Abstract
Rapid and accurate detection of Shiga toxin-producing Escherichia coli (STEC) of all serotypes from patients with diarrhea is critical for medical management and for the prevention of ongoing transmission. In this prospective study, we assessed the performance of a multiplex, real-time PCR assay targeting stx1 and stx2 for the detection of O157 and non-O157 STEC in diarrheal stool samples enriched in Gram-negative broth. We show that the assay is 100% sensitive (95% confidence interval [CI], 89.1% to 100%) and 98.5% specific (95% CI, 90.6% to 99.9%) based on a panel of 40 known STEC-positive specimens and 65 known negative specimens. During a 2-year postvalidation period, the assay detected more positive samples from patients in northern California than did culture and PCR testing performed at a public health reference laboratory, with a positive predictive value of 95.6% (95% CI, 87.6% to 99.1%). Serotyping data showed an incidence rate of 51.2% for non-O157 STEC strains, with 5.8% of patients (1/17) with non-O157 strains and 42.9% (6/14) with O157 strains (P = 0.03) developing hemolytic-uremic syndrome. The findings from this study underscore the recommendations of the CDC for laboratories to test all diarrheal stool samples from patients with acute community-acquired diarrhea for non-O157 STEC in addition to the O157 serotype by using a sensitive assay. Additionally, a survey of 17 clinical laboratories in northern California demonstrated that nearly 50% did not screen all stool specimens for the presence of Shiga toxins, indicating that many clinical microbiology laboratories still do not routinely screen all stool specimens for the presence of Shiga toxins as recommended in the 2009 CDC guidelines.
INTRODUCTION
Shiga toxin-producing Escherichia coli (STEC) infections are a major cause of bacterial gastroenteritis throughout the world and can be complicated by hemorrhagic colitis and hemolytic- uremic syndrome (HUS) (1, 2). Although previously the majority of reported cases were attributed to E. coli O157:H7 and many recent studies indicate higher incidence rates of HUS associated with E. coli O157:H7 (3, 4), non-O157 STEC serotypes are emerging as important etiological agents of both sporadic cases and community outbreaks of diarrhea (3–8). Most notably, in May and June 2011, a large outbreak of E. coli O104:H4-associated diarrheal illness in Germany led to HUS in over 800 patients, many of whom were adults, and ultimately resulted in 54 deaths (5, 9). This outbreak and others have underscored the fact that severe disease and HUS can occur in cases related to non-O157 or O157 E. coli strains and that adults as well as children are susceptible to these complications (8–10). Timely laboratory identification of STEC has important implications for outbreak containment and patient management, including prompt parenteral hydration, monitoring for development of HUS, and avoidance of antibiotics and antidiarrheal agents, which can exacerbate disease (1). Importantly, a number of studies have shown that STEC-related illnesses do not always manifest with fever and bloody diarrhea and are not restricted to a specific season (3, 4, 7, 11); therefore, using such factors in deciding whether to test for STEC leads to missed cases. Given these considerations, the CDC has issued recommendations to test simultaneously for O157 and non-O157 STEC in all stool specimens from patients with acute community-acquired diarrhea (10).
Despite these guidelines, a recent survey by the Washington State Department of Health found that in 2010 only one-third of laboratories in that state performed Shiga toxin assays, with the majority performing exclusively O157 culture (12). Additionally, a recent assessment of physician knowledge and practices regarding STEC found that only 6% of the surveyed physicians specifically ordered testing for non-O157 STEC, while 30% believed that the test is included in a stool test panel and 60% did not consider testing for non-O157 STEC at all (13). These findings indicate that proper diagnosis and management of non-O157 STEC infections depend on improved physician awareness as well as improved laboratory practices for detection of STEC.
The assays recommended by the CDC for non-O157 STEC detection include enzyme immunoassays (EIAs) for Shiga toxin antigens and nucleic acid-based methods for detection of the corresponding genes (stx1 and stx2) (10). A number of EIAs have been approved by the Food and Drug Administration (FDA) for the diagnosis of human STEC infections and have the ability to detect non-O157 STEC in addition to E. coli O157, in contrast to sorbitol-MacConkey agar (SMAC) culture, which detects only E. coli O157 (7, 11). However, EIAs can have relatively high false-positive rates and have been reported to miss E. coli O157 (7, 10, 14). A number of nucleic acid-based methods, including multiplex, real-time PCR assays, have also been described (10, 15–20). Additionally, a Luminex-based molecular assay targeting 15 stool pathogens including STEC was recently approved by the FDA (21). While these assays are more sensitive than EIAs (14, 22), many of them require the use of fluorescently labeled probes, which can be prohibitively expensive for screening assays that would need to be performed thousands of times in order to accommodate the large numbers of stool specimens that are routinely submitted to many clinical microbiology laboratories.
Here, we present a sensitive multiplex, real-time PCR assay for stx1 and stx2 that uses affordable methodology for DNA extraction and real-time PCR and can be employed as a screening tool for overnight enrichment broths in order to accomplish timely reporting of STEC infections to treating physicians. We describe the performance of the assay and the epidemiology of O157 and non-O157 STEC over 2 years in a major U.S. academic center in northern California.
MATERIALS AND METHODS
Ethics.
This study was approved by the institutional review board of Stanford University.
Patient population and data collection.
The study was performed at the Stanford Hospital and Clinics (SHC) clinical microbiology laboratory between July 2010 and July 2012. A total of 4,900 consecutive stool specimens submitted for culture were tested for the presence of stx1 and stx2 with a multiplex, real-time PCR assay developed and validated by the laboratory, as outlined below. The specimens were submitted from adult and pediatric inpatient and outpatient facilities and emergency departments. Clinical data were collected from electronic medical records.
Stool specimen processing.
For each stool sample, 10 μl (liquid specimens) or a lightly coated sterile swab (solid specimens) was inoculated in Gram-negative (GN) broth (BD Diagnostics, Sparks, MD) and incubated at 35 to 37°C for 18 to 24 h. On the following day, 10 μl of GN broth culture was transferred into 90 μl PCR-grade water; the mixture was incubated at ∼100°C for 10 min to lyse the bacteria, immediately cooled, and centrifuged at 12,600 × g for 15 s before an aliquot of supernatant was removed for the PCR assay.
Multiplex, real-time PCR assay design and validation.
The screening and confirmatory multiplex PCR assays simultaneously detect the stx1 and stx2 genes (Table 1). Candidate primer sets were created using Clone Manager Professional Suite (Science and Educational Software, Cary, NC) and NCBI reference sequence NC_002695 (Escherichia coli O157:H7 strain Sakai). Selected primers were aligned with stx1 (n = 23) and stx2 (n = 28) gene sequences deposited in the NCBI database to ensure that the primers ubiquitously detected Shiga toxin genes from different E. coli strains. Additionally, the primers were queried for nonspecific binding using NCBI BLASTN. The stx2 forward and reverse primers in the screening primer set were as described previously (23, 24), with minor modifications.
Table 1.
Primers used in this study
| Primer mix | Primer name | Target | Sequence (reference no.) | Reference positiona | Product length (bp) | Tm (range) (°C) |
|---|---|---|---|---|---|---|
| Screening | STX1 FWD 03 | Stx1 | GCGGTTACATTGTCTGGTGA | 2925319–2925338 | 255 | 79 (77–80) |
| STX1 REV 03 | AGAACGYCCACTGAGATCAT | 2925084–2925103 | ||||
| STX2 FWD 01 | Stx2 | CATGACAACGGACAGCAGTT (24) | 1267351–1267370 | 311 | 81 (80–83) | |
| STX2 REV 02 | CGGAAGCACATTGCTGATT (23) | 1267642–1267660 | ||||
| Confirmatory | STX1 FWD 02 | Stx1 | TCGCTTTGCTGATTTTTCACA | 2925361–2925380 | 129 | 78 (77–79) |
| STX1 REV 02 | ATGGCGATTTATCTGCATCC | 2925252–2925271 | ||||
| STX2 FWD 03 | Stx2 | TCATCATATCTGGCGTTAATGG | 1267439–1267460 | 140 | 78 (77–79) | |
| STX2 REV 01 | GACAGTGCCWGACGAAATTCTC | 1267557–1267578 |
Reference sequence NC_002695 (Escherichia coli O157:H7 strain Sakai).
Validation was performed using 65 known STEC-negative samples and 40 known STEC-positive samples. The absence of E. coli O157 in the negative specimens was based on lack of isolation of E. coli O157 on O157 CHROMagar (BD Diagnostics, Sparks, MD). Fifty of the 65 negative samples were clinical stool specimens for which no stool pathogens were identified by culture. Additionally, 15 clinical stool specimens that were culture positive for Shigella and Salmonella species were tested to evaluate the analytical specificity of the assay. These species included Shigella sonnei, Shigella dysenteriae, Shigella flexneri, Salmonella enterica serotype Typhi, and groups B, C, D, and K. The 40 known STEC-positive specimens included 25 culture-negative stool specimens spiked with control E. coli O157:H7 ATCC 43895 (American Type Culture Collection [ATCC], Manassas, VA) and 15 STEC isolates from the California Department of Public Health (CDPH) laboratory, representing common serotypes circulating in California, namely, stx1-positive E. coli serotypes O121, O103, O145, and O26, stx2-positive E. coli O121, and stx1/stx2-double-positive E. coli O111.
PCR mixtures included 3 μl of DNA extract, 5 μl of 2× FastStart SYBR Green Master (Roche Applied Science, Indianapolis, IN), and 2 μl of screening or confirmatory primer mix (Table 1). The final concentration of each primer was 0.5 μM. The reactions were run on a Rotor-Gene 6000 real-time cycler (Qiagen, Germantown, MD), using the following cycling parameters: 95°C for 5 min and 40 cycles of 95°C for 15 s, 60°C for 30 s, and 72°C for 30 s, followed by melting with ramping from 70°C to 90°C in 0.2°C increments. A positive-control E. coli O157 sample (ATCC 43895), carrying stx1 and stx2, was processed and run at the same time as patient samples, to control for extraction and positivity. A negative-control sample (PCR-grade water) also was included in each PCR run. A separate real-time PCR with universal 16S rRNA primers 1312F (5′-AAGYYGGAATCGCTAGTAATCG-3′) and 1366R (5′-CCGGGAACGTATTCACCG-3′) was performed for each extract, as a control for DNA extraction and amplification. A sample was considered positive for stx if it had an amplicon with a melting temperature (Tm) within the expected range for each primer set (Table 1).
Positive results were reported to the ordering physician and the infection control department, typically within 24 to 36 h after specimen collection. Additionally, 1 ml of positive GN broth was sent on an ice pack to the Santa Clara County Public Health Department (SCCPHD) for E. coli O157 screening on O157 CHROMagar. O157 screen-negative broths (transported and stored at 4°C) were sent to the CDPH for further testing, which included stx detection in broth by conventional PCR assay (16) and culture of the broth for isolation and identification of E. coli using conventional media, followed by stx PCR assay for each E. coli isolate to confirm the presence of STEC. O157 screen-positive isolates were sent from the SCCPHD to the CDPH on a nutrient agar slant at room temperature, for testing for the presence of stx and H flagellar genes by conventional PCR assays (25) and for serotyping using antisera.
If the CDPH work-up results were negative for stx, then a real-time PCR assay was set up in our laboratory with the confirmatory primer mix (Table 1), using the original DNA extract. Although initially the confirmatory reaction was performed only for specimens with discrepant results from the screening real-time PCR assay and the CDPH conventional PCR assay, the testing algorithm was subsequently revised to include the confirmatory PCR assay for all screen-positive specimens. For the purposes of this study, the PCR products were also sequenced for specimens that were negative by the CDPH test but positive by the SHC confirmatory real-time PCR assay. Cycle sequencing was performed as previously described (26), using the PCR primers shown in Table 1. The sequences were queried against known stx gene sequences in GenBank, using BLASTN.
Telephone survey of clinical laboratories in Santa Clara County.
A survey of 17 laboratories in Santa Clara County in northern California was performed in August 2012. The surveyed laboratories included three commercial laboratories and 14 community or academic hospitals. The following specific questions were asked: (i) whether the laboratory performed screening for Shiga toxin for all submitted stool specimens or only on request, (ii) what Shiga toxin test was used and whether specimens had to be sent to a reference laboratory, and (iii) whether E. coli O157 screening was performed for all stool specimens or on request and what test was used.
Statistical analysis.
All statistical analyses, including determinations of sensitivity, specificity, positive predictive value, and 95% confidence intervals (CIs) and Student's t test analysis, were performed using Prism (GraphPad Software, La Jolla, CA). Categorical variables were analyzed using Fisher's exact test. P values of ≤0.05 were considered statistically significant.
RESULTS
Multiplex, real-time stx PCR assay development and validation.
Screening and confirmatory primer sets (Table 1) were designed for a real-time PCR assay with melting curve analysis (Fig. 1). In silico alignment with the sequences of 23 stx1 gene sequences and 28 stx2 gene sequences deposited in GenBank revealed perfect matches of the stx1 and stx1 primers, respectively, with all strains that were queried. The only exception was STX1 FWD 02 in the confirmatory reaction, which had a mismatch in the first 5′ nucleotide for 12 of the 23 queried strains. NCBI BLASTN queries revealed that stx1 primers shared 100% identity with toxin A of Shigella dysenteriae. Additionally, STX2 FWD 03 and STX2 REV 01 were found to share 100% identity with Aeromonas caviae Shiga toxin 2, whereas STX1 REV 03 was found to share 100% identity with Aeromonas caviae Shiga toxin 1. Of the 65 STEC-negative samples tested for primer validation, 64 were negative by the screening and confirmatory real-time PCR assays (specificity, 98.5% [95% CI, 91.7% to 99.9%]). One of the culture-negative clinical specimens tested positive with both primer mixes, with the screening assay indicating the presence of stx1; however, no further evaluation was pursued. No cross-reactivity with non-dysenteriae Shigella or Salmonella species was identified. Both the screening and confirmatory real-time PCR assays detected the stx genes in the 40 known positive samples (sensitivity, 100% [95% CI, 91.2% to 100%]).
Fig 1.
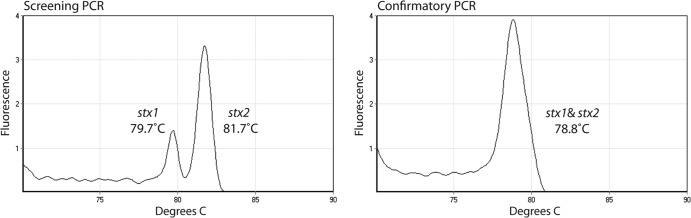
Melting curve profiles of the SYBR green amplicons obtained in multiplex, real-time PCR assays with the screening and confirmatory primer mixes. Representative melting curves for E. coli O157 (ATCC 43895) are shown. Peaks corresponding to specific products are labeled. The two peaks for stx1 and stx2 with the screening primer mix have melting temperature (Tm) ranges of 77 to 80°C and 80 to 83°C, respectively. The stx1 and stx2 amplicons for the confirmatory primer mix have similar Tm values and appear as a single peak at 77 to 79°C.
Performance of the multiplex, real-time stx PCR assay in a U.S. hospital.
Of 4,900 stool specimens tested, 81 (1.65%) were positive, from 69 unique patients. The 69 diagnostic specimens were submitted to the CDPH, which confirmed stx presence in 57/69 (82.6%) specimens. The specimens for which stx test results were reported as negative by the CDPH were assayed with the confirmatory primer set; 8 specimens tested positive, 3 specimens tested negative, and 1 specimen was no longer available. Sequencing and GenBank alignment of the 8 amplified products demonstrated stx1 alone in 5 specimens, stx2 alone in 1 specimen, and stx1 plus stx2 in 2 specimens. Thus, results for 65/69 screen-positive specimens were confirmed by the CDPH or the in-house confirmatory real-time PCR assay. After exclusion of the sample that was unavailable for confirmation, a positive predictive value of 95.6% was calculated (95% CI, 87.6% to 99.1%).
STEC was isolated and serotyped by the CDPH for 43 specimens, and E. coli O157 was identified for 21 (48.8%). The non-O157 E. coli serotypes isolated from the remaining 22 (51.2%) specimens included six cases of O111 (13.9%), four of O26 (9.3%), four of O103 (9.3%), three of O121 (6.9%), one of O76, and one of O rough; the O antigen could not be specifically identified for 3 E. coli isolates. After multiple attempts at the public health laboratory, STEC isolates could not be cultured for 22 of 64 confirmed stx-positive GN broths, two of which were culture positive for Aeromonas hydrophila at both the SHC clinical microbiology laboratory and the CDPH laboratory. Importantly, the presence of stx1 in both GN broths was confirmed by the CDPH.
Comparison of clinical characteristics of patients with O157 and non-O157 infections.
To examine whether O157-mediated STEC infections had greater severity and a greater likelihood of leading to HUS in our patient population, we performed chart reviews for patients with culture-confirmed STEC infections, with specific attention to the presence of bloody diarrhea, fever, hospitalization, and a diagnosis of HUS. Anamnestic histories were available for 16 of 21 patients with E. coli O157 infections and 18 of 22 with non-O157 STEC infections. A patient with Crohn's disease flare, a patient with metastatic cancer and a concurrent O157 infection, and a patient with an E. coli O121:NM infection who was admitted for psychiatric treatment were excluded from the analysis of hospital admissions. In all, 14 O157 cases and 17 non-O157 cases were analyzed further, revealing statistically significantly larger proportions of patients with blood in the stool and HUS in the E. coli O157 group (Table 2). All cases of HUS occurred in pediatric patients. Based on melting temperature profiles in the screening PCR assay (Fig. 1), the STEC isolates in 6 of the 7 cases of HUS were positive for stx2 (5 carrying stx2 alone and 1 carrying both stx genes; data not shown), consistent with prior reports linking the presence of stx2 with more-severe disease (27). Documented fever and admission to the hospital also were more frequent in O157 cases, although these differences were not statistically significant.
Table 2.
Clinical and demographic characteristics of patients with confirmed stx-positive stool specimens
| Characteristic | Value for: |
Pc | |
|---|---|---|---|
| O157 | Non-O157 | ||
| No. of patients | 21 | 22 | |
| Mean age (range) (yr) | 10.0 (0–51) | 19.7 (1–72) | 0.10 |
| History available (no./total no. [%]) | 14/21 (76.2)a | 17/22 (77.3)b | |
| Blood in stool (no./total no. [%]) | 13/14 (92.9) | 10/17 (58.8) | 0.04 |
| Febrile (no./total no. [%]) | 9/14 (64.3) | 7/17 (41.2) | 0.28 |
| Hospitalized (no./total no. [%]) | 12/14 (85.7) | 10/17 (58.8) | 0.13 |
| HUS (no./total no. [%]) | 6/14 (42.9) | 1/17 (5.8) | 0.03 |
Two patients with E. coli O157 had alternative diagnoses (colon cancer and Crohn's disease) and were excluded from further analysis.
One patient with non-O157 STEC was admitted for psychiatric treatment, not treatment of gastroenteritis, and was excluded from further analysis.
The two-sided Fisher exact test was used except for mean age, where the two-sided Student t test was used.
Laboratory practices for non-O157 E. coli testing in northern California.
Given the large proportions of non-O157 E. coli serotypes identified, we wanted to assess laboratory practices regarding the use of Shiga toxin testing in our geographic area. Seventeen clinical microbiology laboratories were surveyed, of which only 1 (5.9%) reported screening all specimens for E. coli O157 and Stx, 8 (47%) reported screening all stool specimens with an Stx test, and the remaining 8 (47%) reported testing for Stx only on request. In all, 9 (53%) of 17 laboratories performed Stx screening on all stool specimens, most frequently with an immunoassay (7 [77.8%] of 9 laboratories). Regarding O157 screening practices, 4 (23.5%) of 17 laboratories reported screening all specimens for E. coli O157, two laboratories reported subjecting specimens to O157 testing only if positive for Stx, six laboratories (35.3%) performed O157 testing only on request or in the presence of a bloody specimen, and five laboratories (29.4%) did not perform O157 testing at all.
DISCUSSION
The study presented here reports the validation and performance of a sensitive multiplex, real-time PCR assay for the detection of Shiga toxin genes in overnight enrichment broth cultures of clinical stool specimens. The assay employs the intercalating dye SYBR green, which is a widely used and relatively inexpensive (less than $1 per assay) real-time PCR reagent, making it possible to screen the large numbers of stool samples routinely submitted to clinical laboratories. This in turn allows reporting of preliminary positive results to treating clinicians within 24 to 36 h after specimen collection, while the results of serotyping by a public health laboratory are pending. The assay also provides an algorithm for confirmation of presumed positive results with an independent set of primers that target different regions of the stx1 and stx2 genes. In the 2 years that the assay has been performed routinely on all stool samples sent to our laboratory, it has identified more stx-positive clinical specimens than the conventional PCR method used by the public health laboratory (16). The positive predictive value of the assay was calculated to be 95.6% (95% CI, 87.6% to 99.1%), based on the combined results of confirmation by the CDPH or testing with the in-house confirmatory primer set and Sanger sequencing.
The primers used in this assay showed no cross-reactivity with non-dysenteriae Shigella or Salmonella species in the validation phase, although both stx1 primer sets share 100% identity with toxin A of S. dysenteriae. This cross-reactivity has been reported for other Shiga toxin assays but it is unlikely to be observed frequently in northern California, since fewer than 50 cases of S. dysenteriae per year are reported in the United States (28). Additionally, one stx1 primer in the screening PCR assay and both stx2 primers in the confirmatory PCR assay were found to share 100% identity with Aeromonas caviae Shiga toxin 1 and toxin 2, respectively. However, Aeromonas isolates rarely carry the stx2 gene (29), and the remaining stx1 primers did not show appreciable identity with Aeromonas stx sequences. After the assay was implemented, two stx1-positive specimens were identified that grew Aeromonas hydrophila but not STEC. The SHC and the CDPH had concordant results for the presence of stx1 by PCR assay, the presence of Aeromonas hydrophila by culture, and the absence of isolated E. coli. One possible explanation is horizontal acquisition of stx by Aeromonas hydrophila, which has been reported (29). Alternatively, the Aeromonas-positive specimens may have been coinfected with a strain of STEC that could not be cultured, since mixed infections of Aeromonas with stool pathogens have been described (30). Because this possibility could not be excluded and the CDPH was able to confirm stx positivity, these samples were considered true-stx-positive samples. Review of additional clinical specimens that were culture positive for Aeromonas (n = 19), Campylobacter (n = 54), or Plesiomonas (n = 1) did not reveal more dual-positive samples.
One of the limitations of our assay is that it requires overnight enrichment culture, which does not allow same-day notification of treating physicians. Although real-time PCR assays for use on direct stool specimens have been described (15), it is unclear whether they have equivalent sensitivity, compared to assays with overnight enrichment broths, especially since some STEC organisms, including O157 and O111, can have infectious doses of <100 organisms (2, 10). Another limitation of the assay is that it does not provide serotype information. In this study, enrichment broths that resulted in positive test results were immediately forwarded to the public health laboratory for further testing, including culture and serotyping. This allowed the STEC organism to be identified by the reference laboratory for 66.2% of stx-positive enrichment broths. The CDPH laboratory was unable to isolate the STEC organism for 22 (33.8%) of 65 specimens that were confirmed to be stx positive by the in-house confirmatory test and/or the CDPH assay. Possible reasons may be decreased viability during transport, low numbers of organisms below the sensitivity limit for STEC culture conditions, or antibiotic treatment at the time of specimen collection.
To our knowledge, this is the first report from California describing testing practices for STEC at an academic institution and comparing the incidence rates of O157 versus non-O157 STEC with the revised CDC guidelines from 2009. Similar numbers of O157 and non-O157 E. coli strains were isolated (21 versus 22 cases), with O26, O76, O103, O111, and O121 accounting for the non-O157 serotypes. These serotypes are among the six most common non-O157 STEC bacteria reported in association with diarrhea in the United States, in addition to O45 and O145 (10). Our finding that 22 (51.2%) of 43 serotypeable STEC strains were non-O157 is consistent with recent reports from Massachusetts, Connecticut, and Minnesota (3, 4, 7). In all of those studies, many of the non-O157 STEC illnesses were complicated by hemorrhagic colitis and hospital admission, although two of the reports found that O157 infections tended to be more severe. In the study presented here, the proportions of patients with bloody diarrhea and HUS were significantly larger for O157 than non-O157 STEC cases. Thus, our data confirm that O157 infections more frequently present with HUS, although the large number of non-O157 cases that were complicated by fever and bloody diarrhea and required hospitalization also clearly emphasizes the need for timely identification of non-O157 STEC. Given the higher risk of HUS with O157, the stx-testing algorithm of a laboratory can incorporate testing of stx-positive GN broths either by plating on selective medium or by real-time PCR assay (31), in order to provide next-day notification of treating physicians regarding the presence of this pathogen.
Findings from this study, as well as epidemiological data from recent non-O157 STEC outbreaks (6, 8, 9), support the CDC recommendations that all stool specimens from cases of community-acquired diarrhea be tested simultaneously for O157 and non-O157 STEC, regardless of patient age, time of year, and presence of blood in the stool (10). Importantly, however, these guidelines have not been uniformly adopted by clinical microbiology laboratories in the United States, as indicated by a recent survey from Washington State (12). The authors of that study showed that nearly 50% of the 71,000 stool specimens that collectively were processed by the surveyed laboratories did not undergo screening for non-O157 STEC. However, approximately one-third of the surveyed laboratories did adopt Shiga toxin testing following the 2009 CDC guidelines, which was associated with increased reporting of non-O157 STEC infections statewide. In fact, non-O157 organisms accounted for ∼40% of reported STEC infections in 2010, compared to only 6% in 2005 (12), which is closer to findings reported elsewhere in the United States and worldwide (2–4). At the CDPH in California, the number of non-O157 STEC infections (n = 269) surpassed that of O157 STEC infections (n = 228) in 2011 (W. Probert, CDPH, personal communication).
The findings of the Washington State Department of Health study are largely supported by the results of a smaller-scale survey performed in northern California and presented in this report. Reasons for not implementing Shiga toxin testing that were cited in the Washington State Department of Health study included cost and procedural changes (12). Although adopting a real-time PCR assay is generally associated with added cost, the one described here is relatively inexpensive and can be easily implemented in laboratories with the capacity to perform nucleic acid tests. The assay incorporates an independent primer mix for same-day confirmation of stx positivity and allows reporting of preliminary stx positivity to treating clinicians within 24 to 36 h after specimen submission. It also can be used as part of a real-time PCR panel for testing of community-acquired diarrhea that includes other pathogens such as Salmonella, Shigella, and Campylobacter and as part of an algorithm that allows identification of the most common STEC serotypes encountered in a given population (31).
In summary, we describe a simple multiplex, real-time PCR assay for sensitive detection of O157 and non-O157 STEC serotypes from stool samples enriched in GN broth. The high incidence of non-O157 STEC strains at our institution underscores the CDC recommendations for laboratories in northern California to test all diarrheal stool samples for O157 and non-O157 STEC serotypes.
ACKNOWLEDGMENTS
We thank Divinia Samson and the staff of the SHC clinical microbiology laboratory for technical assistance. We thank the CDPH for providing reagents and Will Probert for sharing data.
Footnotes
Published ahead of print 10 July 2013
REFERENCES
- 1.Paton JC, Paton AW. 1998. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin. Microbiol. Rev. 11:450–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gyles CL. 2007. Shiga toxin-producing Escherichia coli: an overview. J. Anim. Sci. 85:E45–E62 [DOI] [PubMed] [Google Scholar]
- 3.Hadler JL, Clogher P, Hurd S, Phan Q, Mandour M, Bemis K, Marcus R. 2011. Ten-year trends and risk factors for non-O157 Shiga toxin-producing Escherichia coli found through Shiga toxin testing, Connecticut, 2000–2009. Clin. Infect. Dis. 53:269–276 [DOI] [PubMed] [Google Scholar]
- 4.Hedican EB, Medus C, Besser JM, Juni BA, Koziol B, Taylor C, Smith KE. 2009. Characteristics of O157 versus non-O157 Shiga toxin-producing Escherichia coli infections in Minnesota, 2000–2006. Clin. Infect. Dis. 49:358–364 [DOI] [PubMed] [Google Scholar]
- 5.Bielaszewska M, Mellmann A, Zhang W, Kock R, Fruth A, Bauwens A, Peters G, Karch H. 2011. Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: a microbiological study. Lancet Infect. Dis. 11:671–676 [DOI] [PubMed] [Google Scholar]
- 6.Brown JA, Hite DS, Gillim-Ross LA, Maguire HF, Bennett JK, Patterson JJ, Comstock NA, Watkins AK, Ghosh TS, Vogt RL. 2012. Outbreak of Shiga toxin-producing Escherichia coli serotype O26:H11 infection at a child care center in Colorado. Pediatr. Infect. Dis. J. 31:379–383 [DOI] [PubMed] [Google Scholar]
- 7.Hermos CR, Janineh M, Han LL, McAdam AJ. 2011. Shiga toxin-producing Escherichia coli in children: diagnosis and clinical manifestations of O157:H7 and non-O157:H7 infection. J. Clin. Microbiol. 49:955–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaffzin JK, Coronado F, Dumas NB, Root TP, Halse TA, Schoonmaker-Bopp DJ, Lurie MM, Nicholas D, Gerzonich B, Johnson GS, Wallace BJ, Musser KA. 2012. Public health approach to detection of non-O157 Shiga toxin-producing Escherichia coli: summary of two outbreaks and laboratory procedures. Epidemiol. Infect. 140:283–289 [DOI] [PubMed] [Google Scholar]
- 9.Frank C, Werber D, Cramer JP, Askar M, Faber M, an der Heiden M, Bernard H, Fruth A, Prager R, Spode A, Wadl M, Zoufaly A, Jordan S, Kemper MJ, Follin P, Muller L, King LA, Rosner B, Buchholz U, Stark K, Krause G. 2011. Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N. Engl. J. Med. 365:1771–1780 [DOI] [PubMed] [Google Scholar]
- 10.Gould LH, Bopp C, Strockbine N, Atkinson R, Baselski V, Body B, Carey R, Crandall C, Hurd S, Kaplan R, Neill M, Shea S, Somsel P, Tobin-D'Angelo M, Griffin PM, Gerner-Smidt P. 2009. Recommendations for diagnosis of Shiga toxin-producing Escherichia coli infections by clinical laboratories. MMWR Recomm. Rep. 58(RR-12):1–14 [PubMed] [Google Scholar]
- 11.Gavin PJ, Peterson LR, Pasquariello AC, Blackburn J, Hamming MG, Kuo KJ, Thomson RB., Jr 2004. Evaluation of performance and potential clinical impact of ProSpecT Shiga toxin Escherichia coli microplate assay for detection of Shiga toxin-producing E. coli in stool samples. J. Clin. Microbiol. 42:1652–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stigi KA, Macdonald JK, Tellez-Marfin AA, Lofy KH. 2012. Laboratory practices and incidence of non-O157 Shiga toxin-producing Escherichia coli infections. Emerg. Infect. Dis. 18:477–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clogher P, Hurd S, Hoefer D, Hadler JL, Pasutti L, Cosgrove S, Segler S, Tobin-D'Angelo M, Nicholson C, Booth H, Garman K, Mody RK, Gould LH. 2012. Assessment of physician knowledge and practices concerning Shiga toxin-producing Escherichia coli infection and enteric illness, 2009, Foodborne Diseases Active Surveillance Network (FoodNet). Clin. Infect. Dis. 54(Suppl 5):S446–S452 [DOI] [PubMed] [Google Scholar]
- 14.Pulz M, Matussek A, Monazahian M, Tittel A, Nikolic E, Hartmann M, Bellin T, Buer J, Gunzer F. 2003. Comparison of a Shiga toxin enzyme-linked immunosorbent assay and two types of PCR for detection of Shiga toxin-producing Escherichia coli in human stool specimens. J. Clin. Microbiol. 41:4671–4675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grys TE, Sloan LM, Rosenblatt JE, Patel R. 2009. Rapid and sensitive detection of Shiga toxin-producing Escherichia coli from nonenriched stool specimens by real-time PCR in comparison to enzyme immunoassay and culture. J. Clin. Microbiol. 47:2008–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paton AW, Paton JC. 1998. Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J. Clin. Microbiol. 36:598–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jothikumar N, Griffiths MW. 2002. Rapid detection of Escherichia coli O157:H7 with multiplex real-time PCR assays. Appl. Environ. Microbiol. 68:3169–3171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshitomi KJ, Jinneman KC, Weagant SD. 2006. Detection of Shiga toxin genes stx1, stx2, and the +93 uidA mutation of E. coli O157:H7/H-using SYBR Green I in a real-time multiplex PCR. Mol. Cell. Probes 20:31–41 [DOI] [PubMed] [Google Scholar]
- 19.Bellin T, Pulz M, Matussek A, Hempen HG, Gunzer F. 2001. Rapid detection of enterohemorrhagic Escherichia coli by real-time PCR with fluorescent hybridization probes. J. Clin. Microbiol. 39:370–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chui L, Couturier MR, Chiu T, Wang G, Olson AB, McDonald RR, Antonishyn NA, Horsman G, Gilmour MW. 2010. Comparison of Shiga toxin-producing Escherichia coli detection methods using clinical stool samples. J. Mol. Diagn. 12:469–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Claas E, Burnham CAD, Mazulli T, Templeton K, Topin F. 2013. Performance of the xTAGR Gastrointestinal Pathogen Panel (GPP), a multiplex molecular assay for simultaneous detection of bacterial, viral and parasitic causes of infectious gastroenteritis. J. Microbiol. Biotechnol. 23:7. 10.4014/jmb.1212.12042 [DOI] [PubMed] [Google Scholar]
- 22.Chui L, Lee MC, Malejczyk K, Lim L, Fok D, Kwong P. 2011. Prevalence of Shiga toxin-producing Escherichia coli as detected by enzyme-linked immunoassays and real-time PCR during the summer months in northern Alberta, Canada. J. Clin. Microbiol. 49:4307–4310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belanger SD, Boissinot M, Menard C, Picard FJ, Bergeron MG. 2002. Rapid detection of Shiga toxin-producing bacteria in feces by multiplex PCR with molecular beacons on the Smart Cycler. J. Clin. Microbiol. 40:1436–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilmour MW, Chui L, Chiu T, Tracz DM, Hagedorn K, Tschetter L, Tabor H, Ng LK, Louie M. 2009. Isolation and detection of Shiga toxin-producing Escherichia coli in clinical stool samples using conventional and molecular methods. J. Med. Microbiol. 58:905–911 [DOI] [PubMed] [Google Scholar]
- 25.Gannon VP, D'Souza S, Graham T, King RK, Rahn K, Read S. 1997. Use of the flagellar H7 gene as a target in multiplex PCR assays and improved specificity in identification of enterohemorrhagic Escherichia coli strains. J. Clin. Microbiol. 35:656–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinsky BA, Samson D, Ghafghaichi L, Baron EJ, Banaei N. 2009. Comparison of real-time PCR and conventional biochemical methods for identification of Staphylococcus lugdunensis. J. Clin. Microbiol. 47:3472–3477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boerlin P, McEwen SA, Boerlin-Petzold F, Wilson JB, Johnson RP, Gyles CL. 1999. Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J. Clin. Microbiol. 37:497–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.CDC 2008. Shigella surveillance: annual summary, 2006. CDC, Atlanta, GA [Google Scholar]
- 29.Alperi A, Figueras MJ. 2010. Human isolates of Aeromonas possess Shiga toxin genes (stx1 and stx2) highly similar to the most virulent gene variants of Escherichia coli. Clin. Microbiol. Infect. 16:1563–1567 [DOI] [PubMed] [Google Scholar]
- 30.Pablos M, Remacha MA, Rodriguez-Calleja JM, Santos JA, Otero A, Garcia-Lopez ML. 2010. Identity, virulence genes, and clonal relatedness of Aeromonas isolates from patients with diarrhea and drinking water. Eur. J. Clin. Microbiol. Infect. Dis. 29:1163–1172 [DOI] [PubMed] [Google Scholar]
- 31.Gonzales TK, Kulow M, Park DJ, Kaspar CW, Anklam KS, Pertzborn KM, Kerrish KD, Ivanek R, Dopfer D. 2011. A high-throughput open-array qPCR gene panel to identify, virulotype, and subtype O157 and non-O157 enterohemorrhagic Escherichia coli. Mol. Cell. Probes 25:222–230 [DOI] [PubMed] [Google Scholar]


