Abstract
For optimal antiviral therapy, the hepatitis C virus (HCV) genotype needs to be determined, as it remains a strong predictor of sustained viral response. In this study, we assessed the number of HCV genotyping results that could not be determined using the commercially available line probe assay (LiPA) (Versant hepatitis C virus genotype 2.0 assay) in a large international panel of samples from 9,874 HCV-positive patients. In-house sequencing assays targeting the 5′ untranslated region (UTR), core region, NS3 region, and NS5B region of the HCV genome and phylogenetic analyses were used to resolve these LiPA failures. Among all cases, the genotypes of 51 samples (0.52%) could not be determined with the LiPA. These undetermined results were observed more frequently among samples from non-European regions (mainly the Arabian Peninsula). The use of sequencing assays coupled with phylogenetic analysis provided reliable genotype results for 86% of the LiPA failures, which exhibited higher rates of genotypes 4, 5, and 6 than did LiPA-resolved genotypes. As expected, the 5′ UTR was not sufficiently variable for clear discrimination between genotypes 1 and 6, but it also resulted in errors in classification of some genotype 3 and 4 cases using well-known Web-based BLAST programs. This study demonstrates the low frequency of genotyping failures with the Versant hepatitis C virus genotype 2.0 assay (LiPA) and also underlines the need for a complex combination of sequences and phylogenetic analyses in order to genotype these particular HCV strains correctly.
INTRODUCTION
Hepatitis C virus (HCV) infection is a leading cause of chronic liver disease and affects approximately 120 million to 210 million people worldwide (1, 2). Each year, over 250,000 people die from HCV-related chronic liver diseases, such as end-stage cirrhosis and hepatocellular carcinoma (3, 4). Most infections with HCV can be cured if treatment is available, and the emergence of new antiviral drugs that directly target HCV will greatly improve treatment outcomes.
The HCV genome is characterized by extremely high sequence diversity and HCV strains are classified into genetically distinct groups, which are known as genotypes when differences at the nucleotide level range from 31% to 33% or as subtypes when differences range from 20% to 25%; genetic difference below these values define quasispecies (5–7). The HCV genotype (and to a lesser extent, the subtype) must be determined prior to initiation of antiviral treatment because the genotype affects the choice of agents and the duration of therapy, as well as the prognosis for eradicating the virus (8, 9). HCV typing and subtyping can be performed using various methods, including direct sequence analysis, reverse hybridization, and genotype-specific reverse transcription (RT)-PCR. Several regions of the HCV genome can be analyzed to classify strains accurately into specific genotypes. The 5′ untranslated region (UTR) is the region of choice for detecting and quantifying HCV RNA, due to its high level of conservation. For this reason, it often has been used by virological laboratories for routine genotyping of HCV, although it now has been clearly demonstrated that it is difficult to distinguish genotype 6 from genotype 1 and to distinguish subtypes within genotypes 1, 2, and 3 in this region (10). However, nucleotide sequencing coupled with phylogenetic analysis of genomic regions that are more varying, such as the core/E1 and NS5B regions, has been recommended for HCV genotyping in consensus proposals (7). The reverse-hybridization Versant HCV genotype 1.0 assay (line probe assay [LiPA]) (Bayer HealthCare, Eragny, France), which is based on a 5′ UTR segment, has been upgraded and improved in version 2.0 by the addition of core sequence information (11). With this updated version, amplification failures were described for 1.5 to 2.1% of cases and rates could be lowered after retesting but, according to those reports, 4.6% and 22.8% of results could not be resolved at the genotype and subtype levels, respectively (11, 12). The present study aims (i) to evaluate the number of LiPA (version 2.0) failures in a large panel of samples from Europe and from other parts of the world and (ii) to investigate whether the genotypes of these “difficult-to-type” samples corresponded to particular HCV strains that could be typed by using a classic sequencing approach.
MATERIALS AND METHODS
Clinical samples.
A total of 9,874 HCV genotype analyses of samples from Europe and other parts of the world were performed by the CERBA laboratory between January 2011 and May 2012. Viral loads were measured with the COBAS AmpliPrep/COBAS TaqMan HCV quantitative test, version 1.0 (Roche Diagnostics, Meylan, France), and levels needed to be higher than 500 IU/ml for the genotype analysis to be performed. All blood samples were centrifuged shortly after collection, and plasma was stored at −80°C before being stored and shipped on dry ice.
Versant HCV genotype 2.0 assay (LiPA).
A Versant HCV genotype 2.0 assay (INNO-LiPA HCV 2.0) was performed for all samples according to the manufacturer's instructions. RNA was isolated from 200 μl of plasma by using an m2000sp instrument (Abbott Molecular, Rungis, France). Extracted RNA was resuspended in 70 μl of buffer. RT-PCR was performed in a single tube, producing two distinct biotinylated DNA fragments of 240 and 270 bp, representing the 5′ UTR and the core region, respectively. After denaturation, the biotinylated DNA PCR products were hybridized to membrane-bound oligonucleotide probes specific for the 5′ UTR and the core region of HCV genotypes 1 to 6. Each strip contained three control lines (conjugate, amplification from the 5′ UTR, and amplification from the core region) and 22 DNA probe lines containing sequences specific for HCV genotypes 1 to 6. After automated hybridization steps, all strips were dried and scanned; results were interpreted using Bayer LiPA-Scan HCV software (Innogenetics, Ghent, Belgium), according to the currently valid interpretation chart. When the profile obtained did not match any of the reference patterns, the genotype was considered indeterminate by the software. Frozen samples were sent to the Grenoble hospital virology laboratory for sequencing.
Sequencing methods.
Total nucleic acids were extracted from 1 ml of plasma using the generic protocol of the EasyMag system (bioMérieux, Marcy l'étoile, France) and eluted in a volume of 50 μl. RT-PCR amplifications of the 5′ UTR, the core region, and two different regions of NS5B were performed with a one-step protocol using the Qiagen OneStep RT-PCR kit (Qiagen, Courtaboeuf, France), following the manufacturer's instructions. The NS3 protease region was amplified as described by Besse et al., using a nested PCR when necessary (13). All of the primers used are listed in Table 1. Bidirectional sequencing of the amplicons was then performed with a CEQ 2000XL system (Beckman Coulter, Roissy, France).
Table 1.
Primers used for sequencing analyses
| Gene | Primer name | Sensea | Sequence (5′ to 3′)b | Position numbering in H77 | Reference |
|---|---|---|---|---|---|
| 5′ UTR | KY80 | F | GCAGAAAGCGTCTAGCCATGGCGT | 68–91 | 13 |
| KY78 | R | CTCGCAAGCACCCTATCAGGCAGT | 288–311 | ||
| Core | C954 | F | ACTGCCTGATAGGGTGCTTGCGAG | 288–311 | 14 |
| C410 | R | ATGTACCCCATGAGGATCGGC | 732–752 | ||
| NS3 | NS3-1 | F | M13,c ATGGARAAGAARRTYATYRTITGGG | 3276–3300 | 15 |
| NS3-2 | F | M13,c ATGGARAYYAAGVTYATYACITGGG | |||
| NS3-1 | R | M13,c CTYTTICCRCTICCIGTIGGIGCRTG | 4026–4051 | ||
| Nest-S3-1 | F | M13,c ATCTTICTIGGICCIGCYGA | 3369–3388 | ||
| Nest-S3-2 | F | M13,c ATACTICTIGGICCIGCIGA | |||
| Nest-S3-1 | R | M13,c GCIACYTGRTAIGTITGIGG | 3999–4018 | ||
| NS5B | HC1 | F | TGGGGATCCCGTATGATACCCGCTTTGA | 8245–8275 | 16 |
| HC2 | R | GGCGGAATTCCTGGTCATAGCCTCCGTGAA | 8616–8645 | ||
| HC3 | F | TATGAYACCCGCTGYTTTGACTC | 8256–8278 | ||
| HC5 | R | GCTAGTCATAGCCTCCGT | 8619–8636 | ||
| NA1 | F | TTCACGGAGGCTATGACYAGG | 8617–8638 | ||
| NA2 | R | CGCGCATGMGACASGCTGTGA | 9283–9304 |
F, forward; R, reverse.
Y indicates C or T, R indicates A or G, V indicates A, C, or G, I indicates deoxyinosine, used as a “universal” nucleotide replacing A, G, C, or T.
M13 universal primers.
Phylogenetic analysis.
Phylogenetic analysis was performed after alignment of the 5′ UTR, core region, NS3, and two amplified regions of NS5B and then sequences were compared with reference sequences of genotypes 1 to 7 using the neighbor-joining method (17) in MEGA5 software (18). The reliability of the phylogenetic clustering was evaluated using bootstrap analysis with 1,000 replicates. Reference sequences identified by genotype or subtype, country of identification, strain name, and PubMed number were extracted from the European HCV database (http://euhcvdb.ibcp.fr/euHCVdb/).
BLAST analysis.
BLAST searches were performed in the GenBank HCV genotyping database (http://www.ncbi.nlm.nih.gov/projects/genotyping/formpage.cgi), in the Los Alamos HCV sequence database (19) (http://hcv.lanl.gov/content/sequence/BASIC_BLAST/basic_blast.html), and in the hepatitis C viral database of the Viral Bioinformatics Resource Center (VBRC) (http://www.hcvdb.org/blast.asp) for all amplified regions. The Max Planck Institute Informatik Geno2Pheno database (http://www.geno2pheno.org/) also was used for the NS3 and NS5B regions.
Criteria for final decisions on HCV genotypes.
The final decision to assign an HCV genotype was made preferentially according to the NS5B sequencing results when the results of the four BLAST analyses and the phylogenetic analysis matched. When NS5B amplification could not be obtained or when discrepancies appeared in the results, 5′ UTR, core, and NS3 sequences were used, and a minimum of six consistent BLAST results obtained for two different regions was required to assign an HCV genotype.
Statistical analysis.
Student's t test was used to compare HCV viral loads. Independence between qualitative parameters was assessed using the χ2 test.
RESULTS
Versant HCV genotype 2.0 assay (LiPA) failures.
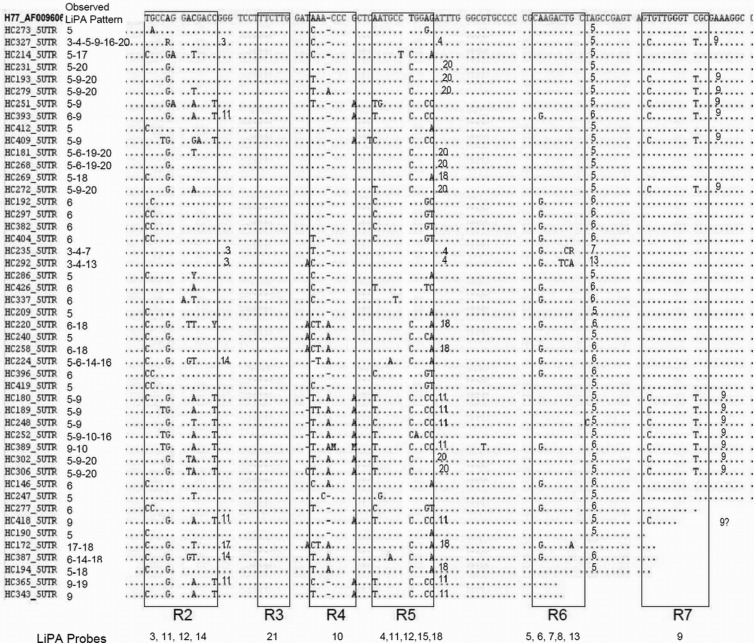
Between January 2011 and May 2012, 51 (0.52%) of the 9,874 HCV genotype analyses performed in the CERBA laboratory using the LiPA could not be resolved because the results could not be interpreted. Twenty-one analyses corresponded to a single positive line in the 5′ UTR, 14 to a double-line profile, and the others to three or more lines (for example, see Fig. 1). Except for variable region 1, which was not sequenced with our primers, 5′ UTR sequencing confirmed most positive lines found with the LiPA (Fig. 2). For seven samples, the sequencing results did not reveal a sequence that perfectly matched the observed positive probes, possibly because mixed infections were not detected. On the other hand, line 11 was never detected in this study's samples, whereas sequencing results showed that it should have been detected in eight cases. All of these observed profiles could not be interpreted using the manufacturer's interpretative chart. Forty-nine percent of these samples came from outside Europe, representing 0.61% of the foreign HCV genotyping analyses conducted at the CERBA laboratory. The failure rates were 0.45% and 0.27% for samples from Europe and Maghreb, respectively, whereas the rates were significantly higher for samples from sub-Saharan Africa (1.66%) and the Arabian Peninsula (29.41%) (Table 2). Using LiPA-recommended procedures, no amplification failures occurred.
Fig 1.
Four examples of LiPA strips and optical density graphs obtained with LiPA-Scan that cannot be interpreted with the manufacturer's interpretative chart. (A) Single positive line 5 (sample HC273). (B) Single positive line 6 (sample HC192). (C) Three positive lines, 5, 9, and 20 (sample HC302). (D) Four positive lines, 5, 9, 10, and 16, and an absence of core amplification (sample HC252). The x axis corresponds to the lines of the different probes of the Inno-LiPA strip. y axis values are optical densities expressed in arbitrary units.
Fig 2.
Alignment of the 5′ UTR sequences of the 47 samples amplified in this region, with the H77 HCV strain (GenBank accession no. AF009606) as a reference. The variable regions used for the design of LiPA probes, as described by Stuyver et al. (20), are boxed and designated R2 to R7. The R1 region, which was the basis for probe lines 16 and 19, was not in the region amplified with this study's primers. The R2 to R7 regions formed the basis for the other LiPA probes, as indicated at the bottom. The observed LiPA profile for each sample is indicated in the second column. The expected reactivity for each sample, given the sequence, is indicated in the sequence alignment by the LiPA line number to the right of the box.
Table 2.
Demographic and clinical features of the patients
| Characteristic | All HCV genotype analyses (n = 9,874) | HCV genotypes resolved with LiPA (n = 9,823) | LiPA failures (n = 51) | Pa |
|---|---|---|---|---|
| Age (yr [mean ± SD]) | 52 ± 14 | 52 ± 14 | 54 ± 15 | NS |
| Gender (n [%]) | ||||
| Male | 5,604 (57) | 5,604 (57) | 29 (57) | NS |
| Female | 4,270 (43) | 4,270 (43) | 22 (43) | |
| HCV RNA viral load (log IU/ml [mean ± SD]) | 5.87 ± 0.91b | 5.87 ± 0.92c | 5.47 ± 1.08d | NS |
| Native country (n [%]) | ||||
| Arabian Peninsula | 17 (0.2) | 12 (0.1) | 5 (9.8) | <0.001 |
| Sub-Saharan Africa | 661 (6.7) | 650 (6.6) | 11 (21.6) | <0.001 |
| Maghreb | 3,393 (34.4) | 3,384 (34.5) | 9 (17.6) | <0.02 |
| Europe | 5,803 (58.8) | 5,777 (58.8) | 26 (50.9) | NS |
| Genotype (n [%]) | ||||
| 1 | 5,540 (56) | 5,530 (56) | 10 (19.6) | <0.001 |
| 2 | 1,772 (18) | 1,760 (17.8) | 12 (23.5) | NS |
| 3 | 1,217 (12.3) | 1,212 (12.3) | 5 (9.8) | NS |
| 4 | 1,250 (12.7) | 1,238 (12.5) | 12 (23.5) | <0.02 |
| 5 | 61 (0.6) | 59 (0.6) | 2 (3.9) | <0.01 |
| 6 | 27 (0.3) | 24 (0.2) | 3 (5.9) | <0.001 |
| Not determined | 7 | 7 |
NS, not significant.
Mean calculated for 4,011 samples.
Mean calculated for 3,975 samples.
Mean calculated for 36 samples.
Amplification of the four different HCV genomic regions.
As presented in Table 3, a total of 38 (74.5%) of the 51 failed LiPA samples gave sequences that could be interpreted in the NS5B region, 29 (56%) with the first set of primers (HC1/HC2), 3 with the second set of NS5B primers (HC3/HC5), and 6 with the third set of NS5B primers (NA1/NA2). Thirteen samples (25.5%) could not be correctly amplified in the NS5B region. Their mean viral load was 4.9 ± 2.6 log IU/ml, compared with 5.7 ± 2.7 log IU/ml for samples amplified in NS5B (P = 0.064).
Table 3.
BLAST and phylogenetic analyses
| Sample | Country | Viral load (log IU/ml) | Genotype found in indicated regiona |
|||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5′ UTR |
Core |
NS3 |
NS5B HC |
NS5B NA |
Final | |||||||||||||||||||||
| GB | LA | VBRC | MEGA | GB | LA | VBRC | MEGA | GB | LA | VBRC | G2P | MEGA | GB | LA | VBRC | G2P | MEGA | GB | LA | VBRC | G2P | MEGA | ||||
| HC146 | Burundi | 6.1 | 1b | 1b | 1b | 6 | / | / | / | / | / | / | / | 1a | 1a | 1a | 1a | 1 | 1 | |||||||
| HC171 | France | 6.6 | / | / | / | / | / | / | / | / | / | / | 1a | 1a | 1a | 1a | 1 | 1 | ||||||||
| HC383 | Algeria | U | / | / | / | / | / | / | / | / | / | / | — | — | — | 1b | 1b | 1b | 1b | 1b | 1 | |||||
| HC231 | Ivory Coast | 5.5 | 1a | 1b | 1a | 5 | 1a | 1a | 1a | 1a | 1a | 1a | 1a | 1a | 1c | 1a | 1a | 1a | 1a | 1 | 1 | |||||
| HC190 | France | 6.1 | 1a | 1g | 1g | 1 | 1c | 1g | 1g | 1 | / | / | / | / | 1a | 1g | 1g | 1a | 1g | 1 | ||||||
| HC209 | France | 6.8 | 1a | 1a | 1g | 1 | 1c | 1g | 1g | 1 | / | / | / | / | 1a | 1g | 1g | 1a | 1g | 1 | ||||||
| HC337 | France | 3.9 | 1b | 1b | 1b | 1 | — | — | — | — | — | — | — | 1b | 1b | 1b | 1b | 1 | 1 | |||||||
| HC273 | France | U | 1c | 1b | 1b | ? | 1a | 1a | 1a | 1a | 1a | 1a | 1a | 1 | 1c | 1a | 1a | 1a | 1a | 1 | 1 | |||||
| HC412 | Gabon | 5.2 | 1a | 1g | 1g | 1 | — | — | — | / | / | / | / | 1a | 1g | 1g | 1a | 1g | 1 | |||||||
| HC247 | Morocco | U | 1a | 1a | 1a | 6 | 1b | 1b | 1b | 1a | 1b | 1b | 1b | 1 | 1b | 1b | 1d | 1b | 1b | 1 | 1 | |||||
| HC343 | Algeria | U | 2a | 2 | 2a | 2 | — | — | — | / | / | / | / | — | — | — | 2i | 2i | 2i | 2c | 2 | 2 | ||||
| HC393 | Algeria | U | 2a | 2 | 2a | 2 | — | — | — | / | / | / | / | 2k | 2k | 2k | 2k | 2k | 2 | |||||||
| HC180 | France | 4.2 | 2a | 2a | 2a | 2 | 2a | 2a | 2a | 2 | — | — | — | — | 2a | 2a | 2a | 2a | 2a | 2 | ||||||
| HC189 | France | U | 2a | 2a | 2a | 2 | 2i | 2i | 2i | 2 | 2i | 2i | 2i | 2 | 2 | — | — | — | 2i | 2i | 2i | 2c | 2 | 2 | ||
| HC248 | France | 4.7 | 2a | 2a | 2a | 2 | 2c | 2c | 2c | 2 | 2c | 2 | 2c | 2 | 2c | 2c | 2c | 2c | 2c | 2c | 2 | |||||
| HC251 | France | 4.5 | 2a | 2a | 2a | 2 | 2i | 2i | 2i | 2 | 2i | 2 | 2i | 2 | 2 | — | — | — | — | — | — | 2 | ||||
| HC302 | France | 6.3 | 2a | 2l | 2a | 2 | 2i | 2 | 2i | 2 | — | — | — | — | 2a | 2l | 2b | 2k | 2 | 2 | ||||||
| HC365 | France | 3.6 | 2a | 1b | 1b | 2 | — | — | — | — | — | — | — | 2a | 2k | 2a | 2a | 2 | 2 | |||||||
| HC409 | France | 5.4 | 2a | 2 | 2a | 2 | 2k | 1b/2kb | 2k | 2 | 2c | 2 | 2c | 2 | 2 | 2a | 2k | 2k | 2k | 2 | 2 | |||||
| HC272 | Madagascar | 4.6 | 2b | 2l | 2a | ? | — | — | — | 5a | 2 | 2a | 2 | 2 | — | — | — | — | — | — | 2 | |||||
| HC252 | Morocco | U | 2a | 2q | 2a | 2 | 2i | 2c | 5a/2ic | 2 | / | / | / | / | — | — | — | — | — | — | 2 | |||||
| HC418 | Morocco | 3.4 | 2a | 2 | 2a | 2 | 2i | 2i/6pd | 2i | 2 | 2i | 2 | 2i | 2 | 2 | — | — | — | 2 | |||||||
| HC192 | France | 7 | 1a | 1b | 1b | ? | 3k | 3a | 3k | 3 | 3b | 4f | 3b | 3 | 3 | 3k | 3h | 3a | 3a | 3 | 3 | |||||
| HC297 | Oman | 5.2 | 1a | 1b | 1b | ? | / | / | / | / | / | / | / | — | — | — | 3a | 3f | 3a | 3k | 3 | 3 | ||||
| HC396 | Oman | 4.6 | 1a | 1b | 1b | ? | 3k | 3a | 1a | 3 | 6h | 3 | 6n | 6 | 3 | 3k | 5 | 6j | 3a | 3 | 3 | |||||
| HC404 | Oman | 6.2 | 1b | 1b | 1a | ? | 3k | 3a | 1g | 3 | / | / | / | / | 5a | 3h | 3a | 3a | 3 | 3 | ||||||
| HC419 | Oman | 4.9 | 1c | 3 | 1b | ? | 3k | 3a | 3k | 3 | / | / | / | / | 3b | 3 | 1a | 3a | 3 | 3 | ||||||
| HC148 | Cameroon | U | / | / | / | / | / | / | / | / | / | / | 4a | 4h | 4f | 4a | 4 | 4 | ||||||||
| HC387 | Burkina Faso | 5.1 | 4a | 4a | 4a | 4 | — | — | — | 4a | 4a | 4a | 4 | 4a | 4a | 4a | 4a | 4a | 4 | 4 | ||||||
| HC220 | Burundi | 6.3 | 3k | 4k | 3k | 4 | 4a | 4k | 4k | 4 | / | / | / | / | 4a | 4k | 4k | 4a | 4k | 4 | ||||||
| HC193 | Cameroon | U | 1c | 4f | 4f | ? | — | — | — | 4a | 4f | 4f | 4 | 4f | — | — | — | — | — | — | 4 | |||||
| HC172 | France | 4.5 | 3k | 4k | 4k | 4 | — | — | — | 4a | 4k | 4k | 4 | 4k | 4a | 4k | 4k | 4a | 4k | 4 | ||||||
| HC194 | France | 5.8 | 4a | 4a | 4d | 4 | — | — | — | — | — | — | — | 4a | 4c | 4a | 4a | 4c | 4 | |||||||
| HC224 | France | U | 4a | 4a | 4a | 4 | 4a | 4e | 4f | 4 | 4a | 4a | 4a | 4 | 4a | 4a | 4a | 4a | 4a | 4a | 4 | |||||
| HC240 | France | U | 1a | 4g | 4k | 4 | 4a | 4o | 4d | 4 | 4a | 4o | 4a | 4 | 4o | 4a | 4o | 4d | 4a | 4o | 4 | |||||
| HC258 | France | U | 3k | 4k | 4k | 4 | — | — | — | 4a | 4k | 4k | 4 | 4k | 4a | 4k | 4k | 4a | 4k | 4 | ||||||
| HC269 | France | 6.1 | 1a | 4p | 4d | 4 | 4a | 4b | 4b | 4 | 1a | 4b | 4b | 4 | 4 | 4a | 4b | 4b | 4a | 4 | 4 | |||||
| HC214 | Gabon | 6.5 | 4a | 4a | 4a | 4 | 4a | 4e | 4f | 4 | 4a | 4 | 4a | 4 | 4 | 4a | 4a | 4a | 4a | 4 | 4 | |||||
| HC286 | Morocco | 6.9 | 1c | 1a | 1g | 1 | — | — | — | 4a | 4 | 4k | 4 | 4k | 4a | 4r | 4k | 4a | 4r | 4 | ||||||
| HC181 | France | U | 5a | 5a | 5a | 5 | 5a | 5a | 5a | 5a | 5a | 5a | 5a | 5 | 5a | 5a | 5a | 5a | 5a | 5a | 5 | |||||
| HC268 | France | 6.6 | 1a | 5a | 1a | 5 | 5a | 5a/2ae | 2a | 5a | 5a | 5a | 5a | 5 | 5a | 5a | 5a | 5a | 5a | 5a | 5 | |||||
| HC235 | France | 6.3 | 1b | 6d | 1b | 6 | 6d | 6e | 6e | 6e | 6b | 6d | 6e | 6 | 6e | — | — | — | — | — | — | 6 | ||||
| HC292 | France | 7 | 1b | 1a | 1b | 6 | / | / | / | / | / | / | / | — | — | — | 6d | 6e | 6e | 6d | 6 | 6 | ||||
| HC426 | French Guiana | 5.7 | 1b | 1a | 1b | ? | / | / | / | / | / | / | / | — | — | — | 3k | 6a | 6a | 6b | 6 | 6 | ||||
| HC279 | Cameroon | 5.3 | 5a | 4f | 4f | ? | — | — | — | / | / | / | / | — | — | — | — | — | — | ? | ||||||
| HC420 | Cameroon | U | — | — | — | — | — | — | / | / | / | / | — | — | — | / | / | / | ? | |||||||
| HC327 | France | 6.2 | 1b | 4f | 4f | ? | — | — | — | — | — | — | — | — | — | — | — | — | — | ? | ||||||
| HC277 | Morocco | 3.1 | 1b | 3h | 1a | ? | / | / | / | / | / | / | / | — | — | — | / | / | / | ? | ||||||
| HC382 | Oman | 6.4 | 1a | 1b | 1b | ? | / | / | / | / | / | / | / | — | — | — | / | / | / | 1? | ||||||
| HC306 | France | 4.2 | 2a | 2l | 2a | 2 | — | — | — | / | / | / | / | — | — | — | — | — | — | 2? | ||||||
| HC389 | Morocco | U | 2a | 2q | 2a | 2 | / | / | / | / | / | / | / | — | — | — | / | / | / | 2? | ||||||
–, amplification failure; /, insufficient quantity; ?, HCV genotype could not be determined with certainty; GB, GenBank HCV genotyping database; LA, Los Alamos HCV sequence database; VBRC, hepatitis C viral database of the Viral Bioinformatics Resource Center; G2P, Max Planck Institute Informatik Geno2Pheno database; MEGA, phylogenetic analysis using MEGA5 software; U, unknown viral load.
GenBank accession no. AY070214, hepatitis C virus strain 747 polyprotein gene, partial coding sequences.
GenBank accession no. EF026073, intergenotypic (genotypes 2 and 5) recombinant.
GenBank accession no. DQ155560, hepatitis C virus strain D3 polyprotein gene, partial coding sequences.
GenBank accession no. JF343786, recombinant hepatitis C virus SA13(5′UTR-NS2)/JFH1_A1022G,K1119R, complete genome.
Forty-seven (98%) of 48 samples gave sequences that could be interpreted in the 5′ UTR with the KY80/KY78 set of primers. For 6 of those samples, the 5′ UTR was the only region that could be amplified and therefore the genotype could not be attributed with certainty. One sample could not be amplified with these primers or with any others. Three samples were not tested in the 5′ UTR because not enough plasma was available.
Twenty-five (61%) of 41 samples were successfully sequenced in the core region with the C954/C410 set of primers. Sixteen samples could not be amplified with these primers. Ten samples were not tested in the core region because not enough plasma was available.
Twenty-three (79.3%) of 29 samples were successfully sequenced in the NS3 protease region by employing the pangenotypic assay described by Besse et al. (13). Six samples could not be amplified with these primers. Twenty-two samples were not tested in the NS3 region because not enough plasma was available.
Sequence analysis.
Concomitant amplification of the NS5B and core regions was obtained for 21 samples. At the genotype level, NS5B BLAST results perfectly matched the core results in 17 (81%) of 21 cases and the results of phylogenetic analysis in 100% of cases (Table 3).
Eighteen samples could be amplified in the NS5B and NS3 regions. Fifteen of them (83%) matched perfectly at the genotype level according to BLAST analysis. For two samples (HC192 and HC269), only one BLAST analysis of the four did not match in the NS3 region; in the third case (HC396), BLAST results for both regions were mixed between genotype 6 (4/8 results), genotype 5 (1/8 results), and genotype 3 (3/8 results), which was also found by phylogenetic analysis.
NS5B BLAST results matched the 5′ UTR BLAST results in 18 (58%) of 31 cases. Discordant results were observed for (i) one NS5B genotype 2 case that was identified as genotype 1 in two of the three 5′ UTR BLAST results (HC365), (ii) all (n = 5) genotype 3 samples in the study, which were classified as genotype 1 in most 5′ UTR BLAST results, (iii) six NS5B genotype 4 cases that were classified as genotype 1 or 3 in the 5′ UTR results, and (iv) one of two genotype 5 cases and the three genotype 6 cases in the study, which were mainly classified as genotype 1.
By using phylogenetic analysis, the entire core, NS3, and NS5B sequences could be assessed without any errors in the final decision. According to 5′ UTR analysis, 13/41 samples could not be classified and results were misinterpreted in 5 cases when NS5B sequencing analysis was used as a reference. Samples that were not identified with the LiPA did not cluster particularly within the same genotype and were dispersed among reference sequences except for the five samples originating from the Sultanate of Oman and one sample from France (HC192) (see Fig. S1 in the supplemental material). They were all phylogenetically related in the 5′ UTR as well as in the core and NS5B regions when results were available (HC192, HC369, HC404, and HC419) and clustered within a separate branch for all genotype 3 sequences. Finally, following the decision criteria fixed for this study, 44 (86%) of 51 HCV genotypes not defined by the LiPA could be determined by sequence analysis, which showed that INNO-LiPA failures could occur with all genotypes but were found more frequently with genotypes 4, 5, and 6 (Table 2).
DISCUSSION
In a large panel of patient samples from all over the world, the HCV genotype could not be determined using the LiPA in 0.52% of cases because the observed pattern was not included in the manufacturer's interpretative chart; however, 86% of these LiPA failures could be resolved using a sequencing approach with common HCV sets of primers. These failures were mostly encountered with genotypes 4, 5, and 6 and in samples from sub-Saharan Africa and especially from the Arabian Peninsula. The number of samples involved is too small to determine whether these factors were linked or independent, however.
The first study using the second-generation line probe assay for the 5′ UTR already showed 11% undetermined LiPA profiles and a particularly high rate of subtyping errors (11% specificity) with samples from West Africa (20). The same version of the INNO-LiPA HCV assay including core sequence hybridization was used to evaluate HCV genotypes for 136 French blood donors and 326 HCV-positive plasma samples in a Belgian study, which exhibited 2 and 7 nonamplifiable samples, respectively, and no or 15 (4.6%) results that could not be clarified (11, 12). More recently, two cases of amplifiable samples giving uninterpretable LiPA results were observed in the Netherlands, and the authors underlined the close resemblance of these two viral strains to viruses of genotypes 2 and 3 originating from non-Western countries (21). The failure of some quantitative RT-PCR assays to detect or to amplify correctly African strains of HCV or HIV has been reported frequently (22–24), and the LiPA may suffer from the same type of bias.
These viral strains also are more difficult to amplify with the classic NS5B primers. Even with the use of three sets of primers covering two different regions, the percentage of samples amplified in the NS5B region in this study (74.5%) was lower than the values normally observed in our laboratory (90% with HC3/HC5 primers during the same period of time) or described in other studies (82 to 97.3%) (10, 25). This could be explained by the greater variety in the geographical origins of the viruses and the use of primers not suitable for these peculiar strains. For these samples, the most conserved region, 5′ UTR, exhibits a higher amplification rate (98%). Discrepancies between amplification rates in the 5′ UTR and NS5B region have been described and were associated mainly with lower levels of viremia and higher polymorphism rates in the annealing regions of the primers (16, 25, 26). In our study, the viral loads of the samples that could not be amplified in NS5B were not statistically smaller than those of amplified samples, and genomic variability in the amplified region appears to provide the most likely explanation for amplification failure. Errors in HCV genotype 6 classification using NS5B sequencing were observed in Asia (27, 28). In our study, no genotype 6 could be amplified with our two first sets of NS5B primers but one could be resolved with core and NS3 amplifications and the two others using another downstream region of NS5B. As already described, we found poor discrimination of genotype 6 samples using only 5′ UTR sequencing.
Phylogenetic analysis of these HCV strains exhibiting LiPA failure showed that they were distributed among different genotypes but were difficult to subtype by NS3 and NS5B sequence phylogenetic analysis, probably due to the high variability in comparison with the reference strains. For example, sequences obtained from the five samples from the Sultanate of Oman were grouped, whatever the genomic region being considered, but were clearly different from the genotype 3 reference strains.
Sequencing of the 5′ UTR is well known to misclassify HCV subtypes, and we now also demonstrate that it can lead to misclassification of genotypes using widely available Web-based BLAST programs, in particular for genotype 3 (14 of 15 analyses) and to a lesser extent for genotype 4 (10 of 33 cases). Genotypes were less frequently misclassified by the Web-based BLAST programs using the core, NS3, or NS5B regions, but this emphasizes the need to include non-European and non-American HCV strains in the international databases and to use phylogenetic analysis to analyze uncommon or previously unreported genotypes.
Indeed, by using pangenotypic sequencing of the NS3 protease region with one set of three primers, 79% of these “difficult” samples could be amplified (13). Analyses with four BLAST algorithms exhibited only 17% ambiguities and an error rate of 1/23 cases (4%) when the criterion that three of four BLAST results should match was applied.
Finally, a sequential approach using three sets of NS5B primers targeting two different regions followed by sequence analysis of the NS3 region can be envisioned. Sequencing of the 5′ UTR should be avoided as it is the source of too much interpretation error. In our study, this strategy gave 74.5% HCV genotype resolution with NS5B and 84% when NS3 was added and the missing sera were taken into account (43/44 cases [98%] when they were discarded).
In this study, discrepancies were observed between 5′ UTR and NS3 or NS5B results in 12 cases, which raises questions about the possibility of intergenotypic recombinant viruses. This hypothesis is unlikely, however, because none of the cases corresponds to what has been described in terms of the genotype involved or recombination points (29).
This study has some limitations. We assumed that the samples for which the INNO-LiPA provided a genotype were correctly classified, and results were not confirmed by sequencing analysis. In addition, problems genotyping HCV may result from mixed infections in the patients' plasma samples. Unfortunately, our sequencing approach based on Sanger technology is not well suited to addressing this point specifically.
In conclusion, with a low rate of HCV genotyping results that could not be resolved, reverse hybridization (the INNO-LiPA 2.0 assay) remains a very convenient method in clinical practice, as it is produces a high rate of amplification and is relatively easy to perform. Special attention should be paid to samples from sub-Saharan Africa and the Arabian Peninsula and to genotypes 4, 5, and 6, which are less common in Western countries. Sequencing assays could be used to resolve uninterpretable LiPA results, but sequencing of multiple regions was needed to deal with more frequent amplification failure for these samples and the low discriminatory power of the 5′ UTR sequences. For these difficult samples, bioinformatic tools and particularly databases are crucial for interpreting the sequences obtained.
Supplementary Material
ACKNOWLEDGMENT
We thank Linda Northrup for revision of the English manuscript.
Footnotes
Published ahead of print 24 April 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.00586-13.
REFERENCES
- 1.Calvaruso V, Craxi A. 2012. 2011 European Association of the Study of the Liver hepatitis C virus clinical practice guidelines. Liver Int. 32(Suppl 1):2–8 [DOI] [PubMed] [Google Scholar]
- 2.Shepard CW, Finelli L, Alter MJ. 2005. Global epidemiology of hepatitis C virus infection. Lancet Infect. Dis. 5:558–567 [DOI] [PubMed] [Google Scholar]
- 3.Chevaliez S. 2011. Virological tools to diagnose and monitor hepatitis C virus infection. Clin. Microbiol. Infect. 17:116–121 [DOI] [PubMed] [Google Scholar]
- 4.El-Serag HB. 2012. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 142:1264–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kieffer TL, Kwong AD, Picchio GR. 2010. Viral resistance to specifically targeted antiviral therapies for hepatitis C (STAT-Cs). J. Antimicrob. Chemother. 65:202–212 [DOI] [PubMed] [Google Scholar]
- 6.Simmonds P. 1999. Viral heterogeneity of the hepatitis C virus. J. Hepatol. 31(Suppl 1):54–60 [DOI] [PubMed] [Google Scholar]
- 7.Simmonds P, Bukh J, Combet C, Deleage G, Enomoto N, Feinstone S, Halfon P, Inchauspe G, Kuiken C, Maertens G, Mizokami M, Murphy DG, Okamoto H, Pawlotsky JM, Penin F, Sablon E, Shin IT, Stuyver LJ, Thiel HJ, Viazov S, Weiner AJ, Widell A. 2005. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology 42:962–973 [DOI] [PubMed] [Google Scholar]
- 8.Doyle JS, Aspinall E, Liew D, Thompson AJ, Hellard ME. 2013. Current and emerging antiviral treatments for hepatitis C infection. Br. J. Clin. Pharmacol. 75:931–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCown MF, Rajyaguru S, Kular S, Cammack N, Najera I. 2009. GT-1a or GT-1b subtype-specific resistance profiles for hepatitis C virus inhibitors telaprevir and HCV-796. Antimicrob. Agents Chemother. 53:2129–2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy DG, Willems B, Deschenes M, Hilzenrat N, Mousseau R, Sabbah S. 2007. Use of sequence analysis of the NS5B region for routine genotyping of hepatitis C virus with reference to C/E1 and 5′ untranslated region sequences. J. Clin. Microbiol. 45:1102–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouchardeau F, Cantaloube JF, Chevaliez S, Portal C, Razer A, Lefrere JJ, Pawlotsky JM, De Micco P, Laperche S. 2007. Improvement of hepatitis C virus (HCV) genotype determination with the new version of the INNO-LiPA HCV assay. J. Clin. Microbiol. 45:1140–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verbeeck J, Stanley MJ, Shieh J, Celis L, Huyck E, Wollants E, Morimoto J, Farrior A, Sablon E, Jankowski-Hennig M, Schaper C, Johnson P, Van Ranst M, Van Brussel M. 2008. Evaluation of Versant hepatitis C virus genotype assay (LiPA) 2.0. J. Clin. Microbiol. 46:1901–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Besse B, Coste-Burel M, Bourgeois N, Feray C, Imbert-Marcille BM, Andre-Garnier E. 2012. Genotyping and resistance profile of hepatitis C (HCV) genotypes 1–6 by sequencing the NS3 protease region using a single optimized sensitive method. J. Virol. Methods 185:94–100 [DOI] [PubMed] [Google Scholar]
- 14.Pham DA, Leuangwutiwong P, Jittmittraphap A, Luplertlop N, Bach HK, Akkarathamrongsin S, Theamboonlers A, Poovorawan Y. 2009. High prevalence of hepatitis C virus genotype 6 in Vietnam. Asian Pac. J. Allergy Immunol. 27:153–160 [PubMed] [Google Scholar]
- 15.Sandres-Saune K, Deny P, Pasquier C, Thibaut V, Duverlie G, Izopet J. 2003. Determining hepatitis C genotype by analyzing the sequence of the NS5b region. J. Virol. Methods 109:187–193 [DOI] [PubMed] [Google Scholar]
- 16.Nakatani SM, Santos CA, Riediger IN, Krieger MA, Duarte CA, Mdo Carmo Debur Carrilho FJ, Ono SK. 2011. Comparative performance evaluation of hepatitis C virus genotyping based on the 5′ untranslated region versus partial sequencing of the NS5B region of Brazilian patients with chronic hepatitis C. Virol. J. 8:459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406–425 [DOI] [PubMed] [Google Scholar]
- 18.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuiken C, Yusim K, Boykin L, Richardson R. 2005. The Los Alamos hepatitis C sequence database. Bioinformatics 21:379–384 [DOI] [PubMed] [Google Scholar]
- 20.Stuyver L, Wyseur A, van Arnhem W, Hernandez F, Maertens G. 1996. Second-generation line probe assay for hepatitis C virus genotyping. J. Clin. Microbiol. 34:2259–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molenkamp R, Harbers G, Schinkel J, Melchers WJ. 2009. Identification of two hepatitis C virus isolates that failed genotyping by Versant LiPA 2.0 assay. J. Clin. Virol. 44:250–253 [DOI] [PubMed] [Google Scholar]
- 22.Bourlet T, Signori-Schmuck A, Roche L, Icard V, Saoudin H, Trabaud MA, Tardy JC, Morand P, Pozzetto B, Ecochard R, Andre P. 2011. HIV-1 load comparison using four commercial real-time assays. J. Clin. Microbiol. 49:292–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chevaliez S, Bouvier-Alias M, Castera L, Pawlotsky JM. 2009. The Cobas AmpliPrep-Cobas TaqMan real-time polymerase chain reaction assay fails to detect hepatitis C virus RNA in highly viremic genotype 4 clinical samples. Hepatology 49:1397–1398 [DOI] [PubMed] [Google Scholar]
- 24.Clarke JR, Galpin S, Braganza R, Ashraf A, Russell R, Churchill DR, Weber JN, McClure MO. 2000. Comparative quantification of diverse serotypes of HIV-1 in plasma from a diverse population of patients. J. Med. Virol. 62:445–449 [DOI] [PubMed] [Google Scholar]
- 25.Laperche S, Lunel F, Izopet J, Alain S, Deny P, Duverlie G, Gaudy C, Pawlotsky JM, Plantier JC, Pozzetto B, Thibault V, Tosetti F, Lefrere JJ. 2005. Comparison of hepatitis C virus NS5b and 5′ noncoding gene sequencing methods in a multicenter study. J. Clin. Microbiol. 43:733–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nolte FS, Green AM, Fiebelkorn KR, Caliendo AM, Sturchio C, Grunwald A, Healy M. 2003. Clinical evaluation of two methods for genotyping hepatitis C virus based on analysis of the 5′ noncoding region. J. Clin. Microbiol. 41:1558–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chao DT, Abe K, Nguyen MH. 2011. Systematic review: epidemiology of hepatitis C genotype 6 and its management. Aliment. Pharmacol. Ther. 34:286–296 [DOI] [PubMed] [Google Scholar]
- 28.Chinchai T, Noppornpanth S, Bedi K, Theamboonlers A, Poovorawan Y. 2006. 222 base pairs in NS5B region and the determination of hepatitis C virus genotype 6. Intervirology 49:224–229 [DOI] [PubMed] [Google Scholar]
- 29.Morel V, Fournier C, Francois C, Brochot E, Helle F, Duverlie G, Castelain S. 2011. Genetic recombination of the hepatitis C virus: clinical implications. J. Viral Hepat. 18:77–83 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.