Abstract
We describe two atypical cases of Kingella kingae infection in children diagnosed by PCR, one case involving a soft tissue abscess and one case a femoral Brodie abscess. Both patients had concomitant human rhinovirus infection. K. kingae strains, isolated from an oropharyngeal swab, were characterized by multilocus sequence typing and rtxA sequencing.
CASE REPORTS
Patient 1 was a healthy 14-month-old girl who was admitted to our tertiary care center because mobilization of her elbow had been painful for 2 days, and an induration of the superior anterior side of her arm was noted, without skin redness. She presented with a 5-day history of febrile viral upper respiratory tract (URT) infection with a maximal temperature of 39.7°C. A biologic inflammatory syndrome was noted, with 14,400 leukocytes/mm3, 574,000 platelets/mm3, 5.36 g/liter of fibrinogen (normal value, 2 to 4 g/liter), and a C-reactive protein (CRP) level of 47 mg/liter (normal value, 0 to 10 mg/liter). X-ray analysis and ultrasonography of the left upper arm were performed, but neither bone lesion, subperiostal abscess (Fig. 1a), nor joint effusion was reported. However, a soft tissue collection with a hypoechogenic center measuring 22 mm by 5 mm was identified in the anterior muscular compartment of the left arm (Fig. 1b). Surgery was performed for debridement before treatment with antibiotics. Blood cultures, as well as the aerobic and anaerobic cultures of the purulent collection, were sterile, despite inoculation into blood culture vials, but Kingella kingae-specific real-time PCR (1) gave positive results in the abscess. In addition, K. kingae was isolated from an oropharyngeal swab, as previously described (2). The isolated strain was susceptible to amoxicillin and cefamandole but resistant to nalidixic acid and lincomycin, as determined by the disc diffusion method (3). Intravenous antibiotic therapy was therefore initiated immediately after bacteriological sampling, with cefamandole (140 mg/kg of body weight/day) for 5 days. The postoperative evolution was favorable, with no more fever and no more pain during mobilization of the upper limb. On day 3, the biologic inflammatory syndrome decreased; the leukocyte count was 8,200 cells/mm3, the fibrinogen level was 4.32 g/liter, and the CRP level was 10 mg/liter. She was discharged on day 5 and given oral antibiotic therapy with amoxicillin (100 mg/kg/day) for 5 more days. Her clinical and biologic features were still normal 3 months later at the control examination.
Fig 1.
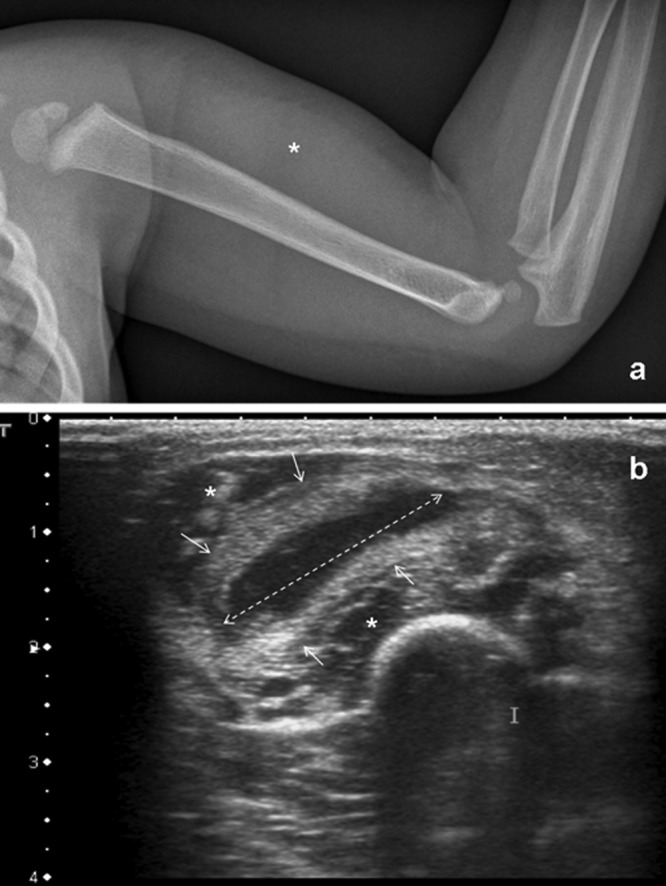
(a) Upper limb X-ray analysis (lateral view) demonstrates anterior soft tissue swelling (∗) but without elbow effusion or bone lesion. (b) Ultrasound analysis of the upper limb (axial view) demonstrates an anterior soft tissue collection (arrow) with a hypoechoic center measuring 22 by 5 mm in diameter (dotted arrow). This collection is located between the two heads of the biceps brachii muscle (∗).
With the use of multilocus sequence typing (MLST) analysis (4), the K. kingae isolate was shown to belong to sequence type 14 (ST14) and to harbor rtxA14 of hemolytic RTX toxin. Unfortunately, because of the low DNA load in the abscess sample and the length of the MLST genes, we failed to amplify all six MLST genes directly on the DNA extract of the abscess. However, three genes (aroE, cpn60, and recA) were successfully studied and displayed the same alleles as those of the oropharyngeal strain. These partial results emphasized the hypothesis that the abscess strain was identical to the strain found in the oropharynx.
Patient 2 was a healthy 21-month-old girl who was hospitalized at our hospital for suspicion of osteoarticular infection (OAI), with a 10-day history of pain in her knee, with limping for 5 days. Her parents reported that the pain had increased and caused insomnia during the last 2 days. She did not have a fever at home, and no other sign except rhinitis was observed during this episode. Ibuprofen was given at home to reduce the pain. At admission, her temperature was 36.9°C, a joint effusion of her right knee was clinically observed, and she experienced pain in her distal femoral metaphysis, without local inflammatory signs or joint amplitude limitation. Her biologic features were as follows: 13,600 leukocytes/mm3, 420,000 platelets/mm3, and a CRP level of <10 mg/liter. X-ray analysis of the right knee showed a metaphyseal lytic lesion with surrounding sclerosis, located in the distal femur, adjacent to the physis, consistent with a Brodie abscess (Fig. 2). Before treatment with antibiotics, surgical debridement of the bony lesion was performed; aerobic and anaerobic cultures of this lesion remained negative, despite inoculation into blood culture vials, but K. kingae-specific real-time PCR gave positive results. The K. kingae strain was also successfully isolated from an oropharyngeal swab and was susceptible to amoxicillin and cefamandole but resistant to nalidixic acid and lincomycin. The patient received a 5-day intravenous cefamandole (140 mg/kg/day) treatment according to our protocol, followed by oral amoxicillin (100 mg/kg/day) for 5 weeks. The clinical outcome was favorable, with a normal CRP level (<10 mg/liter) on day 3 and discharge on day 6. Three months later, the patient's clinical and biologic features were still normal.
Fig 2.
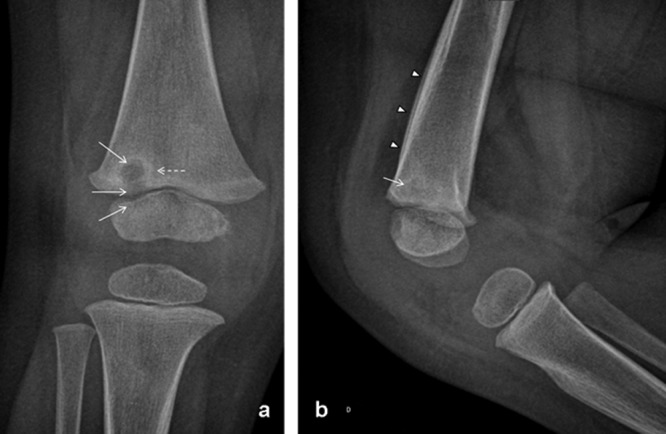
Knee X-ray analyses (anteroposterior [a] and lateral views) demonstrate a round lytic lesion (arrow) circled by a well-defined sclerotic margin (dotted arrow), located in the inferior metaphysis of the femur, adjacent to the physis (arrow). The lateral view also shows a unilamellar inferoanterior periosteal reaction (arrowhead).
The K. kingae strain belonged to ST25 and harbored rtxA1. Unfortunately, because of the very low DNA load in the abscess sample, we failed to obtain a sequence for any MLST gene from the abscess DNA extract.
Finally, because clinical respiratory infection was noted, viral screening was performed for both patients by use of the FilmArray respiratory panel (BioFire Diagnostics, Salt Lake City, UT), according to the manufacturer's recommendations (5). Interestingly, none of the 18 respiratory viruses sought was detected, except human rhinovirus (HRV), which was identified in both cases.
With optimization of conventional culture and development of current molecular techniques, such as real-time PCR, K. kingae has become the major pathogen of OAI in children under 4 years of age in several countries (1, 6, 7). Lower limb joints are most frequently infected, with hips and knees involved in 60% and 80% of septic arthritis cases, respectively (8, 9). Osteomyelitis is also a common presentation of K. kingae infections, and lower limbs are involved in 65% of cases (8). Soft tissue reactions have already been described to occur among children with K. kingae OAI (10), such as presternal tumefaction associated with sternum osteomyelitis (11, 12). However, to our knowledge, only one K. kingae soft tissue abscess without OAI had been described; this abscess occurred in an exceptional presentation of a fluctuating mass anterior to the Achilles tendon insertion (13).
Also, Brodie abscess is a form of subacute osteomyelitis consisting of an intramedullary collection surrounded by sclerosis, most frequently described or associated with Staphylococcus aureus (14–16). K. kingae osteomyelitis is most often insidious and diagnosed following a delay of up to 1 month after the initial symptoms appear (8). Although bone reactions were reported on magnetic resonance imaging (MRI) in almost 50% of K. kingae OAI cases (10), to our knowledge, K. kingae osteomyelitis with typical Brodie abscess including surrounding osteosclerosis had never been described previously.
Interestingly, the possibility of isolating the K. kingae strain in the oropharynx during OAI (2) allowed us to characterize the invasive strains by MLST analysis (4). ST14 is distributed worldwide and is involved in different clinical syndromes, such as occult bacteremia or OAI, without specificity (17). The disease spectrum of ST14 can now be enlarged to include soft tissue infection. On the other hand, ST25 is a France-specific clone that, to date, has been involved only in OAI. Furthermore, ST25 was recently involved in an outbreak of osteomyelitis (18). Our case may underline the higher propensity of ST25 to infect bone tissue than joint tissue.
Finally, concomitant URT viral infections are frequently associated with K. kingae invasive infections (6). To our knowledge, the only viruses that have been associated with K. kingae infection are herpes simplex virus and varicella-zoster virus, with only a few reported cases (6, 19), and a large panel of assays for detection of viruses known or suspected to cause URT infection in human had never been performed. Here, we report, for the first time, simultaneously documented HRV infections and K. kingae invasive infections. The presence of HRV in throat samples does not constitute evidence of infection, since this virus may be present in healthy carrier children (20). However, it is noteworthy that both of our patients had symptoms of rhinitis and, moreover, that no other respiratory virus was detected. In addition, HRV has been found to stimulate bacterial adherence to airway epithelial cells (21) and has been temporally associated with bacterial invasive infections, such as pneumococcal diseases, in children (22). Hence, if HRV-Kingella kingae association can be confirmed in a larger study, HRV infection may represent an interesting approach to better understanding the pathophysiology of K. kingae invasive infections.
Footnotes
Published ahead of print 19 June 2013
REFERENCES
- 1.Ilharreborde B, Bidet P, Lorrot M, Even J, Mariani-Kurkdjian P, Liguori S, Vitoux C, Lefevre Y, Doit C, Fitoussi F, Pennecot G, Bingen E, Mazda K, Bonacorsi S. 2009. New real-time PCR-based method for Kingella kingae DNA detection: application to samples collected from 89 children with acute arthritis. J. Clin. Microbiol. 47:1837–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basmaci R, Ilharreborde B, Bidet P, Doit C, Lorrot M, Mazda K, Bingen E, Bonacorsi S. 2012. Isolation of Kingella kingae in the oropharynx during K. kingae arthritis in children. Clin. Microbiol. Infect. 18:E134–E136. 10.1111/j.1469-0691.2012.03799.x [DOI] [PubMed] [Google Scholar]
- 3.Yagupsky P, Katz O, Peled N. 2001. Antibiotic susceptibility of Kingella kingae isolates from respiratory carriers and patients with invasive infections. J. Antimicrob. Chemother. 47:191–193 [DOI] [PubMed] [Google Scholar]
- 4.Basmaci R, Yagupsky P, Ilharreborde B, Guyot K, Porat N, Chomton M, Thiberge JM, Mazda K, Bingen E, Bonacorsi S, Bidet P. 2012. Multilocus sequence typing and rtxA toxin gene sequencing analysis of Kingella kingae isolates demonstrates genetic diversity and international clones. PLoS One 7:e38078. 10.1371/journal.pone.0038078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poritz MA, Blaschke AJ, Byington CL, Meyers L, Nilsson K, Jones DE, Thatcher SA, Robbins T, Lingenfelter B, Amiott E, Herbener A, Daly J, Dobrowolski SF, Teng DH, Ririe KM. 2011. FilmArray, an automated nested multiplex PCR system for multi-pathogen detection: development and application to respiratory tract infection. PLoS One 6:e26047. 10.1371/journal.pone.0026047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yagupsky P. 2004. Kingella kingae: from medical rarity to an emerging paediatric pathogen. Lancet Infect. Dis. 4:358–367 [DOI] [PubMed] [Google Scholar]
- 7.Ceroni D, Cherkaoui A, Ferey S, Kaelin A, Schrenzel J. 2010. Kingella kingae osteoarticular infections in young children: clinical features and contribution of a new specific real-time PCR assay to the diagnosis. J. Pediatr. Orthop. 30:301–304 [DOI] [PubMed] [Google Scholar]
- 8.Dubnov-Raz G, Ephros M, Garty BZ, Schlesinger Y, Maayan-Metzger A, Hasson J, Kassis I, Schwartz-Harari O, Yagupsky P. 2010. Invasive pediatric Kingella kingae infections: a nationwide collaborative study. Pediatr. Infect. Dis. J. 29:639–643 [DOI] [PubMed] [Google Scholar]
- 9.Basmaci R, Lorrot M, Bidet P, Doit C, Vitoux C, Pennecot G, Mazda K, Bingen E, Ilharreborde B, Bonacorsi S. 2011. Comparison of clinical and biologic features of Kingella kingae and Staphylococcus aureus arthritis at initial evaluation. Pediatr. Infect. Dis. J. 30:902–904 [DOI] [PubMed] [Google Scholar]
- 10.Kanavaki A, Ceroni D, Tchernin D, Hanquinet S, Merlini L. 2012. Can. early MRI distinguish between Kingella kingae and Gram-positive cocci in osteoarticular infections in young children? Pediatr. Radiol. 42:57–62 [DOI] [PubMed] [Google Scholar]
- 11.Luegmair M, Chaker M, Ploton C, Berard J. 2008. Kingella kingae: osteoarticular infections of the sternum in children: a report of six cases. J. Child. Orthop. 2:443–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rolle U, Schille R, Hormann D, Friedrich T, Handrick W. 2001. Soft tissue infection caused by Kingella kingae in a child. J. Pediatr. Surg. 36:946–947 [DOI] [PubMed] [Google Scholar]
- 13.Claesson B, Falsen E, Kjellman B. 1985. Kingella kingae infections: a review and a presentation of data from 10 Swedish cases. Scand. J. Infect. Dis. 17:233–243 [DOI] [PubMed] [Google Scholar]
- 14.Abdulhadi MA, White AM, Pollock AN. 2012. Brodie abscess. Pediatr. Emerg. Care. 28:1249–1251 [DOI] [PubMed] [Google Scholar]
- 15.Brailsford JF. 1938. Brodie's abscess and its differential diagnosis. Br. Med. J. 2:119–138.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stephens MM, MacAuley P. 1988. Brodie's abscess. A long-term review. Clin. Orthop. Relat. Res. 234:211–216. [PubMed] [Google Scholar]
- 17.Amit U, Porat N, Basmaci R, Bidet P, Bonacorsi S, Dagan R, Yagupsky P. 2012. Genotyping of invasive Kingella kingae isolates reveals predominant clones and association with specific clinical syndromes. Clin. Infect. Dis. 55:1074–1079 [DOI] [PubMed] [Google Scholar]
- 18.Bidet P, Collin E, Basmaci R, Courroux C, Prisse V, Dufour V, Bingen E, Grimprel E, Bonacorsi S. 2013. Investigation of an outbreak of osteoarticular infections caused by Kingella kingae in a childcare center using molecular techniques. Pediatr. Infect. Dis. J. 32:558–560 [DOI] [PubMed] [Google Scholar]
- 19.Amir J, Yagupsky P. 1998. Invasive Kingella kingae infection associated with stomatitis in children. Pediatr. Infect. Dis. J. 17:757–758 [DOI] [PubMed] [Google Scholar]
- 20.Jansen RR, Wieringa J, Koekkoek SM, Visser CE, Pajkrt D, Molenkamp R, de Jong MD, Schinkel J. 2011. Frequent detection of respiratory viruses without symptoms: toward defining clinically relevant cutoff values. J. Clin. Microbiol. 49:2631–2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishizuka S, Yamaya M, Suzuki T, Takahashi H, Ida S, Sasaki T, Inoue D, Sekizawa K, Nishimura H, Sasaki H. 2003. Effects of rhinovirus infection on the adherence of Streptococcus pneumoniae to cultured human airway epithelial cells. J. Infect. Dis. 188:1928–1939 [DOI] [PubMed] [Google Scholar]
- 22.Peltola V, Heikkinen T, Ruuskanen O, Jartti T, Hovi T, Kilpi T, Vainionpaa R. 2011. Temporal association between rhinovirus circulation in the community and invasive pneumococcal disease in children. Pediatr. Infect. Dis. J. 30:456–461 [DOI] [PubMed] [Google Scholar]


