Abstract
Pathogenic bacteria produce several virulence factors that help them establish infection in permissive hosts. Bacterial toxins are a major class of virulence factors and hence are attractive therapeutic targets for vaccine development. Here, we describe the development of a rapid, sensitive, and high-throughput assay that can be used as a versatile platform to measure the activities of bacterial toxins. We have exploited the ability of these toxins to cause cell death via apoptosis of sensitive cultured cell lines as a readout for measuring toxin activity. Caspases (cysteine-aspartic proteases) are induced early in the apoptotic pathway, and so we used their induction to measure the activities of Clostridium difficile toxins A (TcdA) and B (TcdB) and binary toxin (CDTa-CDTb), Corynebacterium diphtheriae toxin (DT), and Pseudomonas aeruginosa exotoxin A (PEA). Caspase induction in the cell lines, upon exposure to toxins, was optimized by toxin concentration and intoxication time, and the specificity of caspase activity was established using a genetically mutated toxin and a pan-caspase inhibitor. In addition, we demonstrate the utility of the caspase assay for measuring toxin potency, as well as neutralizing antibody (NAb) activity against C. difficile toxins. Furthermore, the caspase assay showed excellent correlation with the filamentous actin (F-actin) polymerization assay for measuring TcdA and TcdB neutralization titers upon vaccination of hamsters. These results demonstrate that the detection of caspase induction due to toxin exposure using a chemiluminescence readout can support potency and clinical immunogenicity testing for bacterial toxin vaccine candidates in development.
INTRODUCTION
Microorganisms cause pathogenesis by means of a wide variety of molecules called virulence factors. A large number of divergent microbial pathogens synthesize toxins that are recognized as primary virulence factors that affect the metabolism and cause damage to eukaryotic cells, many times with lethal effects to the host (1, 2). Major symptoms associated with diseases, such as diphtheria, whooping cough, cholera, anthrax, and dysentery, are all related to the activities of toxins produced by bacteria. In recognition of their central role in these and other diseases, bacterial toxins have become attractive targets in the development of vaccines (1, 3). Bacterial toxins affect susceptible host cells by a variety of modes of action: damage to cell membranes, inhibition of protein synthesis, activation of immune responses leading to cellular damage, causing direct cell lysis, and facilitation of bacterial spread through tissues (4). Organisms, such as Clostridium difficile, Corynebacterium diphtheriae, and Pseudomonas aeruginosa, secrete toxins that are involved in different ways in the pathogenesis of disease. C. difficile toxins, for example, cause cellular toxicity through the glucosylation of Rho G-protein and ADP-ribosylation of actin, while C. diphtheriae and P. aeruginosa toxins catalyze the transfer of ADP-ribose to elongation factor 2 to block host cell protein synthesis, leading to the death of their target cells (5–8). The clostridial toxin TcdB of C. difficile inactivates the small GTPases Rho, Rac, and Cdc42, which have been shown to trigger cell death via apoptosis (2, 9–11).
Apoptosis is a fundamental feature of all animal cells and is essential for normal development and tissue homeostasis, whereas unregulated apoptosis can create an imbalance in normal cell proliferation processes (4, 7). Apoptosis is characterized by the presence of distinct morphological and biochemical features (12). Morphologically, it can be characterized by DNA fragmentation, membrane blebbing, cell rounding, cytoskeletal collapse, and the formation of membrane-bound apoptotic vesicles that are rapidly eliminated by phagocytosis (13). Biochemical features of apoptotic cell death include the activation of a family of intracellular cysteine endopeptidases known as caspases (cysteine-aspartic proteases), which specifically cleave target proteins at a cysteine amino acid that follows an aspartic acid residue (14, 15). Caspases are synthesized as inactive proenzymes, which are converted into active heterodimers by proteolytic cleavage and are responsible for the deliberate disassembly of cells into apoptotic bodies (16). Their activation indicates progression of the cellular apoptotic pathway. The initiator caspases 8 and 9 and the executioner caspase 3 are positioned at crucial junctions in the apoptotic pathways. The activation of the initiator caspases in response to extracellular cytotoxic agents activates the executioner caspase 3, resulting in a series of events that lead eventually to cell lysis and disruption of normal cell processes (8, 12, 16–18).
Bacterial toxins can activate apoptotic pathways and hence, caspases are molecules of particular interest in assay development as potential indicators of apoptosis due to cell exposure to toxins. A number of cultured cell lines undergo apoptosis when exposed to various cytotoxic signals from pathogens or other sources. Caspase activation occurs early in the programmed cell death pathway and thus allows for the early detection of exposure to these toxins. Measurements of caspase activation due to bacterial toxin exposure, or a lack of caspase induction signal, may be used as potency or release tests in vaccine development. The ability to inhibit toxin-induced caspase activation in vitro by animal and human serum neutralizing antibodies is valuable for an evaluation of vaccine efficacy. Conventionally, cell susceptibility to bacterial toxins and neutralizing antibody responses in vitro rely on radioactive cytotoxicity measurements for protein synthesis or are evaluated by microscopic observations of intoxicated cell monolayers. These methods may be subjective, time-consuming, and inherently low throughput, due to the requirements of manual observation and counting of cells (5, 7, 9, 19–22). Because of these limitations, we sought to develop an alternative assay that may be used as a versatile platform for measuring bacterial toxin activity and to evaluate the immunogenicities of toxin-based vaccines. Here, we describe that the cytotoxic activities of several unrelated bacterial toxins can be easily and reliably quantified by measuring in-cell caspase activation levels. The assay is sensitive and makes use of multiwell plates and automated reagent handling systems, allowing for high-throughput quantification of cellular apoptosis due to toxins.
MATERIALS AND METHODS
Bacterial toxins.
Native C. difficile toxins, TcdA from C. difficile strain VPI 10463 (product no. 01A01) and TcdB from VPI 10463 (product no. 01A02), both of which are VPI ribotype 087 strains, were purchased from tgcBIOMICS GmbH (Mainz, Germany) and stored lyophilized at 4°C and −70°C after reconstitution in pyrogen-free sterile water, with limited (≤3 times) freeze-thawing. The TcdA and TcdB toxins were mutated as described in the literature (23–25). These mutations included W101A, D287A, and W519A for TcdA and W102A, D288A, and W520A for TcdB. These point mutations have been demonstrated to destroy the enzymatic activity of glucosylase and substrate binding of toxins. The muted toxins were expressed in Baculovirus spp., purified from cell lysates using ceramic hydroxyapatite type II (Bio-Rad, Hercules, CA) column chromatography, and stored at −70°C. Recombinant His-tagged C. difficile binary CDTa-CDTb toxins were expressed in Escherichia coli, purified from E. coli lysates by affinity chromatography, and buffer exchanged into 50 mM HEPES (pH 7.5), 150 mM NaCl, and stored at −70°C. P. aeruginosa exotoxin A (PEA) and C. diphtheriae toxin (DT) were purchased from EMD Millipore Corporation (Billerica, MA), reconstituted in sterile water to 1 mg/ml, and stored at 4°C according to the manufacturer's recommendation. CRM197, the mutated form of DT, which is produced from a single missense mutation (Gly52 to Glu52) within the fragment A region (26–28), was purchased from MBL International Corporation (Woburn, MA) in a solution of 1 mg/ml, and was stored at −70°C. All toxins had ≥90% purity, as indicated by the accompanying literature of the purchased toxins and by SDS analysis of internally produced material (data not shown).
Cell lines and cell culture media.
Vero cells (African green monkey kidney) were obtained from the American Type Culture Collection (ATCC) (Manassas, VA) and cultured in Dulbecco's minimum essential medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), per ATCC procedures. HeLa cells were obtained from the ATCC and cultured in Eagle's minimum essential medium (EMEM) supplemented with 10% heat-inactivated FBS, per ATCC instructions.
Caspase inhibition reagents.
z-Val-Ala-Asp-fmk (z-VAD-fmk), a cell-permeant pan-caspase inhibitor that irreversibly binds to the catalytic site of caspase proteases and inhibits the induction of apoptosis (Promega, Madison, WI), was reconstituted in dimethyl sulfoxide (DMSO) at 20 mM, stored at −20°C, and used in the assay at a final concentration of 20 μM for the inhibition of caspase, per the manufacturer's recommendations. Serum samples from hamsters immunized with inactivated TcdA and TcdB toxins as previously described (29) were used for C. difficile toxin neutralization assays.
Caspase-Glo 3/7 assay reagent.
The Caspase-Glo 3/7 assay reagent (Promega, Madison, WI) was used for caspase detection in treated cells in vitro. The reagent provides a proluminescent caspase-3/7 substrate, which contains the tetrapeptide sequence DEVD, in combination with luciferase and a cell-lysing agent. The addition of the Caspase-Glo 3/7 reagent directly to the assay well results in cell lysis, followed by caspase cleavage of the DEVD substrate, and the generation of luminescence. The amount of luminescence as displayed on the readout is proportional to the amount of caspase activity in the sample.
Caspase assay optimization and toxin evaluation.
The caspase assay was optimized for several parameters, including toxin concentration, cell seeding density, and serum-toxin preincubation time for C. difficile toxins TcdA and TcdB, binary CDTa-CDTb, and DT and PEA. Vero cells were used to assess caspase activation due to TcdA, TcdB, and binary CDTa-CDTb. HeLa cells were used as target cells for DT and PEA. Various cell input experiments (data not shown) led to the selection of a seeding density of 2.5 × 104 cells/well in a total volume of 100 μl/well of black glass-bottom 96-well tissue culture plates (Thermo Scientific, Rochester, NY) or a 50-μl cell suspension at a seeding density of 3 × 104 cells/ml in a 384-well plate. Cells were incubated at 37°C in 5% CO2 and allowed to grow to ∼90% confluence. The following day, cell supernatants were replaced by toxins diluted in tissue culture medium at a different range of concentrations for each toxin (Fig. 1) and incubated overnight at 37°C in 5% CO2. Caspase-Glo reagent was added directly to individual wells at 30 μl/well in 96-well plates (15 μl/well in 384-well plates), mixed gently on an orbital shaker for about 30 s, and further incubated at 37°C in 5% CO2 for 30 to 60 min prior to reading. Luminescence measurements were obtained using the PerkinElmer 2030 multilabel reader Victor 4× plate reader. The toxin concentrations used for the time course studies were 90% effective concentrations (EC90s) and were 20 ng/ml TcdA, 80 pg/ml TcdB, 1 μg/ml DT, and 10 μg/ml PEA. In these time course experiments, the Caspase-Glo reagent was added as described above at various times after intoxication, depending on the toxin and cell line being evaluated, ranging from 2 to 48 h, to determine the optimum time for toxin exposure and caspase activation. To demonstrate the specificity of the toxin-induced caspase activation, the genetically modified toxins were tested alongside the corresponding active toxins at the same concentrations, or the pan-caspase inhibitor (z-VAD-fmk) was added to the active toxin wells at a concentration of 20 μM.
Fig 1.
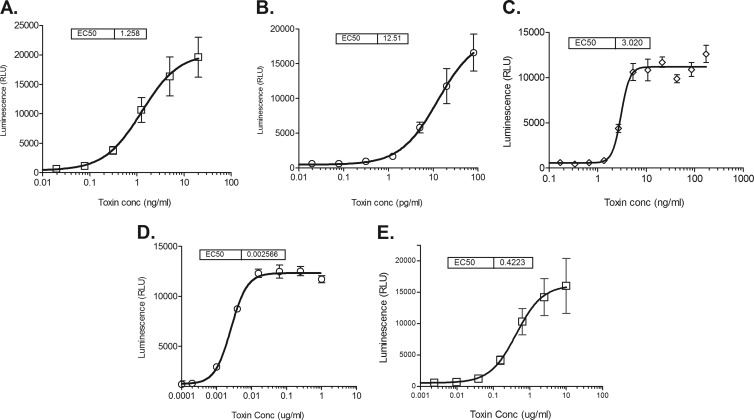
Dose-response curves of in vitro toxin-induced caspase activity: C. difficile TcdA on Vero cells (A), C. difficile TcdB on Vero cells (B), C. difficile binary toxin CDTa-CDTb on Vero cells (C), C. diphtheriae toxin on HeLa cells (D), and P. aeruginosa exotoxin A on HeLa cells (E). Caspase induction was measured in toxin-treated cell cultures using the Caspase-Glo 3/7 assay kit (Promega), and the results are shown as mean luminescence (measured in relative light units [RLU]) ± the standard deviation for 5 replicate wells across 2 assays. The EC50 (50% effective concentration) was calculated by four-parameter logistic regression fitting of the titration curve.
F-actin polymerization assay.
Detection of filamentous actin (F-actin) polymerization was employed as a means to correlate the results with the caspase activation assay data. The F-actin assay was described in detail by Xie et al. (29). Briefly, Vero cell suspensions were added to 384-well plates and incubated overnight at 37°C in 5% CO2. Following incubation, supernatants were replaced by a toxin preincubated with or without neutralizing serum, and plates were returned to the incubator for an additional 48 h (see below for toxin concentrations used in our assay comparisons). Following a series of centrifugations, washes, and incubations, the wells were stained as described in Xie et al. (29) to enable the detection and imaging of F-actin polymerization in the treated cells using a scanning cytometer (Molecular Devices, Sunnyvale, CA).
Toxin neutralization assays.
The EC90s for TcdA and TcdB (4 ng/ml TcdA and 40pg/ml TcdB) were used for demonstrating the neutralization of toxins by preincubation of toxins using serum samples from animals previously hyperimmunized with toxins as described earlier (29). For both caspase and F-actin polymerization assays, Vero cells were seeded at a density of 3 × 104 cells/ml in 50 μl/well of a 384-well plate and were incubated at 37°C in 5% CO2 for 24 h. The following day, supernatants were replaced by preincubated toxins with 1:2 serially diluted immune serum, and cells were further incubated for 24 h for the caspase assay or for 48 h for the F-actin assay. The cytometric F-actin neutralization assay was conducted over a period of four consecutive days, and data acquisition for the assay involved an image of the monolayer acquired using the scanning cytometer. The caspase assay was conducted over a period of three consecutive days, and data acquisition involved the luminescence measurements obtained from the addition of caspase substrate using a luminescence-capable plate reader.
The EC50 was calculated by four-parameter regression fitting of the titration curve using GraphPad Prism 5 for Windows version 5.04, for both the Caspase-Glo and the cytometric assays.
RESULTS
Toxin-induced caspase activation over a range of toxin concentrations was demonstrated in Vero cells for C. difficile toxins (TcdA, TcdB, and binary CDTa-CDTb), and in HeLa cells for C. diphtheriae and P. aeruginosa toxins as shown in Fig. 1. The average 50% effective doses (ED50s) of 5 replicate wells across 2 assays for TcdA and TcdB were calculated to be approximately 1.26 ng/ml and 12.5 pg/ml, respectively. The average ED50 for binary CDTa-CDTb was 3.0 ng/ml. The average ED50s of 5 replicate wells across 2 assays for DT and PEA were found to be approximately 0.0026 μg/ml and 0.42 μg/ml, respectively (Fig. 1).
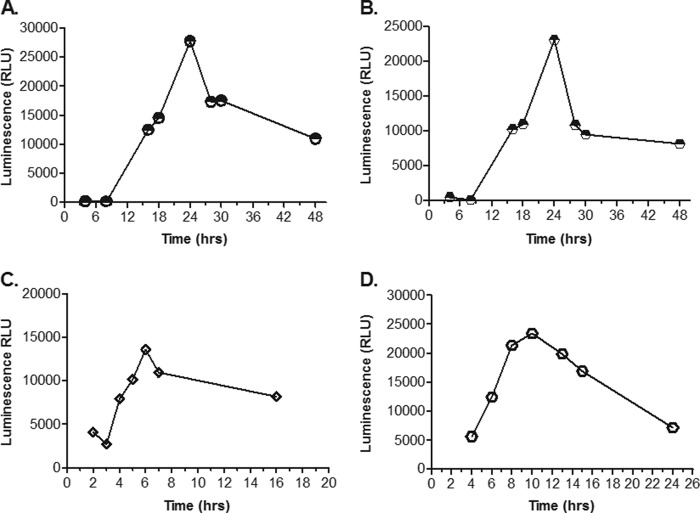
To further optimize the assay, we determined the time course of caspase activation following toxin exposure. Appropriate cell monolayers (seeded 24 h prior at a density of 2.5 × 104 cells/well) were treated with individual toxins at EC90s shown to produce high levels of the caspase activation signal (80 pg/ml of TcdB, 20 ng/ml of TcdA, 1 μg/ml of DT, and 10 μg/ml of PEA). Caspase levels were assessed at various time intervals ranging from 2 to 48 h postintoxication. Preliminary time courses were conducted at fewer and shorter incubation times (2, 4, 5, and 7 h for DT and PEA, and 4, 8, and 16 h for TcdA and TcdB; data not shown). The results showed that the levels of caspase activation increased in a time-dependent manner, and the kinetics varied for each toxin (Fig. 2). TcdA and TcdB showed significant increases at 16 h, peak levels at 24 h, began to decrease at 28 h, and showed a considerable loss of activity by 48 h. DT-induced caspase activation was seen as early as 4 h, with peak levels at 6 h, and it began to decrease thereafter with a significant drop of activity by 16 h. PEA-induced caspase activation peaked around 10 h, noticeably decreased by 14 h, and was followed by an almost complete loss of activity at 24 h.
Fig 2.
Time course of in vitro toxin-induced caspase activity: C. difficile TcdA at 20 ng/ml (A), C. difficile TcdB at 80 pg/ml (B), C. diphtheriae toxin at 1 μg/ml (C), and P. aeruginosa exotoxin A at 10 μg/ml (D). Caspase induction was measured in toxin-treated cell cultures using the Caspase-Glo 3/7 assay kit (Promega), and the results are shown as the mean luminescence (in relative light units [RLU]) for duplicate wells.
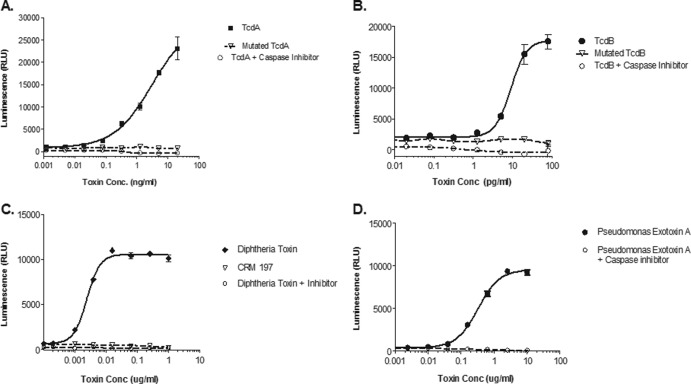
To test the specificity of the toxins' ability to induce caspase, we assayed genetically inactivated toxins (Fig. 3). Toxin mutagenesis has been described in the peer-reviewed literature as a means to decrease or inactivate bacterial toxins and thus to enable their use in the development of vaccines. Previous studies have shown that the introduction of specific mutations in bacterial toxin genes leads to the inactivation of or a decrease in toxicity (23, 27, 30, 31). To test the specificity of the toxins' ability to induce caspase, we assayed genetically altered toxins. All mutant toxins were evaluated using the same titrations as with their corresponding active toxins, and none were assayed for nuclease activity. Genetically inactivated TcdA and TcdB were unable to induce caspase activity at concentrations at which the wild-type toxins clearly demonstrated caspase induction (Fig. 3A and B). Similar data were also obtained with CRM197, a mutant DT (Fig. 3C). Furthermore, we were able to inhibit the caspase signal induced by the four wild-type toxins in the assay by the addition of a caspase inhibitor at all concentrations of toxin tested (Fig. 3A to D). These data demonstrate the specificity of caspase induction by individual toxins.
Fig 3.
Specificity of toxin-induced caspase activity in vitro was demonstrated by addition of 20 μM caspase inhibitor z-VAD-fmk (○) or genetic inactivation of toxin (△) compared to active toxins (closed symbols) for C. difficile TcdA (A), C. difficile TcdB (B), C. diphtheriae toxin (C), and P. aeruginosa exotoxin A (D). Genetically inactivated C. difficile toxins were produced from point mutations in the enzymatic domains of TcdA and TcdB. The inactive C. diphtheriae toxin CRM197 was produced by a single missense mutation (Gly52 to Glu52) within the fragment A region. Caspase induction was measured in toxin-treated cell cultures using Caspase-Glo 3/7 assay kit (Promega), and the results are shown as the mean luminescence (in relative light units [RLU]) ± the standard deviation for 3 replicate wells.
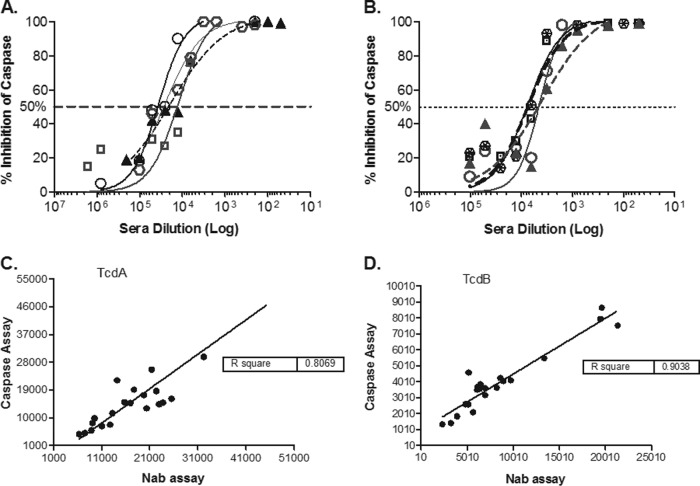
Next, we compared the performance of the caspase assay with that of an established cytometric method (29) to evaluate cellular toxicity caused by toxins. Both assays were conducted to assess the ability of serum antibodies induced by immunization with TcdA and TcdB toxoids to neutralize the proapoptotic activity of the wild-type toxins. Preincubation of TcdA or TcdB toxins with serial dilutions of hyperimmune hamster serum samples (as described in Materials and Methods) protected the cells from toxin-induced cellular toxicity, as measured by the inhibition of caspase activation (Fig. 4A) and by the inhibition of cell rounding via F-actin depolymerization (data not shown). The assays were conducted head to head using identical conditions for both in terms of cell numbers, toxin concentrations, serum dilution, and preincubation of toxins with immune serum samples. Following the incubation of serum samples and toxins on the cells, development of the plates and data acquisition were conducted using parameters that were optimized for each individual assay. The study evaluated toxin neutralization in serum samples collected from 20 individual animals previously immunized with inactivated toxins. Both methods used 384-well plates and a toxin concentration that was previously determined (29) to cause 90% cytotoxicity in the well. Comparable neutralization titers were obtained by both methods with an R2 value of 0.807 for anti-TcdA titers and 0.904 for anti-TcdB titers (Fig. 4B).
Fig 4.
Neutralization of C. difficile toxin-induced caspase activity in vitro by preincubation of toxin with hamster serum containing anti-toxin neutralizing antibodies. The titration of serum samples from a subset of four hamsters vaccinated with inactivated TcdA (A) and TcdB (B) showed a dose-dependent pattern of inhibition of caspase induction by both toxins. A comparison of TcdA (C) and TcdB (D) neutralizing antibody titers in serum samples from 20 vaccinated hamsters determined in the caspase assay (y axis) correlates well with the titers determined in the F-actin cytometric assay (x axis) for both toxins. The results are shown as antibody titers determined from the inverse of the antibody dilution that achieved 50% inhibition of toxin activity at an EC90 dose, calculated using a four-parameter logistical fit of each serum titration curve. Caspase induction was measured in toxin-treated cell cultures using the Caspase-Glo 3/7 assay kit (Promega), and F-actin was measured by a scanning cytometer referenced in Materials and Methods. Both assays were conducted once each at the same time with the same cell culture media, toxins, and sera.
DISCUSSION
Bacterial toxins are attractive targets for drug and vaccine development. Hence, several methods have been reported to assess their functional activity in eukaryotic cells in vitro. Moreover, functional assays are increasingly used to determine the type of immune response induced by toxins, for quality control of toxins as vaccine antigens, and as potency release tests in toxin-based vaccine development. This has led to an increased demand for high-throughput cell-based assays with decreased cycle times. Several assays reported in the literature rely on visual observations of cytopathic effects of toxins, e.g., rounding of cells or loss of viability (5, 7, 9, 19–22). Recently, an elegant high-throughput assay has been reported that can quantitate actin polymerization to measure the effects of C. difficile toxins TcdA and TcdB on Vero cells (29). Other methods available for quantifying the biological (enzymatic) activities of toxins within a cell are often cumbersome, low throughput, and require costly instruments for acquiring measurements related to the effects of toxins in eukaryotic cells. There are several reports in the literature linking C. difficile toxins to apoptosis and the activation of caspases. Carneiro et al. (32) have shown caspase 3 and 9 induction by Western blotting in human intestinal epithelial cell line T84 with TcdA exposure. Similar data have been reported for TcdB in HeLa cells by Qa'Dan et al. (10). Moreover, Hippenstiel et al. (33) showed that TcdA and TcdB mediated rho-inactivation, leading to caspase induction and apoptosis. Since other bacterial toxins are known to cause death of the target cells via apoptosis, we postulated that measuring caspase activation might not only be used to detect the cytotoxic effects of a majority of bacterial toxins but also could be used in the development of a simple luminescent assay allowing for an earlier readout and higher throughput compared to standard cell-based assays that rely on quantifying morphological changes.
The cell lines used in our study were chosen for their sensitivities to the respective toxins and for historical reasons. Vero cells have been shown to be very sensitive to C. difficile toxins and have been used in a published F-actin polymerization assay (19, 29, 33, 34). Since we compared the caspase and F-actin polymerization assay, Vero cells were selected for our caspase studies for TcdA and TcdB toxins. Similarly, diphtheria toxin and Pseudomonas enterotoxins have historically been tested on HeLa cells at our institution, and the use of HeLa cells for studying caspase activation (and thus, apoptosis) has been reported previously (12, 27, 35, 36). Additionally, these cell lines provided the sensitivity needed to carry out the assay rapidly and reliably, leading to their selection as preferred cell substrates. Ultimately, the choice of cell lines depends on the individual toxin source and user needs. We were able to observe caspase induction caused by TcdA, TcdB, and CDTa-CDTb in Vero cells and that caused by DT and PEA in HeLa cells. Interestingly, the kinetics of caspase induction varied with the type of toxin used. DT demonstrated peak caspase induction following intoxication within 6 h, followed by PEA at 10 h. TcdA and TcdB were found to have slower kinetics (peak around 20 h) of caspase induction. This differential kinetics might reflect differences in their modes of action. That is, DT and PEA act on elongation factor, thus shutting down protein synthesis and producing early induction of caspases. In contrast, C. difficile toxins are known to interfere with actin polymerization, which results in detachment and rounding, and may require more time to induce cell death. Hence, it was important to determine the optimal time for caspase detection after exposure to each individual toxin.
We used a pan-caspase inhibitor, z-VAD-fmk, to ascertain the specificities of the signals induced upon toxin exposure. The pan-caspase inhibitor was able to inhibit caspase activity at all concentrations of toxins used. Furthermore, the cells appeared healthy upon visualization, suggesting that the inhibitor was able to prevent cell death induced by TcdA, TcdB, PEA, and DT (data not shown). We also determined the ability of a mutant toxin to induce caspase. Mutations that abolished the ADP-ribosylation activity of DT resulted in a toxin that was incapable of inducing caspase activity (28). Similarly, mutations that led to the loss of the glucosyltransferase activities of TcdA and TcdB resulted in toxins that failed to induce caspase activity (24, 34, 37–39). The data strongly suggest that the biological and enzymatic activities of the toxins are primarily responsible for caspase induction that results in the loss of viability and in cell death. As demonstrated by our studies and results, this assay allows for the differentiation of specific toxins at given concentrations and at different levels of biological activity.
Natural infection with C. difficile or immunization with toxins TcdA and TcdB results in antibody responses that are capable of neutralizing the toxins in vitro, which have shown to be protective against disease in vivo (40). Therefore, we evaluated the ability of the caspase assay to quantify the neutralization titers generated in hamsters upon immunization with toxin. We observed that caspase activation was inhibited by hyperimmune sera raised against TcdA and TcdB, demonstrating the specificity of the assay to reliably detect caspase signals due to bacterial toxins and to detect the neutralization of toxins by specific antibodies. Furthermore, we compared the neutralizing antibody titers generated in the animal study as measured by the caspase assay with those measured in the established neutralizing antibody assay (the actin polymerization cytometric assay described earlier), and we observed excellent correlation between the two assays, with a shorter cycle time of 3 days for caspase assay.
In summary, we present a versatile platform for quantifying the cytotoxic activities of several common bacterial toxins via the measurement of caspase activation as an early indicator of cell death. The signal obtained for caspase activity and therefore, apoptosis, is directly proportional to toxin dose and can be specifically blocked by anti-toxin antibodies at a magnitude relative to the antibody titer. The assay can be performed in a high-throughput format. It is a fast, efficient, and reliable method for evaluating cell apoptosis in response to bacterial toxins, as well as a means to measure neutralizing antibody titers. This assay is a valuable tool for the study of toxin-based vaccine candidates and their efficacy in clinical trials, and for establishing immune correlates to protective antibody levels for bacterial toxins that cause disease.
ACKNOWLEDGMENTS
We recognize the contributions of Rachel Xoconostle for the purification efforts of C. difficile recombinant toxins. We are also grateful to Andy Xie, Tony Kanavage, and Suzanne Cole for helpful discussions and to Joe Joyce and Jon Heinrichs for critical reading of the manuscript.
All authors are current employees of Merck and Co., Inc., and may own stocks of the company.
Footnotes
Published ahead of print 3 July 2013
REFERENCES
- 1.Rappuoli R, Pizza M, Douce G, Dougan G. 1996. New vaccines against bacterial toxins. Adv. Exp. Med. Biol. 397:55–60 [DOI] [PubMed] [Google Scholar]
- 2.Schmitt CK, Meysick KC, O'Brien AD. 1999. Bacterial toxins: friends or foes? Emerg. Infect. Dis. 5:224–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dorner F, McDonel JL. 1985. Bacterial toxin vaccines. Vaccine 3:94–102 [DOI] [PubMed] [Google Scholar]
- 4.Weinrauch Y, Zychlinsky A. 1999. The induction of apoptosis by bacterial pathogens. Annu. Rev. Microbiol. 53:155–187 [DOI] [PubMed] [Google Scholar]
- 5.Morimoto H, Bonavida B. 1992. Diphtheria toxin- and Pseudomonas A toxin-mediated apoptosis. ADP ribosylation of elongation factor-2 is required for DNA fragmentation and cell lysis and synergy with tumor necrosis factor-alpha. J. Immunol. 149:2089–2094 [PubMed] [Google Scholar]
- 6.Burnette WN. 1997. Bacterial ADP-ribosylating toxins: form, function, and recombinant vaccine development. Behring Inst. Mitt. 98:434–441 [PubMed] [Google Scholar]
- 7.Keppler-Hafkemeyer A, Kreitman RJ, Pastan I. 2000. Apoptosis induced by immunotoxins used in the treatment of hematologic malignancies. Int. J. Cancer 87:86–94 [PubMed] [Google Scholar]
- 8.Jenkins CE, Swiatoniowski A, Issekutz AC, Lin TJ. 2004. Pseudomonas aeruginosa exotoxin A induces human mast cell apoptosis by a caspase-8 and -3-dependent mechanism. J. Biol. Chem. 279:37201–37207 [DOI] [PubMed] [Google Scholar]
- 9.Castex F, Corthier G, Jouvert S, Elmer GW, Lucas F, Bastide M. 1990. Prevention of Clostridium difficile-induced experimental pseudomembranous colitis by Saccharomyces boulardii: a scanning electron microscopic and microbiological study. J. Gen. Microbiol. 136:1085–1089 [DOI] [PubMed] [Google Scholar]
- 10.Qa'Dan M, Ramsey M, Daniel J, Spyres LM, Safiejko-Mroczka B, Ortiz-Leduc W, Ballard JD. 2002. Clostridium difficile toxin B activates dual caspase-dependent and caspase-independent apoptosis in intoxicated cells. Cell Microbiol. 4:425–434 [DOI] [PubMed] [Google Scholar]
- 11.Voth DE, Ballard JD. 2005. Clostridium difficile toxins: mechanism of action and role in disease. Clin. Microbiol. Rev. 18:247–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imre G, Heering J, Takeda AN, Husmann M, Thiede B, zu Heringdorf DM, Green DR, van der Goot FG, Sinha B, Dötsch V, Rajalingam K. 2012. Caspase-2 is an initiator caspase responsible for pore-forming toxin-mediated apoptosis. EMBO J. 31:2615–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kochi SK, Collier RJ. 1993. DNA fragmentation and cytolysis in U937 cells treated with diphtheria toxin or other inhibitors of protein synthesis. Exp. Cell Res. 208:296–302 [DOI] [PubMed] [Google Scholar]
- 14.Bossy-Wetzel E, Newmeyer DD, Green DR. 1998. Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J. 17:37–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wyllie AH, Kerr JF, Currie AR. 1980. Cell death: the significance of apoptosis. Int. Rev. Cytol. 68:251–306 [DOI] [PubMed] [Google Scholar]
- 16.Li J, Yuan J. 2008. Caspases in apoptosis and beyond. Oncogene 27:6194–6206 [DOI] [PubMed] [Google Scholar]
- 17.Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J. 1996. Human ICE/CED-3 protease nomenclature. Cell 87:171. 10.1016/50248-4900(97)89273-8 [DOI] [PubMed] [Google Scholar]
- 18.Salvesen GS, Riedl SJ. 2008. Caspase mechanisms. Adv. Exp. Med. Biol. 615:13–23 [DOI] [PubMed] [Google Scholar]
- 19.Sundriyal A, Roberts AK, Ling R, McGlashan J, Shone CC, Acharya KR. 2010. Expression, purification and cell cytotoxicity of actin-modifying binary toxin from Clostridium difficile. Protein Expr. Purif. 74:42–48 [DOI] [PubMed] [Google Scholar]
- 20.Brito GA, Fujji J, Carneiro-Filho BA, Lima AA, Obrig T, Guerrant RL. 2002. Mechanism of Clostridium difficile toxin A-induced apoptosis in T84 cells. J. Infect. Dis. 186:1438–1447 [DOI] [PubMed] [Google Scholar]
- 21.Sullivan NM, Pellett S, Wilkins TD. 1982. Purification and characterization of toxins A and B of Clostridium difficile. Infect. Immun. 35:1032–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brito GA, Carneiro-Filho B, Oriá RB, Destura RV, Lima AA, Guerrant RL. 2005. Clostridium difficile toxin A induces intestinal epithelial cell apoptosis and damage: role of Gln and Ala-Gln in toxin A effects. Dig. Dis. Sci. 50:1271–1278 [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Sun X, Zhang Y, Li S, Chen K, Shi L, Nie W, Kumar R, Tzipori S, Wang J, Savidge T, Feng H. 2012. A chimeric toxin vaccine protects against primary and recurrent Clostridium difficile infection. Infect. Immun. 80:2678–2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teichert M, Tatge H, Schoentaube J, Just I, Gerhard R. 2006. Application of mutated Clostridium difficile toxin A for determination of glucosyltransferase-dependent effects. Infect. Immun. 74:6006–6010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jank T, Giesemann T, Aktories K. 2007. Clostridium difficile glucosyltransferase toxin B-essential amino acids for substrate binding. J. Biol. Chem. 282:35222–35231 [DOI] [PubMed] [Google Scholar]
- 26.Mitamura T, Umata T, Nakano F, Shishido Y, Toyoda T, Itai A, Kimura H, Mekada E. 1997. Structure-function analysis of the diphtheria toxin receptor toxin binding site by site-directed mutagenesis. J. Biol. Chem. 272:27084–27090 [DOI] [PubMed] [Google Scholar]
- 27.Kageyama T, Ohishi M, Miyamoto S, Mizushima H, Iwamoto R, Mekada E. 2007. Diphtheria toxin mutant CRM197 possesses weak EF2-ADP-ribosyl activity that potentiates its anti-tumorigenic activity. J. Biochem. 142:95–104 [DOI] [PubMed] [Google Scholar]
- 28.Giannini G, Rappuoli R, Ratti G. 1984. The amino-acid sequence of two non-toxic mutants of diphtheria toxin: CRM45 and CRM197. Nucleic Acids Res. 12:4063–4069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie J, Zorman J, Indrawati L, Horton M, Soring K, Antonello JM, Zhang Y, Secore S, Miezeiewski M, Wang S, Kanavage AD, Skinner JM, Rogers I, Bodmer JL, Heinrichs JH. 2013. Development and optimization of a novel assay to measure neutralizing antibodies against Clostridium difficile toxins. Clin. Vaccine Immunol. 20:517–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun X, He X, Tzipori S, Gerhard R, Feng H. 2009. Essential role of the glucosyltransferase activity in Clostridium difficile toxin-induced secretion of TNF-alpha by macrophages. Microb. Pathog. 46:298–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rappuoli R. 1994. Toxin inactivation and antigen stabilization: two different uses of formaldehyde. Vaccine 12:579–581 [DOI] [PubMed] [Google Scholar]
- 32.Carneiro BA, Fujii J, Brito GA, Alcantara C, Oria RB, Lima AA, Obrig T, Guerrant RL. 2006. Caspase and bid involvement in Clostridium difficile toxin A-induced apoptosis and modulation of toxin A effects by glutamine and alanyl-glutamine in vivo and in vitro. Infect. Immun. 74:81–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hippenstiel S, Schmeck B, N′Guessan PD, Seybold J, Krüll M, Preissner K, Eichel-Streiber CV, Suttorp N. 2002. Rho protein inactivation induced apoptosis of cultured human endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 283:L830–L838. 10.1152/ajplung.00467.2001 [DOI] [PubMed] [Google Scholar]
- 34.Tian JH, Fuhrmann SR, Kluepfel-Stahl S, Carman RJ, Ellingsworth L, Flyer DC. 2012. A novel fusion protein containing the receptor binding domains of C. difficile toxin A and toxin B elicits protective immunity against lethal toxin and spore challenge in preclinical efficacy models. Vaccine 30:4249–4258 [DOI] [PubMed] [Google Scholar]
- 35.Ogura K, Yahiro K, Tsutsuki H, Nagasawa S, Yamasaki S, Moss J, Noda M. 2011. Characterization of Cholix toxin-induced apoptosis in HeLa cells. J. Biol. Chem. 286:37207–37215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Venter BR, Kaplan NO. 1976. Diphtheria toxin effects on human cells in tissue culture. Cancer Res. 36:4590–4594 [PubMed] [Google Scholar]
- 37.Spyres LM, Daniel J, Hensley A, Qa'Dan M, Ortiz-Leduc W, Ballard JD. 2003. Mutational analysis of the enzymatic domain of Clostridium difficile toxin B reveals novel inhibitors of the wild-type toxin. Infect. Immun. 71:3294–3301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Busch C, Hofmann F, Selzer J, Munro S, Jeckel D, Aktories K. 1998. A common motif of eukaryotic glycosyltransferases is essential for the enzyme activity of large clostridial cytotoxins. J. Biol. Chem. 273:19566. [DOI] [PubMed] [Google Scholar]
- 39.Lanis JM, Barua S, Ballard JD. 2010. Variations in TcdB activity and the hypervirulence of emerging strains of Clostridium difficile. PLoS Pathog. 6:e1001061. 10.1371/journal.ppat.1001061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giannasca PJ, Warny M. 2004. Active and passive immunization against Clostridium difficile diarrhea and colitis. Vaccine 22:848–856 [DOI] [PubMed] [Google Scholar]