Abstract
The sensitivity of microscopy for the diagnosis of tuberculosis (TB) is around 50% but decreases by about 15% in patients with suspected TB who are coinfected with HIV. Here, we compared the accuracies of three microscopy methods for processing sputum smears (concentration by centrifugation with or without N-acetyl-l-cysteine [NALC] and concentration by filtration on a polycarbonate membrane) to that of culture on Ogawa-Kudoh medium as the gold standard method. Sputum samples were obtained from 432 patients with suspected pulmonary TB, of whom 60% were infected with HIV. Analysis was performed using the first specimen. Compared to the gold standard culture, the small-membrane-filter (SMF) method was the most sensitive microscopic method. In HIV-infected TB patients, the sensitivity of the SMF method was significantly higher than those for centrifugation of sputum samples with or without NALC treatment (61.9%, 47.6%, and 45.2%, respectively; P = 0.001). Similarly, in TB patients without HIV infection, the sensitivity of the SMF method was significantly higher than those for centrifugation of sputum samples with or without NALC treatment (81.8%, 63.6%, and 57.5%, respectively; P = 0.001). In the two study groups, TB patients with or without HIV, no significant differences between the specificities of the three methods were observed. Handling of the second sputum sample similarly by centrifugation with or without NALC and by the SMF method increased positivities by 13%, 11%, and 4%, respectively. The overall agreement between microscopy and culture was above 90% for all groups. Microscopic evaluation of the sputum samples treated with NALC compared to those not treated with NALC did not show any increase in sensitivity. Altogether, the sensitivity of the SMF method is higher than those of the other two microscopic methods studied without a loss of specificity.
INTRODUCTION
Diagnosing tuberculosis (TB) in HIV-infected patients still presents a major challenge since clinical and radiological findings are often atypical (1, 2), and many cases of smear-negative pulmonary TB are undiagnosed (3). Sputum smear microscopy, a low-cost method, is important in resource-limited scenarios, where cultures are not available, but it provides a lower sensitivity in HIV-infected patients with suspected TB (4–6).
Filtration on a polycarbonate membrane, the small-membrane-filter (SMF) method, was proved to have greater sensitivity and specificity than direct microscopy, even after centrifugation of sputum samples (7, 8), but the HIV status of the populations was not reported in those studies.
Under the routine conditions of a bacteriology laboratory, we evaluated this method in patients with suspected TB, most of whom were infected with HIV, along with bacilloscopy after centrifugation with or without decontamination. The three methods were compared to culture on solid medium (Ogawa-Kudoh [OK]), considered the gold standard for TB diagnosis in our setting, and their sensitivities, specificities, and Cohen's kappa coefficients were calculated.
MATERIALS AND METHODS
Design and study setting.
A prospective study was conducted between September 2011 and January 2012 in Manaus, Amazonas, Brazil; the patients were recruited from Fundação de Medicina Tropical Doutor Heitor Vieira Dourado (FMT-HVD) and Policlínica Cardoso Fontes, the regional reference center for TB.
Enrollment.
Patients suspected of having pulmonary TB were eligible and were enrolled consecutively as they attended the clinics. The collection of clinical and demographic data was conducted prospectively using a standardized questionnaire. A pulmonary tuberculosis case was defined as a patient with positive sputum microscopy and/or a positive culture or a patient with suggestive clinical and radiological findings treated as TB.
Sample collection.
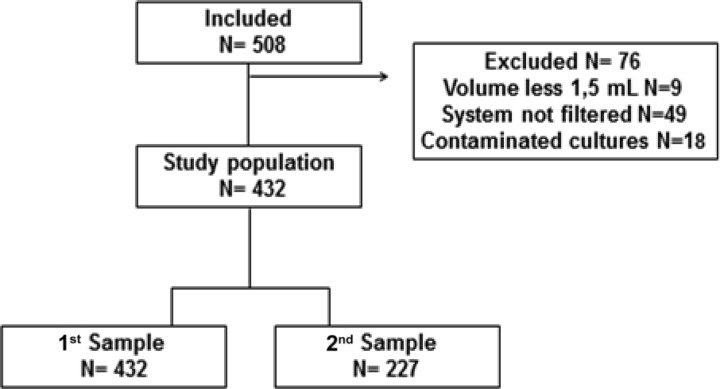
Two sputum samples on 2 consecutive days were requested from each patient, but patients were included even if they had only one sputum sample (Fig. 1). A total of 508 patients provided the first sample, but the second was provided by only 227 patients. Sputum samples with a volume of ≥1.5 ml were included in the study. In the case of HIV-positive subjects, blood samples were drawn for CD4 T-cell counts and HIV plasma viral load tests via the routine laboratory service of the FMT-HVD. Sample size calculations were based on an estimated prevalence of 13.1%, a sensitivity of 89.0%, and a specificity of 99.6%. The precision of the estimates was based on 95% confidence intervals (CIs) and a beta value of 20%. For a power of 80%, we needed 451 samples. These expectations were based on a previous small-membrane-filter study (7).
Fig 1.
The study population and samples.
Centrifugation of sputum smears.
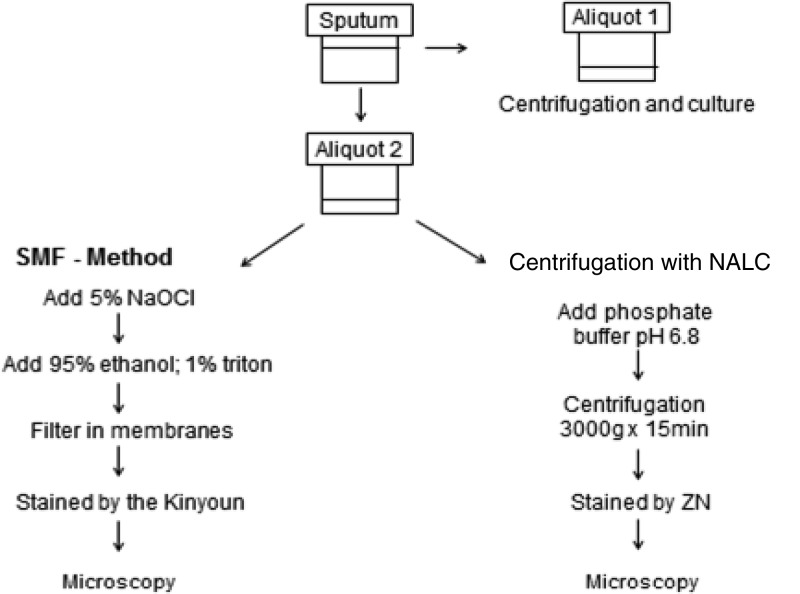
The smears were prepared using a 500-μl aliquot after centrifugation at 3,000 × g at 4°C for 15 min and were stained by the Ziehl-Neelsen (ZN) method (Fig. 2).
Fig 2.
Processing of sputum specimens.
Centrifugation of sputum smears with NALC.
The second aliquot was digested with N-acetyl-l-cysteine (NALC) using a solution of 5% NaOH–2.9% sodium citrate in a volume corresponding to 10% of the sputum sample, and it was left to rest for 15 min at 20°C. Later, the sample was divided into two digested aliquots (9). In the first digested aliquot, phosphate buffer (pH 6.8) was added up to 50 ml, and after centrifugation at 3,000 × g, the solution was left to rest for 15 min at 4°C. The smear was then stained by the ZN method.
Characteristics of the filtration system.
The system described by Fennelly et al. (7) was used with the following minor changes: (i) 10 vacuum system stainless steel filtration points controlled by manual shutoff valves; (ii) a prefilter nylon membrane of 25 mm with a pore size of 30 μm; (iii) a filter with a white polycarbonate membrane of 13 mm with a pore size of 0.8 μm (Millipore); and (iv) an electric vacuum pump for applying negative pressure (5 mm Hg).
SMF method.
Using the second digested aliquot, twice the aliquot volume of 5% NaOCl was added. The sample was homogenized for 15 min, 95% ethanol–1% Triton X-100 in a volume equal to the volume of the mixture was added, and the mixture was again homogenized for 15 s. After the passage of sputum though the filter systems, the valves were closed, and the membranes still in the filter were stained with the Kinyoun method (10). The membranes were then transferred to microscope slides, and a coverslip was placed on each slide for microscopic reading.
Reading and interpretation of microscopy.
The slides were observed under a bright-field microscope with a 100× immersion objective to detect acid-fast bacilli (AFB) in 100 fields and interpreted on a semiquantitative scale.
OK culture medium.
Culture on OK medium was used as the gold standard method. The first sputum aliquots were decontaminated by the OK method (11) and plated onto solid culture medium. The cultures were incubated at 37°C and read for up to 8 weeks, after which the culture results were reported as negative. The culture results were reported as positive when the growth of a CFU occurred, with the presence of AFB confirmed by microscopy.
Identification of Mycobacteria isolates.
Isolates of Mycobacteria tuberculosis were identified using the criteria of morphology, pigmentation of colonies, microscopy, a niacin test, and a growth inhibition test in medium with para-nitrobenzoic acid and in medium with thiophene-2-carboxylic hydrazide.
Statistical analysis.
The study population was characterized with simple descriptive statistics, and the patients with and without tuberculosis were compared with the Wilcoxon rank sum test, t test, χ2 test, and Fisher's exact test accordingly. The sensitivities, specificities, and predictive values of the three tests were estimated with 95% confidence intervals and were determined by comparing those individually with the culture results of the first sputum sample. The McNemar test was used to compare the sensitivities of the different microscopy methods. To calculate the sensitivity increase when two samples were used, positive results of the second sample from patients whose first sputum test was negative were used as the numerator, and the total number of patients diagnosed as having TB was the denominator. Cohen's kappa coefficient was used to determine the agreement between culture results and 3 other diagnostic methods. The data were analyzed with Epi Info (version 3.5.3).
Ethical aspects.
This study was approved by the Research Ethics Committee of the FMT-HVD, protocol 1845/2011 of 9 September 2011. Each patient signed an informed consent form.
RESULTS
Population and samples.
Fig. 1 shows that of the 508 eligible patients with suspected TB, 76 were excluded from the analysis: 1.8% (9/508) had a sample volume of <1.5 ml, 9.6% (49/508) had samples that the system failed to filter, and 3.5% (18/508) had a contaminated culture. Table 1 summarizes the characteristics of 432 patients included in the analysis. The mean age of the patients was 39 years, and 60% (261/432) were HIV infected. Based on all specimen culture results available (for the 1st or 2nd specimen), the prevalence of pulmonary tuberculosis in the eligible group of patients was 19.4% (95% CI, 15.9% to 23.4%; n = 84/432). From those, 50% (42/84) were HIV positive. The prevalence of tuberculosis in the HIV-infected patients was 16.1% (95% CI, 12.0% to 20.9%; n = 42/261). Moreover, in 9 cases, the patients had positive bacilloscopy results but a negative culture. The HIV-infected patients with positive TB cultures had lower mean CD4 counts than the patients with negative cultures (mean CD4 count, 65 versus 279; P < 0.001).
Table 1.
Characteristics of patients by TB culture results
| Characteristic | TB culture results |
Total (n = 432) | P value | |
|---|---|---|---|---|
| Positive (n = 84) | Negative (n = 348) | |||
| Age (mean [SD]a) (yr) | 34.3 (11.4) | 40.1 (14.5) | 39 (14.2) | <0.001 |
| Gender, male (%) | 63.1 | 63.5 | 63.4 | 0.944 |
| Outpatient clinic (%) | 73.8 | 87.9 | 85.2 | <0.001 |
| New TB case (%) | 96.4 | 97.1 | 97.0 | 0.984 |
| HIV positive (%) | 58.3 | 60.9 | 60.4 | 0.664 |
| CD4 cells, if HIV positive (mean [SD]) | 65 (54) | 279 (231) | 239 (226) | <0.001 |
SD, standard deviation.
Cohen's kappa coefficient between the gold standard and microscopy methods.
Table 2 shows the kappa coefficients between cultures and sputum smears performed by the 3 different methods. In the HIV-infected group, the kappa results were considered moderate (k = 0.55 and 0.578, respectively).
Table 2.
Microscopy compared to solid culture methods in HIV-infected and -uninfected patient groups
| Group | Culture | Centrifugation |
Centrifugation-NALC |
SMF |
|||
|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | ||
| HIV infected (n = 261) | Positive | 19 | 23 | 20 | 22 | 26 | 16 |
| Negative | 2 | 217 | 2 | 217 | 3 | 216 | |
| % agreement | 90.4 | 90.8 | 92.7 | ||||
| Kappa | 0.555 | 0.578 | 0.692 | ||||
| HIV uninfected (n = 171) | Positive | 19 | 14 | 21 | 12 | 26 | 7 |
| Negative | 1 | 137 | 1 | 137 | 7 | 131 | |
| % agreement | 91.2 | 92.3 | 92.9 | ||||
| Kappa | 0.669 | 0.720 | 0.737 | ||||
| Total (n = 432) | Positive | 38 | 37 | 41 | 34 | 52 | 23 |
| Negative | 3 | 354 | 3 | 354 | 10 | 347 | |
| % agreement | 90.7 | 91.4 | 92.8 | ||||
| Kappa | 0.607 | 0.643 | 0.714 | ||||
Performance of microscopy methods.
As shown in Table 3, when we computed results obtained by testing only the first specimen, the sensitivity obtained using the SMF method was higher than the sensitivity using centrifugation with or without NALC (70.6% versus 54.6%, P < 0.001, and 70.6% versus 50.6%, P < 0.001, respectively). The SMF method presented the highest sensitivities for the HIV-negative patient group and for the HIV-positive patient group (81.8% and 61.4%, respectively, P < 0.001). There were no statistically significant differences between the sensitivities of microscopy after centrifugation with or without NALC (P = 0.246).
Table 3.
Performance of microscopy methods compared to that of solid culture in HIV-infected and -uninfected patient groups for results of the first specimen
| Microscopy methoda | Microscopy results (% [95% CI]) for: |
||
|---|---|---|---|
| HIV-positive patients (n = 261) | HIV-negative patients (n = 171) | Total (n = 432) | |
| Centrifugation | |||
| Sensitivity | 45.2 (28.9–61.4) | 57.5 (39.2–75.9) | 50.6 (38.6–62.6) |
| Specificity | 99 (97.3–100) | 99.3 (97.7–100) | 99.1 (98.0–100) |
| PPV | 90.4 (75.5–100) | 95.00 (82.9–100) | 92.6 (83.4–100) |
| NPV | 89.6 (85.4–93.8) | 91.7 (87.2–96.1) | 90.5 (87.5–93.5) |
| Centrifugation with NALC | |||
| Sensitivity | 47.6 (31.3–63.9) | 63.6 (45.7–81.5) | 54.6 (42.7–66.6) |
| Specificity | 99 (97.3–100) | 99.3 (97.7–100) | 99.1 (98.0–100) |
| PPV | 90.9 (76.6–100) | 95.4 (84.4–100) | 93.1 (84.6–100) |
| NPV | 90.0 (85.8–94.2) | 92.8 (88.6–97.0) | 91.2 (88.2–94.1) |
| SMF | |||
| Sensitivity | 61.9 (46.0–77.7) | 81.8 (67.1–96.4) | 70.6 (59.7–81.6) |
| Specificity | 98.5 (96.5–100) | 96.7 (93.7–99.8) | 97.4 (95.7–99.2) |
| PPV | 89.6 (76.8–100) | 84.3 (70.2–98.5) | 85.4 (75.9–95.0) |
| NPV | 92.5 (88.7–96.2) | 96.1 (92.8–99.5) | 94.0 (91.5–96.6) |
CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value.
When the performances of all three processing methods using the results from specimens 1 and 2 (Table 4) were analyzed, similar results were obtained. Losses of sensitivity for the NALC and the SMF methods were observed, but no significant differences were seen (P = 0.05).
Table 4.
Performance of microscopy methods compared to that of solid culture in HIV-infected and -uninfected patient groups for 432 patients, considering positivity of the first and second specimens
| Microscopy methoda | Microscopy results (% [95% CI]) for: |
||
|---|---|---|---|
| HIV-positive patients (n = 261) | HIV-negative patients (n = 171) | Total (n = 432) | |
| Centrifugation | |||
| Sensitivity | 46.9 (32.5–61.7) | 57.1 (39.4–73.7) | 51.2 (40.0–62.3) |
| Specificity | 99.1 (96.6–99.9) | 99.3 (96.0–99.9) | 99.1 (97.5–99.8) |
| PPV | 92 (73.9–98.8) | 95.2 (76.1–99.2) | 93.5 (82.1–98.6) |
| NPV | 89.0 (84.3–92.7) | 90.0 (84.0–94.3) | 89.4 (85.9–92.3) |
| Centrifugation with NALC | |||
| Sensitivity | 49.0 (34.4–63.7) | 62.9 (44.9–78.5) | 54.8 (43.5–65.7) |
| Specificity | 98.6 (95.9–99.7) | 99.3 (96.0–99.9) | 98.9 (97.1–99.7) |
| PPV | 88.9 (70.8–97.5) | 95.7 (78.0–99.3) | 92.0 (80.8–97.7) |
| NPV | 89.3 (84.6–93.0) | 91.2 (85.5–95.2) | 90.1 (86.6–92.9) |
| SMF | |||
| Sensitivity | 57.1 (42.2–71.2) | 74.3 (56.7–87.5) | 64.3 (53.1–74.5) |
| Specificity | 98.6 (95.9–99.7) | 94.9 (89.7–97.9) | 97.1 (94.8–98.6) |
| PPV | 90.3 (74.2–97.9) | 78.8 (61.1–91.0) | 84.4 (73.1–92.2) |
| NPV | 90.9 (86.38–94.3) | 93.5 (88.0–97.0) | 91.9 (88.6–94.4) |
CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value.
A second specimen was provided by 227 patients, and 47 of these had a positive culture. The second specimen showed increases of 13% (6/47) positivity for microscopy after centrifugation with NALC, 11% (5/47) positivity for microscopy after centrifugation without NALC, and 4% (2/47) positivity with the SMF method. Table 5 shows the performance of each microscopy method for 227 patients who delivered a 1st and 2nd specimen.
Table 5.
Performance of microscopy methods compared to that of solid culture in HIV-infected and -uninfected patient groups for 227 patients who delivered first and second specimens
| Microscopy methoda | Microscopy results (% [95% CI]) for: |
||
|---|---|---|---|
| HIV-positive patients (n = 163) | HIV-negative patients (n = 64) | Total (n = 227) | |
| Centrifugation | |||
| Sensitivity | 31.3 (16.1–50.0) | 60.0 (32.2–83.6) | 40.4 (26.4–55.7) |
| Specificity | 99.2 (95.8–99.9) | 98.0 (89.1–99.7) | 98.9 (96.0–99.8) |
| PPV | 90.9 (58.7–98.5) | 90.0 (55.5–98.3) | 90.5 (69.6–98.6) |
| NPV | 85.5 (78.9–90.7) | 89.0 (77.4–95.8) | 86.4 (81.0–90.8) |
| Centrifugation with NALC | |||
| Sensitivity | 49.0 (34.4–63.7) | 73.3 (44.9–92.1) | 53.2 (38.1–67.9) |
| Specificity | 98.5 (94.6–99.8) | 98.0 (89.1–99.7) | 98.3 (95.2–99.6) |
| PPV | 87.5 (61.6–98.1) | 91.7 (61.5–98.6) | 89.3 (71.8–97.6) |
| NPV | 87.8 (81.3–92.6) | 92.3 (81.4–97.8) | 88.9 (83.7–92.9) |
| SMF | |||
| Sensitivity | 53.1 (34.8–70.9) | 73.3 (44.9–92.1) | 59.6 (44.3–73.6) |
| Specificity | 98.5 (94.6–99.8) | 93.9 (83.1–98.7) | 97.2 (93.6–99.1) |
| PPV | 89.5 (68.8–98.4) | 78.6 (49.2–95.1) | 84.9 (68.1–94.8) |
| NPV | 89.6 (83.4–94.1) | 92.0 (80.75–97.7) | 90.2 (85.1–94.0) |
CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value.
DISCUSSION
In general, all TB diagnosis methods have poorer performance and reduced sensitivities for patients coinfected with TB and HIV. A rapid diagnostic tool for assisting in the diagnosis of TB in HIV-infected patients is needed in order to improve the prognosis of such patients. In our study, the sensitivity of sputum smear microscopy with NALC treatment in the HIV-infected patient group was lower (47.6%) than the sensitivity of 61.8% in a study that used a CD4 cell count of >200 cells/mm3 as an eligibility criterion (12) and also lower than the 72% found in a study using fluorescence microscopy (13). On the other hand, we found sensitivity similar to that of a study that used the hot Ziehl-Neelsen method (14) and also similar to that of a study of outpatients with suspected TB in a reference laboratory in South Africa (15), a country with high TB and HIV burdens. The differences observed are likely to be due to variations in patient populations and to the methodologies used in each study.
In our study, the specificities of smear microscopy after centrifugation with and without NALC were virtually the same in the HIV-infected and -uninfected patient groups. The specificities of the smears after centrifugation with NALC among the infected and noninfected HIV groups (99% and 99.3%, respectively) are consistent with results from other studies (14, 16, 17).
The SMF method had a sensitivity of 61.9% for HIV-infected patients in our study, higher than those for the other microscopic methods used. The sensitivity of 81.8% in HIV-negative subjects was similar to the sensitivities of 80% and 89% reported by Smithwick and Stratigos (8) and Fennelly et al. (7), respectively.
The Xpert MTB/RIF assay (Cepheid Inc., Sunnyvale, CA) is a rapid molecular diagnostic test that has been endorsed by the WHO. A recent systematic review found that this assay had a sensitivity of 80% in an HIV-positive population (18), higher than the sensitivity of 67% for the SMF method in our study. However, the Xpert assay is considerably more expensive than other methods used for tuberculosis diagnosis and cannot be used to monitor treatment because it does not distinguish between viable and nonviable microorganisms. The SMF method could be an important alternative, especially in peripheral health care settings.
The SMF method may have performed better if a more sensitive culture medium had been used. Limitations of the cultures used probably decreased the measured specificity of the SMF method. Additional information for the microscopy-positive culture-negative cases was sought. Of these, 3 also had positive sputum smears after centrifugation with and without NALC, and of this group, 2 were HIV-infected patients, one of whom died of sepsis and the other was diagnosed with pulmonary TB after a 3rd positive sputum culture and was under treatment for TB. Of the 6 patients who only had positive SMF method results, 4 were diagnosed with pulmonary TB based on clinical and radiological findings and responded to TB treatment, and 2 were diagnosed by culture of the 3rd specimen, one with pulmonary and extrapulmonary TB and one with pulmonary TB. These findings suggest that sputum cultures performed on the first sample in this study were not sensitive enough to detect TB in all cases due to intrinsic limitations with solid media and TB cultures in general.
The Brazilian Ministry of Health suggests that 2 samples be submitted for bacilloscopy (without centrifugation or decontamination) (19) because of the limited sensitivity of microscopy. Due to the higher sensitivity of the SMF method for the first sample, our results suggest that the second sputum sample, which was often missing, may be less important when this method is used.
The study was performed in routine settings, and some limitations might have influenced the sensitivities and specificities of the studied microscopy methods. One of them could be the use of a solid culture medium, which has a lower sensitivity than liquid media such as MGIT (20). Second, quality control of the SMF readings was not performed mainly because the bacillus staining of the SMF fades away after 24 h. As the two staining methods use the same stain, it might be possible that the remaining hypochlorite and/or Triton X-100 in the membrane fades the stain.
The SMF process takes twice as long as the 15 min for microscopy after centrifuging, and safety issues concerning the handling of samples are similar to those for microscopy. The SMF method requires greater technical expertise than the Xpert MTB/RIF assay but is more affordable for places where the number of specimens to be tested does not allow investments in costly equipment.
With respect to technical difficulties, the cleaning of SMF material used in this study was laborious because disposable components were not available, and due to technical problems, 10% of the sputum samples could not be filtered. The clogged membranes might have occurred due to the lack of some air in the filter holder. It was not due to the thickness of samples as most of them were salivary samples. The observed pitfalls led to improvements in the filtration system with the use of disposable components and adjustments to the filtration process.
In conclusion, the sensitivity of the SMF method was superior to the sensitivities of all other microscopic techniques applied without any loss of specificity and may be a useful approach for the diagnosis of TB in HIV-infected patients, reducing morbidity and improving the patients' prognoses. Treatment of the specimens with NALC did not increase the sensitivity of smear microscopy with centrifugation, indicating that the addition of such a procedure is not necessary. We believe that the SMF method provides considerable advantages for the detection of TB infection in HIV-positive patients, especially considering that this GeneXpert system might not be appropriate for monitoring patient treatments.
ACKNOWLEDGMENTS
We thank Richard Antony for his comments and the English revision of the manuscript.
This study was partially supported by contracts CNPq/INCT 573548/2008-0 and PPSUS/FAPEAM 05/2010.
Footnotes
Published ahead of print 26 June 2013
REFERENCES
- 1.Lawn SD, Wood R. 2011. Tuberculosis in antiretroviral treatment services in resource-limited settings: addressing the challenges of screening and diagnosis. J. Infect. Dis. 204(Suppl 4):S1159–S1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schutz C, Meintjes G, Almajid F, Wilkinson RJ, Pozniak A. 2010. Clinical management of tuberculosis and HIV-1 co-infection. Eur. Respir. J. 36:1460–1481 [DOI] [PubMed] [Google Scholar]
- 3.Davis JL, Worodria W, Kisembo H, Metcalfe JZ, Cattamanchi A, Kawooya M, Kyeyune R, den Boon S, Powell K, Okello R, Yoo S, Huang L. 2010. Clinical and radiographic factors do not accurately diagnose smear-negative tuberculosis in HIV-infected inpatients in Uganda: a cross-sectional study. PLoS One 5:e9859. 10.1371/journal.pone.0009859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Getahun H, Harrington M, O'Brien R, Nunn P. 2007. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet 369:2042–2049 [DOI] [PubMed] [Google Scholar]
- 5.Hargreaves NJ, Kadzakumanja O, Whitty CJ, Salaniponi FM, Harries AD, Squire SB. 2001. “Smear-negative” pulmonary tuberculosis in a DOTS programme: poor outcomes in an area of high HIV seroprevalence. Int. J. Tuberc. Lung Dis. 5:847–854 [PubMed] [Google Scholar]
- 6.Perkins MD, Cunningham J. 2007. Facing the crisis: improving the diagnosis of tuberculosis in the HIV era. J. Infect. Dis. 196(Suppl 1):S15–S27 [DOI] [PubMed] [Google Scholar]
- 7.Fennelly KP, Morais CG, Hadad DJ, Vinhas S, Dietze R, Palaci M. 2012. The small membrane filter method of microscopy to diagnose pulmonary tuberculosis. J. Clin. Microbiol. 50:2096–2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smithwick RW, Stratigos CB. 1981. Acid-fast microscopy on polycarbonate membrane filter sputum sediments. J. Clin. Microbiol. 13:1109–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hadad DJ, Morais CG, Vinhas SA, Fennelly KP, Dietze R, Nascimento CP, Palaci M. 2012. Evaluation of processing methods to equitably aliquot sputa for mycobacterial testing. J. Clin. Microbiol. 50:1440–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância Epidemiológica 11 December 2012, posting date Manual nacional de vigilância laboratorial da tuberculose e outras micobactérias. Ministério da Saúde, Brazil: http://portal.saude.gov.br/portal/arquivos/pdf/manual_laboratorio_tb_3_9_10.pdf [Google Scholar]
- 11.Kudoh S, Kudoh T. 1974. A simple technique for culturing tubercle bacilli. Bull. World Health Organ. 51:71–82 [PMC free article] [PubMed] [Google Scholar]
- 12.Matee M, Mtei L, Lounasvaara T, Wieland-Alter W, Waddell R, Lyimo J, Bakari M, Pallangyo K, von Reyn CF. 2008. Sputum microscopy for the diagnosis of HIV-associated pulmonary tuberculosis in Tanzania. BMC Public Health 8:68. 10.1186/1471-2458-8-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cattamanchi A, Davis JL, Worodria W, den Boon S, Yoo S, Matovu J, Kiidha J, Nankya F, Kyeyune R, Byanyima P, Andama A, Joloba M, Osmond DH, Hopewell PC, Huang L. 2009. Sensitivity and specificity of fluorescence microscopy for diagnosing pulmonary tuberculosis in a high HIV prevalence setting. Int. J. Tuberc. Lung Dis. 13:1130–1136 [PMC free article] [PubMed] [Google Scholar]
- 14.Cattamanchi A, Dowdy DW, Davis JL, Worodria W, Yoo S, Joloba M, Matovu J, Hopewell PC, Huang L. 2009. Sensitivity of direct versus concentrated sputum smear microscopy in HIV-infected patients suspected of having pulmonary tuberculosis. BMC Infect. Dis. 9:53. 10.1186/1471-2334-9-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilkinson D, Sturm AW. 1997. Diagnosing tuberculosis in a resource-poor setting: the value of sputum concentration. Trans. R. Soc. Trop. Med. Hyg. 91:420–421 [DOI] [PubMed] [Google Scholar]
- 16.Kivihya-Ndugga LE, van Cleeff MR, Githui WA, Nganga LW, Kibuga DK, Odhiambo JA, Klatser PR. 2003. A comprehensive comparison of Ziehl-Neelsen and fluorescence microscopy for the diagnosis of tuberculosis in a resource-poor urban setting. Int. J. Tuberc. Lung Dis. 7:1163–1171 [PubMed] [Google Scholar]
- 17.Steingart KR, Ng V, Henry M, Hopewell PC, Ramsay A, Cunningham J, Urbanczik R, Perkins MD, Aziz MA, Pai M. 2006. Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: a systematic review. Lancet Infect. Dis. 6:664–674 [DOI] [PubMed] [Google Scholar]
- 18.Steingart KR, Sohn H, Schiller I, Kloda LA, Boehme CC, Pai M, Dendukuri N. 2013. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst. Rev. 1:CD009593. 10.1002/14651858.CD009593.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância Epidemiológica, Brazil. 11 December 2012, posting date Manual de recomendações para o controle da tuberculose no Brasil; Ministério da Saúde, Brazil: http://portal.saude.gov.br/portal/arquivos/pdf/manual_de_recomendacoes_tb.pdf [Google Scholar]
- 20.Chew WK, Lasaitis RM, Schio FA, Gilbert GL. 1998. Clinical evaluation of the Mycobacteria Growth Indicator Tube (MGIT) compared with radiometric (Bactec) and solid media for isolation of Mycobacterium species. J. Med. Microbiol. 47:821–827 [DOI] [PubMed] [Google Scholar]