Abstract
Shiga toxin-converting bacteriophages (Stx phages) carry the stx gene and convert nonpathogenic bacterial strains into Shiga toxin-producing bacteria. Previous studies have shown that high densities of free and infectious Stx phages are found in environments polluted with feces and also in food samples. Taken together, these two findings suggest that Stx phages could be excreted through feces, but this has not been tested to date. In this study, we purified Stx phages from 100 fecal samples from 100 healthy individuals showing no enteric symptoms. The phages retrieved from each sample were then quantified by quantitative PCR (qPCR). In total, 62% of the samples carried Stx phages, with an average value of 2.6 × 104 Stx phages/g. This result confirms the excretion of free Stx phages by healthy humans. Moreover, the Stx phages from feces were able to propagate in enrichment cultures of stx-negative Escherichia coli (strains C600 and O157:H7) and in Shigella sonnei, indicating that at least a fraction of the Stx phages present were infective. Plaque blot hybridization revealed lysis by Stx phages from feces. Our results confirm the presence of infectious free Stx phages in feces from healthy persons, possibly explaining the environmental prevalence observed in previous studies. It cannot be ruled out, therefore, that some positive stx results obtained during the molecular diagnosis of Shiga toxin-producing Escherichia coli (STEC)-related diseases using stool samples are due to the presence of Stx phages.
INTRODUCTION
Shiga toxin-producing Escherichia coli (STEC) strains are pathogens involved in food-borne outbreaks that may cause serious intestinal and systemic disease (1). The main virulence factor of STEC is the production of Shiga toxin. The genes coding for Shiga toxin (stx) and its variants are harbored in the genomes of temperate bacteriophages (Stx phages) (2, 3). Stx phages can be associated with the pathogenic subset of locus of enterocyte effacement (LEE)-positive STEC strains but also with LEE-negative STEC isolates from diverse serogroups (4).
Stx phages are highly mobile genetic elements. They are involved in the pathogenic profile of their bacterial hosts, in the development of the genome plasticity of host bacteria, in the regulation of pathogenicity factors, and in the survival and dissemination of stx in the environment (2, 5, 6). One or more Stx phages may be present in the genome of an STEC strain, and their incorporation can lead to the emergence of new pathogenic strains, as observed in recent outbreaks (7, 8). Stx phages are a heterogeneous group both genetically and morphologically, as almost any phage with an stx operon is referred to as an Stx phage. Despite their heterogeneity, most Stx phages conserve lambdoid-like regulation of lysis-lysogeny cycles. Activation of the lytic cycle culminates in bacterial cell lysis and release of Shiga toxin. However, free infectious Stx phages are also released after cell lysis, contributing to the spread of stx. Released Stx phages may infect new bacterial cells and convert them into pathogens, thereby playing an important role in the evolution of STEC strains (9).
Free Stx phages have been found in several environments with fecal pollution (10–14). A fraction of the Stx phages found free in the environment are infectious and potentially able to transfer the stx gene to nonpathogenic strains (12), thus converting them into Stx producers. This could occur in environments that are polluted with feces carrying Stx phages either as a consequence of induction of the lytic cycle of the Stx phages from an STEC reservoir or as a consequence of direct excretion of these phages in feces.
This is the first paper to isolate and quantify free Stx phages from feces of healthy humans in order to establish whether free Stx phages are directly present in feces and contribute to the environmental pool of stx genes. We evaluated the capacity of infectious Stx phages detected in feces to propagate in bacterial hosts.
MATERIALS AND METHODS
Fecal samples.
This study was performed on fecal samples from 100 individuals aged 6 months to 102 years who attended the Hospital de la Santa Creu i Sant Pau (Barcelona, Spain) over a 10-month period. Stool samples were processed according to conventional protocols to isolate enteropathogenic bacteria (Salmonella, Shigella, Yersinia, Campylobacter, Vibrio, toxigenic Clostridium difficile, and enteropathogenic E. coli), and they were microscopically examined fresh and after staining for protozoa. Immunochromatography was used, when required, to detect rotavirus or adenovirus. Only samples that were negative for these pathogens were included in the study. None of the patients selected had been involved in a food-borne outbreak or had reported any severe gastrointestinal pathology.
Strains, bacteriophages, and media.
Laboratory E. coli strain C600, E. coli O157:H7 ATCC 43888, and Shigella sonnei strain 866 (15) were used as stx-negative bacterial hosts to detect and propagate Stx2 phages. E. coli strain WG5 (ATCC 700078) (16) was used as a host to enumerate somatic coliphages according to standard ISO procedures (16). The E. coli C600 (pGEM::stx2) (17) construct was used for the standards in the stx quantitative PCR (qPCR) assay. Luria-Bertani (LB) broth or LB agar was used for culturing of bacteria and phage assays.
Purification of bacteriophages.
Fecal samples were homogenized at a 1:5 (wt/vol) dilution in phosphate-buffered saline (PBS) by magnetic stirring for 15 min. The samples were then centrifuged at 3,000 × g, and the supernatant was filtered through low-protein-binding 0.22-μm-pore-size membrane filters (Millex-GP; Millipore, Bedford, MA). When necessary, several filter units were used to filter the whole volume. When necessary for DNA extraction, phages were 100-fold concentrated by means of protein concentrators (100-kDa Amicon ultracentrifugal filter units; Millipore, Bedford, MA) according to the manufacturer's instructions. The samples were treated with DNase (100 units/ml of the phage lysate) to eliminate DNA outside the phage particles. After treatment, DNase was inactivated by heating.
Bacteriophage propagation in enrichment cultures.
Bacteriophages purified from 20 of the stool samples were used to evaluate the infectivity of the Stx phages present in the samples. Homogenate containing the phages was prepared as described above. Five milliliters of the phage suspension was used to enumerate the Stx phages present in the samples. Five milliliters of the phage suspension was mixed with 0.5 ml of a log-phase culture of each host strain (E. coli C600, E. coli O157:H7 [stx negative], or S. sonnei 866), and LB broth was added to a final volume of 20 ml. The cultures were incubated by shaking for 18 h at 37°C. Phages were purified from the supernatants of the enrichment cultures, and phage DNA was extracted and used as the template for quantification of stx gene copies (GC) by qPCR. Stx phages were considered propagated when a significant (P < 0.05) increase in GC was observed in the supernatant from the enrichment cultures compared to the original homogenate.
Plaque assay.
One milliliter of the supernatant from the enrichment cultures that had previously been filtered and DNase treated was used to screen infectious Stx phages by the spot test method. Briefly, 0.5 ml of an exponentially growing host bacterium culture, containing approximately 2 × 108 cells per ml, was added to 100 μl of 0.1 M CaCl2 and incubated for 30 min at 37°C. The suspension was then mixed with 3 ml of LB top agar containing 5% sterile glycerol (18), poured onto LB agar plates, gently mixed, spread onto the LB agar plates, and allowed to dry. A 10-μl drop of each phage suspension was then carefully spotted onto the bacterial lawn. When the suspension had dried, the plates were incubated (right side up) for 18 h at 37°C. A 10-fold serial dilution of the phage suspension was performed for phage enumeration. One hundred microliters of each dilution was mixed with 100 μl of 0.1 M CaCl2 and 500 μl of a log-phase culture of the host strains and incubated for 30 min at 37°C.
Screening for plaques of lysis generated by Stx phages.
To determine the presence of stx2, the plaques were transferred onto a nylon membrane (Hybond-N+; Amersham Pharmacia Biotech, Spain) according to standard procedures (19). The membranes were hybridized at 65°C with a digoxigenin (DIG)-labeled stx2 A probe prepared as described previously (15). Stringent hybridization was achieved with the DIG DNA labeling and detection kit (Roche Diagnostics, Barcelona, Spain), according to the manufacturer's instructions.
Phage DNA extraction.
DNA from purified Stx phages was isolated by proteinase K digestion and phenol-chloroform (1:1, vol/vol) treatment (19). The phenol-chloroform–phage lysate mixture was added to Phase Lock Gel tubes (5-Prime; VWR International, Spain) and centrifuged according to the manufacturer's instructions. DNA from the supernatant was precipitated by using 100% ethanol and 3 M sodium acetate, and the volume was adjusted to 50 μl. The purified DNA was evaluated by agarose (0.8%) gel electrophoresis, and bands were visualized by ethidium bromide staining. The concentration and purity of extracted phage DNA were determined by using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Thermoscientifics, Wilmington, DE).
PCR procedures.
The oligonucleotides used are summarized in Table 1. Conventional PCRs were performed by using a GeneAmp 2700 PCR system (Applied Biosystems, Barcelona, Spain). When necessary, PCR products were purified by using a QIAquick gel extraction kit (Qiagen Inc., Valencia, CA), according to the manufacturer's instructions.
Table 1.
Oligonucleotides used in this study
| Oligonucleotide | Sequence (5′–3′) | Characteristic | Size (bp) | Reference |
|---|---|---|---|---|
| S2Aup | ATGAAGTGTATATTATTTA | stx2 A fragment | 979 | 42 |
| S2Alp | TTCTTCATGCTTAACTCCT | |||
| UP378 | GCGTTTTGACCATCTTCGT | 378-bp stx2 A fragment | 378 | 12 |
| LP378 | ACAGGAGCAGTTTCAGACAG | |||
| 27f | AAGAGTTTGATCCTGGCTCAG | Eubacterial 16S rRNA gene | 1,503 | 43 |
| 1492r | TACGGCTACCTTGTTACGACTT | |||
| STX-Any f | ACGGACAGCAGTTATACCACTCT | Real-time PCR for stx2 | 65 | 17 |
| STX-Any r | ACGTTCCGGAATGCAAATCAG | |||
| STX-Any probe | FAM-CCAGCGCTGCGACACG-NFQ |
Real-time qPCR assays for stx2 were used to quantify stx GC in the phage DNA. All primers and TaqMan hydrolysis probes used in the qPCR assays were commercially synthesized by Applied Biosystems. Minor-groove binding probes with a FAM (6-carboxyfluorescein) reporter and a nonfluorescent quencher (NFQ) (Table 1) were used. The primers and probes (900 nM for primers and 200 nM for the TaqMan probe) were used under standard conditions with a Step-One real-time PCR system (Applied Biosystems, Spain), as previously described (17). To prepare the standard curves, a 378-bp fragment of stx2 subunit A was cloned into a pGEM vector. The stx qPCR assay has an efficiency of 94% to 100% and a detection limit of 5.29 stx copies (17). To screen for PCR inhibition, the DNA isolated from the samples was spiked with dilutions of the standard, and the results were compared to the true number of GC of stx in the standards. No inhibition of the PCR by the samples was detected.
To exclude the possibility that DNA from a nonviral origin was amplified in the qPCR, control experiments were performed during phage DNA extraction. Aliquots of the samples were taken after DNase treatment but before the phage DNA was extracted from the capsid. These aliquots were used as the template for conventional PCR amplification of eubacterial 16S rRNA genes and also for qPCR amplification of stx. These controls had to be negative to confirm that the samples were free of bacterial DNA or nonencapsidated DNA. These control experiments were performed for all the samples tested.
Sequencing.
Sequencing of the PCR amplicons obtained with phage DNA using combinations of primers S2Aup/S2Alp and UP378/LP378 (Table 1) was performed with an ABI Prism BigDye 3.1 Terminator Cycle Sequencing Ready Reaction kit (Applied Biosystems, Barcelona, Spain) in an ABI Prism 3730 DNA analyzer (Applied Biosystems, Barcelona, Spain), according to the manufacturer's instructions. All sequencing was performed in duplicate.
Statistics.
Statistical tests were performed by using Statistical Package for Social Science (SPSS) software. A paired Student t test was used to evaluate the differences between Stx phage GC before and after enrichment. Stx phages were considered to have propagated by enrichment when their levels were significantly higher (P < 0.05) than those quantified before the enrichment. The chi-square test was used to compare the frequency of Stx phages in both adults (aged >19 years) and nonadults (aged ≤19 years).
RESULTS
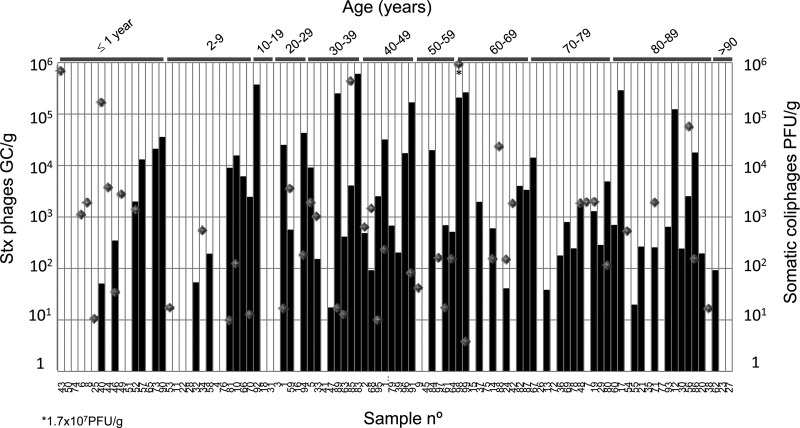
Somatic coliphages are virulent phages that infect E. coli, and they were included as a reference for the densities of free E. coli phages present in the samples. Somatic coliphages were detected in 46% of the samples, with values ranging from 5 to 1.7 × 107 PFU/g of feces, consistent with previous reports (20) (Fig. 1).
Fig 1.
Quantification of Stx2 bacteriophages (GC/g of feces) (black bars) and infectious somatic coliphages (PFU/g) (gray diamonds) in 100 human fecal samples.
Quantification of Stx phages.
Phage DNA was extracted from stool samples. As explained in Materials and Methods, an aliquot of the sample taken after DNase treatment and before desencapsidation was used as a template for the PCR of eubacterial 16S rRNA genes (Table 1) and qPCR of stx2. Both controls were negative, confirming the total removal of nonencapsidated DNA.
The results showed that 62% of the samples contained stx2 in the isolated phage DNA, with an average value of 2.6 × 104 Stx phages/g (from 17.1 to 6.0 × 105). Results for Stx phages/g of feces are presented in relation to the age of the person from whom the stool sample was obtained (Fig. 1). As indicated above, samples were taken from healthy humans who did not present any of the symptoms associated with infection by STEC or any other symptoms that could be associated with an enteric disease (such as renal failure or colitis). All the samples were negative in microbiological studies. Adults (aged >19 years) showed a significantly higher frequency of carriage of Stx phages than nonadults (≤19 years) (χ2 = 7.66; degree of freedom = 1; P = 0.05).
Subunit A of Stx in phage DNA isolated from some of the positive stool samples was amplified by conventional PCR. Amplicons were visualized by agarose gel electrophoresis. Eleven amplicons showed a clear band and were sequenced. Most sequences belonged to stx2, although some showed homology with other stx2 variants (Table 2). Since the fragment of the A subunit sequenced was short (400 to 700 bp) (see the supplemental material), the presence of phages carrying stx2 variants cannot be excluded.
Table 2.
Sequence variants corresponding to the amplicon of the stx2 A subunit obtained from the phage DNA of 11 positive samples
| Sample | GC/g | stx2 variant | Homology (%) | Sequence homologue | GenBank accession no. |
|---|---|---|---|---|---|
| 5 | 9.0 × 103 | stx2c | 95 | E. coli O157:H7 | AB761230 |
| 29 | 3.0 × 102 | stx2 | 95 | E. coli ONT:NM | JX206445 |
| stx2d | E. coli O8:H19 | DQ235775 | |||
| 36 | 1.7 × 102 | stx2 | 95 | E. coli ONT:NM | JX206445 |
| stx2v | E. coli O130:H11 | FM998861 | |||
| stx2d | E. coli O8:H19 | DQ235775 | |||
| 37 | 1.9 × 103 | stx2 | 99 | E. coli ONT:NM | JX206445 |
| stx2v | E. coli O8:H19 | DQ235775 | |||
| 39 | 2.0 × 102 | stx2c | 94 | E. coli O157:H7 | AB761230 |
| 48 | 1.6 × 103 | stx2v | 96 | E. coli O130:H11 | FM998861 |
| stx2d | E. coli O8:H19 | DQ235775 | |||
| 58 | 1.9 × 102 | stx2 | 97 | E. coli O104:H4 | CP003301 |
| 59 | 5.6 × 102 | stx2 | 95 | E. coli O103:H25 | JQ011318 |
| E. coli O104:H4 | CP003301 | ||||
| 61 | 6.8 × 102 | stx2 | 96 | E. coli O104:H4 | CP003301 |
| E. coli O157:H7 | CP001925 | ||||
| 67 | 1.4 × 104 | stx2v | 97 | E. coli O130:H11 | FM998861 |
| stx2d | E. coli O8:H19 | DQ235775 | |||
| 73 | 2.1 × 104 | stx2 | 99 | E. coli ONT:NM | JX206445 |
| stx2v | E. coli O8:H19 | DQ235775 |
Infectivity of Stx phages.
To assess the infectivity of Stx phages, we evaluated their abilities to propagate in three stx-negative bacterial host strains. We compared the log10 densities of Stx phages (stx GC in phage DNA) quantified from the homogenate of the original sample with the densities of Stx phages obtained after the propagation of phages in bacterial enrichment cultures (Table 3).
Table 3.
Increase in densities of Stx phages from stool samples after their propagation in cultures of the three host strains
| Sample | Stx phage density (log10
stx GC in phage DNA) in culture ofa: |
||
|---|---|---|---|
| E. coli C600 | E. coli O157:H7 | S. sonnei 866 | |
| 81 | 3.05 | 3.46 | 3.25 |
| 82 | 0.13 | 0.52 | 1.21 |
| 83 | 0.10 | 0.41 | NP |
| 84 | NP | 0.93 | 0.51 |
| 85 | NP | NP | 0.95 |
| 86 | NP | NP | 0.26 |
| 87 | 1.00 | 1.36 | 0.94 |
| 88 | NP | 0.77 | 1.21 |
| 89 | NP | 0.16 | 0.15 |
| 90 | NP | 0.52 | NP |
| 91 | NP | 0.50 | 0.96 |
| 92 | NP | 0.55 | 0.38 |
| 93 | 1.90 | 2.42 | 0.84 |
| 94 | NP | 0.59 | NP |
| 95 | 0.94 | 1.38 | 0.28 |
| 96 | 0.42 | 0.62 | 0.15 |
| 97 | NP | NP | 3.06 |
| 98 | NP | NP | NP |
| 99 | NP | 0.16 | 0.10 |
| 100 | NP | 1.01 | NP |
NP, not propagated. No significant increase (P > 0.05) was observed in the densities of Stx phages after enrichment culture.
We observed a significant (P < 0.05) increase in the densities of Stx phages after their propagation in at least one of the host strains for 95% of the samples. Only 1 of the 20 samples evaluated (sample 98) showed no propagation of Stx phages. Sample 98 showed the highest levels of somatic coliphages (1.7 × 107 PFU/g) (Fig. 1). The increase in densities indicates that the Stx phages were infective, although the results varied depending on the sample and the host strain. Comparing results between the three hosts used, E. coli C600 showed Stx phage propagation with only 35% of the samples, while E. coli O157:H7 and S. sonnei 866 showed propagation with 80% and 75% of the samples, respectively. Some samples showed a high level of propagation, while others showed only a slight increase. Some samples contained phages able to propagate in all three strains (e.g., sample 81, which showed the highest densities of Stx phages after enrichment in the three strains), while others showed propagation in only one of the strains (e.g., samples 85, 86, and 97 showed propagation of Stx phages only in S. sonnei, or samples 90, 94, and 100 showed propagation only in E. coli O157:H7).
Evaluation of plaques of lysis generated by Stx phages.
The presence of infectious Stx phages in the samples and in the supernatants of the enrichment cultures from the same set of 20 samples described in Table 3 was evaluated by a plaque assay using the three host strains and hybridization with the stx2 probe. Plaques were observed in most plates but were probably formed by non-Stx phages. After hybridization with the stx2 probe, we observed positive signals by using phages from sample 97 propagated in S. sonnei enrichment cultures and using S. sonnei as the host for the plaque assay (Fig. 2). The titer was slightly lower than that reported in Table 3, probably because phages were stored at 4°C for 2 days between the experiments of propagation and the evaluation of plaques of lysis. Ten Stx-positive plaques were excised from the top layer of agar, suspended in 50 μl of double-distilled water, and processed for phage DNA extraction. The presence of stx2 in the phage DNA isolated from the plaques was further confirmed by conventional PCR.
Fig 2.
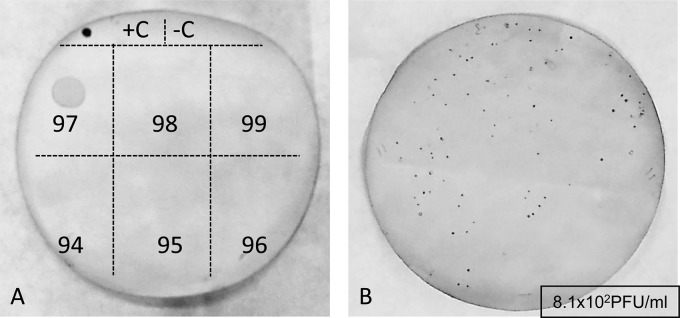
Plaques of Stx phages obtained from the S. sonnei enrichment culture of phages from stool samples and using S. sonnei as host strain. (A) First screening using a drop of phages purified from the supernatants of enrichment cultures of samples 94 to 99. (B) Plaque assay performed with a 1/10 dilution of the phages purified from the supernatant of enrichment of sample 97. Plaques were detected after hybridization with a DIG-stx2 A probe. +C, phage 933W DNA; −C, S. sonnei DNA.
We did not detect any stx-positive lysogens with the host strains used, either in the Stx phage plaques or in the supernatant of the enrichment cultures.
DISCUSSION
The occurrence of infections by STEC bacteria is not of major concern in the Barcelona area, where this study was performed, and only a few cases are reported per year (21). The incidence of such infections is generally low throughout Spain compared to other European countries (22). Despite this low incidence, STEC strains can be isolated from urban sewage in the Barcelona area although at very low densities (23). In addition, and as shown in previous reports, the prevalence of free Stx phages in sewage in Spain is high (11, 12), as in other countries (10, 13, 14, 24, 25).
Some Stx phages detected in sewage are infectious and able to propagate and transduce stx in laboratory host strains (12). Analysis of salad and minced beef samples from the Barcelona area that were suitable for consumption according to European Union norms showed a high prevalence of free Stx phages (26). In several samples, Stx phages proved to be infectious due to their ability to propagate in laboratory E. coli and Shigella strains (26). This and previous studies by our group have focused on Stx2 bacteriophages, but studies are needed to evaluate the occurrence of infectious phages encoding Stx1.
Previous findings suggested that Stx phages circulating in the healthy population could be ingested with certain foods and excreted in feces. They could then be detected in environments contaminated with human or animal feces. We speculated that the origin of Stx phages found in sewage could be a reservoir of lysogenic STEC, or they could be free phages that have been directly excreted in feces. In the present study, we demonstrate that Stx phages are directly excreted in feces, and to the best of our knowledge, this is the first study to provide such evidence. The presence of Stx phages in these samples, however, would not necessarily correspond to an outbreak of a pathogenic STEC strain.
Adults showed a higher frequency of Stx phages than younger individuals. This difference could be attributed to a more complex diet of adults and to the increasing chances of ingestion of Stx phages over a lifetime.
Other points that should also be considered are that susceptibility to hemolytic-uremic syndrome (HUS) is higher for children than for adults (27) and that induction of Stx phages from their bacterial hosts could increase the expression of Stx. Accordingly, large amounts of Stx phages would likely correlate with higher risks of HUS in young individuals. As our stool samples were obtained from healthy individuals without HUS, the incidence of Stx phages in young individuals was not expected to be high.
Our findings are in line with previous studies reporting the spread of STEC in healthy individuals (28–30) and the occurrence of stx genes in stool samples in the general population (31). In two of those studies (29, 31), stx-positive results were obtained in a first screening by PCR. Considering the methods described in those reports, it cannot be excluded that those authors detected Stx phages in addition to the STEC strains isolated.
The Stx phages in some of the samples in our study were infectious and therefore able to transduce the gene. Only one sample showed no propagation, but it had the largest number of lytic phages. Lytic phages other than Stx phages in the homogenate could have caused the lysis of the host strain before the Stx phages had the chance to replicate. As estimated in previous studies (17), the ratio of infectious Stx phages (by plaque blot assay) to total Stx phages (by qPCR) is between 1/10 and 1/1,000, depending on the Stx phage and the bacterial host strain. If we apply this estimate to the stool samples, those with densities of Stx phages exceeding 102 GC/g (Fig. 1) could be expected to present infectious Stx phages.
Although we found infectious Stx phages and positive plaques for Stx phages, we did not detect Stx lysogens, supporting previous findings (26). Generation of Stx lysogens may occur at a low frequency, but not all Stx phages are capable of generating lysogens at the same frequency (15) or lysogenizing the same bacterial host strain (32). Although the host strains selected here have shown good results for Stx phage detection (15, 17, 26, 32), in previous studies, we found that few or no lysogens are generated, even under the best conditions, using Stx phages recently induced from a lysogenic strain (15). Several explanations can be given to explain why we did not detect Stx lysogens: the small number of Stx phages in the fecal samples, the fact that some Stx phages might not be infectious, the possibility that the strains used are not the optimal hosts, and the presence of other lytic, non-Stx phages. These other lytic phages could infect E. coli and cause the lysis of possible Stx lysogens before they can grow and be identified on an agar plate. The low generation of Stx lysogens is the main reason why antibiotic-resistant recombinant Stx phages have been used to study lysogeny in Stx phages. In this case, the use of selective antimicrobial agents would improve the detection of lysogens (32–35). Nevertheless, the fact that some Stx phages from feces in our study were infectious shows that they would be capable of transducing the gene under more favorable conditions. Under such circumstances, nonpathogenic flora could be converted into pathogenic strains by free Stx phages present in the gut (36). The low efficiency of the generation of lysogens with the Stx phages in this study could correlate with the low incidence of STEC infections and HUS in Spain (21, 22). However, our data are insufficient to confirm this hypothesis, and further studies are necessary.
In the light of these findings, another possibility should be considered. Most methods currently used to extract bacterial DNA from feces include the extraction of phage DNA, since most of them include a treatment with proteinase K, which eliminates phage capsid (19). Disease related to STEC is diagnosed by isolating cultivable STEC from fecal samples. Nevertheless, in some cases, cultures from fecal samples could be negative due to previous treatment with antimicrobial agents or to a disease diagnosed late in the course of the disease (37). In these cases, detection of stx using PCR, either conventional multiplex or real-time qPCR (38–40), would be an alternative. The fact that free Stx phages are excreted in the feces of healthy humans indicates that some individuals, as observed for STEC in animals (41), could be shedding free Stx phages, even though they are not colonized by STEC. However, in a clinical setting, a positive stx result from the molecular analysis of DNA in the samples will result in a positive diagnosis of STEC-related illness, even though STEC is not always the cause of disease. This false-positive result may hinder detection of the real pathogen.
In conclusion, taking into account the high prevalence of Stx phages in fecal samples from healthy individuals, diagnostic methods that could detect phage DNA from stool samples are likely unreliable for diagnosis of disease related to STEC strains.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the Spanish Ministry of Education and Science (grants AGL2009-07576 and AGL2012-30880); the Ministry of Education and Science, Instituto de Salud Carlos III, cofinanced by the European Development Regional Fund, A Way To Achieve Europe ERDF; the Spanish Network for Research in Infectious Diseases (grant REIPI RD06/0008/0013); the Generalitat de Catalunya (grant 2009SGR1043); and a project of the RecerCaixa program (La Caixa). Alexandre Martinez-Castillo has an FPI grant from the Ministry of Education and Science.
Footnotes
Published ahead of print 7 June 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01158-13.
REFERENCES
- 1. Erickson MC, Doyle MP. 2007. Food as a vehicle for transmission of Shiga toxin-producing Escherichia coli. J. Food Prot. 70:2426–2449 [DOI] [PubMed] [Google Scholar]
- 2. Herold S, Karch H, Schmidt H. 2004. Shiga toxin-encoding bacteriophages—genomes in motion. Int. J. Med. Microbiol. 294:115–121 [DOI] [PubMed] [Google Scholar]
- 3. O'Brien AD, Newland JW, Miller SF, Holmes RK, Smith HW, Formal SB. 1984. Shiga-like toxin-converting phages from Escherichia coli strains that cause hemorrhagic colitis or infantile diarrhea. Science 226:694–696 [DOI] [PubMed] [Google Scholar]
- 4. Steyert SR, Sahl JW, Fraser CM, Teel LD, Scheutz F, Rasko DA. 2012. Comparative genomics and Stx phage characterization of LEE-negative Shiga toxin-producing Escherichia coli. Front. Cell. Infect. Microbiol. 2:133. 10.3389/fcimb.2012.00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brüssow H, Canchaya C, Hardt WD. 2004. Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion. Microbiol. Mol. Biol. Rev. 68:560–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xu X, McAteer SP, Tree JJ, Shaw DJ, Wolfson EB, Beatson SA, Roe AJ, Allison LJ, Chase-Topping ME, Mahajan A, Tozzoli R, Woolhouse ME, Morabito S, Gally DL. 2012. Lysogeny with Shiga toxin 2-encoding bacteriophages represses type III secretion in enterohemorrhagic Escherichia coli. PLoS Pathog. 8:e1002672. 10.1371/journal.ppat.1002672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. L'Abée-Lund TM, Jørgensen HJ, O'Sullivan K, Bohlin J, Ligård G, Granum PE, Lindbäck T. 2012. The highly virulent 2006 Norwegian EHEC O103:H25 outbreak strain is related to the 2011 German O104:H4 outbreak strain. PLoS One 7:e31413. 10.1371/journal.pone.0031413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bielaszewska M, Mellmann A, Zhang W, Köck R, Fruth A, Bauwens A, Peters G, Karch H. 2011. Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: a microbiological study. Lancet Infect. Dis. 11:671–676 [DOI] [PubMed] [Google Scholar]
- 9. Allison HE. 2007. Stx-phages: drivers and mediators of the evolution of STEC and STEC-like pathogens. Future Microbiol. 2:165–174 [DOI] [PubMed] [Google Scholar]
- 10. Dumke R, Schroter-Bobsin U, Jacobs E, Roske I. 2006. Detection of phages carrying the Shiga toxin 1 and 2 genes in waste water and river water samples. Lett. Appl. Microbiol. 42:48–53 [DOI] [PubMed] [Google Scholar]
- 11. Imamovic L, Ballesté E, Jofre J, Muniesa M. 2010. Quantification of Shiga toxin-converting bacteriophages in wastewater and in fecal samples by real-time quantitative PCR. Appl. Environ. Microbiol. 76:5693–5701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Muniesa M, Jofre J. 1998. Abundance in sewage of bacteriophages that infect Escherichia coli O157:H7 and that carry the Shiga toxin 2 gene. Appl. Environ. Microbiol. 64:2443–2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rooks DJ, Yan Y, McDonald JE, Woodward MJ, McCarthy AJ, Allison HE. 2010. Development and validation of a qPCR-based method for quantifying Shiga toxin-encoding and other lambdoid bacteriophages. Environ. Microbiol. 12:1194–1204 [DOI] [PubMed] [Google Scholar]
- 14. Tanji Y, Mizoguchi K, Akitsu T, Morita M, Hori K, Unno H. 2002. Fate of coliphage in waste water treatment process and detection of phages carrying the Shiga toxin type 2 gene. Water Sci. Technol. 46(11–12):285–289 [PubMed] [Google Scholar]
- 15. Muniesa M, Blanco JE, De Simón M, Serra-Moreno R, Blanch AR, Jofre J. 2004. Diversity of stx2 converting bacteriophages induced from Shiga-toxin-producing Escherichia coli strains isolated from cattle. Microbiology 150:2959–2971 [DOI] [PubMed] [Google Scholar]
- 16. Anonymous 2000. ISO 10705-2: water quality. Detection and enumeration of bacteriophages—part 2: enumeration of somatic coliphages. International Organization for Standardization, Geneva, Switzerland [Google Scholar]
- 17. Imamovic L, Serra-Moreno R, Jofre J, Muniesa M. 2010. Quantification of Shiga toxin 2-encoding bacteriophages, by real-time PCR and correlation with phage infectivity. J. Appl. Microbiol. 108:1105–1114 [DOI] [PubMed] [Google Scholar]
- 18. Santos SB, Carvalho CM, Sillankorva S, Nicolau A, Ferreira EC, Azeredo J. 2009. The use of antibiotics to improve phage detection and enumeration by the double-layer agar technique. BMC Microbiol. 9:148. 10.1186/1471-2180-9-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 20. Grabow WOK, Neubrech TE, Holtzhausen CS, Jofre J. 1995. Bacteroides fragilis and Escherichia coli bacteriophages. Excretion by humans and animals. Water Sci. Technol. 31(5-6):223–230 [Google Scholar]
- 21. Generalitat de Catalunya Agència Catalana de Seguretat Alimentària 2011. Informe de la situació d'Escherichia coli verotoxigen a Catalunya. Generalitat de Catalunya Agència Catalana de Seguretat Alimentària, Barcelona, Spain: http://www.gencat.cat/salut/acsa/html/ca/dir2839/ecvt_catalunya_06-2011.pdf [Google Scholar]
- 22. European Centre for Disease Prevention and Control, European Food Safety Authority 2011. Shiga toxin/verotoxin-producing Escherichia coli in humans, food and animals in the EU/EEA, with special reference to the German outbreak strain STEC O104. ECDC, Stockholm, Sweden [Google Scholar]
- 23. Blanch AR, García-Aljaro C, Muniesa M, Jofre J. 2003. Detection, enumeration and isolation of strains carrying the stx2 gene from urban sewage. Water Sci. Technol. 47(3):109–116 [PubMed] [Google Scholar]
- 24. Muniesa M, Jofre J. 2000. Occurrence of phages infecting Escherichia coli O157:H7 carrying the Shiga toxin 2 gene in sewage from different countries. FEMS Microbiol. Lett. 183:197–200 [DOI] [PubMed] [Google Scholar]
- 25. Yan Y, Shi Y, Cao D, Meng X, Xia L, Sun J. 2011. Prevalence of Stx phages in environments of a pig farm and lysogenic infection of the field Escherichia coli O157 isolates with a recombinant converting phage. Curr. Microbiol. 62:458–464 [DOI] [PubMed] [Google Scholar]
- 26. Imamovic L, Muniesa M. 2011. Quantification and evaluation of infectivity of Shiga toxin-encoding bacteriophages in beef and salad. Appl. Environ. Microbiol. 77:3536–3540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karch H, Tarr PI, Bielaszewska M. 2005. Enterohaemorrhagic Escherichia coli in human medicine. Int. J. Med. Microbiol. 295:405–418 [DOI] [PubMed] [Google Scholar]
- 28. Fujihara S, Arikawa K, Aota T, Tanaka H, Nakamura H, Wada T, Hase A, Nishikawa Y. 2009. Prevalence and properties of diarrheagenic Escherichia coli among healthy individuals in Osaka City, Japan. Jpn. J. Infect. Dis. 62:318–323 [PubMed] [Google Scholar]
- 29. Hong S, Oh KH, Cho SH, Kim JC, Park MS, Lim HS, Lee BK. 2009. Asymptomatic healthy slaughterhouse workers in South Korea carrying Shiga toxin-producing Escherichia coli. FEMS Immunol. Med. Microbiol. 56:41–47 [DOI] [PubMed] [Google Scholar]
- 30. Alikhani MY, Mirsalehian A, Fatollahzadeh B, Pourshafie MR, Aslani MM. 2007. Prevalence of enteropathogenic and Shiga toxin-producing Escherichia coli among children with and without diarrhoea in Iran. J. Health Popul. Nutr. 25:88–93 [PMC free article] [PubMed] [Google Scholar]
- 31. Urdahl AM, Solheim HT, Vold L, Hasseltvedt V, Wasteson Y. 2013. Shiga toxin-encoding genes (stx genes) in human fecal samples. APMIS 121:202–210 [DOI] [PubMed] [Google Scholar]
- 32. Serra-Moreno R, Acosta S, Hernalsteens JP, Jofre J, Muniesa M. 2006. Use of the lambda Red recombinase system to produce recombinant prophages carrying antibiotic resistance genes. BMC Mol. Biol. 7:31. 10.1186/1471-2199-7-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cornick NA, Helgerson AF, Mai V, Ritchie JM, Acheson DW. 2006. In vivo transduction of an Stx-encoding phage in ruminants. Appl. Environ. Microbiol. 72:5086–5088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. James CE, Stanley KN, Allison HE, Flint HJ, Stewart CS, Sharp RJ, Saunders JR, McCarthy AJ. 2001. Lytic and lysogenic infection of diverse Escherichia coli and Shigella strains with a verocytotoxigenic bacteriophage. Appl. Environ. Microbiol. 67:4335–4337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schmidt H, Bielaszewska M, Karch H. 1999. Transduction of enteric Escherichia coli isolates with a derivative of Shiga toxin 2-encoding bacteriophage phi3538 isolated from Escherichia coli O157:H7. Appl. Environ. Microbiol. 65:3855–3861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gamage SD, Strasser JE, Chalk CL, Weiss AA. 2003. Nonpathogenic Escherichia coli can contribute to the production of Shiga toxin. Infect. Immun. 71:3107–3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Paton AW, Paton JC. 1999. Direct detection of Shiga toxigenic Escherichia coli strains belonging to serogroups O111, O157, and O113 by multiplex PCR. J. Clin. Microbiol. 37:3362–3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Grys TE, Sloan LM, Rosenblatt JE, Patel R. 2009. Rapid and sensitive detection of Shiga toxin-producing Escherichia coli from nonenriched stool specimens by real-time PCR in comparison to enzyme immunoassay and culture. J. Clin. Microbiol. 47:2008–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Monday SR, Beisaw A, Feng PC. 2007. Identification of Shiga toxigenic Escherichia coli seropathotypes A and B by multiplex PCR. Mol. Cell. Probes 21:308–311 [DOI] [PubMed] [Google Scholar]
- 40. Paton JC, Paton AW. 2003. Methods for detection of STEC in humans: an overview. In Philpott D, Ebel F. (ed), Methods in molecular medicine, vol 73 Escherichia coli: Shiga toxin methods and protocols. Humana Press Inc, Totowa, NJ: [DOI] [PubMed] [Google Scholar]
- 41. Chase-Topping M, Gally D, Low C, Matthews L, Woolhouse M. 2008. Super-shedding and the link between human infection and livestock carriage of Escherichia coli O157. Nat. Rev. Microbiol. 6:904–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Muniesa M, de Simon M, Prats G, Ferrer D, Panella H, Jofre J. 2003. Shiga toxin 2-converting bacteriophages associated with clonal variability in Escherichia coli O157:H7 strains of human origin isolated from a single outbreak. Infect. Immun. 71:4554–4562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sander M, Schmieger H. 2001. Method for host-independent detection of generalized transducing bacteriophages in natural habitats. Appl. Environ. Microbiol. 67:1490–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.