of the Johns Hopkins University School of Medicine. Institutional review board approval to use archived discarded deidentified samples was granted, since obtaining consent was deemed both impractical and unnecessary (Johns Hopkins Medicine protocol NA_00021376).
Blood samples from patients.
For evaluation of a 4-color 384-well platform designed to detect and to distinguish P. falciparum, P. vivax, P. ovale, and P. malariae malaria, 148 samples from the aforementioned cohort of patients with microscopic diagnoses of clinical malaria (72 P. falciparum samples, 38 P. vivax samples, 17 P. ovale samples, 9 P. malariae samples, and 12 Plasmodium sp. samples) were available, including DNA previously extracted from blood samples from 17 patients with malaria (11 P. ovale samples, 5 P. malariae samples, and 1 indeterminate Plasmodium sp. sample). Since 12 samples were not identified microscopically to the species level, 136 samples obtained from 100 patients with malaria (50 P. falciparum cases, 24 P. vivax cases, 17 P. ovale cases, and 9 P. malariae cases) were used for assessment of clinical sensitivity and specificity. The non-Plasmodium panel used for assessment of specificity included 11 discarded deidentified blood samples, i.e., 1 sample spiked with Trypanosoma brucei rhodesiense, 4 samples from patients with no known bloodstream infection, 2 samples from patients with microscopy-confirmed babesiosis, and 4 blood smear-negative samples obtained during monitoring of parasitemia in 2 patients with recent P. falciparum malaria.
For extension of the 4-plex assay to a 5-plex 96-well platform that also detects and distinguishes P. knowlesi, 10 EDTA-treated blood samples were obtained from 10 Malaysian patients with P. knowlesi, as confirmed by microscopy and nested PCR assays (5). A subset of available samples was used to assess the sensitivity and specificity of the 5-plex assay and included 33 blood samples from patients with microscopy-confirmed clinical malaria (7 P. falciparum samples, 7 P. vivax samples, 5 P. ovale samples, 4 P. malariae samples, and 10 P. knowlesi samples) and 4 samples from patients without malaria (2 with bacteremia and 2 with no known bloodstream infection).
Preparation of DNA.
DNA was prepared from archived EDTA-anticoagulated blood samples with the automated QIAsymphony SP system (Qiagen Inc., Valencia, CA). Blood samples (800 μl to 1 ml) were brought to 1 ml with 1× phosphate-buffered saline before extraction with a QIAsymphony DNA Midi kit; the final elution volume was 200 μl. Blood samples of insufficient volume were extracted with spin columns and a DNeasy Blood & Tissue kit (Qiagen); the elution volume was 200 μl, which then was concentrated to 100 μl.
DNA was prepared from 152 blood samples (141 Plasmodium samples and 11 non-Plasmodium samples), 121 by the QIAsymphony system and 31 by the Qiagen DNeasy Blood & Tissue kit. The MagNA Pure System or MagNA Pure Compact System was used for the 17 previously extracted samples.
Development of multiplex 5′ nuclease (TaqMan) quantitative real-time PCR assays.
We initially selected amplification targets for P. falciparum, P. vivax, P. malariae, and P. ovale based on prior publications (7) and available gene sequences in GenBank. We verified specific 18S rRNA gene primers and probes only for P. falciparum (8); hence, we targeted the apical membrane antigen 1 (AMA1) gene in P. vivax and the plasmepsin genes in P. ovale (GenBank accession no. AF001209), P. malariae (GenBank accession no. AF001210), and P. knowlesi (GenBank accession no. XM_002260879.1). We used the human beta-actin (ACTB) gene as an internal amplification control. We optimized multiplex reactions using uniform conditions with AlleleID version 6.1 software (Premier Biosoft International, Palo Alto, CA).
The primers (forward and reverse) and probes targeting 18S rRNA (P. falciparum) (8), AMA1 (P. vivax), and plasmepsin (P. ovale, P. malariae, and P. knowlesi) are shown in Table 1. The forward and reverse primers for ACTB were GTGCTCAGGGCTTCTTGTCC and CCATGTCGTCCCAGTTGGT, respectively, and the probe was Texas Red- or hexachloro-6-carboxyfluorescein (HEX)-ACCCATGCCCACCATCACGCCC-black hole quencher 1 (BHQ1). Amplification using primers for P. ovale based on plasmepsin 4 and using SYBR green allowed detection of all 9 samples initially tested; however, only 4 could be detected using a probe based on this sequence. To clarify potential genetic heterogeneity, 1,000-bp sections of the P. ovale plasmepsin gene were amplified from these 4 blood samples by qPCR; these sections were cloned, sequenced, and aligned with reference sequences to identify conserved and variable regions and to guide query and design of new primers/probes using AlleleID.
Table 1.
Primers and probes for detection and identification of Plasmodium species
| Species | Primer or probe (amplicon length [bp])a | Sequenceb |
|---|---|---|
| P. falciparum | Primer, forward | CCACATCTAAGGAAGGCAGCAG |
| Primer, reverse | CCTCCAATTGTTACTCTGGGAAGG | |
| Probe (145) | Cy5-CCCACCATTCCAATTACAA-BHQ1 | |
| P. vivax | Primer, forward | ACGCCAAGTTCGGATTATGG |
| Primer, reverse | CCGTCATTTCTTCTTCATACTGAG | |
| Probe (157) | TET-TTGATCTGAGGCACTCGCTCCG-BHQ1 | |
| P. ovale | Primer, forward | TATCCTCGGAGCACCATTTATGAG |
| Primer, reverse | ACGGCAAAACCGACCCTCTC | |
| Probe (81) | FAM-ACTTCTCCGTTTTCG-BHQ1 | |
| P. malariae | Primer, forward | CCAACAATACATACACATTAGAACC |
| Primer, reverse | GTAGGATATAAAGCATACACAAAGTG | |
| Probe (145) | Texas Red-ATCTAGTAATGGCTCC-BHQ1 | |
| P. knowlesi | Primer, forward | TAACATGGTAATCATACATAAGG |
| Primer, reverse | TAAGGAAATGCCAACTCTTG | |
| Probe (117) | TAMRA-TCAGCCAACAACACTTACAG-BHQ1 |
PCR amplification targets were 18S (small-subunit) rRNA (P. falciparum) (8), AMA1 (P. vivax), plasmepsin 4 (P. ovale [GenBank accession no. AF001209] and P. malariae [GenBank accession no. AF001210]), and strain H chromosome 13 plasmepsin (P. knowlesi [GenBank accession no. XM_002260879.1]).
TET, tetrachloro-6-carboxyfluorescein; FAM, 6-carboxyfluorescein; TAMRA, 6-carboxytetramethylrhodamine.
Multiplex qPCR was first performed using a C1000 thermal cycler with a CFX384 real-time PCR (4-color) system (Bio-Rad, Hercules, CA) with 384-well plates. Initial evaluations of primers were conducted with reaction mixtures containing 1 μl DNA template, 200 nM each primer, and iQ SYBR green supermix (Bio-Rad). Reaction mixtures that contained probes were prepared with 1 μl blood DNA, 200 nM each primer and probe, and 2× iQ Multiplex Powermix (Bio-Rad), in a final total volume of 10 μl. Each qPCR run included a human blood DNA positive-control sample for each of the 4 major Plasmodium species (courtesy of David Sullivan), 2 to 4 human blood DNA negative-control samples, 2 to 4 control samples without template (water only), plasmid standards for quantitation of each Plasmodium sp. (diluted to provide 105, 103, 101, and 100 copies/μl DNA input), and validation or unknown samples in duplicate. Quantitative PCR assays for ACTB were run separately, since the CFX384 real-time PCR system is a 4-color system. Standard two-step qPCR was performed, with initial denaturation at 95°C for 3 min followed by 40 cycles of denaturation at 95°C for 10 s and annealing/extension at 55°C for 30 s. All samples were run in duplicate wells, and most assays were reproduced at least twice during the course of assay development and optimization.
To establish a consistent baseline and accurate cycle threshold (CT) and end relative fluorescence unit (RFU) values, we applied the baseline with curve fit algorithm to the data for each sample, using Bio-Rad CFX384 Manager software. Curves generated using this algorithm were manually inspected and corrected if the logarithmic features expected with DNA amplification were not observed or if any systematic technical errors were detected. Endpoint analysis was conducted with Bio-Rad CFX384 system software. Different tolerances, i.e., percentages of the difference between the maximum RFU value and the average RFU value for 2 to 4 negative-control samples for each fluor on the plate over the last 5 qPCR cycles, were calculated. Each tolerance value was added to the average negative-control RFU value over the last 5 cycles to establish a potential cutoff value above which a sample would be considered positive. Various cutoffs were examined, and sensitivity and specificity were maximized using receiver operating characteristic curves.
To extend the multiplex assay to include P. knowlesi, a probe and a primer pair were designed to recognize a specific portion of the P. knowlesi strain H chromosome 13 plasmepsin gene (GenBank accession no. XM_002260879.1) (Table 1). After initial testing in a singleplex assay, P. knowlesi identity was confirmed by melting curve analysis. The analytical sensitivity and linearity of P. knowlesi DNA standards were determined using a plasmid containing the region of the P. knowlesi plasmepsin gene targeted by the assay. The P. knowlesi assay was then integrated into the 4-plex assay for P. falciparum, P. ovale, P. vivax, and P. malariae, which required adaptation of the previously optimized 4-plex assay to a 5-plex assay using the Bio-Rad iCycler iQ5 multicolor real-time PCR detection system for 96-well plates. For the iQ5 system, each reaction mixture included 2.5 μl of DNA, 12.5 μl of iQ Multiplex Powermix (Bio-Rad), and primers and probes at final concentrations of 200 nM each. Runs included duplicates of template, plasmid standard, DNA positive- and negative-control, and no-template control samples. Standard two-step qPCR was again performed, and analysis was similarly accomplished using Bio-Rad iQ5 software.
Correlation of multiplex PCR and microscopy findings.
Multiplex qPCR testing was performed with masking (blinding) with respect to the microscopy (smear) results. As the primary analysis of the performance of our qPCR assay, we considered microscopy the gold standard. Samples for which qPCR results did not agree with smear results were considered discordant. Discordant samples were retested with at least one additional PCR method, i.e., our multiplex qPCR assay, an alternative target PCR assay used by the Mayo Clinic Reference Laboratory (9), and 2 singleplex assays for P. falciparum (targeting the 18S [1/4 targets in our multiplex assay] and cytochrome B [CYTB] [8] genes), and melting curve analysis (the CYTB target for P. falciparum melts at 76°C and P. vivax AMA1 at 82.5°C). As a secondary analysis, we considered samples with identical results with two different DNA targets as true-positive PCR results, since assigning a species is not always possible with microscopy. In a final analysis, we assessed the performance of clinical microscopy versus composite PCR testing for accurate identification of clinical malaria to the species level.
Quantitation of parasitemia.
Comparisons of quantitation of parasitemia by microscopy versus qPCR testing were performed for samples for which parasitemia had been determined by microscopy. Microscopic parasitemia values of <1% and <0.5% were considered 0.5% and 0.25%, respectively, in tests of correlations between microscopic and molecular parasitemia results.
RESULTS
Analytical sensitivity and specificity.
The 4-plex assay detected 105 to 100 copies of Plasmodium spp.; P. falciparum contains four to six 18S rRNA gene copies per parasite, and each of the other species contains one gene (AMA1 or the specific plasmepsin gene target) copy or more per parasite. Detection was linear (R2 ≥ 0.99) and efficient (>85% to <115%). The assay did not detect the targets in blood spiked with Trypanosoma brucei rhodesiense. The analytical limit of sensitivity for each Plasmodium species component of the 5-plex assay was 10 to 100 copies/μl, similar to that of the 4-plex qPCR, and detection was linear from 101 to 105 copies.
Clinical sensitivity.
Among blood samples from patients with microscopically identified P. falciparum malaria that were tested with the 4-plex assay, 95.8% (69/72 samples) were qPCR positive for P. falciparum (discordant qPCR results: P. malariae, 1 sample; negative, 2 samples). One discordant sample was confirmed as P. malariae on retesting and alternative target PCR testing (results of singleplex assays and melting curve analyses for P. falciparum also were negative). Of the two initially negative samples by multiplex qPCR, one was confirmed as P. falciparum by multiplex qPCR retesting, alternative target PCR testing, and our singleplex P. falciparum assays. The other remained negative for P. falciparum on repeat multiplex qPCR testing but was confirmed as P. falciparum by alternative target PCR assays and our singleplex P. falciparum assays.
Among blood samples from patients with microscopically identified P. vivax malaria that were tested with the 4-plex assay, 89.5% (34/38 samples) were qPCR positive for P. vivax (discordant qPCR results: P. ovale, 2 samples; P. falciparum, 1 sample; negative, 1 sample). Of the two samples that were initially identified as P. ovale by multiplex qPCR, one was confirmed as P. ovale by both multiplex qPCR retesting and alternative target PCR testing and was negative for P. falciparum by singleplex PCR assays. The other sample was confirmed as P. ovale by multiplex qPCR retesting, could not be identified to the species level by alternative target PCR testing, and was P. falciparum negative by singleplex assays. The sample initially identified as P. falciparum by multiplex qPCR testing was confirmed to be P. vivax by multiplex qPCR retesting, by alternative target PCR testing, and by singleplex PCR testing with melting curve analysis. The sample that was initially negative by multiplex qPCR testing remained negative by multiplex qPCR retesting and by alternative target PCR testing.
Of blood samples from 17 patients with P. ovale malaria by microscopy, 94.1% (16/17 samples) were positive for P. ovale by the 4-plex qPCR assay (discordant result: negative, 1 sample). This sample was confirmed as P. ovale by alternative target PCR testing. Of blood samples from 9 patients with P. malariae malaria by microscopy, 100% (9/9 samples) were positive for P. malariae by the 4-plex qPCR assay.
All samples that were tested in duplicate amplified human ACTB. If the analyses were restricted to 1 sample per patient, then the sensitivity of multiplex qPCR for microscopic P. falciparum was 94.0% (47/50 samples) and that for P. vivax was 91.7% (22/24 samples) (P. ovale and P. malariae values were unchanged, since only 1 sample was available for each patient). The clinical sensitivity of the 5-plex assay for both P. falciparum and P. vivax microscopy-confirmed malaria was 100% (7/7 samples), that for P. ovale was 100% (5/5 samples), that for P. malariae was 100% (4/4 samples), and that for P. knowlesi was 100% (10/10 samples).
Clinical specificity.
The clinical specificities of the 4-plex qPCR assay for detection of P. falciparum, P. vivax, P. ovale, and P. malariae in blood samples from patients with microscopy-confirmed malaria were 98.6% (70/71 samples), 99.0% (104/105 samples), 98.4% (124/126 samples), and 99.3% (133/134 samples), respectively. One P. vivax-smear-positive sample was initially detected as P. falciparum by our multiplex assay; repeat testing with the multiplex assay, alternative-target PCR testing, singleplex assays, and melting curve analyses all indicated P. vivax (i.e., the multiplex qPCR P. falciparum result was a false-positive finding). One P. falciparum-smear-positive sample that was initially positive for P. falciparum and P. vivax by our multiplex qPCR assay was positive only for P. falciparum with repeat testing with the multiplex assay, alternative target PCR testing, singleplex assays, and melting curve analyses (i.e., the multiplex P. vivax result was a false-positive finding). The two P. vivax-smear-positive samples identified as P. ovale by the multiplex assay were confirmed on retesting; further, although one sample could not be identified to the species level by alternative target PCR testing, the other was identified as P. ovale (both likely true P. ovale cases). Finally, the P. falciparum-smear-positive sample identified as P. malariae by the multiplex qPCR assay was confirmed as P. malariae on retesting and by alternative target PCR testing (likely a true P. malariae case).
No Plasmodium DNA was detected in blood samples from patients with no known bloodstream infection or patients with confirmed babesiosis (clinical specificity of 100% for patients without malaria). The persistence of P. falciparum DNA was detected in one smear-negative blood sample from a patient who had been smear positive for P. falciparum 48 h earlier. The clinical specificity of the 5-plex assay for both P. falciparum and P. vivax microscopy-confirmed malaria was 100% (30/30 samples), that for P. ovale was 100% (32/32 samples), that for P. malariae was 100% (33/33 samples), and that for P. knowlesi was 100% (27/27 samples).
Performance of multiplex qPCR versus microscopy for the diagnosis of microscopy-confirmed clinical malaria.
Table 2 summarizes the performance data for our multiplex qPCR assay, relative to microscopy, for the diagnosis of malaria. In primary data analysis, the sensitivities of the 4-plex qPCR assay for microscopically identified P. falciparum, P. vivax, P. ovale, and P. malariae malaria were 95.8% for P. falciparum, 89.5% for P. vivax, 94.1% for P. ovale, and 100% for P. malariae, and the specificities were 98.6% for P. falciparum, 99.0% for P. vivax, 98.4% for P. ovale, and 99.3% for P. malariae. Using the 5-plex assay, the sensitivity and specificity for P. knowlesi were both 100%.
Table 2.
Initial performance of multiplex PCR versus microscopy for detection of microscopy confirmed clinical malariaa
| Microscopy result (n) | PCR performance (% [95% CI]) |
|||
|---|---|---|---|---|
| Sensitivity | Specificity | Positive predictive value | Negative predictive value | |
| P. falciparum (72) | 95.8 (88.3–99.1) | 98.6 (92.4–100) | 98.6 (92.3–100) | 95.9 (88.5–99.1) |
| P. vivax (38) | 89.5 (75.2–97.1) | 99 (84.8–100) | 97.1 (85.1–99.9) | 96.3 (90.8–99) |
| P. ovale (17) | 94.1 (71.3–99.9) | 98.4 (94.4–99.8) | 88.9 (65.3–98.6) | 99.2 (95.6–100) |
| P. malariae (9) | 100 (66.4–100) | 99.3 (95.9–100) | 90 (55.5–99.7) | 100 (97.3–100) |
| P. knowlesi (10) | 100 (69.2–100) | 100 (87.2–100) | 100 (69.2–100) | 100 (87.2–100) |
Results for species other than P. knowlesi were from testing of 143 samples with a 4-plex 384-well assay. Results for P. knowlesi were based on testing of a 37-sample subset with a 5-plex 96-well assay. CI, confidence interval.
Performance of multiplex qPCR versus microscopy after resolution of qPCR-microscopy discrepancies.
In secondary data analysis (Table 3), the sensitivities of the 4-plex qPCR assay for microscopically identified P. falciparum, P. vivax, P. ovale, and P. malariae malaria were at least 97.2% (69/71 samples) for P. falciparum, 97.1% (34/35 samples) for P. vivax, 94.7% (18/19 samples) for P. ovale, and 100% (10/10 samples) for P. malariae, and the specificities were 98.6% (71/72 samples) for P. falciparum, 99.1% (107/108 samples) for P. vivax, 100% (124/124 samples) for P. ovale, and 100% (133/133 samples) for P. malariae.
Table 3.
Final performance of multiplex PCR versus microscopy for detection of microscopy-confirmed clinical malaria after resolution of discrepanciesa
| Microscopy result (n) | PCR performance (% [95% CI]) |
|||
|---|---|---|---|---|
| Sensitivity | Specificity | Positive predictive value | Negative predictive value | |
| P. falciparum (71) | 97.2 (90.2–99.7) | 98.6 (92.5–100) | 98.6 (92.3–100) | 97.3 (90.5–99.7) |
| P. vivax (35) | 97.1 (85.1–99.9) | 99.1 (94.9–100) | 97.1 (85.1–99.9) | 99.1 (94.9–100) |
| P. ovale (19) | 94.7 (74.0–99.9) | 100 (97.1–100) | 100 (81.5–100) | 99.2 (95.6–100) |
| P. malariae (10) | 100 (69.2–100) | 100 (97.3–100) | 100 (69.2–100) | 100 (97.3–100) |
| P. knowlesi (10) | 100 (69.2–100) | 100 (87.2–100) | 100 (69.2–100) | 100 (87.2–100) |
Results for species other than P. knowlesi were from testing of 143 samples with a 4-plex 384-well assay. Results for P. knowlesi were based on testing of a 37-sample subset with a 5-plex 96-well assay.
Additionally, 91.7% of samples (11/12 samples) of uncertain species by microscopy were definitively identified to the species level by multiplex qPCR, including 9 P. falciparum samples (confirmed by multiplex qPCR testing twice, alternative target PCR testing, and our singleplex assays), 1 P. ovale sample, and 1 P. vivax sample. The remaining sample of uncertain species might have been a coinfection. It was initially qPCR positive for P. vivax and P. ovale; repeat multiplex qPCR testing, singleplex assays, and melting curve analyses suggested P. falciparum/P. vivax coinfection, but alternative target PCR testing showed only P. vivax.
Performance of clinical microscopy versus composite PCR (≥2 assays) for identifying microscopy-confirmed clinical malaria to the species level.
Finally, with the benchmark standard of PCR confirmed by ≥2 assays, the sensitivity of microscopy for identifying P. falciparum to the species level would be at best 88.8% (71/80 samples), since 9 P. falciparum infections were reported as indeterminate species and 1 was reported as P. malariae (Table 4). Similarly, the clinical sensitivities for identifying P. vivax and P. ovale to the species level would be ≤97.2% (35/36 samples) and ≤85.0% (17/20 samples), respectively, since 1 P. vivax sample and 1 P. ovale sample were reported as indeterminate species and 2 P. ovale samples were erroneously reported as P. vivax. Finally, the sensitivity of microscopy for identifying P. malariae to the species level would be ≤90.0% (9/10 samples), since 1 P. malariae sample was reported as P. falciparum. The specificities of microscopy for accurately identifying P. falciparum, P. vivax, P. ovale, and P. malariae to the species level (95.9% [71/74 samples], 89.0% [105/118 samples], 92.5% [124/134 samples], and 92.4% [133/144 samples], respectively) were similarly worse than the specificities of our multiplex assay, particularly for P. vivax.
Table 4.
Performance of clinical microscopy versus composite (≥1 target and retesting) PCR for species-level identification of clinical malariaa
| PCR result (n) | Microscopy performance (% [95% CI]) |
|||
|---|---|---|---|---|
| Sensitivity | Specificity | Positive predictive value | Negative predictive value | |
| P. falciparum (80) | 88.8 (79.7–94.7) | 95.9 (88.6–99.2) | 95.9 (88.6–99.2) | 88.8 (79.7–94.7) |
| P. vivax (37) | 97.3 (85.8–99.9) | 89.7 (82.8–94.6) | 75.0 (60.4–86.4) | 99.1 (94.9–100) |
| P. ovale (20) | 85.0 (62.1–96.8) | 92.5 (86.7–96.4) | 63.0 (42.4–80.6) | 97.6 (93.3–99.5) |
| P. malariae (10) | 90.0 (55.5–99.7) | 92.4 (86.7–96.1) | 45.0 (23.1–68.5) | 99.3 (95.9–100) |
| P. knowlesi (10) | 100 (69.2–100) | 100 (87.2–100) | 100 (69.2–100) | 100 (87.2–100) |
Results for species other than P. knowlesi were from testing of 143 samples with a 4-plex 384-well assay. Results for P. knowlesi were based on testing of a 37-sample subset with a 5-plex 96-well assay.
Quantitation of parasitemia.
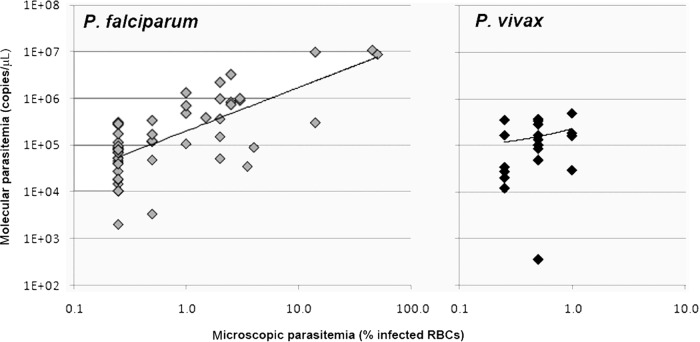
Quantitation of parasitemia by microscopy and qPCR was performed for 52 confirmed P. falciparum samples and 19 confirmed P. vivax samples. The median microscopic parasitemia value (and interquartile range [IQR]) for P. falciparum was 0.5% (IQR, 0.25% to 2.0%), and that for P. vivax was 0.5% (0.25% to 0.5%). The median molecular parasitemia value for P. falciparum was 1.2 × 105 copies/μl (IQR, 5.1 × 104 to 4.1 × 105 copies/μl), and that for P. vivax was 1.3 × 105 copies/μl (IQR, 3.2 × 104 to 2.4 × 105 copies/μl). In contrast to the median and IQR values, the minimum and maximum values differed for both microscopic (P. falciparum, 0.5% to 50%; P. vivax, 0.25% to 1%) and molecular (P. falciparum, 2.0 × 103 to 1.1 × 107 copies/μl; P. vivax, 3.5 × 102 to 4.9 × 105 copies/μl) parasitemia findings. The correlation between microscopic and molecular parasitemia findings (Fig. 1) was statistically significant for P. falciparum (Spearman correlation, P < 0.001) but not for P. vivax (P = 0.155).
Fig 1.
Clinical microscopic (lower limit, 0.5% or 1%) versus molecular parasitemia for P. falciparum (Spearman rank correlation, P < 0.0001) and P. vivax (Spearman rank correlation, P = 0.155). RBCs, red blood cells.
DISCUSSION
The gold standard for the diagnosis of clinical malaria has been microscopy. However, microscopy is subjective and laborious, requires expertise gained by rigorous training and experience, and cannot effectively support large studies. RDTs based on immunochromatographic antigen-detection systems have been implemented in some diagnostic laboratories as a complement to microscopy. Although they are rapid, simple, and easy to interpret, RDTs target proteins specific to P. falciparum or P. vivax or those common to all Plasmodium species (2) and cannot specifically identify P. malariae, P. ovale, and P. knowlesi (2, 10). Further, commercially available RDTs vary widely in diagnostic sensitivity (e.g., lacking sensitivity for some strains of P. falciparum and up to 50% of P. knowlesi) (11) and specificity (e.g., P. vivax in patients with coinfections and high P. falciparum parasitemia levels). Lastly, RDTs cannot be used to monitor therapy, because they are qualitative (2).
Molecular assays offer an attractive alternative approach. Conventional PCR assays are time-consuming, and nested conventional PCR assays enable identification to the species level but with a high risk of cross-contamination. A few real-time PCR assays have been described (10). However, most target one (most commonly P. falciparum) or two of the four major Plasmodium species, with similarly limited evaluation of specimens from patients with clinical malaria. For example, several assays only distinguish P. falciparum from other Plasmodium species (12, 13). One assay cannot detect P. malariae (14) and additionally could miss variants of P. ovale (15, 16). Clinical specificity is not ensured, since blood samples from patients with P. ovale and P. malariae are difficult to obtain and infrequently tested. Recently, a multiplex PCR system targeting 18S small-subunit rRNA genes and capable of detecting all 5 known species of malaria in humans was reported, using nested PCR as the molecular gold standard (17). However, these results have yet to be corroborated outside Malaysia; further, the group tested only 2 clinical samples from patients with P. malariae and 1 from a patient with P. ovale malaria.
We describe multiplex quantitative real-time PCR assays for malaria that are sensitive and specific for detection of the 4 major Plasmodium species as well as the recently recognized species P. knowlesi. Detection and definitive species-level identification of P. falciparum and P. knowlesi are important, since both species are likely to be clinically urgent (18) and misdiagnosis of P. knowlesi as P. malariae has been associated with excess mortality due to delayed treatment with intravenous artesunate therapy (19). Furthermore, relapses have occurred from failure to administer antihypnozoite treatment to patients with P. vivax parasites that were microscopically misidentified as P. knowlesi (20). Multiplex quantitative real-time PCR assays can be conducted rapidly (<4.5 h from DNA preparation to PCR amplification), and the 384-well (4 species) and 96-well (5 species) formats both enable high throughput, as required for large clinical and epidemiologic studies. The assays are quantitative over a wide range of parasitemia levels and can detect as few as 1 to 6 parasites/μl of blood, well below the limit of detection of microscopy.
Since increased blood volume improves sensitivity and automated extraction supports high throughput, we extracted DNA from a large volume (usually 1 ml) of blood with an automated platform. Further, we chose multiplex real-time qPCR, since this automated, quantitative, closed-system format supports high throughput and decreases contamination (increases specificity). We also obtained and tested a large number of blood samples from patients with clinically suspected and clinical microbiology laboratory-confirmed microscopic malaria, and we enriched our specimen repository with additional samples from patients with rare P. ovale, P. malariae, and P. knowlesi infections. We compared qPCR to the existing standard of microscopy as our primary analysis but we also completed extensive additional testing to adjudicate discordant qPCR and microscopy results, since microscopy can be insensitive, relative to other methods (3), and identification to the species level is not always possible.
Resolution of discordant results and testing by qPCR of specimens from patients with malaria caused by indeterminate Plasmodium species (secondary analysis) showed improved sensitivity and specificity for all 4 Plasmodium targets in the 4-plex qPCR assay versus microscopy. However, this is likely a significant underestimate, since we did not test samples from patients who had epidemiologically and clinically suspected malaria but had negative blood smears, due to effective prophylaxis or treatment. Of paramount clinical importance, both the sensitivity and specificity of detecting P. falciparum were higher with our qPCR assay (97.2% and 98.6%, respectively) than with microscopy (88.8% and 95.9%, respectively). The multiplex qPCR assay was able to identify additional infections with P. falciparum that had not been identified to the species level by microscopy, which could improve clinical care by identifying infections more likely to be severe. We also found that infections with less-common species of malaria may be reported as more-common, morphologically similar species when there is doubt, such as reporting of P. ovale as P. vivax or P. malariae as P. falciparum. Although epidemiologic histories could suggest the species (e.g., P. ovale is much more likely than P. vivax in a patient from West Africa) (21), clinical and reference laboratories rarely are provided such important information about pretest probability. Furthermore, epidemiologic histories also may be misleading, since recent work has shown that P. vivax merozoites do not absolutely require Duffy surface antigens to invade erythrocytes (22) and clinical P. vivax malaria can occur in some Duffy-negative individuals (23). These findings highlight the importance of developing and applying species-specific molecular assays to support rigorous scientific enquiry in addition to clinical care.
Improved sensitivity and specificity of multiplex qPCR versus microscopy for the detection of clinical and subclinical malaria are plausible. In a study in which nested PCR was considered the gold standard, multiplex qPCR was comparable to microscopy for 30 symptomatic patients (24); additionally, 2 P. ovale infections that were microscopically misdiagnosed as P. vivax were correctly identified by multiplex qPCR. Although assessment of the clinical sensitivity and specificity for P. malariae was not possible (no patient had P. malariae infection), the assay is now used routinely for identification of malaria to the species level in Alberta, Canada (24). In addition, a multiplex assay based on similar primers (7) was found to aid proper microscopic identification in a study of 89 patients with predominantly P. falciparum malaria (only 6 P. vivax cases, 3 P. ovale cases, and 1 P. malariae case) evaluated by a large, university-based, clinical microbiology laboratory in Switzerland (25). In that study, the multiplex qPCR assay correctly identified to the species level P. falciparum initially identified as P. malariae and P. ovale initially classified as P. vivax and detected mixed infections with low levels of parasitemia.
Because our study population included predominantly symptomatic returning travelers (individuals lacking preexisting protective immunity) presenting for routine clinical care, microscopic parasitemia was reported most often for P. falciparum malaria and levels were relatively low. Further, quantitation of microscopic parasitemia was performed in accordance with clinical practice (no quantitation for findings of <0.5% or <1%). Consequently, data that would allow correlation of microscopic and molecular parasitemia were limited. Nevertheless, since the average normal erythrocyte count for adults is >4.0 × 106 cells/μl of blood and there are 6 copies of 18S rRNA genes per P. falciparum cell, a median molecular parasitemia value of 1.2 × 105 copies/μl of blood would be expected for a median microscopic parasitemia value of <0.5%, consistent with what we observed. It also is not surprising that we detected a statistically significant correlation between microscopy and qPCR results for P. falciparum but not for P. vivax, since the latter had a smaller sample size and highly restricted range of parasitemia levels (many cases at the lowest levels reported by clinical microbiology laboratories). Since the lowest microscopy category includes parasitemia levels that likely vary >1 order of magnitude, it is not surprising that molecular parasitemia levels were highly variable in this group. Finally, since all samples were obtained from patients with clinical malaria that had been diagnosed by microscopy, we could not challenge the lower limit of detection of our assay for the diagnosis of subclinical malaria or submicroscopic parasitemia.
Precise correlation of molecular and microscopic parasitemia findings could be assessed optimally in a large cohort of patients monitored longitudinally for symptomatic and asymptomatic parasitemia, with documentation of treatment and with 1 ml of blood being obtained at each visit. Although less clinically relevant, microscopic and molecular parasitemia levels ideally also would be correlated for other Plasmodium species. We have prospectively evaluated the described 18S rRNA gene P. falciparum target in a clinical trial of chemoprophylaxis for the prevention of infection with P. falciparum (26) and found it equivalent to microscopy (minimum of 200 oil-immersion fields per thick blood film reviewed). Our 18S rRNA gene P. falciparum target also was assessed in a >2,000-person cohort followed for 6 months in a hypoendemic area of Tanzania (8). Using latent-class analysis, we found it to be more sensitive than microscopy, RDTs, and a CYTB target for the detection of symptomatic and asymptomatic P. falciparum malaria, despite extraction of blood DNA from small volumes (∼25 μl of blood on filter paper) rather than the large volumes (∼1 ml) reported here.
In summary, we describe the development of multiplex qPCR assays to detect and to differentiate the four major species of malaria and emerging P. knowlesi and subsequent evaluation with a large panel of blood samples from symptomatic patients with clinical microbiology laboratory-confirmed microscopic malaria. The assays described have many potential applications, including support of large epidemiologic studies and trials of new vaccines or therapies, sensitive and specific detection of malaria with low parasitemia levels (such as clinical malaria in returning travelers and subclinical malaria in refugees) (27), and accurate identification to the species level of malaria diagnosed by another method (such as microscopy or RDT). Adapting our assays to other platforms, such as loop-mediated isothermal amplification, could make them cost-effective, point-of-care tests for use in poor regions in which malaria is endemic (28).
ACKNOWLEDGMENTS
We thank the staff of the Johns Hopkins University clinical microbiology laboratory, Peggy Althaus at the Duke University clinical microbiology laboratory, David Sullivan at the Johns Hopkins Bloomberg School of Public Health, and Yee Ling Lau and Mun Yik Fong at the University of Malaya, both supported by University of Malaya High Impact Research grant UM-MOHE (UM.C/625/1/HIR/MOHE/CHAN/14/3), for provision of blood from patients with microscopy-confirmed malaria. Additionally, we thank Lynne Sloan and Bobbi Pritt at the Mayo Clinic for provision of DNA extracted from patients with microscopy-confirmed malaria and for confirmatory testing of samples with discordant microscopy and qPCR results by alternative target PCR. We thank Emily Clemens for preliminary work on development of the assay.
This study was supported by the National Institute of Allergy and Infectious Diseases (NIAID) (grant K23-AIO83931 to M.E.R.) and the HIV Prevention Trials Network (HPTN) sponsored by the NIAID, National Institute of Child Health and Human Development, National Institute on Drug Abuse, National Institute of Mental Health, and Office of AIDS Research (grants U01-AI46745, U01-AI48054, and UM1-AI068613).
Footnotes
Published ahead of print 26 June 2013
REFERENCES
- 1.WHO Global Malaria Programme 2011. World malaria report 2011, vol 42 World Health Organization, Geneva, Switzerland [Google Scholar]
- 2.Wilson ML. 2012. Malaria rapid diagnostic tests. Clin. Infect. Dis. 54:1637–1641 [DOI] [PubMed] [Google Scholar]
- 3.Goncalves L, Subtil A, de Oliveira MR, Vdo Rosario Lee PW, Shaio MF. 2012. Bayesian latent class models in malaria diagnosis. PLoS One 7:e40633. 10.1371/journal.pone.0040633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilles H. 1993. Diagnostic methods in malaria. Edward Arnold, London, United Kingdom [Google Scholar]
- 5.Singh B, Kim Sung L, Matusop A, Radhakrishnan A, Shamsul SS, Cox-Singh J, Thomas A, Conway DJ. 2004. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet 363:1017–1024 [DOI] [PubMed] [Google Scholar]
- 6.Singh B, Daneshvar C. 2013. Human infections and detection of Plasmodium knowlesi. Clin. Microbiol. Rev. 26:165–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rougemont M, Van Saanen M, Sahli R, Hinrikson HP, Bille J, Jaton K. 2004. Detection of four Plasmodium species in blood from humans by 18S rRNA gene subunit-based and species-specific real-time PCR assays. J. Clin. Microbiol. 42:5636–5643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schachterle SE, Mtove G, Levens JP, Clemens EG, Shi L, Raj A, Munoz B, Reller ME, West S, Dumler JS, Sullivan D. 2011. Prevalence and density-related concordance of three diagnostic tests for malaria in a region of Tanzania with hypoendemic malaria. J. Clin. Microbiol. 49:3885–3891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swan H, Sloan L, Muyombwe A, Chavalitshewinkoon-Petmitr P, Krudsood S, Leowattana W, Wilairatana P, Looareesuwan S, Rosenblatt J. 2005. Evaluation of a real-time polymerase chain reaction assay for the diagnosis of malaria in patients from Thailand. Am. J. Trop. Med. Hyg. 73:850–854 [PubMed] [Google Scholar]
- 10.Erdman LK, Kain KC. 2008. Molecular diagnostic and surveillance tools for global malaria control. Travel Med. Infect. Dis. 6:82–99 [DOI] [PubMed] [Google Scholar]
- 11.Barber BE, William T, Grigg MJ, Piera K, Yeo TW, Anstey NM. 2013. Evaluation of the sensitivity of a pLDH-based and an aldolase-based rapid diagnostic test for diagnosis of uncomplicated and severe malaria caused by PCR-confirmed Plasmodium knowlesi, Plasmodium falciparum, and Plasmodium vivax. J. Clin. Microbiol. 51:1118–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elsayed S, Plewes K, Church D, Chow B, Zhang K. 2006. Use of molecular beacon probes for real-time PCR detection of Plasmodium falciparum and other Plasmodium species in peripheral blood specimens. J. Clin. Microbiol. 44:622–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farcas GA, Zhong KJ, Mazzulli T, Kain KC. 2004. Evaluation of the RealArt Malaria LC real-time PCR assay for malaria diagnosis. J. Clin. Microbiol. 42:636–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perandin F, Manca N, Calderaro A, Piccolo G, Galati L, Ricci L, Medici MC, Arcangeletti MC, Snounou G, Dettori G, Chezzi C. 2004. Development of a real-time PCR assay for detection of Plasmodium falciparum, Plasmodium vivax, and Plasmodium ovale for routine clinical diagnosis. J. Clin. Microbiol. 42:1214–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bialasiewicz S, Whiley DM, Nissen MD, Sloots TP. 2007. Impact of competitive inhibition and sequence variation upon the sensitivity of malaria PCR. J. Clin. Microbiol. 45:1621–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calderaro A, Piccolo G, Perandin F, Gorrini C, Peruzzi S, Zuelli C, Ricci L, Manca N, Dettori G, Chezzi C, Snounou G. 2007. Genetic polymorphisms influence Plasmodium ovale PCR detection accuracy. J. Clin. Microbiol. 45:1624–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chew CH, Lim YA, Lee PC, Mahmud R, Chua KH. 2012. Hexaplex PCR detection system for identification of five human Plasmodium species with an internal control. J. Clin. Microbiol. 50:4012–4019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.William T, Menon J, Rajahram G, Chan L, Ma G, Donaldson S, Khoo S, Frederick C, Jelip J, Anstey NM, Yeo TW. 2011. Severe Plasmodium knowlesi malaria in a tertiary care hospital, Sabah, Malaysia. Emerg. Infect. Dis. 17:1248–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajahram GS, Barber BE, William T, Menon J, Anstey NM, Yeo TW. 2012. Deaths due to Plasmodium knowlesi malaria in Sabah, Malaysia: association with reporting as Plasmodium malariae and delayed parenteral artesunate. Malar. J. 11:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barber BE, William T, Grigg MJ, Yeo TW, Anstey NM. 2013. Limitations of microscopy to differentiate Plasmodium species in a region co-endemic for Plasmodium falciparum, Plasmodium vivax and Plasmodium knowlesi. Malar. J. 12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller LH, Mason SJ, Clyde DF, McGinniss MH. 1976. The resistance factor to Plasmodium vivax in blacks: the Duffy-blood-group genotype, FyFy. N. Engl. J. Med. 295:302–304 [DOI] [PubMed] [Google Scholar]
- 22.Mendes C, Dias F, Figueiredo J, Mora VG, Cano J, de Sousa B, do Rosario VE, Benito A, Berzosa P, Arez AP. 2011. Duffy negative antigen is no longer a barrier to Plasmodium vivax: molecular evidences from the African West Coast (Angola and Equatorial Guinea). PLoS Negl. Trop. Dis. 5:e1192. 10.1371/journal.pntd.0001192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woldearegai TG, Kremsner PG, Kun JF, Mordmuller B. 2013. Plasmodium vivax malaria in Duffy-negative individuals from Ethiopia. Trans. R. Soc. Trop. Med. Hyg. 107:328–331 [DOI] [PubMed] [Google Scholar]
- 24.Shokoples SE, Ndao M, Kowalewska-Grochowska K, Yanow SK. 2009. Multiplexed real-time PCR assay for discrimination of Plasmodium species with improved sensitivity for mixed infections. J. Clin. Microbiol. 47:975–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dormond L, Jaton-Ogay K, de Valliere S, Genton B, Bille J, Greub G. 2011. Multiplex real-time PCR for the diagnosis of malaria: correlation with microscopy. Clin. Microbiol. Infect. 17:469–475 [DOI] [PubMed] [Google Scholar]
- 26.Deye GA, Miller RS, Miller L, Salas CJ, Tosh D, Macareo L, Smith BL, Fracisco S, Clemens EG, Murphy J, Sousa JC, Dumler JS, Magill AJ. 2012. Prolonged protection provided by a single dose of atovaquone-proguanil for the chemoprophylaxis of Plasmodium falciparum malaria in a human challenge model. Clin. Infect. Dis. 54:232–239 [DOI] [PubMed] [Google Scholar]
- 27.Stauffer WM, Weinberg M, Newman RD, Causer LM, Hamel MJ, Slutsker L, Cetron MS. 2008. Pre-departure and post-arrival management of P. falciparum malaria in refugees relocating from sub-Saharan Africa to the United States. Am. J. Trop. Med. Hyg. 79:141–146 [PubMed] [Google Scholar]
- 28.Lee PW, Ji DD, Liu CT, Rampao HS, do Rosario VE, Lin IF, Shaio MF. 2012. Application of loop-mediated isothermal amplification for malaria diagnosis during a follow-up study in Sao Tome. Malar. J. 11:408. [DOI] [PMC free article] [PubMed] [Google Scholar]