Abstract
Brucellosis is a bacterial zoonotic disease which has been associated with laboratory-acquired infections. No recent reviews have addressed the characteristics of laboratory-acquired brucellosis (LAB). English-language literature was reviewed to identify reports of laboratory exposures to Brucella spp. and LAB cases between 1982 and 2007. Evaluation of 28 case reports identified 167 potentially exposed laboratory workers, of whom 71 had LAB. Nine reports were identified that summarized an additional 186 cases of LAB. Only 18 (11%) exposures were due to laboratory accidents, 147 (88%) exposures were due to aerosolization of organisms during routine identification activities, and the circumstances of 2 (1%) exposures were unknown. Brucella melitensis was the causative agent in 80% (135/167) of the exposures. Workers with high-risk exposures were 9.3 times more likely to develop LAB than workers with low-risk exposures (95% confidence interval [CI], 3.0 to 38.6; P < 0.0001); they were also 0.009 times likelier to develop LAB if they took antimicrobial PEP than if they did not (95% CI, 0 to 0.042; P < 0.0001). The median incubation period in case and summary reports was 8 weeks (range 1 to 40 weeks). Antimicrobial PEP is effective in preventing LAB. The incubation period may be used to identify appropriate serological and symptom surveillance time frames for exposed laboratory workers.
INTRODUCTION
Brucellosis is caused by pathogenic Brucella spp., of which Brucella abortus, B. melitensis, and B. suis most commonly affect humans. Brucellosis is a zoonotic disease that can be severe and may become chronic if untreated or treated improperly; common symptoms include undulant fever, myalgia, arthralgia, night sweats, and malaise (1). Brucella sp. infections can lead to spontaneous abortions and intrauterine fetal death in pregnant women but have not been associated with birth defects (2). Though the disease was once considered an occupational disease in the United States due to endemicity in domestic herd animals, control measures have substantially reduced the burden of animal disease and thus human disease (3, 4). Three primary sources of human infection are thought to exist in the United States today—consumption of unpasteurized dairy products consumed in or imported from a country where brucellosis is endemic (4), contact with meat or tissues of infected wild animals (5), and laboratory exposures to Brucella isolates (6).
Brucellosis is a frequently reported laboratory-acquired infection (6–9). Characteristics of the organism and the disease contribute to the associated risk in a laboratory setting. Brucella spp. are readily aerosolized and have an infective dose of 10 to 100 organisms (10). In addition, because brucellosis is uncommon in the United States and patients often present with nonspecific signs and symptoms, clinicians may not suspect brucellosis, include it as a differential diagnosis, or notify the laboratory (11); also, laboratory workers may not be familiar with the organism. This can lead to exposures to Brucella spp. in clinical laboratories during culturing and isolation of clinical specimens (12–14). Also, proper safety precautions (15, 16) for Brucella isolates may not be observed in laboratories that rarely receive highly pathogenic organisms.
Despite the publication of laboratory safety measures (15, 16) and postexposure recommendations (17), laboratory exposures and laboratory-acquired brucellosis (LAB) cases continue to occur (18, 19). In the United States, roughly 120 cases of brucellosis are reported annually. No national surveillance system specifically identifies laboratory-acquired cases (20); therefore, the annual incidence of brucellosis resulting from laboratory transmission is not known. A few reviews of laboratory-acquired infections have been published; however, these reviews do not describe exposures leading to laboratory-acquired brucellosis in detail (6, 8, 21).
A literature review of Brucella spp. laboratory exposure case reports and summary reports was performed to determine the characteristics of laboratory exposures, better define high risk activities, and identify evidence-based time points for serological and symptom monitoring.
MATERIALS AND METHODS
Search strategy.
A search was conducted in PubMed and ISI Web of Knowledge databases for laboratory exposure case and summary reports. The search was restricted to English language reports but allowed reports from outside the United States. Several searches were conducted using a combination of keywords: “brucellosis + laboratory,” “brucellosis + laboratory-acquired,” “Brucella + laboratory + exposure,” “brucellosis + laboratory infection,” and “Brucella + exposure.” This search strategy resulted in 32 case reports and 14 summary articles. Manual examination of reference lists from the located articles identified nine additional case reports and nine summary articles. A laboratory infection bibliography was used to locate older articles not in PubMed (22); four case reports and two summary reports were identified. In total, 45 case reports and 25 summary articles were found. These were narrowed down to include articles published in the 25 years prior to the publication of the Centers for Disease Control and Prevention (CDC) postexposure prophylaxis (PEP) recommendations in January 2008 (17). Articles published after this date widely referenced the CDC publication; the time frame was chosen to reduce reporting bias introduced by these recommendations. Reports describing the same exposure were excluded. This resulted in 28 case reports (Table 1) and 9 summary reports (Table 2) published between 1982 and 2007.
Table 1.
Case reports describing laboratory exposures to Brucella spp. and laboratory-acquired brucellosis
| Author(s) (reference) | Yr published | Country | No. of cases/no. exposed | No. receiving PEP | No. with risk level |
|||
|---|---|---|---|---|---|---|---|---|
| High | Low | None | Unknown | |||||
| Gossens et al. (42)a | 1983 | Belgium | 1/1 | 0 | 0 | 0 | 0 | 1 |
| Young (33)a | 1983 | U.S. | 2/2 | 0 | 2 | 0 | 0 | 0 |
| Elidan et al. (46)a | 1985 | Israel | 1/1 | 0 | 0 | 0 | 0 | 1 |
| Montes et al. (45) | 1986 | Spain | 1/1 | 0 | 0 | 0 | 0 | 1 |
| Al-Aska and Chagla (31)a | 1989 | Saudi Arabia | 4/4 | 0 | 4 | 0 | 0 | 0 |
| Georgihiou and Young (41)a | 1991 | U.S. | 1/1 | 0 | 1 | 0 | 0 | 0 |
| Young (34)a | 1991 | U.S. | 5/5 | 0 | 0 | 0 | 0 | 5 |
| Batchelor et al. (12)a | 1992 | Kenya/United Kingdom | 2/2 | 0 | 2 | 0 | 0 | 0 |
| Chusid et al. (25)a | 1993 | US | 3/3 | 0 | 0 | 0 | 0 | 3 |
| Kiel and Khan (36) | 1993 | Saudi Arabia | 6/7 | 0 | 1 | 0 | 0 | 6 |
| Gruner et al. (26)a | 1994 | Switzerland | 5/5 | 0 | 5 | 0 | 0 | 0 |
| Martin-Manzuelos et al. (71) | 1994 | Spain | 4/4 | 0 | 0 | 0 | 0 | 4 |
| Wheat et al. (29) | 1995 | United Kingdom | 0/11 | 11 | 11 | 0 | 0 | 0 |
| Arlett (43)a | 1996 | Britain | 1/1 | 0 | 0 | 0 | 0 | 1 |
| Grammont-Cupillard et al. (13)a | 1996 | France | 3/3 | 0 | 3 | 0 | 0 | 0 |
| Zervos and Bostic (38) | 1997 | U.S. | 0/3 | 3 | 2 | 0 | 0 | 1 |
| Brew et al. (64) | 1999 | United Kingdom | 1/1 | 0 | 0 | 1 | 0 | 0 |
| Fiori et al. (32)a | 2000 | Italy | 12/38 | 0 | 11 | 0 | 39 | 0 |
| Yagupsky et al. (39)a | 2000 | Israel | 7/7 | 0 | 2 | 0 | 3 | 2 |
| Memish and Mah (35)a | 2001 | Saudi Arabia | 6/6 | 0 | 0 | 3 | 0 | 3 |
| Memish et al. (37)a | 2001 | Saudi Arabia | 1/1 | 0 | 1 | 0 | 0 | 0 |
| Gannon (30) | 2003 | U.S. | 0/3 | 3 | 3 | 0 | 0 | 0 |
| Noviello et al. (27)a | 2004 | U.S. | 2/2 | 0 | 2 | 0 | 0 | 0 |
| Ozaras et al. (61) | 2004 | Turkey | 1/1 | 0 | 0 | 0 | 0 | 1 |
| Robichaud et al. (28)a | 2004 | Canada | 1/26 | 5 | 6 | 20 | 0 | 0 |
| Wallach et al. (60)a | 2004 | U.S. | 1/1 | 0 | 1 | 0 | 0 | 0 |
| Uhde et al. (44) | 2005 | U.S. | 0/9 | 5 | 6 | 3 | 0 | 0 |
| Maley et al. (14) | 2006 | Australia | 0/44 | 7 | 19b | 25 | 0 | 0 |
Cases described in this report were used to calculate incubation period.
Twelve of these workers were classified as having medium-risk exposure in the original publication.
Table 2.
Summary reports describing laboratory exposures to Brucella spp. and laboratory-acquired brucellosis
| Author(s) (reference) | Yr | Country | Data source | No. of cases/denominatora | No. with risk level |
||
|---|---|---|---|---|---|---|---|
| High | Low | Unknown | |||||
| Grist and Emslie (72) | 1985 | United Kingdom | Survey | 1/5,330* | 1 | 0 | 0 |
| Miller et al. (63) | 1987 | U.S. | Facility review | 18/128† | 18 | ||
| Olle-Goig and Canela-Soler (56)b | 1987 | Spain | Incident report | 28/164‡ | 21 | 7 | 0 |
| Staszkiewicz et al. (73) | 1991 | U.S. | Incident report | 8/26‡ | 5 | 3 | 0 |
| Ergonul et al. (74) | 2004 | Turkey | Facility review | 12/55§ | 12 | ||
| Hasanjani Roushan et al. (75) | 2004 | Iran | Facility review | 38/469¶ | 38 | ||
| Reid (76) | 2005 | Ireland | Facility review | 6/158§ | 6 | ||
| Bouza et al. (62) | 2005 | Spain | Survey | 75/628* | 75 | ||
| Al Dahouk et al. (77) | 2005 | Germany | Facility review | 1/31¶ | 1 | ||
Sources of the denominators varied by article, as follows: *, microbiology laboratory worker respondents to national laboratory safety surveys; †, reported laboratory exposures to any infectious agent at facility; ‡, employees at facility; §, sample from employees at facility based on response or specific criteria; ¶, brucellosis patients admitted to medical facility.
Cases described in this report were used to calculate incubation period.
The summary reports were excluded from the primary analysis, as the exposure information was not clearly defined for each laboratory worker. Exposure information was summarized when available, including number of cases, risk classification, laboratory activities which led to exposure, and symptoms. The available denominators varied and did not necessarily represent those exposed to Brucella (Table 2).
Variable review and classification.
Key variables regarding the case patient, worker demographics, exposure, laboratory activities, prophylaxis, and health outcomes were identified from the case reports. When available, information was collected on the individuals whose clinical sample was the cause of exposure.
Laboratory roles were grouped into similar fields. Physicians and nurses were grouped as health care workers, while microbiologists and laboratory technologists were grouped as microbiologists; other groups were researchers and administrators. Facilities were categorized into four groups: reference laboratories included state or national health and agriculture laboratories, clinical laboratories were first-tier laboratories receiving clinical specimens, research laboratories were those with a primary focus on experimental microbiology, and the last group was vaccine production laboratories.
When risk classification was not clearly stated but there was a clear description of laboratory activities performed, the exposure risk was classified using the CDC's definition of risk. The CDC definition of a high-risk exposure is direct contact with Brucella, work on an open bench with a Brucella isolate or within five feet of such work, or presence in a laboratory during an aerosol-generating event; CDC defines a low-risk exposure as presence in a laboratory during work with a Brucella isolate not meeting the definition of a high-risk exposure (17). Since risk classification is dichotomous, a conservative approach was taken in which workers classified as having a medium-risk exposure (14) were reassigned to the high-risk group for this analysis. Workers who were never in the laboratory, and thus did not meet the high- or low-risk exposure criteria, were classified as having no risk.
Two authors (R.M.T. and M.W.L.) independently assigned exposure risk. Any discordant cases were discussed; when needed, a third author (M.A.G.) made the final determination. Risk was classified as unknown when specific activities, use of personal protective equipment (PPE), or use of a biological safety cabinet (BSC) was not explicitly described. Receipt of antimicrobial PEP was determined by an explicit description of any antimicrobial agent or agents given for any length of time to an exposed worker to prevent infection following a known exposure.
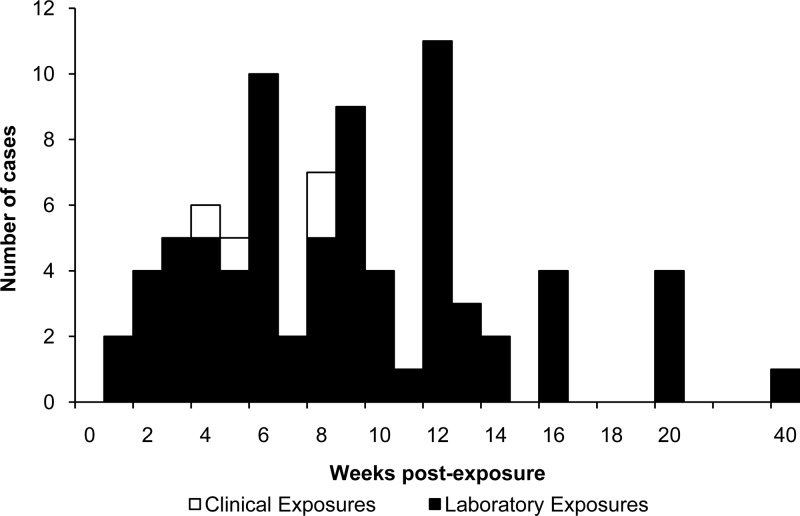
Exposed workers were classified as having laboratory-acquired brucellosis (LAB) if the case report stated that seroconversion occurred, titers indicative of brucellosis were provided (23), or the case was culture confirmed. Brucellosis-consistent symptoms following a known exposure were considered suggestive of infection. Optimal treatment was defined as a treatment regimen consisting of at least two antimicrobial agents effective against Brucella and given for at least 6 weeks (24). Based on this definition, LAB cases were classified as either receiving optimal treatment or not. The relapse rate was calculated for LAB cases for which relapse status and treatment regimen and duration were reported. All identified case reports and summary reports with dates of exposure and symptom onset were used to develop the incubation period curve (Fig. 1).
Fig 1.
Time course of disease onset following occupational exposure to Brucella spp. (n = 80).
The attack rate (AR) for each variable was calculated as the total number of infected workers divided by the total number exposed for the variable. The chi-square test and Fisher's exact test were used to calculate statistical associations. Exact methods were used to calculate odds ratios to adjust for cells with a value of zero. All analyses were performed using SAS 9.2 (SAS Institute, Cary, NC).
RESULTS
Case reports.
In the 28 laboratory exposure case reports, 167 workers were potentially exposed to Brucella spp., 71 (43%) of whom developed LAB (Table 3). More than 48 exposure incidents were described. Four reports described secondary exposures involving the same or related isolates (12, 25–27).
Table 3.
Demographics of laboratory workers exposed to Brucella spp. and laboratory-acquired brucellosis (LAB) cases
| Occupation or facility | No. exposed (n = 167) | No. with LAB (n = 71) |
|---|---|---|
| Occupation | ||
| Microbiologist | 158 | 62 |
| Researcher | 3 | 3 |
| Clinician | 3 | 3 |
| Administrator | 2 | 2 |
| Unknown | 1 | 1 |
| Facility | ||
| Clinical | 142 | 46 |
| Reference | 2 | 2 |
| Research | 15 | 15 |
| Vaccine production | 2 | 2 |
| Unknown | 6 | 6 |
Information about the source of the clinical specimen (the index case) was provided in 7 reports describing 94 exposures. In 5 reports describing 86 exposures, the index cases were immigrants from (27–29) or travelers to (14, 30) a country where the organism is endemic. The index cases in two reports describing eight exposures had consumed unpasteurized dairy products in a country where the organism is endemic (26) or imported the products to the United States (25). Time to bacterial growth was reported for six cases, with a median of 4 days (range 3 to 7).
Microbiologists were most frequently exposed (158 [95%]); however, they had the lowest attack rate (39%, Table 3). Most individuals worked in clinical laboratories, while 19 worked in reference, research, and vaccine production laboratories.
Aerosolized Brucella organisms were implicated as the route of exposure for 155 (93%) exposures, while direct contact with Brucella organisms was implicated in 6 exposures; route of exposure was not reported for another six exposures (Table 4). Only 18 (11%) exposures were due to laboratory accidents which led to both aerosol and direct contact with organisms. These included a broken tube of a culture in liquid medium, injection, splash of liquid medium, skin and conjunctiva contact, and accidental ingestion from mouth pipetting (31–34). The majority of exposures were due to aerosolization of organisms during routine identification activities (147 [88%]).
Table 4.
Sources of Brucella exposure among laboratory workers and subsequent laboratory-acquired brucellosis
| Source | No. exposed (n = 167) | No. with LAB (n = 71) | Attack rate (%) |
|---|---|---|---|
| Route of exposure | |||
| Aerosol | 155 | 59 | 38 |
| Contact/ingestion | 6 | 6 | 100 |
| Unknown | 6 | 6 | |
| Exposure method | |||
| Routine | 147 | 52 | 35 |
| Accidental | 18 | 18 | 100 |
| Unknown | 2 | 1 | |
| Exposure | |||
| Culture | 155 | 63 | 41 |
| Clinical specimens | 9 | 5 | 56 |
| Vaccine | 1 | 1 | 100 |
| Unknown | 2 | 2 | |
| Activity | |||
| Open bench | 82 | 46 | 56 |
| Subculture | 56 | 24 | 43 |
| Gram stain | 46 | 20 | 43 |
| Commercial tests | 30 | 5 | 17 |
| Biochemicals | 44 | 18 | 41 |
| Break | 15 | 15 | 100 |
| Catalase | 13 | 3 | 23 |
| Use of BSC | 11 | 9 | 82 |
| Sniff | 9 | 5 | 56 |
| Misread Gram stain | 3 | 3 | 100 |
| Proximity | |||
| Work with agent | 85 | 49 | 58 |
| No work with agent | 75 | 15 | 20 |
| Not in room | 4 | 4 | 100 |
| Unknown | 7 | 7 | |
| Species | |||
| B. melitensis | 135 | 49 | 36 |
| B. abortus | 13 | 13 | 100 |
| B. suis | 9 | 0 | 0 |
| B. canis | 1 | 1 | 100 |
| Marine species | 1 | 1 | 100 |
| Brucella species | 5 | 5 | 100 |
| Unknown | 3 | 2 | |
| Risk class | |||
| High risk | 82 | 36 | 44 |
| No PEP | 49 | 36 | 73 |
| PEP | 33 | 0 | 0 |
| Low risk | 52 | 4 | 8 |
| No riska | 4 | 4 | 100 |
| Unknown | 29 | 27 | |
| PEP | |||
| Yes | 34 | 0 | 0 |
| No | 128 | 66 | 52 |
| Unknown | 5 | 5 |
“No risk” refers to workers who do not meet the CDC's high- or low-risk exposure criteria (17).
Routine activities which led to exposures included manipulation of an organism outside a BSC, misidentification of an isolate, and unsafe laboratory practices (Table 4). Eighty-five of the 167 (51%) exposed workers directly manipulated an isolate, of whom 77 (91%) reported manipulation of an isolate outside a BSC. Misidentification by commercial identification systems reportedly led to three exposure events (12, 25, 28). These systems did not contain a profile for Brucella spp. in their database at the time of the reports, which resulted in misidentification as Psychrobacter phenylpyruvica. Three employees worked with isolates outside a BSC after misinterpretation of the Gram stain, all of whom developed LAB (27, 35). Nine workers reportedly sniffed a culture plate of Brucella, five of whom developed LAB (AR, 56%) (13, 14, 28, 36–38).
Exposure to Brucella sp. culture was reported for 155 exposed individuals (93%), 63 (41%) of whom developed LAB (Table 4). Ten workers were exposed to other infectious specimens or products, six of whom developed LAB. Nine of these workers were exposed to human or animal clinical specimens in a laboratory. One worker was exposed to animal vaccine in a vaccine development laboratory. Exposure was not reported for two workers.
Seventy-five exposed workers (45%) did not directly manipulate an isolate, and 15 (20%) developed LAB. Of these LAB cases, 11 were involved in a laboratory accident (32), while four non-laboratory workers had never entered the room where Brucella was manipulated (32, 39).
B. melitensis was the causative agent leading to 135 exposures (81%). Reports from countries where B. melitensis is not endemic in domestic animals (40) accounted for 105 of the 135 (78%) exposures to B. melitensis (12, 14, 25–30, 33, 34, 38, 41–43). Twenty-nine exposures were to B. abortus, B. suis, B. canis, unidentified Brucella spp., and a recently identified marine mammal species. The etiologic agent was not specified for three exposures, although subsequent laboratory-acquired infections were reported as brucellosis.
Serological monitoring of exposed workers was described in 6 of 28 case reports and included 72 exposed workers, of which 13 seroconverted (14, 28, 30, 32, 38, 44). A standard or microagglutination assay was used in each instance; however, one individual was treated for brucellosis based on IgM antibody detection using indirect fluorescence, although the Brucella microagglutination test results were negative (not categorized as a LAB case) (44). Variable monitoring timelines were reported, ranging from testing of acute- and convalescent-phase specimens (30) to biweekly testing for 3 months (14, 28, 32). Poor compliance with biweekly monitoring was described in one case report, in which 77% of workers missed two or more serum draws (28). However, another reported that only 18% of serum draws were missed on the same biweekly monitoring timeline (14).
Among the 134 workers classified as having high- or low-risk exposures, those with high-risk exposures were 9.3 times more likely to develop LAB than those with low-risk exposures (95% confidence interval [CI], 3.0 to 38.6; P < 0.0001). Antimicrobial PEP was offered to 36 of the 167 (22%) exposed workers (35 high-risk and 1 unknown-risk exposures) and was accepted by 34 (94%) workers (14, 28–30, 38, 44). The antimicrobial PEP regimen was described for 29 exposed workers; 25 received doxycycline and rifampin, 3 received doxycycline alone, and 1 pregnant worker received trimethoprim-sulfamethoxazole. The median duration of antimicrobial PEP was 2 weeks (range 1 to 6 weeks). Eight of the 34 (24%) workers who received antimicrobial PEP did not complete their prescription due to side effects or perceived low risk of infection (14, 30, 44). None of the workers who took PEP developed infection, including 33 workers with high-risk exposures. Workers classified as having high-risk exposures who took antimicrobial PEP were 0.009 times as likely to develop LAB than those who did not take PEP (95% CI, 0 to 0.042; P < 0.0001).
All LAB cases had positive serology or culture. Sixty-eight (96%) of 71 LAB cases were seropositive and 47 (66%) were culture positive; 44 were both seropositive and culture positive.
Of the 24 seropositive-only cases, culture was not done or not reported for 18; 20 had agglutination titers of ≥1:160, while 4 lacked confirmatory serological evidence of brucellosis but were considered by the original authors to have brucellosis (13, 25, 34). Two of the three culture-positive-only cases had negative serological results reported. Bacterial growth of isolates from LAB cases was slow (n = 17; median, 9.5 days; range, 3 to 42).
Agglutination methods identified 47 LAB cases and were used as secondary assays for another 7. Rose bengal staining and enzyme-linked immunosorbent assay (ELISA) identified 21 cases; all but two were also positive in another assay or by culture. Coombs' test and complement fixation were used as secondary assays for three cases.
The median titer from quantitative assays was 1:1,280 (1:40 to 1:20,480). The median titers did not vary substantially between LAB cases with low-risk exposures (n = 3) and those with high-risk exposures (n = 29) (1:1,280 [1,280 to 10,240] versus 1:640 [1:120 to 1:10,240]).
Symptomatic LAB cases experienced typical nonspecific symptoms, including fever (71%), arthralgia (36%), sweats (32%), headache (22%), myalgia (22%), malaise (22%), and fatigue (14%). Some workers experienced more severe signs, including soft tissue abscesses (some of which involved implants or prostheses) (35, 37), spondylitis or sacroiliitis (26, 33, 35), and neurologic brucellosis (45). Chronic fatigue (34) and permanent hearing loss (46) were also reported.
Seven of 35 (20%) female workers for whom sex was reported were pregnant at the time of exposure, of which six developed LAB (86%). Four of these LAB cases aborted (13, 31, 34, 41); all had positive Brucella antibody titers by agglutination or rose bengal serological tests, and three were culture positive for B. melitensis. Two abortions occurred spontaneously following onset of fever, malaise, and vaginal bleeding (31, 34); one patient was on treatment for 1 month before the abortion occurred. The third LAB patient underwent a therapeutic termination following a diagnosis of disseminated intravascular coagulation; the patient made a full recovery (41). The final LAB patient also underwent a therapeutic abortion following seroconversion and onset of night sweats after sniffing culture plates (13). The reason given for the abortion was the potential risk to the fetus; placental tissues were culture negative.
Individual incubation periods were identified or calculated for 80 LAB cases (12, 13, 17, 18, 25–28, 31–35, 37, 39, 41–43, 46–60). The median time to symptom onset among these case patients was 8 weeks (interquartile range [IQR], 5 to 12 weeks; mean, 9.0 weeks; standard deviation [SD], 5.8 weeks), with a range of 1 to 40 weeks (Fig. 1). Date of seroconversion was reported for 12 workers (13, 28, 32). The median time to seroconversion was 11 weeks (IQR, 9 to 14 weeks; mean, 11.7 weeks; SD, 3.4 weeks), with a range of 8 to 20 weeks.
The antimicrobial agents given for treatment of LAB were described for 59 of the 72 (82%) LAB cases. Six LAB cases received trimethoprim-sulfamethoxazole only, five received tetracycline, and one case received ceftriaxone. The remaining 47 LAB cases received at least two antimicrobial agents: doxycycline and an aminoglycoside (n = 21), doxycycline and rifampin (n = 22), tetracycline or rifampin and trimethoprim-sulfamethoxazole (n = 4), and rifampin and an aminoglycoside (n = 1). A second antimicrobial regimen was described for 10 of 12 cases that experienced relapse. Prescribed antimicrobials included tetracycline with streptomycin (n = 3) and doxycycline with rifampin (n = 4); the remaining three cases received doxycycline alone or in combination with ciprofloxacin, rifampin, or streptomycin.
The duration of treatment was reported for 45 of the 59 (76%) LAB cases with antimicrobial agents described; the duration of a second treatment regimen was provided for 8 of 12 (67%) relapsed cases. The median duration of treatment was 6 weeks for both first and second treatment regimens (mean, 5.7 weeks [SD, 1.8 weeks] for the first treatment; mean, 8.6 weeks [SD, 10.0 weeks] for the second treatment), though the duration varied widely between regimens (3 days to 12 weeks versus 1 to 40 weeks). Workers with LAB who received optimal treatment were less likely to relapse than those who did not (odds ratio, 0.055; 95% CI, 0.0070 to 0.39; P < 0.01). The three cases who relapsed after receiving optimal treatment had been prescribed 6 weeks' treatment with doxycycline and rifampin; the relapse rate for this regimen was 14% (3/21) (36, 37, 61).
Summary reports.
From the 9 summary reports, 186 LAB cases were reported. Microbiologists were most frequently identified with LAB (n = 85, 46%), followed by pathologists (n = 43, 23%). Individuals with other occupations who developed LAB included 11 clinicians (6%), nine animal caretakers (5%), two laboratory maintenance workers (1%), and two administrators (1%).
Of 142 LAB cases for which the route of exposure was described, 121 (85%) were due to aerosol exposure and 2 were due to skin contact; the remainder were unknown or not reported. The most frequently reported source of infection was processing of blood cultures (n = 71), followed by work with known Brucella strains (n = 62) and unknown (n = 4); the source was not reported for 49 cases. A national survey of LAB reported that 60 of 75 cases were linked to major biosafety violations (62).
Serological diagnosis was reported for 139 cases. Species results were reported for 63 LAB cases: 44 (70%) were infected with B. melitensis, 9 (14%) with B. abortus, and 1 (2%) with B. suis; 9 (14%) were culture negative, and the species to which the individuals were exposed were not indicated (63).
LAB cases with reported symptoms (n = 48) experienced symptoms similar to those found in the case reports, though at a higher rate. Frequently reported symptoms included fever (71%), arthralgia (58%), headache (58%), fatigue (56%), sweats (45%), malaise (44%), and myalgia (40%).
DISCUSSION
Of the 167 exposed workers, 71 developed LAB, of whom 12 relapsed. Microbiologists and clinical laboratory staff were more frequently exposed to Brucella spp. than individuals in other occupations (e.g., researchers) and those employed in other laboratory settings, yet the rate of LAB was lower in this group. This review demonstrates that staff other than microbiologists are also at risk when Brucella organisms are manipulated in a laboratory, but exposures likely go unrecognized unless an infection is identified. It may be important to consider brucellosis as a differential diagnosis among employees outside the microbiology laboratory but within the same facility as a known exposure.
Routine diagnostic work with Brucella spp., or proximity to the organism, resulted in the majority of exposures. Unlike laboratory accidents, which provide a clear exposure event, exposures due to routine work lack clearly identified incidents and may not be linked to breaches in laboratory safety protocols (64). Manipulation of unknown isolates in a BSC is considered unnecessary by some (65); however, this review suggests that it is a primary safety measure to prevent LAB, particularly for slow-growing Gram-negative or Gram-variable organisms. In addition, risky diagnostic methods, such as mouth pipetting and sniffing plates, should be prohibited due to the associated risk of infection (13, 14, 28, 36–38).
Though manipulation in a BSC is a primary safety measure, seven cases did occur despite constant use of a BSC with no recognized lapses in biosafety. Air movement from a door causing airflow out of the BSC was identified in one outbreak (35, 37), and unidentified lapses in safety were thought to have caused another (39). These reports, along with the cases reported to have never entered the laboratory (32, 39), demonstrate the need for evaluation of ventilation systems, particularly following highly aerosolizing events.
B. melitensis is not present in a number of countries from which exposures to the species were reported (12, 14, 25–30, 33, 34, 38, 40–43, 66). The index cases from these reports were travelers to and immigrants from countries of endemicity or individuals who consumed unpasteurized dairy products from countries of endemicity. When submitting a specimen from a patient with a history of travel to a country of endemicity or history of consuming unpasteurized milk products, physicians should notify the laboratory of the possibility of Brucella spp.
This review demonstrates that antimicrobial postexposure prophylaxis is highly effective in preventing brucellosis among workers classified with high-risk exposures. Optimal duration of antimicrobial PEP could not be concluded from this review, as duration of PEP was highly variable. However, LAB did not develop in any worker who took antimicrobial PEP. CDC recommends 3 weeks of antimicrobial PEP (17, 70), though 6 weeks has also been recommended (67).
No deaths were reported among the LAB cases, though four pregnant LAB cases experienced abortions. Only a few deaths from LAB have been reported in the literature; most occurred prior to the development of antimicrobial agents. This is consistent with other reviews of LAB (6, 8, 21); the case fatality rate for brucellosis is reportedly 1 to 2%, which usually occurs in chronic cases with endocarditis (1).
A notable finding in this review is the increased effectiveness of optimal treatment of LAB, which consists of two or more effective antimicrobials for 6 weeks or more (68), compared to monotherapy. Studies have shown relapse rates of 0 to 16% for a variety of optimal antimicrobial combinations, such as doxycycline plus rifampin or streptomycin given for 6 weeks (68); the relapse rate of cases receiving optimal therapy from these reports falls in this range.
There are a number of limitations to this review. First, the definitions used to determine risk classification, illness, incubation, and optimal treatment may not accurately represent exposures and cases. However, published recommendations were used to define risk and optimal treatment (17, 24), and only four (6%) cases classified in the original case reports as LAB cases lacked confirmatory laboratory evidence of infection. The date of illness onset is subject to recall bias; thus, the incubation period may be overestimated. Second, the laboratory workers described in these case reports and summaries may not be representative of all laboratory workers occupationally exposed to Brucella spp. It is possible that the number of LAB, especially those where proper precautions were used, is overrepresented, since exposures that did not result in disease are less likely to be reported. Third, the workers who developed LAB may have had risk factors that increased their risk of infection compared to those workers who did not develop LAB. Fourth, exposures may not be recognized even if an employee becomes ill, due to the nonspecific symptoms of brucellosis, lack of awareness of the disease, and difficult diagnosis. Fifth, reports describing exposures in nonclinical laboratories do not provide an accurate attack rate. Exposures in atypical settings likely go unrecognized unless an infection is diagnosed.
Laboratory exposures to Brucella spp. remain a public health problem for which there are practical exposure and infection prevention solutions, which protect against other laboratory-acquired infections. Many of the exposures described above were caused by routine work with clinical specimens where brucellosis was not suspected. Laboratory workers and clinicians should communicate to assess the risk posed to the laboratory staff during the identification of a specimen (69). As a safety precaution, it may be advisable that all unknown specimens be manipulated in a BSC until a highly infectious pathogen is ruled out. If an exposure to Brucella spp. occurs, revised postexposure guidelines are available in the accompanying article (70).
This review demonstrates the effectiveness of antimicrobial postexposure prophylaxis following high-risk exposures, and optimal treatment with combination antimicrobials for at least 6 weeks for individuals who develop brucellosis. The incubation period identified in these reports is valuable to appropriately select a time frame for serological and symptom surveillance. Standardized active surveillance and monitoring should be initiated to protect the health of laboratory workers.
ACKNOWLEDGMENTS
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Published ahead of print 3 July 2013
REFERENCES
- 1.Young EJ. 2000. Brucellosis, p 353–354 In Mandell GL, Bennett JE, Dolin R. (ed), Principles and practice of infectious disease, 5th ed. Churchill Livingstone, Philadelphia, PA [Google Scholar]
- 2.Khan MY, Mah MW, Memish ZA. 2001. Brucellosis in pregnant women. Clin. Infect. Dis. 32:1172–1177 [DOI] [PubMed] [Google Scholar]
- 3.Nicoletti PL. 1989. Relationship between animal and human disease, p 41–51 In Young EJ, Corbel MJ. (ed), Brucellosis: clinical and laboratory aspects. CRC Press, Boca Raton, FL [Google Scholar]
- 4.Chomel BB, DeBess EE, Mangiamele DM, Reilly KF, Farver TB, Sun RK, Barrett LR. 1994. Changing trends in the epidemiology of human brucellosis in California from 1973 to 1992: a shift toward foodborne transmission. J. Infect. Dis. 170:1216–1223 [DOI] [PubMed] [Google Scholar]
- 5.CDC 2009. Brucella suis infection associated with feral swine hunting—three states, 2007–2008. MMWR Morb. Mortal. Wkly. Rep. 58:618–621 [PubMed] [Google Scholar]
- 6.Harding AL, Byers KB. 2006. Laboratory-associated infections: summary and analysis of 3921 cases, p 53–77 In Fleming DO, Hunt DL. (ed), Biological safety: principles and practices, 4th ed. ASM Press, Washington DC [Google Scholar]
- 7.Baron EJ, Miller JM. 2008. Bacterial and fungal infections among diagnostic laboratory workers: evaluating the risks. Diagn. Microbiol. Infect. Dis. 60:241–246 [DOI] [PubMed] [Google Scholar]
- 8.Pike RM. 1979. Laboratory-associated infections: incidence, fatalities, causes, and prevention. Annu. Rev. Microbiol. 33:41–66 [DOI] [PubMed] [Google Scholar]
- 9.Singh K. 2009. Laboratory-acquired infections. Clin. Infect. Dis. 49:142–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pappas G, Panagopoulou P, Christou L, Akritidis N. 2006. Brucella as a biological weapon. Cell Mol. Life Sci. 63:2229–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young EJ. 1989. Relationship between animal and human disease, p 97–126 In Young EJ, Corbel MJ. (ed), Brucellosis: clinical and laboratory aspects. CRC Press, Boca Raton, FL [Google Scholar]
- 12.Batchelor BI, Brindle RJ, Gilks GF, Selkon JB. 1992. Biochemical mis-identification of Brucella melitensis and subsequent laboratory-acquired infections. J. Hosp. Infect. 22:159–162 [DOI] [PubMed] [Google Scholar]
- 13.Grammont-Cupillard M, Berthet-Badetti L, Dellamonica P. 1996. Brucellosis from sniffing bacteriological cultures. Lancet 348:1733–1734 [DOI] [PubMed] [Google Scholar]
- 14.Maley MW, Kociuba K, Chan RC. 2006. Prevention of laboratory-acquired brucellosis: significant side effects of prophylaxis. Clin. Infect. Dis. 42:433–434 [DOI] [PubMed] [Google Scholar]
- 15.American Society for Microbiology 2013, posting date Sentinel level clinical microbiology laboratory guidelines for suspected agents of bioterrorism and emerging infectious diseases. Brucella species. ASM, Washington, DC: http://www.asm.org/images/PSAB/Brucella_July2013.pdf [Google Scholar]
- 16.U.S. Department of Health and Human Services 2010, posting date Biosafety in microbiological and biomedical laboratories, 5th ed. U.S. Department of Health and Human Services, Washington, DC: http://www.cdc.gov/biosafety/publications/bmbl5/BMBL.pdf [Google Scholar]
- 17.CDC 2008. Laboratory-acquired brucellosis—Indiana and Minnesota, 2006. MMWR Morb. Mortal. Wkly. Rep. 57:39–42 [PubMed] [Google Scholar]
- 18.Gerberding JL, Romero JM, Ferraro MJ. 2008. Case records of the Massachusetts General Hospital. Case 34–2008. A 58-year-old woman with neck pain and fever. N. Engl. J. Med. 359:1942–1949 [DOI] [PubMed] [Google Scholar]
- 19.Horvat RT, El Atrouni W, Hammoud K, Hawkinson D, Cowden S. 2011. Ribosomal RNA sequence analysis of Brucella infection misidentified as Ochrobactrum anthropi infection. J. Clin. Microbiol. 49:1165–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CDC 2011. Summary of notifiable diseases—United States, 2009. MMWR Morb. Mortal. Wkly. Rep. 58:1–100 [PubMed] [Google Scholar]
- 21.Pike RM. 1976. Laboratory-associated infections: summary and analysis of 3921 cases. Health Lab. Sci. 13:105–114 [PubMed] [Google Scholar]
- 22.Jemski JV, Phillips GB. 1966. Laboratory infections bibliography. American Association for Laboratory Animal Science, Memphis, TN [Google Scholar]
- 23.Al-Dahouk S, Tomaso H, Nockler K, Neubauer H, Frangoulidis D. 2003. Laboratory-based diagnosis of brucellosis—a review of the literature. Part II: serological tests for brucellosis. Clin. Lab. 49:577–589 [PubMed] [Google Scholar]
- 24.Ariza J, Bosilkovski M, Cascio A, Colmenero JD, Corbel MJ, Falagas ME, Memish ZA, Roushan MR, Rubinstein E, Sipsas NV, Solera J, Young EJ, Pappas G, International Society of Chemotherapy, Institute of Continuing Medical Education of Ioannina 2007. Perspectives for the treatment of brucellosis in the 21st century: the Ioannina recommendations. PLoS Med. 4:e317. 10.1371/journal.pmed.0040317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chusid MJ, Russler SK, Mohr BA, Margolis DA, Hillery CA, Kehl KC. 1993. Unsuspected brucellosis diagnosed in a child as a result of an outbreak of laboratory-acquired brucellosis. Pediatr. Infect. Dis. J. 12:1031–1033 [PubMed] [Google Scholar]
- 26.Gruner E, Bernasconi E, Galeazzi RL, Buhl D, Heinzle R, Nadal D. 1994. Brucellosis: an occupational hazard for medical laboratory personnel. Report of five cases. Infection 22:33–36 [DOI] [PubMed] [Google Scholar]
- 27.Noviello S, Gallo R, Kelly M, Limberger RJ, DeAngelis K, Cain L, Wallace B, Dumas N. 2004. Laboratory-acquired brucellosis. Emerg. Infect. Dis. 10:1848–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robichaud S, Libman M, Behr M, Rubin E. 2004. Prevention of laboratory-acquired brucellosis. Clin. Infect. Dis. 38:e119–122 [DOI] [PubMed] [Google Scholar]
- 29.Wheat PF, Dabbs DJ, Thickett KJ. 1995. Brucella melitensis: an unexpected isolate from cerebrospinal fluid. Commun. Dis. Rep. 5:R56–57 [PubMed] [Google Scholar]
- 30.Gannon CK. 2003. Anatomy of an exposure: a hospital lab's recovery of Brucella melitensis. MLO Med. Lab. Obs. 35:22–25 [PubMed] [Google Scholar]
- 31.Al-Aska AK, Chagla AH. 1989. Laboratory-acquired brucellosis. J. Hosp. Infect. 14:69–71 [DOI] [PubMed] [Google Scholar]
- 32.Fiori PL, Mastrandrea S, Rappelli P, Cappuccinelli P. 2000. Brucella abortus infection acquired in microbiology laboratories. J. Clin. Microbiol. 38:2005–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young EJ. 1983. Human brucellosis. Rev. Infect. Dis. 5:821–842 [DOI] [PubMed] [Google Scholar]
- 34.Young EJ. 1991. Serologic diagnosis of human brucellosis: analysis of 214 cases by agglutination tests and review of the literature. Rev. Infect. Dis. 13:359–372 [DOI] [PubMed] [Google Scholar]
- 35.Memish ZA, Mah MW. 2001. Brucellosis in laboratory workers at a Saudi Arabian hospital. Am. J. Infect. Control 29:48–52 [DOI] [PubMed] [Google Scholar]
- 36.Kiel FW, Khan MY. 1993. Brucellosis among hospital employees in Saudi Arabia. Infect. Control Hosp. Epidemiol. 14:268–272 [DOI] [PubMed] [Google Scholar]
- 37.Memish ZA, Alazzawi M, Bannatyne R. 2001. Unusual complication of breast implants: Brucella infection. Infection 29:291–292 [DOI] [PubMed] [Google Scholar]
- 38.Zervos MJ, Bostic G. 1997. Exposure to Brucella in the laboratory. Lancet 349:651. [DOI] [PubMed] [Google Scholar]
- 39.Yagupsky P, Peled N, Riesenberg K, Banai M. 2000. Exposure of hospital personnel to Brucella melitensis and occurrence of laboratory-acquired disease in an endemic area. Scand. J. Infect. Dis. 32:31–35 [DOI] [PubMed] [Google Scholar]
- 40.OIE 2009, posting date World animal health information database 1.4. http://www.oie.int/wahis_2/public/wahid.php/Wahidhome/Home
- 41.Georghiou PR, Young EJ. 1991. Prolonged incubation in brucellosis. Lancet 337:1543. [DOI] [PubMed] [Google Scholar]
- 42.Goossens H, Marcelis L, Dekeyser P, Butzler JP. 1983. Brucella melitensis: person-to-person transmission? Lancet i:773. [DOI] [PubMed] [Google Scholar]
- 43.Arlett PR. 1996. A case of laboratory acquired brucellosis. Br. Med. J. 313:1130–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uhde K, Chang M, Oda G, Rosen J, Holodniy M, Cody S, Bajani Ari M, Bragg S, Fischer M, Clark T. 2005. Laboratory-acquired brucellosis, poster 162. Abstr. Am. Soc. Trop. Med. Hyg. Annu. Meet. American Society of Tropical Medicine and Hygiene, Deerfield, IL [Google Scholar]
- 45.Montes J, Rodriguez MA, Martin T, Martin F. 1986. Laboratory-acquired meningitis caused by Brucella abortus strain 19. J. Infect. Dis. 154:915–916 [DOI] [PubMed] [Google Scholar]
- 46.Elidan J, Michel J, Gay I, Springer H. 1985. Ear involvement in human brucellosis. J. Laryngol. Otol. 99:289–291 [DOI] [PubMed] [Google Scholar]
- 47.Carmichael LE, Barol SR, Broad RH, Freitag JL. 1968. Human infection with the agent of canine abortion. MMWR Morb. Mortal. Wkly. Rep. 17:285–286 [Google Scholar]
- 48.Demirdal T, Demirturk N. 2008. Laboratory-acquired brucellosis. Ann. Acad. Med. Singapore 37:86–87 [PubMed] [Google Scholar]
- 49.Faigel HC. 1969. Beagle fever, canine brucellosis. Clin. Pediatr. 8:59. [DOI] [PubMed] [Google Scholar]
- 50.Green HN. 1941. Laboratory Infection with Brucella abortus. Br. Med. J. 1:478–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Humphreys FA, Guest WA. 1932. Undulant fever contracted in the laboratory. Can. Med. Assoc. J. 27:616–619 [PMC free article] [PubMed] [Google Scholar]
- 52.Marianelli C, Petrucca A, Pasquali P, Ciuchini F, Papadopoulou S, Cipriani P. 2008. Use of MLVA-16 typing to trace the source of a laboratory-acquired Brucella infection. J. Hosp. Infect. 68:274–276 [DOI] [PubMed] [Google Scholar]
- 53.Mesner O, Riesenberg K, Biliar N, Borstein E, Bouhnik L, Peled N, Yagupsky P. 2007. The many faces of human-to-human transmission of brucellosis: congenital infection and outbreak of nosocomial disease related to an unrecognized clinical case. Clin. Infect. Dis. 45:e135–e140 [DOI] [PubMed] [Google Scholar]
- 54.Nelson KE, Ruben FL, Andersen B. 1975. An unusual outbreak of brucellosis. Arch. Intern. Med. 135:691–695 [PubMed] [Google Scholar]
- 55.O'Brien PJ. 1962. A naturally acquired and a laboratory acquired case of brucellosis. Med. J. Aust. 2:377–378 [Google Scholar]
- 56.Olle-Goig JE, Canela-Soler J. 1987. An outbreak of Brucella melitensis infection by airborne transmission among laboratory workers. Am. J. Public Health 77:335–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Poulou A, Markou F, Xipolitos I, Skandalakis PN. 2006. A rare case of Brucella melitensis infection in an obstetrician during the delivery of a transplacentally infected infant. J. Infect. 53:e39–e41 [DOI] [PubMed] [Google Scholar]
- 58.Schulze zur Wiesch J, Wichmann D, Sobottka I, Rohde H, Schmoock G, Wernery R, Schmiedel S, Dieter Burchard G, Melzer F. 2009. Genomic tandem repeat analysis proves laboratory-acquired brucellosis in veterinary (camel) diagnostic laboratory in the United Arab Emirates. Zoo. Pub. Health 57:315–317 [DOI] [PubMed] [Google Scholar]
- 59.Smith JA, Skidmore AG, Andersen RG. 1980. Brucellosis in a laboratory technologist. Can. Med. Assoc. J. 122:1231–1232 [PMC free article] [PubMed] [Google Scholar]
- 60.Wallach JC, Giambartolomei GH, Baldi PC, Fossati CA. 2004. Human infection with M-strain of Brucella canis. Emerg. Infect. Dis. 10:146–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ozaras R, Celik AD, Demirel A. 2004. Acute hepatitis due to brucellosis in a laboratory technician. Eur. J. Intern. Med. 15:264. [DOI] [PubMed] [Google Scholar]
- 62.Bouza E, Sanchez-Carrillo C, Hernangomez S, Gonzalez MJ. 2005. Laboratory-acquired brucellosis: a Spanish national survey. J. Hosp. Infect. 61:80–83 [DOI] [PubMed] [Google Scholar]
- 63.Miller CD, Songer JR, Sullivan JF. 1987. A twenty-five year review of laboratory-acquired human infections at the National Animal Disease Center. Am. Ind. Hyg. Assoc. J. 48:271–275 [DOI] [PubMed] [Google Scholar]
- 64.Brew SD, Perrett LL, Stack JA, MacMillan AP, Staunton NJ. 1999. Human exposure to Brucella recovered from a sea mammal. Vet. Rec. 144:483. [PubMed] [Google Scholar]
- 65.Yagupsky P, Baron EJ. 2005. Laboratory exposures to brucellae and implications for bioterrorism. Emerg. Infect. Dis. 11:1180–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grist NR, Emslie JAN. 1991. Infections in British clinical laboratories, 1988–1989. J. Clin. Pathol. 44:667–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Young EJ. 1995. An overview of human brucellosis. Clin. Infect. Dis. 21:283–289 [DOI] [PubMed] [Google Scholar]
- 68.Al-Tawfiq JA. 2008. Therapeutic options for human brucellosis. Expert Rev. Anti Infect. Ther. 6:109–120 [DOI] [PubMed] [Google Scholar]
- 69.CDC 2012. Guidelines for safe work practices in human and animal medical diagnostic laboratories. Recommendations of a CDC-convened, biosafety blue ribbon panel. MMWR Morb. Mortal. Wkly. Rep. 61(Suppl.):1–102 [PubMed] [Google Scholar]
- 70.Traxler RM, Guerra MA, Morrow MG, Haupt T, Morrison J, Saah JR, Smith CG, Williams C, Fleischauer AT, Lee PA, Stanek D, Trevino-Garrison I, Franklin P, Oakes P, Hand S, Shadomy SV, Blaney DD, Lehman MW, Benoit TJ, Stoddard RA, Tiller RV, De BK, Bower W, Smith TL. 2013. Review of brucellosis cases from laboratory exposures in the United States in 2008 to 2011 and improved strategies for disease prevention. J. Clin. Microbiol. 51:3132–3136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martin-Mazuelos E, Nogales MC, Florez C, Gomez-Mateos JM, Lozano F, Sanchez A. 1994. Outbreak of Brucella melitensis among microbiology laboratory workers. J. Clin. Microbiol. 32:2035–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grist NR, Emslie J. 1985. Infections in British clinical laboratories, 1982–3. J. Clin. Pathol. 38:721–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Staszkiewicz J, Lewis CM, Colville J, Zervos MJ, Band J. 1991. Outbreak of Brucella melitensis among microbiology laboratory workers in a community hospital. J. Clin. Microbiol. 29:287–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ergonul O, Celikbas A, Tezeren D, Guvener E, Dokuzoguz B. 2004. Analysis of risk factors for laboratory-acquired Brucella infections. J. Hosp. Infect. 56:223–227 [DOI] [PubMed] [Google Scholar]
- 75.Hasanjani Roushan MR, Mohrez M, Smailnejad Gangi SM, Soleimani Amiri MJ, Hajiahmadi M. 2004. Epidemiological features and clinical manifestations in 469 adult patients with brucellosis in Babol, northern Iran. Epidemiol. Infect. 132:1109–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reid AJ. 2005. Brucellosis—a persistent occupational hazard in Ireland. Int. J. Occup. Environ. Health 11:302–304 [DOI] [PubMed] [Google Scholar]
- 77.Al Dahouk S, Nockler K, Hensel A, Tomaso H, Scholz HC, Hagen RM, Neubauer H. 2005. Human brucellosis in a nonendemic country: a report from Germany, 2002 and 2003. Eur. J. Clin. Microbiol. Infect. Dis. 24:450–456 [DOI] [PubMed] [Google Scholar]