Abstract
Attention has been drawn to phage therapy as an alternative approach for controlling pathogenic bacteria such as Flavobacterium psychrophilum in salmonid aquaculture, which can give rise to high mortalities, especially in rainbow trout fry. Recently, phages have been isolated with a broad host range and a strong lytic potential against pathogenic F. psychrophilum under experimental conditions. However, little is known about the fate of phages at environmental conditions. Here, we quantified the dispersal and fate of F. psychrophilum phages and hosts in rainbow trout fry after intraperitoneal injection. Both phages and bacteria were isolated from the fish organs for up to 10 days after injection, and coinjection with both bacteria and phages resulted in a longer persistence of the phage in the fish organs, than when the fish had been injected with the phages only. The occurrence of both phage and bacterium was most prevalent in the kidney and spleen, with only minor occurrence in the brain. The experiment showed that injected phages were rapidly spread in the internal organs of the fish, also in the absence of bacteria. Parallel examination of the regulation of bacteriophage infectivity in controlled laboratory experiments at various environmental conditions showed that pH had only minor effects on long-term (3 months) phage infectivity within a pH range of 4.5 to 7.5, whereas phage infectivity was immediately lost at pH 3. In the absence of host cells, phage infectivity decreased by a factor of 10,000 over 55 days in untreated pond water, while the sterilization and removal of particles caused a 100-fold increase in phage survival relative to the control. In addition, F. psychrophilum-specific phages maintained their infectivity for ∼2 months in glycerol at −80°C, whereas infectivity decreased by a factor 10 when kept in a buffer at 20°C. Only a very small degradation in infectivity was seen when bacteriophages were added and dried on fish feed pellets. Together, these results indicate that application of bacteriophages represents a promising approach for the control of F. psychrophilum infections in trout and suggest fish feed as a potential delivery method.
INTRODUCTION
Disease outbreaks with the bacterium Flavobacterium psychrophilum cause considerable economic losses in salmonid aquaculture worldwide (1). Fish infected with F. psychrophilum have high mortality rates, and fry are especially affected, with mortalities of up to 80 to 90% (2) if left untreated. Until now, commercial vaccines against this fish pathogen have not been available, and treatment with antibiotics is thus required to limit the losses. However, resistance against some of the approved drugs has been found (3), and there is therefore a strong need for alternative treatments.
Phage therapy may be a realistic alternative approach for controlling pathogenic bacteria in aquaculture. Phages have been isolated against important fish pathogens such as Aeromonas salmonicida in brook trout (Oncorhynchus fontinalis) (4), Vibrio harveyi in shrimp (Penaeus monodon) (5), Pseudomonas plecoglossicida in ayu (Plecoglossus altivelis) (6), and Lactococcus garvieae in yellowtail (Seriola quinqueradiata) (7). The aforementioned studies have demonstrated the potential of these specific phages to significantly reduce the impacts of their bacterial hosts on fish mortality.
Bacteriophages and flavobacteria have been shown to be widespread in freshwaters in Denmark and Finland (8, 9). Phages of the fish pathogen Flavobacterium columnare were shown to be lytic and host specific, suggesting they may be good candidates for phage therapy (8). Isolation and characterization of Flavobacterium psychrophilum phages originating from Danish rainbow trout farms revealed an immense genetic and functional diversity of the phage community, which collectively infected 24 of the 27 F. psychrophilum strains that were tested (9). The phages showed highly variable patterns of host range and infection efficiency against F. psychrophilum, and some had a broad host range (infecting 23 of the 27 host strains) and a strong lytic potential against numerous pathogenic F. psychrophilum host strains. This prompted further investigation of the potential of phages in the treatment of disease caused by F. psychrophilum. However, preliminary experiments examining the effects of intraperitoneal (i.p.) injections of 104 F. psychrophilum phage FpV-9 to infected rainbow trout fry (i.p. infected with F. psychrophilum [104 CFU/fish] 24 h before the injection with phages) did not significantly reduce fish mortality relative to untreated controls (unpublished results). Similarly, phage treatment of Atlantic salmon challenged with Aeromonas salmonicida did not enhance fish survival (10), emphasizing the need for a deeper understanding of phage activity and survival in phage-treated fish to optimize the approach. Recently, however, Castillo et al. (11) showed that the addition of F. psychrophilum specific bacteriophages reduced the mortality of trout and salmon after i.p. injection of their host strain F. psychrophilum. The bacteriophages and bacteria were injected together in concentrations of 109 PFU and 108 CFU per fish, respectively. These results demonstrated that phages may be used to protect salmonids from infections with F. psychrophilum, provided that the abundance of phages and the multiplicity of infection are sufficiently high.
The impact of bacteriophage treatment of bacterial pathogens in infected fish depends on the ability of the phages to reach the target organs at sufficient density and lytic potential to control the pathogen. Numerous factors affect the survival and lytic properties of the phages administered to the fish, e.g., delivery mechanism, multiplicity of infection, fish immune response, physical and chemical environment, and development of phage-resistant bacteria. However, little is known about the fate of bacteriophages after addition to infected fish (12, 13), or the key factors regulating phage infectivity in the aquaculture environment.
Application of phages to control fish pathogens in aquaculture therefore requires detailed insight to the efficiency of dispersal and persistence of infective phages in the target organs, as well as the factors that regulates their infectivity. The aim of the present study was to examine the regulation of bacteriophage survival/decay in controlled laboratory experiments at various environmental conditions and to quantify the distribution and fate of Flavobacterium psychrophilum phages and their hosts in rainbow trout. The results demonstrated that phages can reach and proliferate in infected organs of F. psychrophilum, thus emphasizing that phages may be used to reduce infections by this pathogen.
MATERIALS AND METHODS
Bacterial strains.
The Flavobacterium psychrophilum strain used in the present study was a well-characterized Danish strain 950106-1/1 (serotype Fd, ribotype A, 3.3-kb plasmid, virulent) (14, 15).
The strain was stored at −80°C in tryptone yeast extract salts (TYES) media (16) with 15 to 20% glycerol and was subcultured in agitated cultures at 15°C. Strains were taken directly from −80°C and incubated in TYES for a minimum of 48 h before further inoculations were made for liquid cultures in TYES. The incubation of bacterial cultures for experimental infection was done according to Madsen and Dalsgaard (14).
Bacteriophages.
The bacteriophages FpV-4 and FpV-9 used here were isolated from pond water samples collected at Danish freshwater rainbow trout farms (9). Both phages have been described as lytic and infective to F. psychrophilum, infecting a broad range of strains of this pathogen (9). High titer concentrates of the phages were purified (9) and kept in SM-buffer (50 mM Tris-Cl [pH 7.5], 99 mM NaCl, 8 mM MgSO4, 0.01% gelatin) at 5°C.
Plaque assays.
A plaque assay is an agar overlay method (17, 18) used to quantify the titer of lytic and infective bacteriophages as PFU in a sample. We used a modified version of the double-layer method (17): 100 μl of bacteriophage dilutions (10-fold serial dilutions in SM buffer) was mixed with 300 μl of F. psychrophilum cells in the exponential growth phase (i.e., at an optical density at 525 nm [OD525] of 0.3 to 0.5 for a 48-h-old culture) and incubated at 15°C for ∼30 min to allow the phages to absorb to the bacterial cells. A total of 4 ml of 48°C top agar (TYES broth with 0.4% agar) was added, and the mixture was poured onto a cold TYES agar plate, which was immediately incubated at 15°C. After incubation of the plates for 3 to 5 days at 15°C, the plaques were counted to estimate the abundance of lytic bacteriophages. The assay samples were kept for 3 to 4 weeks, at which point the plaques were recounted.
Spot test.
The spot test method (9, 19) is used to detect bacteriophages in liquid samples and is a quick method to screen a large set of samples; in the present study, organ samples were diluted in SM buffer with added chloroform (see the description of the qualitative and quantitative registration of bacteriophages below). Triplicate 10-μl samples of each organ dilutions were added to TYES agar plates with a lawn of F. psychrophilum. For preparation of the bacterial lawn, 300 μl of F. psychrophilum cells in the exponential growth phase (an OD525 of 0.3 to 0.5 of a 48-h-old culture) was added to 4 ml of 48°C top agar (TYES broth with 0.4% agar), and the mixture was poured onto a cold TYES agar plate and immediately incubated at 15°C to solidify. After the addition of phage, the agar plates were placed at 15°C. After 3 days of incubation, plaque formation was visible, and the lytic response (i.e., the presence of lytic phages) detected. In some cases, the number of PFU in the spot could be determined. The PFU were recounted on the plates after approximately 2 to 3 weeks of incubation.
Dispersal of F. psychrophilum and FpV-9 in juvenile rainbow trout.
An infection experiment was carried out to investigate the dispersal, distribution, and decay of F. psychrophilum and the lytic bacteriophage FpV-9 in juvenile rainbow trout (2 to 5 g) when infected by intraperitoneal (i.p.) injection. An i.p. injection method was used because it has been shown to be a reproducible infection method resulting in high mortalities when it comes to F. psychrophilum in comparison to a bath infection method, where mortalities are lower and the reproducibility not as high (14). In every treatment, a total of 50 μl was injected into the peritoneal cavity of the fish. A total of four treatments were established in duplicate aquaria each containing approximately 25 fish. In one aquarium, fish were injected with sterile TYES media serving as controls, and the other aquaria contained (i) fish injected with phage FpV-9 (+FpV-9), (ii) fish injected with phage FpV-9 and F. psychrophilum (+FpV-9 and F. psychrophilum), and (iii) fish injected with F. psychrophilum alone (+ F. psychrophilum). The bacteriophage FpV-9 was added in a total titer of 107 PFU fish−1, whereas the bacterium was added in a total concentration of ∼104 CFU fish−1.
The fish originated from a hatchery with an IPN-free status and with no records of F. psychrophilum infections in fry. They were acclimatized at the aquarium facilities for at least 1 month before the experiments. F. psychrophilum or other fish pathogens were not found when doing a bacteriological investigation of 20 random sampled fish. The experimental infections were carried out as described by Madsen and Dalsgaard (14). To eliminate cross-contamination, the groups of fish were injected in the listed order, starting with the injection of the fish representing the control group and ending with the aquarium-only added F. psychrophilum. The injections of bacteria and phage were performed at T = 0. Prior to injection, all fish were anesthetized with 3-aminobenzoic acid ethyl ester (MS-222, Sigma catalog no. A-5040).
The fish were fed dry commercial pellets (Ecostart, BioMar A/S) 1% biomass per day and kept at 13°C in 30-liter tanks with separate recirculation of water and air supply for each tank. Water was changed twice a week. The content of ammonia, nitrite, and nitrate in the water was monitored by ammonia test strips (Hach, catalog no. 27553-25) and nitrate/nitrite test strips (Hach, catalog no. 27454-25) to ensure that the biological filters were functioning during the experiments. The aquaria and the condition of the fish were monitored at least twice per day, and moribund and dead fish were collected and counted. Moribund fish were killed by an overdose of MS-222 in water.
During the experiments, two live fish were collected from each tank 1, 5, 10, 24, 29, 48, 72, 99, and 168 h after i.p. injection and killed by an overdose of MS-222 for analyses of bacteria and phages. Weight and length were measured in all fish upon sampling, and samples for detection of F. psychrophilum and phages were collected from the spleen, kidney, brain, and scrape from abdominal cavity. Qualitative methods (streaking on plates and spot test, respectively) were used. In addition to the qualitative measurements, the abundance of infective FpV-9 was determined to obtain a quantitative measure of phage survival in one of the organs over time. Dead fish during the experiment were also sampled. The experiment was terminated after 240 h, where the surviving fish were killed by an overdose of MS-222, and organs from all of the surviving fish were sampled as described above.
Qualitative bacteriological analysis.
Samples were collected from organs, streaked onto TYES agar and blood agar (BA) plates, and incubated at 15°C for 5 days up to 3 to 4 weeks. Yellow colonies that showed growth on TYES agar but not on BA was identified as F. psychrophilum by a species-specific PCR with DNA primers against a sequence of the 16SrRNA gene (20).
Qualitative and quantitative registration of bacteriophages.
Samples for qualitative and quantitative registration of bacteriophages were collected immediately after the samples for the bacteriological analysis from the same organs. This was done by transferring the organs to Eppendorf tubes with 300 μl of SM buffer and 15 μl of chloroform. Only the kidney samples were weighed to allow a quantitative registration of weight-specific phage abundance.
All samples for registration of bacteriophages were whirly mixed, killed with chloroform (as described above), and stored at 5°C. Within 24 h, the samples were sonicated for 15 s at 20 kHz to homogenize the organs prior to the PFU quantification. Preliminary tests verified that 15 s of sonication was sufficient to obtain a homogenized mixture without affecting phage infectivity (unpublished results).
Qualitative examination of the presence of phages was done in the spleen, kidney, brain, and peritoneal cavity by triplicate spot tests of the homogenized organs. Spot tests were designated as positive, if spots were seen in one or more of the triplicate samples. In addition to the spot test, a quantitative examination of abundance of infectious phages was done in kidney samples where the weight-specific phage abundance was quantified by plaque assay after dilution of the homogenized sample.
Sensitivity of bacteriophages to selected pH values.
pH values of 3, 4.5, 6, and 7.5 were used to determine whether the bacteriophages were affected by exposure to different pH values. The values were chosen in accordance with the range of pHs (3–7) obtained in starved fish (21). Triplicate tubes were prepared with 9.9 ml of SM buffer adjusted to the respective pH values using 0.1 M HCl. At T = 0, 100 μl of either FpV-4 or FpV-9 was added to a total volume of 10 ml (∼107 phages ml−1, final concentration), and the tubes were wrapped in foil to eliminate any possible decay in response to light exposure and kept at 15°C. Then, 1-ml samples were taken with regular intervals (from hourly sampling the first 24 h to a sampling frequency of 1 to 3 weeks after the first week). The last sample was collected after ∼3 months. Plaque assays were performed on every sample to register the change in abundance of infectious phages over time.
Degradation of bacteriophages in pond water.
The purpose of this experiment was to study changes in phage infectivity over time in trout farm pond water in the absence of host cells and to examine the influence of particles and biological processes for the rate of phage decay in natural environments. The FpV-9 phage (0.5 × 106 to 1 × 106 PFU ml−1) was added to triplicate 10-ml tubes with untreated, autoclaved, or 0.2-μm-pore-size-filtered pond water, and the phage abundances were monitored regularly over 55 days of incubation in the dark at 20°C.
Degradation of bacteriophages on fish feed pellets.
Feeding pellets are potential vehicles for the delivery of phages to infected fish, and the purpose of this experiment was to monitor the loss of phage infectivity over time after the addition and desiccation on feeding pellets. At time zero, 30 sterile 50-ml tubes with 1 ml of fish feed pellets (0.8 mm) were added 150 μl of FpV-9 dilution corresponding to 4.3 × 107 phages. The tubes were shaken gently to make sure that all of the added liquid was absorbed by the food pellets and left at room temperature (∼20°C) for 30 min with the lid off (placed in a sterile cabinet) for the moisture to evaporate. The dried samples were incubated in the tubes in the dark at room temperature, and subsamples were collected regularly for 60 days. For every sampling, triplicate tubes were harvested by adding 5 ml of SM buffer, followed by homogenization by sonication (twice for 20 s each time) while cooled on ice. Immediately after this, the homogenized samples were diluted, and the abundance of infectious phages was determined by plaque assays.
Sensitivity of bacteriophages to glycerol and SM buffer.
For examination of the decay of phages at different storage conditions, the abundance of infectious FpV-9 phages was monitored during incubation in glycerol at −80°C and in SM buffer at 20°C. A total of 106 PFU of phage FpV-9 ml−1 were added to either glycerol or SM buffer in triplicates, followed by incubation at −80 and 20°C, respectively, and triplicate samples were collected at regular intervals for up to 55 days.
Statistics.
For all of the decay experiments, the exponential rate of infectivity loss was calculated by linear regression of the decrease in PFU over time after log transformation of the data. The statistical significance of the obtained rates (i.e., if the slope of PFU versus time was significantly different from 0) was tested using the Student t test.
RESULTS
Dispersal of F. psychrophilum and FpV-9 in juvenile rainbow trout.
Within the first hour after i.p. injection, phage FpV-9 was spread to all the organs of the sampled fish; however, the persistence of the phage over time varied considerably between the organs (Fig. 1 to 4). Already by 24 h after injection phages were only isolated in fewer than half of the peritoneal cavity and spleen samples, whereas they persisted for a longer period in the kidney samples.
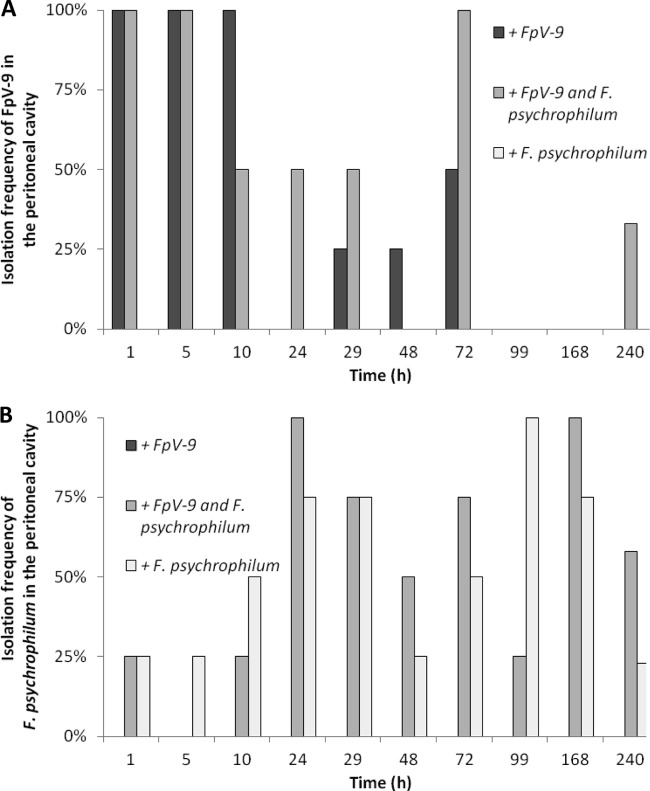
Fig 1.
Isolation frequency of FpV-9 (A) and F. psychrophilum (B) from the peritoneal cavity over time in the sampled fish injected with phage alone (+FpV-9), phage and pathogen (+FpV-9 and F. psychrophilum), and pathogen alone (+F. psychrophilum). Four fish were sampled at each time point, except for the last sampling, which consisted of 12 to 14 fish.
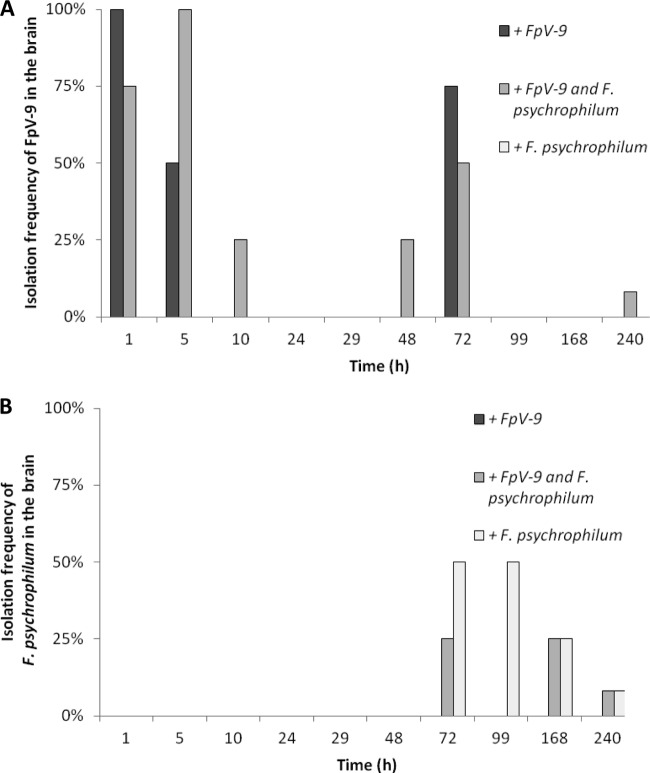
Fig 4.
Isolation frequency of FpV-9 (A) and F. psychrophilum (B) from the brain over time in the sampled fish injected with phage alone (+FpV-9), phage and pathogen (+FpV-9 and F. psychrophilum), and pathogen alone (+F. psychrophilum). Four fish were sampled at each time point, except for the last sampling, which consisted of 12 to 14 fish.
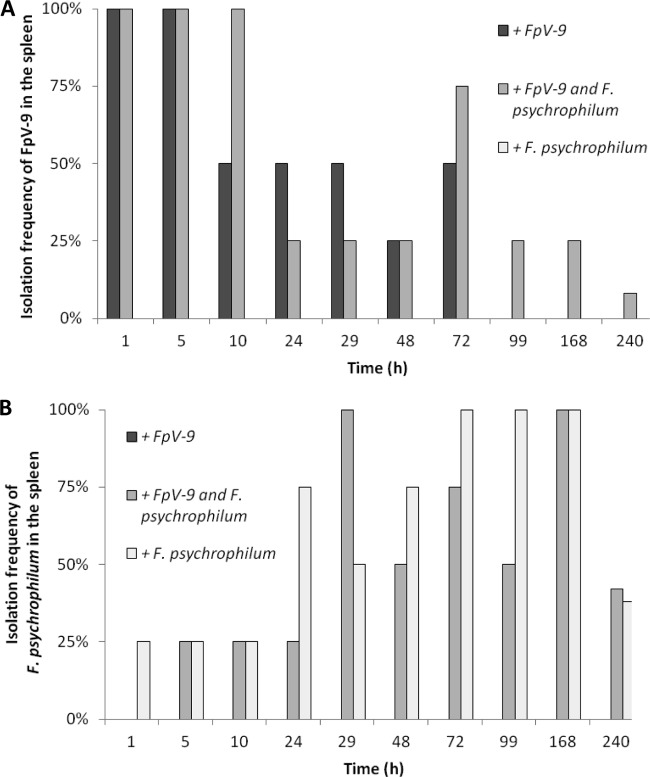
Fig 2.
Isolation frequency of FpV-9 (A) and F. psychrophilum (B) from the spleen over time in the sampled fish injected with phage alone (+FpV-9), phage and pathogen (+FpV-9 and F. psychrophilum), and pathogen alone (+F. psychrophilum). Four fish were sampled at each time point, except for the last sampling, which consisted of 12 to 14 fish.
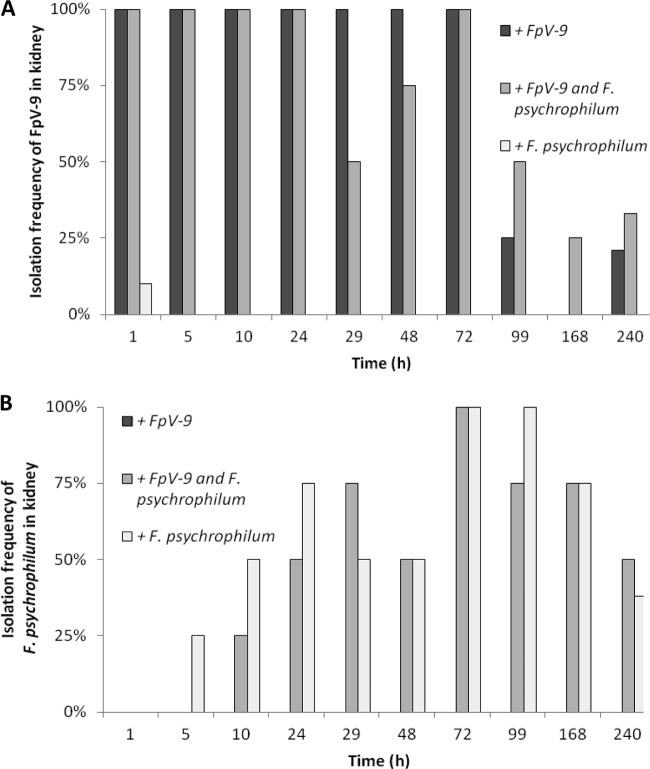
When fish were infected with FpV-9 in the absence of the pathogen, the phages were found in all of the different organs in 50 to 100% of the fish 72 h after injection. In the kidney, where phage recovery were best, phages were found in 25% of the samples after 99 h and in 21% after 240 h, where the experiment were terminated (Fig. 3A). In the group of fish where the phage was injected along with its host strain (+FpV-9 and F. psychrophilum), the phages persisted for longer time in the organs and were found in 33% of the peritoneal cavity and kidney samples and in 8% of the brain and spleen samples by the end of the experiment (240 h, Fig. 1A to 4A). The injected F. psychrophilum was also spread in the fish organs, with increasing occurrences over time during the first 100 h, reaching 100% occurrence in the kidney, spleen, and peritoneal cavity and 50% occurrence in the brain. By the end of the experiment, F. psychrophilum occurrence decreased to 38% of the kidney and spleen samples, 8% of the brain samples, and 33% of the peritoneal cavity samples (Fig. 1B to 4B). There was no difference between the development in F. psychrophilum occurrence in the two treatments, and FpV-9 addition did therefore not reduce the frequency of F. psychrophilum detection in the organs.
Fig 3.
Isolation frequency of FpV-9 (A) and F. psychrophilum (B) from the kidney over time in the sampled fish injected with phage alone (+FpV-9), phage and pathogen (+FpV-9 and F. psychrophilum), and pathogen alone (+F. psychrophilum). Four fish were sampled at each time point, except for the last sampling, which consisted of 12 to 14 fish.
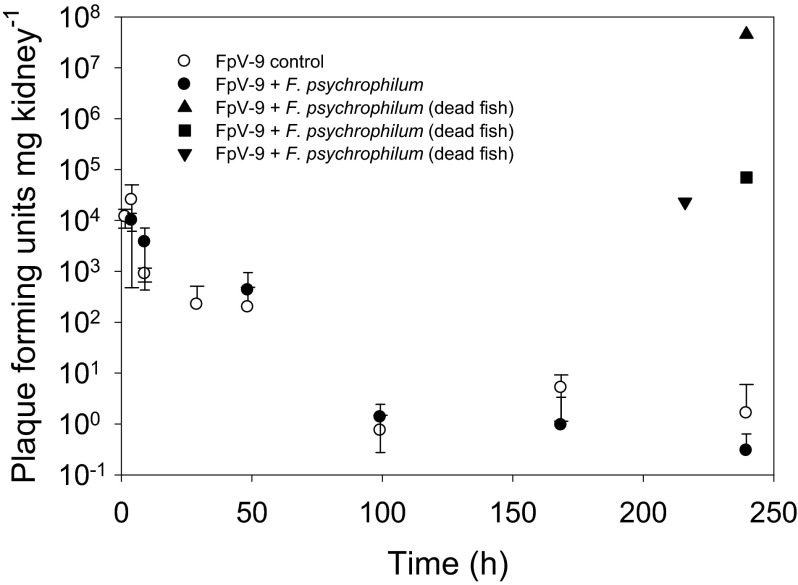
The quantitative analysis of phage persistence in the kidney supported the qualitative observations (Fig. 5 and Table 1). The abundance of phages decreased exponentially (0.037 to 0.045 h−1, P < 0.01) from ∼104 PFU mg of kidney−1 after 1 h to 1 to 10 PFU mg of kidney−1 after 100 h but remained above the detection limit throughout the experiment. The amount of phages in the kidney was not influenced by the presence of F. psychrophilum; however, the density of phage FpV-9 had increased to between 2.3 × 104 and 4.5 × 107 PFU mg of kidney−1 in fish which had died from F. psychrophilum infection, suggesting proliferation of phages in the organ of these fish.
Fig 5.
Average kidney-specific bacteriophage density (PFU mg of kidney−1). Each data point represents the average of kidney samples from four fish. Three of the data points represent the FpV-9 density in the kidneys of fish that died before the end of the experiment.
Table 1.
Decay rates of F. psychrophilum phages in the trout kidney, in buffer at various pHs, and in pond watera
| Condition | Mean FpV-9 decay rate ± SDb |
|---|---|
| Kidney | |
| +F. psychrophilum | 0.037 ± 0.010* h−1 |
| Control | 0.045 ± 0.009* h−1 |
| Buffer | |
| pH 3.0 | 1 |
| pH 4.5 | 0.038 ± 0.004* day−1 |
| pH 6.0 | 0.002 ± 0.003 day−1 |
| pH 7.5 | 0.004 ± 0.003 day−1 |
| Pond water | |
| Untreated | 0.112 ± 0.066* day−1 |
| Autoclaved | 0.076 ± 0.009* day−1 |
| Filtered | 0.072 ± 0.008* day−1 |
The decay rates were determined for F. psychrophilum phages (i) inside the rainbow trout kidney after i.p. injections, (ii) during incubations in buffer at various pHs, and (iii) in untreated, autoclaved, and 0.2-μm-pore-size-filtered pond water, respectively.
*, a decrease in phage infectivity over time that was statistically significant (i.e., the slope was significantly different from 0; P < 0.05).
Phage decay rates.
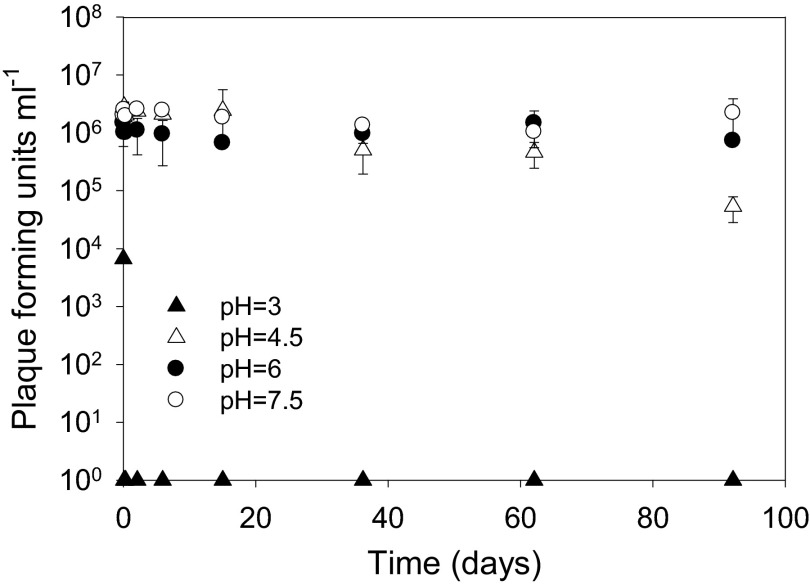
pH influenced the decay rates of FpV-9 (Fig. 6 and Table 1). Although the infectivity of the bacteriophage FpV-9 decreased at a rate of 0.038 ± 0.004 day−1 (r2 = 0.92, P < 0.01) at pH 4.5, the infectivity did not decrease significantly over the 3-month incubation at pH 6 and 7.5 (Fig. 6). Similar results were obtained for the phage FpV-4, with a decay rate of 0.037 ± 0.006 day−1 (r2 = 0.81, P < 0.01) at pH 4.5 (data not shown). Both bacteriophages immediately lost their infectivity when incubated at pH 3.
Fig 6.
Change in infectivity of the bacteriophage FpV-9 over time at selected pH values.
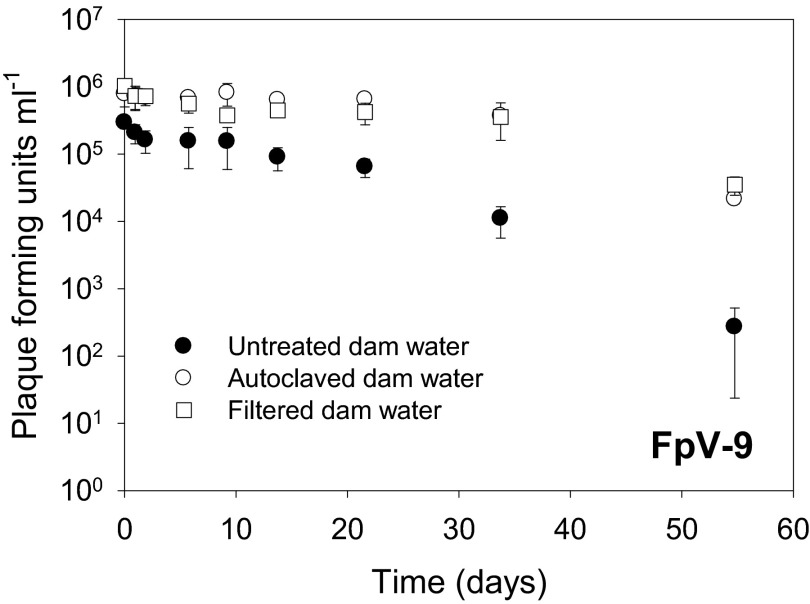
In untreated pond water, the decay rate of phages in pond water was high (0.112 ± 0.066 day−1, r2 = 0.97, P < 0.01) compared to the rates obtained in parallel particle-free and sterilized samples (0.076 ± 0.009 day−1 and 0.072 ± 0.008 day−1, respectively [r2 = 0.91, P < 0.01]) (Fig. 7 and Table 1), demonstrating that filtration and autoclaving the pond water reduced phage decay. After a 50% loss of infectivity immediately after coating on the fish feed pellets, the coated phages decayed at a rate of 0.036 ± 0.011 day−1 (r2 = 0.62, P < 0.05) (Fig. 8 and Table 2) over the subsequent 2-month incubation. This indicated that FpV-9 can tolerate long periods of desiccation and still maintain a high infectivity.
Fig 7.
Change in infectivity over time of bacteriophage FpV-9 in untreated, particle-free (0.2-μm-pore-size-filtered), and autoclaved fish pond water collected at an aquaculture site.
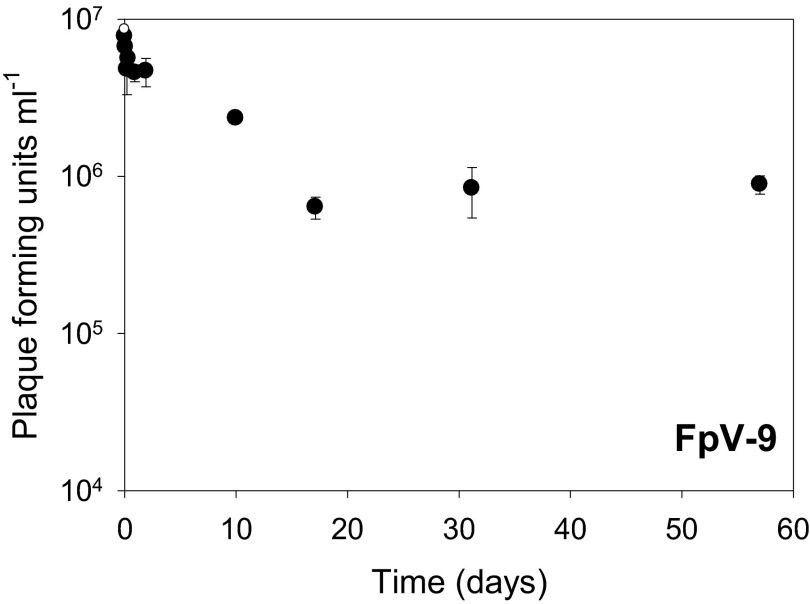
Fig 8.
Phage infectivity over time after coating on fish feed pellets. Degradation of bacteriophages on fish feed pellets. By regression of the log transformed values, a slope of −0.6 × 10−3 for the whole period of the experiment was determined. This indicates that only a relatively small degradation of the added bacteriophages, FpV-9, occurred and that this phage can tolerate long periods of drying out and still conserve its infectivity.
Table 2.
Decay rates of F. psychrophilum phages during storage on fish feed pellets, in glycerol/buffer, and in buffera
| Condition | Temp (°C) | Mean FpV-9 decay rate ± SD day−1b |
|---|---|---|
| Feeding pellets | 20 | 0.036 ± 0.011* |
| Glycerol/buffer | −80 | 0.001 ± 0.001 |
| Buffer | 20 | 0.036 ± 0.002* |
Decay rates were determined for F. psychrophilum phages during storage on fish feed pellets at 20°C, in glycerol/buffer at −80°C, and in buffer at 20°C.
*, a decrease in phage infectivity over time that was statistically significant (i.e., the slope was significantly different from 0; P < 0.05).
Persistence of bacteriophages in glycerol and SM buffer.
The phages kept in 50% glycerol and 50% SM buffer at −80°C did not show significant decay during the investigation period of 55 days (Table 2), whereas phages kept in SM buffer at 20°C decayed at a rate of 0.036 ± 0.002 day−1 (r2 = 0.97, P < 0.001) (Table 2).
DISCUSSION
The potential of bacteriophages to control F. psychrophilum infections as an alternative to antibiotic treatment has received considerable attention the past few years. In a preliminary experiment, the addition of phages to F. psychrophilum-infected rainbow trout did not have significant effects on fish survival (unpublished results). In these experiments, rainbow trout fry were injected i.p. with 104 CFU/fish and, after 24 h, injected i.p. with 104 PFU/fish of bacteriophages FpV-9 or FpV-10 (9), parallel to control groups injected with sterile media or without injections. Random-sampling-based determination of phage occurrence in fish organs over time in the experiment showed a rapid loss of phage activity in the organs and, after 5 days, no infective phages were recovered. These data raised questions about the fate of the phages in the fish after injection and to what extend the number of phages that reaches the target organs is sufficient to ensure the required encounter rate between phage and host to allow significant phage proliferation and subsequently pathogen control in the infected organs.
The present experiment addressed these questions, investigating the abundance and dispersal of phages in various fish organs after infection. Further, the phage addition was increased to 107 PFU fish−1, representing a 1,000-fold-higher initial phage abundance than in the preliminary experiment.
In the group injected with both phages and bacteria, phages were still present in all of the organs of some of the fish 10 days after infection, whereas it was only possible to isolate phages from a few kidney samples in the fish that was only treated with phages. The results demonstrated that addition of a higher phage titer resulted in an efficient dispersal of phages to all of the investigated organs and a prolonged persistence of infective phages in the organs compared to our preliminary experiment. Furthermore, the extended persistence of phages in the presence of the pathogen host, suggested that phages were in fact produced in the infected organs by infection and lysis of the pathogen, thus maintaining the phage population for a longer period of time than in the absence of the host. This conclusion was supported by the highly elevated abundances of phages in the kidney of fish that had died from F. psychrophilum infection. This observation suggested that phage proliferation was significantly enhanced in fish where infection had reached a more severe stage, with higher densities of bacteria, and thus increased encounters between phage and pathogen. However, the fact that the fraction of fish which contained phages decreased over time, even in the presence of host cells, and that the occurrence of the pathogen was unaffected by the addition of phages, clearly showed that phages were not capable of controlling the pathogen under these experimental conditions.
We suggest that the fast initial phage decay in the organs, in combination with the delay in pathogen infection and proliferation in the organs, reduced the encounter rate between phages and hosts during the critical phase of infection, and thus prevented efficient phage proliferation and pathogen control. In the cases of high phage abundance in the fish that died from the infection, the production of phages apparently occurred at a stage of infection where the effects of the pathogen were irreversible.
Efficient phage control of F. psychrophilum has recently been demonstrated in a small-scale challenge experiment (11), where the addition of 109 PFU of F. psychrophilum phage fish−1 significantly reduced the mortality of salmon (Salmo salar) and trout (Oncorhynchus mykiss) during 15-day incubations. Together, these results demonstrate that F. psychrophilum bacteriophages do have the potential to survive and proliferate in fish organs and to provide protection against F. psychrophilum infections. It is obvious, however, that both the dose of phages added and the timing between pathogen development and phage addition are crucial factors influencing the efficiency of the phage control of the pathogen in the infected organs.
Our results are in accordance with previous findings of high abundances of phages in dead Ayu fish (6), which suggested that the phages only provided little protection against naturally occurring infections, when the fish were already in a late stage of the disease process (6), and the authors argued that phages possibly only offer disease protection in an early stage of infection. In accordance with that, the observation of high concentrations of P. plecoglossicida phages in healthy fish originating from a pond where P. plecoglossicida infection prevailed (22), suggested that in natural environments, phages may be present in fish also during the early stage of infection, possibly preventing the pathogen from establishing an infection.
Although phages were rapidly spread to fish organs after addition, it was also obvious that the rate of decay in the organs was fast (0.037 to 0.045 h−1). In an attempt to elucidate important factors for phage decay, we therefore measured decay rates at various environmental conditions. The experiments testing the pH tolerance of the bacteriophages FpV-4 and FpV-9 showed that the pH was very important for the infectivity of the phages. At pH 3, the bacteriophages immediately lost their infectivity, and also a pH of 4.5 significantly increased phage decay rates relative to at pH 6 and 7.5. These results confirmed other studies on pH sensitivity in F. psychrophilum phages (12), which showed complete loss of infectivity at pH 2 and 3 and significantly reduced infectivity at pH 4 and 11 during short-term (1 h) incubations. If the bacteriophages are going to be delivered to the fish via the feed, the pH in the stomach may be essential for the ability of the phages to survive the stomach passage. Bucking and Wood (23) found that the pH profile of the stomach contents in rainbow trout increased from a resting pH of 2.7 to 4.9 within 1 h after the ingestion of a meal and stayed at that level for almost 12 h. In addition, these researchers found the intestine to be much more alkaline than the stomach, with recorded pH values of >7.5 both before and after feeding. In our study even at pH 4.5 the phage decay rate was 25 to 30 times lower than in the organs, suggesting that other factors, such as irreversible adsorption to the fish tissue were regulating phage infectivity.
The reduced phage decay rate in filtered pond water compared to untreated controls, also suggested that nonspecific adsorption to surfaces increased phage decay, as also observed in similar experiments with various phages of fish pathogens (24, 12). Similarly, autoclavation of the pond water increased phage survival, which may be an indication of a biological degradation of phages in untreated pond water.
Interestingly, the tested phage (FpV-9) can tolerate long periods of desiccation and still maintain a high infectivity, since the bacteriophages coated on fish feed pellets showed relatively low degradation (0.036 ± 0.011 day−1) of the added phage FpV-9 during long-term incubations. In fact, the stability of the phage on the pellets was similar to that of the phages kept in a buffer at 20°C. Storing the phages in glycerol at −80°C turned out to be an effective long-term storage option for the phage with insignificant phage decay over a 3-month period of incubation.
The results suggest that a diet with phage additives might be a method for delivery of phages to F. psychrophilum-infected fish. This is further supported by the fact that the bacteriophages maintained infectivity at pH levels of 4.5, and other authors have shown that the pH in the stomach rises to this level when fish have been fed (23), meaning that the phages seem to be able to survive the stomach pH if they are provided along with the diet. Due to the relatively fast decay of phages in the organs, it is, however, crucial to further investigate the dose and frequency of phage addition in order to optimize the encounter rate between phages and pathogen during the initial part of the infection.
Here we have examined the dispersal of phages after i.p. injection. The next step will be to investigate whether F. psychrophilum bacteriophages can survive the conditions in vivo in the gastrointestinal lumen of the fish (including the potentially low pH during digestion) and reach the internal organs through this route. Other authors have shown that bacteriophages can survive on feed pellets and have used oral administration of phages impregnated on the feed for treating P. plecoglossicida-infected ayu with success (6). It will also be highly relevant to investigate different feeding regimes when it comes to administering bacteriophages in this way, including the prophylactic administration of phages. The administration of phages via the feed enables the treatment of a whole fish population against a specific pathogen without affecting the normal intestinal microflora (25).
The present study has documented the potential of phages to spread to inner organs of rainbow trout after i.p. injection and to proliferate and maintain infectivity for up to 10 days. Although previous studies have found evidence for significantly reduced salmonid mortality after treatment with F. psychrophilum-specific phages in small-scale experiments (11), further studies are needed to evaluate the potential of phage therapy for F. psychrophilum control and enhanced fish survival under conditions simulating normal fish farm conditions using phage-coated feed.
ACKNOWLEDGMENTS
The study was supported by the Directorate for Food, Fisheries, and Agribusiness and the Danish Council for Independent Research (FNU-09-072829) and by EU-IRSES project AQUAPHAGE.
We thank Kirsten Kaas and Lene Gertman for excellent technical support.
Footnotes
Published ahead of print 7 June 2013
REFERENCES
- 1. Nematollahi A, Decostere A, Pasmans F, Haesebrouck F. 2003. Flavobacterium psychrophilum infections in salmonid fish. J. Fish Dis. 26:563–574 [DOI] [PubMed] [Google Scholar]
- 2. Jensen Aa P, Henriksen NH, Michelsen K, Madsen L, Dalsgaard I. 2003. Prevention of RTFS (rainbow trout fry syndrome) and reduction in the amount of used antibiotics in Danish rainbow trout hatcheries and fry farms. DFU report 124-03 Danish Institute for Fisheries Research, Copenhagen, Denmark: (In Danish.) [Google Scholar]
- 3. Bruun MS, Schmidt AS, Madsen L, Dalsgaard I. 2000. Antimicrobial resistance patterns in Danish isolates of Flavobacterium psychrophilum. Aquaculture 187:201–212 [Google Scholar]
- 4. Imbeault S, Parent S, Lagacé M, Uhland CF, Blais J-F. 2006. Using bacteriophages to prevent furunculosis caused by Aeromonas salmonicida in farmed brook trout. J. Aquat. Anim. Health 18:203–214 [Google Scholar]
- 5. Vinod MG, Shivu MM, Umesha KR, Rajeeva BC, Krohne G, Karunasagar I, Karunasagar I. 2006. Isolation of Vibrio harveyi bacteriophage with a potential for biocontrol of luminous vibriosis in hatchery environments. Aquaculture 255:117–124 [Google Scholar]
- 6. Park SC, Nakai T. 2003. Bacteriophage control of Pseudomonas plecoglossicida infection in ayu Plecoglossus altivelis. Dis. Aquat. Organ. 53:33–39 [DOI] [PubMed] [Google Scholar]
- 7. Nakai T, Sugimoto R, Park K-H, Mori K, Nishioka T, Maruyama K. 1999. Protective effects of bacteriophage on experimental Lactococcus garvieae infection in yellowtail. Dis. Aquat. Organ. 37:33–41 [DOI] [PubMed] [Google Scholar]
- 8. Laanto E, Sundberg L-R, Bamford JKH. 2011. Phage specificity of the freshwater fish pathogen Flavobacterium columnare. Appl. Environ. Microbiol. 77:7868–7872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stenholm AR, Dalsgaard I, Middelboe M. 2008. Isolation and characterization of bacteriophages infecting the fish pathogen Flavobacterium psychrophilum. Appl. Environ. Microbiol. 74:4070–4078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Verner-Jeffreys D, Algoet M, Pond MJ, Virdee HK, Bagwell NJ, Roberts EG. 2007. Furunculosis in Atlantic salmon (Salmo salar L.) is not readily controllable by bacteriophage therapy. Aquaculture 270:475–484 [Google Scholar]
- 11. Castillo D, Higuera G, Villa M, Middelboe M, Dalsgaard I, Madsen L, Espejo RT. 2012. Diversity of Flavobacterium psychrophilum and the potential use of its phages for protection against bacterial cold water disease in salmonids. J. Fish Dis. 35:193–201 [DOI] [PubMed] [Google Scholar]
- 12. Kim JH, Gomez DK, Nakai T, Park SC. 2010. Isolation and identification of bacteriophages infecting ayu Plecoglossus altivelis altivelis specific Flavobacterium psychrophilum. Vet. Microbiol. 140:109–115 [DOI] [PubMed] [Google Scholar]
- 13. Prasad Y, Arpana Kumar D, Sharma AK. 2011. Lytic bacteriophages specific to Flavobacterium columnare rescue catfish, Clarias batrachus (Linn.) from columnaris disease. J. Environ. Biol. 32:161–168 [PubMed] [Google Scholar]
- 14. Madsen L, Dalsgaard I. 1999. Reproducible methods for experimental infection with Flavobacterium psychrophilum in rainbow trout (Oncorhynchus mykiss). Dis. Aquat. Organ. 36:169–176 [DOI] [PubMed] [Google Scholar]
- 15. Madsen L, Dalsgaard I. 2000. Comparative studies of Danish Flavobac-terium psychrophilum isolates: ribotypes, plasmid profiles, serotypes and virulence. J. Fish Dis. 23:211–218 [Google Scholar]
- 16. Holt RA, Rohovec JS, Fryer JL. 1993. Bacterial coldwater disease, p 3–23 In Englis V, Roberts RJ, Bromage NR. (ed), Bacterial diseases of fish. Blackwell Scientific Publications, Oxford, United Kingdom [Google Scholar]
- 17. Adams MH. 1959. Bacteriophages. Interscience Publishers, New York, NY [Google Scholar]
- 18. Kutter E, Sulakvelidze A. 2003. Bacteriophage as antibiotics: molecular biology and applications. CRC Press, Inc, Boca Raton, FL [Google Scholar]
- 19. Kutter E, Sulakvelidze A. 2005. Bacteriophages: biology and applications. CRC Press, Inc, Boca Raton, FL [Google Scholar]
- 20. Wiklund T, Madsen L, Bruun MS, Dalsgaard I. 2000. Detection of Flavobacterium psychrophilum from fish tissue and water samples by PCR amplification. J. Appl. Microbiol. 88:299–307 [DOI] [PubMed] [Google Scholar]
- 21. Jobling M, Hjelmeland K. 1992. Ernæring og fordøjelse, p 233–257 In Djøving K, Reimers E. (ed), Fiskens fysiologi. John Grieg, Forlag, Norway [Google Scholar]
- 22. Nakai T, Park SC. 2002. Bacteriophage therapy of infectious diseases in aquaculture. Res. Microbiol. 153:13–18 [DOI] [PubMed] [Google Scholar]
- 23. Bucking C, Wood CM. 2009. The effect of postprandial changes in pH along the gastrointestinal tract on the distribution of ions between the solid and fluid phases of chime in rainbow trout. Aquaculture Nutr. 15:282–296 [Google Scholar]
- 24. Park SC, Shimamura I, Fukunaga M, Mori K-I, Nakai T. 2000. Isolation of bacteriophages specific to a fish pathogen, Pseudomonas plecoglossicida, as a candidate for disease control. Appl. Environ. Microbiol. 66:1416–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oliveira J, Castilho F, Cunha A, Pereira MJ. 2012. Bacteriophage therapy as a bacterial control strategy in aquaculture. Aquaculture Int. 20:879–910 [Google Scholar]