Abstract
Malolactic fermentation (MLF) is a biochemical transformation conducted by lactic acid bacteria (LAB) that occurs in wine at the end of alcoholic fermentation. Oenococcus oeni is the main species responsible for MLF in most wines. As in other fermented foods, where bacteriophages represent a potential risk for the fermentative process, O. oeni bacteriophages have been reported to be a possible cause of unsuccessful MLF in wine. Thus, preparation of commercial starters that take into account the different sensitivities of O. oeni strains to different phages would be advisable. However, currently, no methods have been described to identify phages infecting O. oeni. In this study, two factors are addressed: detection and typing of bacteriophages. First, a simple PCR method was devised targeting a conserved region of the endolysin (lys) gene to detect temperate O. oeni bacteriophages. For this purpose, 37 O. oeni strains isolated from Italian wines during different phases of the vinification process were analyzed by PCR for the presence of the lys gene, and 25 strains gave a band of the expected size (1,160 bp). This is the first method to be developed that allows identification of lysogenic O. oeni strains without the need for time-consuming phage bacterial-lysis induction methods. Moreover, a phylogenetic analysis was conducted to type bacteriophages. After the treatment of bacteria with UV light, lysis was obtained for 15 strains, and the 15 phage DNAs isolated were subjected to two randomly amplified polymorphic DNA (RAPD)-PCRs. By combining the RAPD profiles and lys sequences, 12 different O. oeni phages were clearly distinguished.
INTRODUCTION
Malolactic fermentation (MLF) is a secondary fermentation that occurs in wine after the alcoholic fermentation. This fermentation can take place spontaneously through the action of the population of lactic acid bacteria (LAB) present in the wine or by the use of commercial starters, mainly belonging to the species Oenococcus oeni, the principal LAB species responsible for MLF in wine (1, 2).
As in other fermented foods, like dairy products, in which bacteriophages represent a potential risk for the fermentative process (3, 4), bacteriophages have also been reported to be a possible cause of unsuccessful MLF in wine (5, 6). Therefore, preparation of commercial starters that take into account the different sensitivities of O. oeni strains to different phages has been proposed (5, 7). However, under laboratory conditions, O. oeni has a very low growth rate, and its poorly visible colonies are hardly produced in agar medium, hampering the accurate detection of O. oeni phages, which has contributed to the low number of studies conducted until now on this topic. For instance, while PCR-based approaches to detect bacteriophages have been used in bacteria of the genera Lactobacillus and Lactococcus present in dairy products (8–11), such methods have never been applied to detect phages infecting O. oeni. In the present study, a new PCR method for the detection of bacteriophages of O. oeni without the need for a sensitive indicator strain and for a growth step on solid medium to confirm the presence of prophages was developed.
Until now, typing of O. oeni bacteriophages has been conducted by morphological characterization, structural-protein composition, host range analysis, and/or restriction enzyme analysis (5, 12–15). The last approach (15) was the first DNA-based technique that allowed the grouping of different O. oeni phage types.
Bacteriophages of O. oeni present in wines were first observed by Sozzi et al. (12) by electron microscopy (EM) and were subsequently described as being different in size (5). Later, EM analyses of four O. oeni phages showed that they were very similar, exhibiting a Bradley type B morphology, with the presence of a head, a tail, and a base plate (13). Similar results were reported after EM analysis of 10 phages isolated from wines (14), which also showed similar morphology with slight differences in the head size, tail length, and presence of a base plate. These phages were divided into four groups based on structural-protein composition, host range, and restriction enzyme analysis. Likewise, comparable morphologies and structural protein profiles have also been reported in 17 O. oeni mitomycin C (mit C)-induced phages from different O. oeni strains, which were further divided into six groups based on their enzymatic restriction patterns (15).
Endolysins, also termed lysins, are enzymes encoded by most double-stranded DNA phage genomes that lyse bacterial cell walls and cause host death (16, 17). Detection of the lys gene has recently been used to evaluate phages infecting Lactobacillus helveticus (11). However, unlike some other LAB bacteriophages, a complete O. oeni phage sequence is not yet available. Only partial sequences of four different temperate phages containing attP (a phage attachment site) have been reported, and for only one of them, termed fOg44, does the available sequence cover almost 50% of the whole genome (18).
In the present report, the presence of temperate bacteriophages in 37 O. oeni strains isolated from wines was tested with a newly developed PCR assay based on the amplification of the endolysin (lys) gene in the bacterial genome and subsequent randomly amplified polymorphic DNA (RAPD) analysis. A total of 15 O. oeni phages, which were classified into 12 different groups, are described for the first time.
MATERIALS AND METHODS
Bacterial-strain isolation and DNA extraction.
A total of 37 O. oeni strains (Table 1) were isolated from wines from wineries around the southeast Piedmont (Italy). Wines were sampled during and at the end of MLF. One milliliter of each wine was serially diluted in sterile physiological water (0.9% NaCl) and plated on MRS agar medium (VWR, Milan, Italy) containing 0.1 mg/ml cycloheximide (Sigma) for yeast growth inhibition. The plates were incubated at 30°C for 10 days, and then colonies were randomly picked, inoculated in MRS broth, and incubated again at 30°C. DNA was extracted from 2 ml of bacterial culture in exponential phase (19).
Table 1.
O. oeni strains and their identified prophages analyzed in this study
| O. oeni strain | Wine sourcea | Wineryb | Prophage |
|---|---|---|---|
| OE1 | Grignolino (after AF) | 1 | fOE1 |
| OE2 | Grignolino (after AF) | 1 | fOE2 |
| OE3 | Grignolino (after AF) | 1 | fOE3 |
| OE4 | Grignolino (after MLF) | 1 | fOE4c |
| OE6 | Arneis (during MLF) | 2 | |
| OE7 | Arneis (during MLF) | 2 | |
| OE9 | Arneis (during MLF) | 2 | fOE9c |
| OE10 | Arneis (during MLF) | 2 | |
| OE11 | Arneis (during MLF) | 2 | fOE11c |
| OE12 | Arneis (during MLF) | 2 | |
| OE13 | Arneis (during MLF) | 2 | |
| OE14 | Arneis (during MLF) | 2 | |
| OE15 | Arneis (during MLF) | 2 | |
| OE16 | Arneis (during MLF) | 2 | |
| OE17 | Arneis (during MLF) | 2 | fOE17 |
| OE18 | Arneis | 2 | |
| OE19 | Arneis | 2 | |
| OE20 | Chardonnay | 2 | fOE20 |
| OE21 | Chardonnay | 2 | fOE21 |
| OE22 | Barbera (after MLF) | 3 | fOE22 |
| OE23 | Barbera (during MLF) | 4 | fOE23c |
| OE24 | Nebbiolo (during MLF) | 4 | fOE24c |
| OE25 | Nebbiolo (after ALF) | 4 | fOE25 |
| OE26 | Nebbiolo (during MLF) | 4 | fOE26 |
| OE28 | Nebbiolo (after ALF) | 2 | |
| OE29 | Nebbiolo (after MLF) | 2 | fOE29 |
| OE30 | Nebbiolo (after ALF) | 2 | fOE30c |
| OE31 | Nebbiolo (after MLF) | 2 | fOE31 |
| OE32 | Nebbiolo (during MLF) | 5 | fOE32c |
| OE33 | Nebbiolo (during MLF) | 5 | fOE33 |
| OE34 | Nebbiolo (during MLF) | 5 | fOE34 |
| OE35 | Nebbiolo (during MLF) | 5 | fOE35 |
| OE36 | Dolcetto | 6 | fOE36c |
| OE37 | Chardonnay | 7 | fOE37 |
| OE38 | Chardonnay | 7 | fOE38c |
| OE39 | Barbera (during MLF) | 3 | |
| OE40 | Chardonnay (during MLF) | 2 | fOE40c |
AF, alcoholic fermentation.
The wineries of origin are all situated in different localities in the southeastern Piedmont (Italy).
Prophage did not respond to UV treatment.
Bacterial-species characterization and strain typing.
Identification at the species level was conducted using a species-specific PCR for O. oeni targeting the mle gene (20). The species was also confirmed by sequencing the 16S rRNA gene using the universal primers 68f-1387r (21). The sequences obtained were compared to the O. oeni sequences available in the GenBank database.
Strain typing was done by multiplex RAPD-PCR analysis as described previously (22) using 0.5 μM primer Coc and 0.25 μM primer On2, respectively. The amplicons were resolved by electrophoresis in 1.2% agarose gels, and the images were acquired with Quantity One 4.1 software (Bio-Rad, Milan, Italy).
Similarities between sequences were calculated using the Dice coefficient, and grouping was performed by cluster analysis (unweighted pair group mean average [UPGMA]), using Bionumerics software (Applied Maths, Belgium).
Detection of lysogenic O. oeni bacteriophages by amplification of the endolysin (lys) gene.
Primers were designed after comparison of the few available lys sequences of O. oeni bacteriophages (fOg44, GenBank accession number gi|57281901; fOgPSU1, GenBank accession number gi|50057020; 10 MC, GenBank accession number gi|4105634; and fOg30, GenBank accession number gi|51035320). The primer set Lys20_fw (5′-ATCTCGGCTTTATCGGCTTT-3′) and Lys1143_rv (5′-TACGGATCCGGAAACCTTTA-3′), amplifying a fragment of 1,160 bp, was designed with the use of Primer3 software (http://frodo.wi.mit.edu/).
PCRs were performed in 25 μl containing 0.13 μl of Perfect Taq DNA Polymerase (5 Prime; Eppendorf, Milan, Italy), 2.5 μl of 10× Taq buffer, 0.5 μl of 10 mM deoxynucleoside triphosphates (dNTPs), 1 μl of 15 μM MgCl2, 0.5 μl (each) of 10 μM primer, and 1 μl of template. Amplifications were performed as follows: 95°C for 3 min and 35 cycles of 95°C for 45 s, 62°C for 45 s, and 72°C for 1 min, followed by a 72°C cycle for 10 min. PCR fragments were visualized in a standard 1% agarose gel stained with ethidium bromide and visualized under UV light.
Sequencing and phage lys gene sequence comparison.
PCR products were purified with a PCR Extract Mini Kit (5 Prime) and sequenced (BMR Genomics, University of Padova, Padua, Italy). Sequence analysis and comparisons were performed using the BLAST program available from the National Center for Biotechnology Information (NCBI). Multialignment and phylogenetic trees were generated by Bionumerics software using the Dice correlation coefficient and the UPGMA algorithm.
Prophage induction with UV light.
The transition from lysogeny to lytic development was induced by UV light as described previously (23) with some modifications. Briefly, O. oeni strains stored at −80°C were inoculated in MRS medium with 20% tomato juice added (MRSTJ) and incubated at 25°C. Once exponential growth (optical density at 600 nm [OD600], 0.1 to 0.3; measured using a DU730 spectrophotometer [Beckman Coulter, Brea, CA]) was reached, 5 ml of bacterial culture was transferred to sterile tubes and centrifuged at 6,000 × g for 10 min at room temperature. The cell pellets were resuspended in 5 ml of sterile 0.1 M MgSO4 and irradiated with a Sankyo Denki (Japan) G30T8 germicidal lamp for 25 s at 52-cm distance. Then, the cell suspension was transferred to a new sterile tube containing 5 ml of double-strength MRSTJ medium and incubated at 25°C in the dark. Bacterial growth was monitored every 4 h for 24 to 32 h or until there was a marked decrease in the absorbance reading between irradiated and nonirradiated cells.
Prophage induction with mitomycin C.
An aqueous stock solution of 500 μg/ml of mit C was prepared and sterilized through a 0.22-μm cellulose acetate membrane (VWR International, Milan, Italy). Sterile aqueous stock and working (1:50) mit C solutions were preserved at 4°C in the dark.
Bacterial strains were grown in MRSTJ medium, pH 4.5 (7), with and without the addition of 1 μg/ml of mit C, and the optical density was measured at 600 nm. Cultures were incubated at 25°C in the dark. The absorbance was read every 2 h for up to 6 h and then after 24 h. In lysogenic strains, there was a marked decrease in the absorbance reading between the control and the culture treated with mit C. The bacterial cultures were centrifuged at 3,000 × g for 12 min at 4°C to remove the bacteria and cell fragments from the medium, and the supernatant containing phages was filter sterilized as described above and stored at 4°C.
Total counts of phages by epifluorescence microscopy.
The phage count was determined using epifluorescence as described previously (24). Slides were examined under a Leica microscope (DM2500; Leica Microsystems Srl., Milan, Italy) with filter H3 for SYBR green I (excitation wavelength, 420 to 490 nm). Leica application suite software ver. 2.5.0 was used for image analysis and phage counting.
Bacteriophage typing by RAPD-PCR.
The O. oeni strains that were positive in the PCR assays were grown in MRS broth and treated with UV light to induce the prophages, as described above. The culture was then centrifuged for 10 min at 4°C at 6,000 × g, and the supernatants were filtered (0.2 μm) to eliminate bacterial cells. Two milliliters of these filtered lysates was treated with DNase (Omega Biotech, VWR, Milan, Italy) and RNase (5 Prime; Eppendorf, Milan, Italy) at 1-μg/ml final concentration to remove any trace of bacterial nucleic acids. Phages were recovered by adding 1 M NaCl and 10% polyethylene glycol (PEG) 6000 and incubating overnight on ice. After centrifugation at 13,000 × g for 1 h at 4°C, the phages were resuspended in 40 μl SM buffer (100 mM NaCl, 8 mM MgSO4, 50 mM Tris-HCl, pH 7.5).
Total DNA from phage lysates was obtained by standard procedures (25). Bacteriophage DNA was used as a template for two RAPD-PCRs according to the method of Giraffa and Neviani (26) with primer M13 (27) alone and then in combination with primer Lys 20_fw, which was specifically designed based on the available lys gene sequences. PCR profiles were visualized in 1.2% agarose gels stained with ethidium bromide. The reproducibility of the PCR fingerprinting was assessed by running the same DNA three times. The RAPD fingerprints obtained were analyzed with Bionumerics software. A similarity matrix was calculated on the basis of the Pearson correlation coefficient, and the corresponding dendrogram was generated with the UPGMA clustering algorithm.
Nucleotide sequence accession numbers.
The 25 sequences obtained were deposited in GenBank under accession numbers KC292225 to KC292249.
RESULTS
PCR screening and phage characterization by lys prophage sequences.
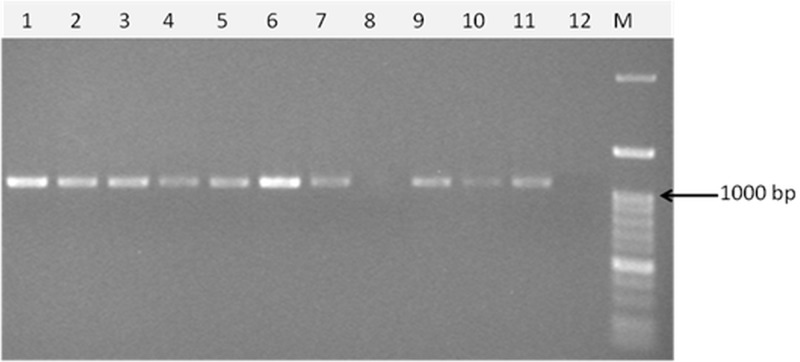
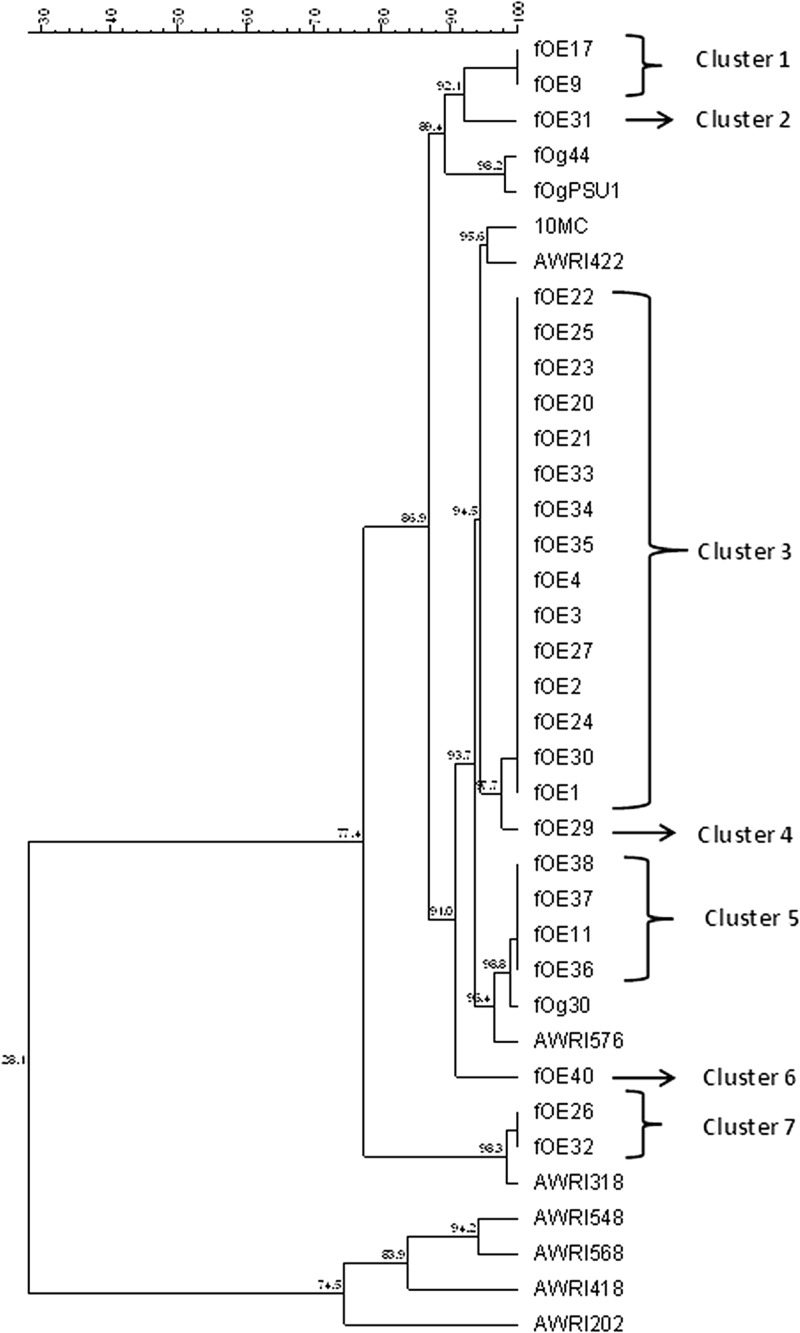
A simple PCR method to detect temperate O. oeni bacteriophages was devised by targeting a conserved region of the lys gene after sequence alignment of the only four available O. oeni bacteriophage partial sequences. Analysis of 37 O. oeni bacterial strains for the presence of the lys sequence showed that 25 gave a band of the expected size, 1,160 bp (Table 2 and Fig. 1). BLAST analysis of the 25 sequences obtained confirmed that they corresponded to lys sequences of O. oeni bacteriophages. Recently, multiple distinct bacteriophage sequences were detected in the sequenced genome of O. oeni strains, including lys gene sequences of remnant phages in O. oeni, AWRI202, AWRI318, AWRI418, AWRIB422, AWRIB548, AWRIB568, and AWRI576 (28). Phylogenetic analysis of the lys sequences amplified here and those of O. oeni bacteriophages and the remnant phages available in GenBank showed that the sequences detected in the present report grouped into seven clusters (Fig. 2). Clusters 3 (14 sequences) and 4 (1 sequence) have 94.5% similarity to the bacteriophages 10 MC and AWRI422. Cluster 5 (4 sequences) has 98.8% similarity to bacteriophage fOg30 and 96.4% similarity with AWRI576. Lower similarities to previously reported sequences were recorded for clusters 1 (2 sequences) and 2 (1 sequence), which were 89.4% similar to bacteriophages fOg44 and fOgPSU1, and cluster 6 (1 sequence), which was 91% similar to bacteriophages fOg30, 10 MC, AWRI422, and AWRI576. Finally, cluster 7 (2 sequences) showed 98.3% similarity to AWRI318. The lys sequences of AWRI202, AWRI418, AWRI 548, and AWRI568 (28) form a completely separate branch.
Table 2.
Presence of the lys gene in O. oeni strains and induction of phages using different detection methods
| O. oeni strain | Prophage | UV inductiona | Mit C inductiona | PCRa | lys geneb |
|---|---|---|---|---|---|
| OE1 | fOE1 | + | − | + | KC292244 |
| OE2 | fOE2 | + | + | + | KC292239 |
| OE3 | fOE3 | + | − | + | KC292245 |
| OE4 | fOE4 | − | − | + | KC292234 |
| OE6 | − | − | − | ||
| OE7 | − | − | − | ||
| OE9 | fOE9 | − | − | + | KC292249 |
| OE10 | − | − | − | ||
| OE11 | fOE11 | − | − | + | KC292237 |
| OE12 | − | − | − | ||
| OE13 | − | − | − | ||
| OE14 | − | − | − | ||
| OE15 | − | − | − | ||
| OE16 | − | − | − | ||
| OE17 | fOE17 | + | − | + | KC292240 |
| OE18 | − | − | − | ||
| OE19 | − | − | − | ||
| OE20 | fOE20 | + | − | + | KC292229 |
| OE21 | fOE21 | + | + | + | KC292230 |
| OE22 | fOE22 | + | − | + | KC292225 |
| OE23 | fOE23 | − | − | + | KC292228 |
| OE24 | fOE24 | − | − | + | KC292242 |
| OE25 | fOE25 | + | + | + | KC292226 |
| OE26 | fOE26 | + | + | + | KC292235 |
| OE28 | − | − | − | ||
| OE29 | fOE29 | + | + | + | KC292246 |
| OE30 | fOE30 | − | − | + | KC292243 |
| OE31 | fOE31 | + | − | + | KC292241 |
| OE32 | fOE32 | − | − | + | KC292247 |
| OE33 | fOE33 | + | + | + | KC292231 |
| OE34 | fOE34 | + | + | + | KC292232 |
| OE35 | fOE35 | + | − | + | KC292233 |
| OE36 | fOE36 | − | − | + | KC292238 |
| OE37 | fOE37 | + | + | + | KC292236 |
| OE38 | fOE38 | − | − | + | KC292227 |
| OE39 | − | − | − | ||
| OE40 | fOE40 | − | − | + | KC292248 |
| Total no. positive | 15 | 8 | 25 |
+, present; −, absent.
GenBank accession numbers.
Fig 1.
Agarose gel electrophoresis of a PCR-amplified fragment of the lys gene in O. oeni. M, marker (100-bp DNA ladder H3 RTU; Genedirex). The arrow marks 1,000-bp size.
Fig 2.
Dendrogram obtained by alignment of the lys gene sequences found in this study (labeled fOE) with the lys sequences of O. oeni bacteriophages fOg44 (gi|57281901), fOgPSU1 (gi|50057020), 10 MC (gi|4105634), and fOg30 (gi|51035320) available in GenBank and the lys sequences contained in O. oeni genomes (28): AWRIB422 (gb|ALAG01000013.1), AWRIB548 (gb|ALAH01000027.1), AWRIB568 (gb|ALAJ01000015.1), AWRI576 (gb|ALAK01000018.1), AWRI418 (gb|ALAE01000014.1), AWRI202 (gb|AJTO01000030.1), and AWRI318 (gb|ALAD01000011.1). Sequence alignment and phylogenetic analysis were performed with Bionumerics software using multiple-sequence alignment and the UPGMA algorithm. The similarity distances are shown at each node.
Phage induction by UV and mitomycin C treatment.
Thirty-seven O. oeni strains were tested for phage induction by using UV or mitomycin C (Table 2). Treatment with mitomycin C induced a lytic cycle in 8 strains, while UV light treatment resulted in 15 positive strains. Thus, among the 25 lysogenic strains detected by PCR, 10 contained prophages that cannot be induced into the lytic cycle under the conditions applied. Neither UV nor mitomycin C lytic induction was observed in any of the bacteria in which the lys gene was not amplified, confirming the accurate specificity of the PCR method.
RAPD characterization.
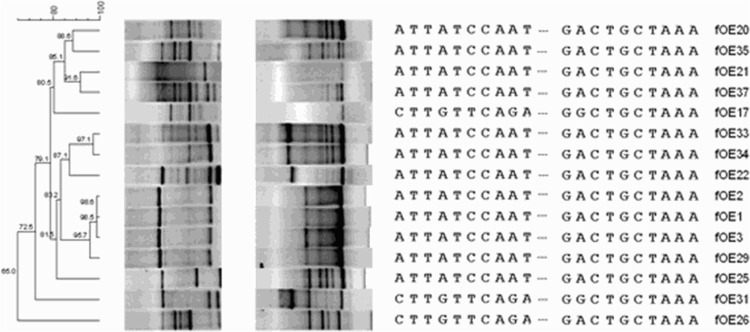
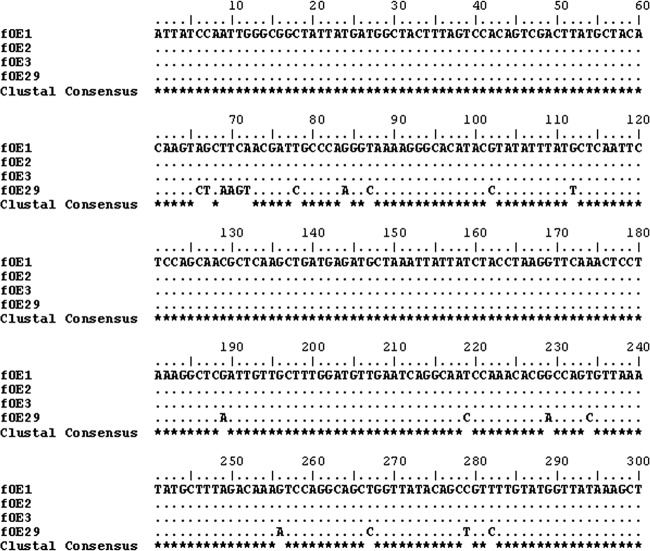
Phages were induced by UV light and purified as described in Materials and Methods. At the end of the treatment with UV, the phage count was estimated by epifluorescence at 1010 phage/ml, 1 log unit higher than that reported to be needed for optimal reproducibility (29). The 15 phage DNAs were subjected to two RAPD-PCRs, one using only the random primer M13 and, to increase the sensitivity of the profiles (22), a second one with primers M13 and Lys20_fw. A dendrogram created by combining the two RAPD-PCR data sets and the partial lys sequences (Fig. 3) showed a more complex phage-typing profile that allowed us to clearly discriminate 12 of the 15 phages tested. Major similarities (98.6%) were found between phages fOE1, fOE2, and fOE3, which branched close to fOE29, to which they showed 95.7% similarity. A more detailed comparison of their lys sequences allowed even better discrimination between them (Fig. 4), so that fOE29 differs from fOE1, fOE2, and fOE3, all of which have identical sequences in the region analyzed. Therefore, fOE1, fOE2, and fOE3 were considered similar, while fOE29 was considered different. fOE34 and fOE33, which showed 97.1% similarity after RAPD-PCR analysis (Fig. 3) and whose lys sequences are identical (Fig. 2), were considered to belong to the same group. Thus, with the comparison of RAPD profiles and lys sequences, it was possible to clearly individuate 12 different phages.
Fig 3.
(Left) Phylogenetic tree obtained by analyzing the composite data from two RAPD profiles and lys gene partial sequences using Bionumerics software with the UPGMA algorithm. The similarity distance is reported at each node. (Middle and right) RAPD-PCR with the M13 primer (left gel), RAPD-PCR with M13 plus Lys primers (right gel), and a final alignment of 813-bp partial sequences of lys genes (only the first and last 10 nucleotides are shown for each sequence).
Fig 4.
Alignment of the lys sequences of the phages fOE1, fOE2, fOE3, and fOE29 using ClustalW. Dots indicate that the nucleotide is the same as in the first sequence; stars highlight the consensus sequence.
DISCUSSION
Sluggish or incomplete MLF can be a problem in the vinification process, and it has been suggested that MLF may be interrupted, delayed, or completely inhibited by the actions of phages active against LAB (5). Later, it was concluded that phages did not represent a critical problem for MLF, because they should be inactivated by wine conditions, such as low pH (<3.5) and sulfur dioxide content (13). However, interference by phages with MLF in a wine of pH 3.23 has been observed (6). Likewise, another study (7) concluded that wine composition may affect the infective capacity of phages and that, as previously suggested (5), there is a need to inoculate a mixture of bacterial strains with different sensitivities to avoid starter culture failure and problems during MLF.
The use of bacteria as the starter for malolactic fermentation in wine is a widespread practice in oenology, but, although O. oeni is the principal LAB species responsible for this fermentation (30, 31), there are few reports about the sensitivity of O. oeni strains to bacteriophages. Methods for detection of lysogeny in the species have been reported by different authors and are based on induction of phages with mitomycin C and the observation of phages by EM (5, 6, 13, 32). However, although a protocol taking into account the sequence of the lys gene has recently been successfully applied to evaluate phages infecting L. helveticus (11), no PCR methods have been described to identify phages infecting O. oeni.
In the present study, after analysis of the few available O. oeni bacteriophage partial sequences, a PCR method based on the detection of the lys gene was designed and set up for the study of O. oeni carrying bacteriophages. When the method was applied to 37 O. oeni strains, it was shown that 25 (67.6%) of them were lysogenic, as demonstrated by amplification of the lys gene. Of these, the lytic cycle was induced in 15 strains (60% of the lysogenic strains; 40.5% of the total strains studied) by UV exposure and in only 8 strains by mitomycin C treatment.
Until now, the percentages of lysogeny in O. oeni have been analyzed after mitomycin C induction with variable results, 33.3%, 45%, and 63% (7, 32, 33), and it has been reported that O. oeni strains that were very sensitive to infection were previously suspected to be prophage free as a result of the mitomycin test (7). Here, the mitomycin C induction achieved was lower (21.6%) than that with UV treatment (40.5%), demonstrating that lysis of bacteria carrying a prophage cannot always be induced in the same way by external agents, as has been previously reported by Shan et al. (34), who showed that, by using mitomycin C and norfloxacin as inducing agents in Clostridium difficile, two phages were induced only by norfloxacin, nine only by mitomycin C, and three by both antibiotics.
However, noninducible phages have been frequently detected in bacterial genomes (35, 36), a fact that was first reported after mitomycin C lysis induction in lactic streptococci (37) and later in Lactobacillus species (38, 39). In this regard, it has also been reported that prophage induction in bacteriophages infecting members of the genus Bifidobacterium varied depending on the growth medium (40). In any case, the specific molecular analysis applied in this work showed a higher percentage of O. oeni lysogeny (67.6%) than any previously published data based on prophage induction by external agents (7, 32, 33). Nevertheless, it should be noted that some phage remnants have been found in the pangenome of bacteria (28, 41), and further studies should be conducted on the strains that possess lys genes but are not inducible to properly characterize phages.
Phylogenetic analysis based on the sequences of lys genes amplified from 25 O. oeni strains showed the presence of seven distinct clusters (Fig. 2). Interestingly, lys gene sequences are identical between the strains of a single cluster, confirming that this high homology makes the lys gene a suitable target for phylogenic analysis. The sequences obtained were unique and have not been previously identified, as none of them had 100% homology with any of the O. oeni bacteriophages already described. The largest cluster found here included 13 sequences with 94.5% similarity to the bacteriophages 10 MC and AWRI422, followed by cluster 5, which contains 4 sequences with 98.8% similarity to bacteriophage fOg30 and 96.4% similarity to AWRI576, while cluster 7 (2 sequences) shows 98% similarity to AWRI318.
Phage DNAs obtained after UV induction were analyzed by two RAPD-PCRs. Analysis of RAPD profiles and the partial lys sequence of each phage allowed the discrimination of 12 different groups of phages (Fig. 3).
In conclusion, this study shows that this newly developed method is more sensitive in detecting bacteriophages than traditional nonmolecular methods, and therefore, it is a useful and rapid tool to perform screenings to assess the presence of prophages in the O. oeni genome. The study also demonstrates that lysogeny is high among the O. oeni population.
ACKNOWLEDGMENTS
This work was supported by grant Det. Dir. no. 4 (3 October 2007) from the Regione Piemonte (Italy) and by BIODATI project DM 16101/7301/08 from the Italian Ministry of Agriculture, Food and Forestry.
Footnotes
Published ahead of print 31 May 2013
This article is dedicated to the memory of Tomaso Sozzi, who was a driving force in food bacteriology and a pioneer in the study of phages present in wine and, thus, in understanding the world of bacteriophages.
REFERENCES
- 1. Davis CR, Wibowo DJ, Lee TH, Fleet GH. 1986. Growth and metabolism of lactic acid bacteria during and after malolactic fermentation of wines at different pH. Appl. Environ. Microbiol. 51:539–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Van Vuuren H, Dicks LMT. 1993. Leuconostoc oenos: a review. Am. J. Enol. Vitic. 44:99–112 [Google Scholar]
- 3. De Antoni G, Zago M, Vasek O, Giraffa G, Briggiler Marcó M, Reinheimer J, Suárez V. 2010. Lactobacillus plantarum bacteriophages isolated from Kefir grains: phenotypic and molecular characterization. J. Dairy Res. 77:7–12 [DOI] [PubMed] [Google Scholar]
- 4. Mercanti D, Carminati D, Reinheimer J, Quiberoni A. 2011. Widely distributed lysogeny in probiotic lactobacilli represents a potentially high risk for the fermentative dairy industry. Int. J. Food Microbiol. 144:503–512 [DOI] [PubMed] [Google Scholar]
- 5. Sozzi T, Gnaegi F, D'Amico N, Hose H. 1982. Difficultés de fermentation malolactique du vin dues à des bactériophages de Leuconostoc oenos. Rev. Suisse Vitic. Arboric. Hortic. 14:17–23 [Google Scholar]
- 6. Henick-Kling T, Lee TH, Nicholas DJD. 1986. Inhibition of bacterial growth and malolactic fermentation in wine by bacteriophages. J. Appl. Bacteriol. 61:287–293 [Google Scholar]
- 7. Poblet-Icart M, Bordons A, Lonvaud-Funel A. 1998. Lysogeny of Oenococcus oeni (syn. Leuconostoc oenos) and study of their induced bacteriophages. Curr. Microbiol. 36:365–369 [DOI] [PubMed] [Google Scholar]
- 8. Labrie S, Moineau S. 2000. Multiplex PCR for detection and identification of lactococcal bacteriophages. Appl. Environ. Microbiol. 66:987–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O'Sullivan D, Ross RP, Fitzgerald GF, Coffey A. 2000. Investigation of the relationship between lysogeny and lysis of Lactococcus lactis in cheese using prophage-targeted PCR. Appl. Environ. Microbiol. 66:2192–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zago M, De Lorentiis A, Carminati D, Comaschi L, Giraffa G. 2006. Detection and identification of Lactobacillus delbrueckii subsp. lactis bacteriophage by PCR. J. Dairy Res. 73:146–153 [DOI] [PubMed] [Google Scholar]
- 11. Zago M, Rosetti L, Reinheimer J, Carminati D, Giraffa G. 2008. Detection and identification of Lactobacillus helveticus bacteriophages by PCR. J. Dairy Res. 75:196–201 [DOI] [PubMed] [Google Scholar]
- 12. Sozzi T, Maret R, Poulin JM. 1976. Mise en évidence de bactériophages dans le vin. Separatum Experientia 32:568–569 [DOI] [PubMed] [Google Scholar]
- 13. Davis C, Silveira NFA, Fleet GH. 1985. Occurrence and properties of bacteriophages of Leuconostoc oenos in Australian wines. Appl. Environ. Microbiol. 50:872–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arendt EK, Hammes WP. 1992. Isolation and characterization of Leuconostoc oenos phages from German wines. Appl. Microbiol. Biotechnol. 37:643–646 [Google Scholar]
- 15. Santos R, Vieira G, Santos MA, Paveia H. 1996. Characterization of temperate bacteriophages of Leuconostoc oenus and evidence for two prophage attachment sites in the genome of starter strain PSU-1. J. Appl. Bacteriol. 81:383–392 [Google Scholar]
- 16. Bernhardt TG, Wang IN, Struck DK, Young R. 2002. Breaking free: “protein antibiotics” and phage lysis. Res. Microbiol. 153:493–501 [DOI] [PubMed] [Google Scholar]
- 17. Young R. 1992. Bacteriophage lysis: mechanism and regulation. Microbiol. Mol. Biol. Rev. 56:430–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schmitt MJ, São-José C, Santos MA. 2009. General outline of oenophage genome organization, p 101–103 In König H, Unden G, Fröhlich J. (ed), Biology of microorganisms on grapes, in must and wine. Springer, New York, NY [Google Scholar]
- 19. Arena ME, Manca de Nadra MC, Muñoz R. 2002. The arginine deiminase pathway in the wine lactic acid bacterium Lactobacillus hilgardii X1B: structural and functional study of the arcABC genes. Gene 301:61–66 [DOI] [PubMed] [Google Scholar]
- 20. Zapparoli G, Torriani S, Pesente P, Dellaglio F. 1998. Design and evaluation of malolactic enzyme gene targeted primers for rapid identification and detection of Oenococcus oeni in wine. Lett. Appl. Microbiol. 27:243–246 [DOI] [PubMed] [Google Scholar]
- 21. Marchesi JR, Sato T, Weightman AJ, Martin TA, Fry JC, Hiom SJ, Wade WG. 1998. Design and evaluation of useful bacterial-specific PCR primers that amplify genes coding for bacterial 16S rRNA genes. Appl. Environ. Microbiol. 64:795–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reguant C, Bordons A. 2003. Typification of Oenococcus oeni strains by multiplex RAPD-PCR and study of population dynamics during malolactic fermentation. J. Appl. Microbiol. 95:344–353 [DOI] [PubMed] [Google Scholar]
- 23. Raya RR, Hébert EM. 2009. Isolation of phage via induction of lysogens, p 23–32 In Clokie MRJ, Kropinski AM. (ed), Bacteriophages: methods and protocols, vol 1 Isolation, characterization, and interactions. Humana Press, Totowa, NJ [Google Scholar]
- 24. Zago M, Scaltriti E, Fornasari ME, Rivetti C, Grolli S, Giraffa G, Ramoni R, Carminati D. 2012. Epifluorescence and atomic force microscopy: two innovative applications for studying phage-host interactions in Lactobacillus helveticus. J. Microbiol. Methods 88:41–46 [DOI] [PubMed] [Google Scholar]
- 25. Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory Press, New York, NY [Google Scholar]
- 26. Giraffa G, Neviani E. 2000. Molecular identification and characterisation of food-associated lactobacilli. Ital. J. Food Sci. 12:403–423 [Google Scholar]
- 27. Huey B, Hall J. 1989. Hypervariable DNA fingerprinting in Escherichia coli: minisatellite probe from bacteriophage M13. J. Bacteriol. 171:2528–2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Borneman AR, McCarthy JM, Chambers PJ, Bartowsky EJ. 2012. Comparative analysis of the Oenococcus oeni pan genome reveals genetic diversity in industrially-relevant pathways. BMC Genomics 13:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gutiérrez D, Martín-Platero AM, Rodríguez A, Martínez-Bueno M, García P, Martínez B. 2011. Typing of bacteriophages by randomly amplified polymorphic DNA (RAPD)-PCR to assess genetic diversity. FEMS Microbiol. Lett. 322:90–97 [DOI] [PubMed] [Google Scholar]
- 30. Lafon-Lafourcade S, Carre E, Riberau-Gayon P. 1983. Occurrence of lactic acid bacteria during different stages of vinification and conservation of wines. Appl. Environ. Microbiol. 46:874–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fleet GH, Lafon-Lafourcade S, Riberau-Gayon P. 1984. Evolution of yeast and lactic acid bacteria during fermentation and storage of Bordeaux wines. Appl. Environ. Microbiol. 48:1034–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arendt EK, Lonvaud A, Hammes WP. 1991. Lysogeny in Leuconostoc oenos. J. Gen. Microbiol. 137:2135–2139 [DOI] [PubMed] [Google Scholar]
- 33. Huang CM, Asmundson RV, Kelly WJ. 1996. Characterization of a temperate phage isolated from Leuconostoc oenos strain 1002. Appl. Microbiol. Biotechnol. 45:472–476 [Google Scholar]
- 34. Shan J, Patel KV, Hickenbotham PT, Nale JY, Hargreaves KR, Clokie MRJ. 2012. Prophage carriage and diversity within clinically relevant strains of Clostridium difficile. Appl. Environ. Microbiol. 78:6027–6034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Canchaya C, Proux C, Fournous G, Bruttin A, Brüssow H. 2003. Prophage genomics. Microbiol. Mol. Biol. Rev. 67:238–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Casjens S. 2003. Prophages and bacterial genomics: what have we learned so far? Mol. Microbiol. 49:277–300 [DOI] [PubMed] [Google Scholar]
- 37. Whitehead HR, Harkness WL. 1952. The influence of bacteriophage in cheese manufacture. The effect of an extended ripening period. Aust. J. Dairy Technol. 7:3–5 [Google Scholar]
- 38. Desiere F, Pridmore RD, Brüssow H. 2000. Comparative genomics of the late gene cluster from Lactobacillus phages. Virology 275:294–305 [DOI] [PubMed] [Google Scholar]
- 39. Ventura M, Canchaya C, Pridmore D, Brüssow H. 2004. The prophages of Lactobacillus johnsonii NCC 533: comparative genomics and transcription analysis. Virology 320:229–242 [DOI] [PubMed] [Google Scholar]
- 40. Ventura M, Turroni F, Lima-Mendez G, Foroni E, Zomer A, Duranti S, Giubellini V, Bottacini F, Horvath P, Barrangou R, Sela DA, Mills DA, van Sinderen D. 2009. Comparative analyses of prophage-like elements present in bifidobacterial genomes. Appl. Environ. Microbiol. 75:6929–6936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bon E, Delaherche A, Bilhère E, De Daruvar A, Lonvaud-Funel A, Le Marrec C. 2009. Oenococcus oeni genome plasticity is associated with fitness. Appl. Environ. Microbiol. 75:2079–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]