Abstract
In this study, we investigated whether probiotic lysates can modify the tight-junction function of human primary keratinocytes. The keratinocytes were grown on cell culture inserts and treated with lysates from Bifidobacterium longum, Lactobacillus plantarum, Lactobacillus reuteri, Lactobacillus fermentum, or Lactobacillus rhamnosus GG. With the exception of L. fermentum (which decreased cell viability), all strains markedly enhanced tight-junction barrier function within 24 h, as assessed by measurements of transepithelial electrical resistance (TEER). However, B. longum and L. rhamnosus GG were the most efficacious, producing dose-dependent increases in resistance that were maintained for 4 days. These increases in TEER correlated with elevated expression of tight-junction protein components. Neutralization of Toll-like receptor 2 abolished both the increase in TEER and expression of tight-junction proteins induced by B. longum, but not L. rhamnosus GG. These data suggest that some bacterial strains increase tight-junction function via modulation of protein components but the different pathways involved may vary depending on the bacterial strain.
INTRODUCTION
The concept of using “probiotic” bacteria to benefit human health is well established. Ingestion of probiotic bacteria has been claimed to prevent or treat a variety of disorders of the gut, ranging from travelers' diarrhea to the chronic relapsing inflammatory condition Crohn's disease (1, 2) through mechanisms that are incompletely understood. However, evidence suggests that one mechanism may be via protection or augmentation of gut epithelial barrier function (3, 4, 5). This is provided for in the most part by tight junctions (TJs), which are multiprotein complexes sealing the paracellular space between adjacent epithelial cells and limiting transport through this pathway to small, hydrophilic molecules and ions (reviewed in reference 6). The importance of the TJ seal is demonstrated under conditions where the gut barrier is compromised. For example, elevated gut permeability has been observed in inflammatory bowel disease, apparently due to aberrant expression of essential TJ proteins such as claudin isoforms, occludin, or ZO-1 (7, 8). Although direct evidence in humans is still lacking, defects in TJs could allow greater ingress of antigens leading to the inflammatory responses associated with this condition.
Probiotic bacteria, particularly members of the genus Lactobacillus, enhance or protect epithelial barrier function in vitro and in animal models, via modulation of TJs. In this respect, studies using the enterocyte cell line Caco-2 demonstrated that certain probiotic strains inhibited cytokine and hydrogen peroxide-mediated disturbances of transepithelial electrical resistance (TEER), a measure of TJ function (9, 10). Furthermore, in rodents, dextran sulfate-induced gut hyperpermeability was reduced by feeding the rats a probiotic mixture containing Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus casei, Lactobacillus bulgaricus, and Streptococcus thermophilus (11). The reduction in gut permeability appeared to be due to probiotic-induced upregulation of the TJ proteins occludin, ZO-1, and selected claudin isoforms. There is also growing evidence that lactobacilli influence gut permeability in humans; a recent clinical study demonstrated that a strain of L. plantarum affected relocation of ZO-1 and occludin in duodenal cells within 6 h of administration (12). Thus, probiotics may have utility as therapeutic interventions by increasing TJ function under conditions where gut barrier function is aberrant.
Recently, investigations into the utility of probiotics to benefit health have moved beyond the gut, and in particular, the use of lactobacilli and bifidobacteria as topical therapies for skin has received attention (13–16). The skin and the gut have much in common; they both support taxonomically diverse microbiotas, act as a barrier between internal and external environments, and are subject to breaches in that barrier. Investigations have suggested that probiotics may be of value as treatments for skin: L. plantarum improved tissue repair in a burned mouse model and prevented infections in burns and chronic leg ulcers (13–15). Application of B. longum to healthy human skin reduced water loss (16), and we previously demonstrated that specific lactobacilli can inhibit adherence of the skin pathogen Staphylococcus aureus to primary human keratinocytes (17). Thus, the limited amount of work in this area suggests that probiotic bacteria may be of considerable use as agents to protect/enhance the skin's barrier function, but in general, the mechanisms underlying these effects are unexplored.
Until recently, the skin barrier was thought to function entirely through the tough, waterproof outer layer of the epidermis, the stratum corneum. However, recent work has demonstrated that TJs exist between keratinocytes in the first living layer of the epidermis, the stratum granulosum (18) and that these are critical to the skin barrier. This was exemplified by a knockout mouse deficient in claudin 1 expression, which dies of excessive transepidermal water loss within 24 h of birth (18). Therefore, in the present study, we used a primary human keratinocyte model to investigate whether lysates from probiotic bacteria can influence TJs, the molecular changes accompanying this, and the possible mechanisms involved.
MATERIALS AND METHODS
Preparation of probiotic lysates.
All probiotic strains (Bifidobacterium longum ATCC 51870, Lactobacillus plantarum ATCC 10241, Lactobacillus reuteri ATCC 55730, Lactobacillus rhamnosus Goldin and Gorbach [GG] ATCC 53103, and Lactobacillus fermentum ATCC 14932) were purchased from LGC Ltd., Middlesex, United Kingdom) and were routinely grown to stationary phase in Wilkins-Chalgren broth or on Wilkins-Chalgren agar at 37°C in a Mark 3 anaerobic work station (Don Whitley Scientific, United Kingdom). Cultures were adjusted spectrophotometrically to approximately 108 CFU/ml and then centrifuged (15,000 × g for 10 min; 10 ml), washed three times in 1× phosphate-buffered saline (PBS), and then concentrated in 1 ml of keratinocyte basal medium (Promocell, Heidelberg, Germany). The sample was then lysed using a bead beater (FastPrep FP120; Thermo Electron Corporation, United Kingdom) and filter sterilized to remove any remaining whole bacteria. Finally, 100 μl of this lysate was used to treat keratinocyte cultures.
Primary keratinocyte cell culture and measurement of TEER.
Normal human epidermal keratinocytes (NHEK) were obtained and cultured as previously described by Prince et al. (17). For experiments measuring TJ function, cells were plated on 12-well, permeable polycarbonate Thincert cell culture inserts with a 0.4-μm pore size (Greiner Bio-one Ltd., United Kingdom). NHEK were grown in keratinocyte basal medium (Promocell, Heidelberg, Germany) until confluent. At this point, the medium was replaced with CNT-02-3DP5 high-calcium medium (CELLnTEC Advanced Cell Systems, Switzerland), which induces TJ formation. TJ function was measured using an epithelial voltmeter fitted with chopstick electrodes (World Precision Instruments Ltd., United Kingdom). All experiments were repeated at least three times with triplicate wells within individual experiments. In some experiments, lysates of probiotic bacteria were added to the apical side of the inserts, and the TEER was measured at various times posttreatment.
Measurement of NHEK viability using an MTT assay.
MTT [3-(4,5-dimethylthiazol-2-yl)diphenyltetrazolium bromide; Sigma-Aldrich Ltd., Poole, United Kingdom] was prepared as a stock solution of 5 mg/ml in phosphate-buffered saline. NHEK were grown to confluence in 96-well plates and then treated with bacterial lysates for 24 h. Medium was then replaced with fresh medium containing 10% MTT. The plates were incubated for 4 h at 37°C, and the medium was replaced with dimethyl sulfoxide. The absorbance of each well was then read at 570 nm in a plate reader.
Extraction of protein from NHEK and immunoblotting.
Protein was extracted from NHEK cells according to the method described by McLaughlin et al. (19). Briefly, cells from a single Thincert were scraped into 100 μl extraction buffer (NaCl [120 mM], HEPES [pH 7.5; 25 mM], Triton X-100 [1% {vol/vol}], EDTA [2 mM], NaF [25 mM], NaVO4 [1 mM], SDS [0.2% {wt/vol}]) containing aprotinin (10 μg/ml), leupeptin (10 μg/ml), and pepstatin A (10 μg/ml) and then incubated on ice for 30 min. Following centrifugation in a microcentrifuge, the supernatant was recovered in a clean Eppendorf tube and used for analysis of TJ protein expression. SDS-PAGE was performed according to the method of Laemmli (20), and proteins were electrophoretically transferred onto polyvinylidene difluoride (PVDF) membranes. These were subsequently washed, blocked in 5% (wt/vol) skim milk, and incubated with the primary antibodies overnight; the antibodies were rabbit anti-claudin 1 and anti-claudin 4, mouse anti-occludin, mouse anti-ZO-1, and mouse anti-β-actin (all purchased from Invitrogen, Paisley, United Kingdom). The membranes were subsequently washed and incubated with horseradish peroxidase-conjugated secondary antibodies. The immunoblots were developed using enhanced chemiluminescence (Amersham, Bucks, United Kingdom), and densitometry was performed as described by McLaughlin et al. (19).
Inhibition of TLR-2.
For inhibition of TLR2, keratinocytes were pretreated with recombinant human anti-TLR2 antibody at 10 μg/ml (Abcam, United Kingdom) for 1 h before stimulation with probiotic lysates.
Statistical analyses.
All experiments were performed a minimum of three times and analyzed using SPSS software version 20. For experiments comparing two treatments, an independent-samples t test was used to statistically analyze all data. For experiments comparing two or more treatments, a one-way analysis of variance (ANOVA) was used to statistically analyze all data. Results were considered statistically significant at a P value of <0.05. Data are expressed as means ± standard errors of the means (SEM) for at least 3 samples for each experiment.
RESULTS
Probiotic lysates are well tolerated by NHEK.
We initially investigated whether any of the test lysates affected the viability of NHEK. To this end, an MTT assay was performed on NHEK that had been incubated with bacterial lysates for 24 h. The data in Fig. 1 illustrate that, with the exception of lysates of L. fermentum, none of the lysates of the strains significantly affected the viability of NHEK. The L. fermentum lysate induced a 52% ± 10.3% (P = <0.01, n = 3) reduction in NHEK viability following 24 h of incubation.
Fig 1.
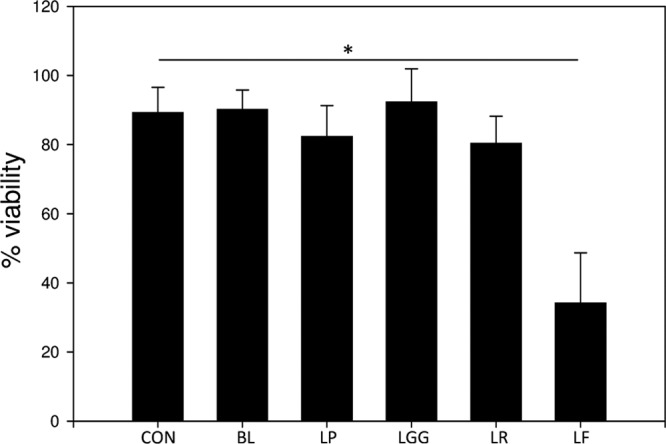
Lysates of probiotic bacteria have strain-dependent effects on keratinocyte viability. Human primary epidermal keratinocytes were incubated with lysate made from 108 CFU/ml bacteria for 24 h. Following exposure, the viability of keratinocytes was measured using an MTT assay. The viability of keratinocytes incubated in the presence of lysates of B. longum (BL), L. plantarum (LP), L. reuteri (LR), or L. rhamnosus Goldwin and Gorbach (LGG) was not significantly different from that of untreated cells (CON). However, keratinocyte cultures treated with a lysate of L. fermentum (LF) had reduced viability compared to controls (∼50% reduction in viability; P < 0.01). *, statistical significance.
Probiotic bacteria augment TJ function in NHEK.
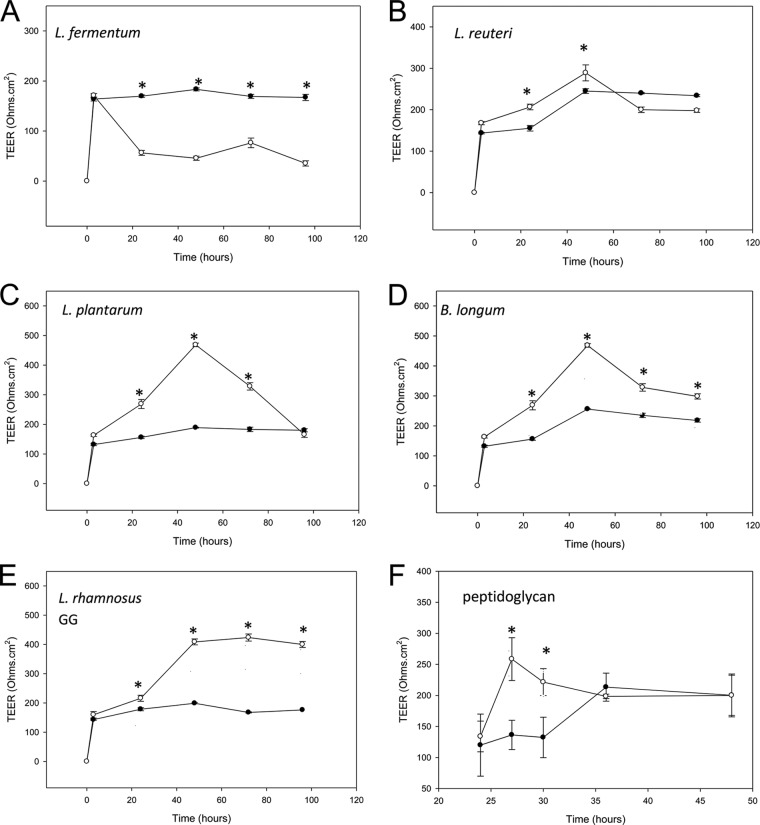
Normal human epidermal keratinocytes develop TJs when they are transferred from medium containing low calcium concentrations (∼0.2 mM) into medium containing high calcium (∼1.8 mM). This “calcium switch” induces assembly of TJs, which can be detected as a rise in the TEER of the cells with time. TEER reaches a peak 48 h after the calcium switch and then drops slightly to reach a steady state at 72 h (Fig. 2).
Fig 2.
Lysates of probiotic bacteria enhance tight-junction barrier function with strain-specific effects. Human primary keratinocytes were induced to form TJs, and the TEER of the monolayers was monitored with time in control, untreated monolayers (filled circles) and treated monolayers (open circles). In control monolayers, TEER developed with time after the calcium switch and reached a peak of around 200 ± 50.4 Ω · cm2. With the exception of L. fermentum (A), which decreased the TEER relative to control monolayers, lysates of all the probiotic bacteria increased TEER over control levels in a strain-dependent manner (L. reuteri [B] and L. plantarum [C]). B. longum (D) and L. rhamnosus GG (E) lysates produced the greatest and most sustained effects. Peptidoglycan from S. aureus (used at 0.01 μg/ml) (F) also induced increased TEER, but its effects occurred more rapidly than those of probiotic lysates and were not sustained. *, statistical significance.
When 100 μl of lysate from 108 CFU/ml probiotic bacteria was added to the apical chamber of the Thincert, significant strain-dependent differences in the development of TEER between untreated and treated NHEK were observed. In agreement with the observation that lysates of L. fermentum reduce cell viability, there was a significant decrease in TEER in NHEK treated with this lysate compared to the TEER of untreated cells (Fig. 2A; P < 0.005). In contrast, lysates of L. reuteri initially increased the TEER at 24 h after the calcium switch, but this subsequently declined back to levels not significantly different from that of control (untreated) cells (Fig. 2B). L. plantarum also induced an increase in TEER over that of control cells which was sustained for 48 h after the calcium switch. However, at 72 h, the TEER was not significantly different from that of untreated NHEK (Fig. 2C). The largest increases in TEER were observed in NHEK treated with B. longum and L. rhamnosus GG lysates. Lysates from both these strains produced increases in TEER that were ∼300 ohms · cm2 higher than in control cells (Fig. 2D and E; P < 0.05). Furthermore, these increases were sustained, and the TEER in treated cells was still significantly greater in treated than untreated NHEK at 72 h after the calcium switch. Interestingly, a major ligand of Gram-positive bacteria, peptidoglycan, also induced an increase in TEER. However, this occurred much more rapidly than with whole bacterial lysates, with significantly increased TEER being noted within 3 h of addition of the peptidoglycan. However, TEER dropped to levels identical to that of the control within 24 h (Fig. 2F).
Since lysates of B. longum and L. rhamnosus GG were the most efficacious, they were used for further investigation.
B. longum and L. rhamnosus GG produce dose-dependent effects on TEER in keratinocytes.
To further define the effects of B. longum and L. rhamnosus GG on TJ function in keratinocytes, TEER was measured in the presence of 100 μl lysate made from bacteria at 106, 104, and 102 CFU/ml. At 106 CFU/ml, lysates from both strains were still able to produce TEERs that were significantly higher than those in untreated cells (Fig. 3A and B; P < 0.005). Additionally, lysates of L. rhamnosus GG produced from 104 CFU/ml were also able to elicit an increase in TEER (Fig. 3B). However, lower concentrations of either strain resulted in lysates that were unable to produce TEERs significantly different from those of control cells (Fig. 3A and B).
Fig 3.
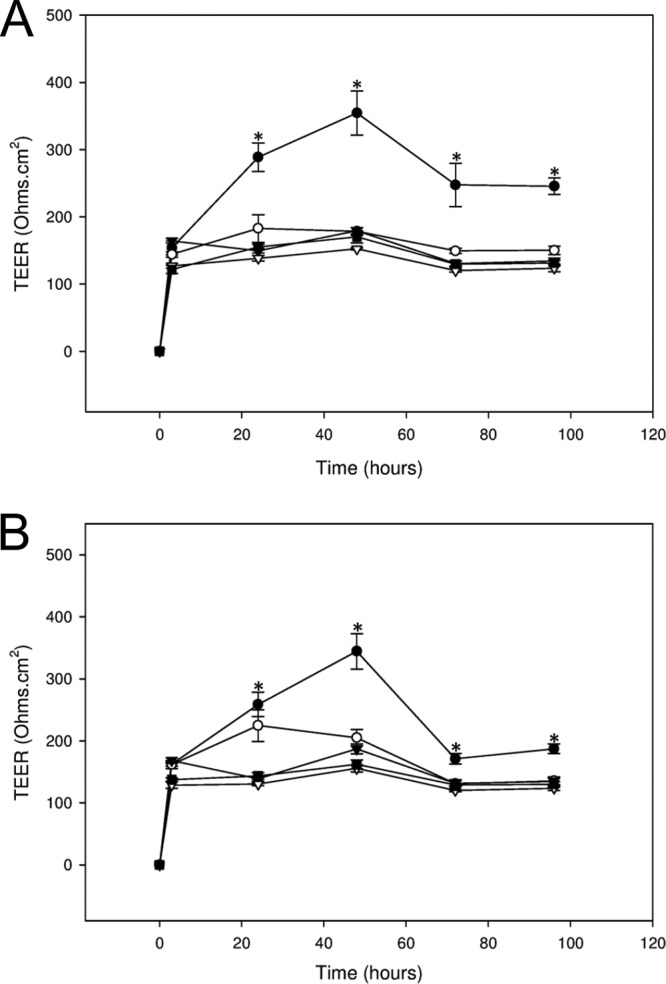
Lysates of probiotic bacteria have dose-dependent effects on TEER in human keratinocytes. Human keratinocytes were untreated (▽) or treated with lysates made from B. longum (BL [A]) or L. rhamnosus GG (LGG [B]) at concentrations of 108 (●), 106 (○), 104 (▼), and 102 (■) CFU/ml, and the effects on TEER were measured with time. B. longum was effective only at a concentration of 108 CFU/ml. However, L. rhamnosus GG was also effective at 106 and 104 CFU/ml. *, statistical significance.
Probiotic lysates modulate the expression of specific TJ proteins.
Evidence suggests that TJ function is often reflected in the expression levels of particular proteins involved in the complexes (19, 21, 22). The main TJ proteins expressed by keratinocytes include claudin 1, claudin 4, ZO-1, and occludin. Therefore, we used immunoblotting to investigate the expression of these proteins in untreated NHEK versus NHEK treated with probiotic lysates for 72 h.
Treatment of NHEK with a lysate of B. longum produced significant increases in all four TJ proteins (Fig. 4A and B). However, L. rhamnosus GG induced increases in the protein levels of claudin 1, occludin, and ZO-1 only (Fig. 4A and B). There was no significant change in the levels of claudin 4 in L. rhamnosus GG-treated NHEK versus control cells. However, in general, increases in protein expression elicited by L. rhamnosus GG were greater than those induced by B. longum (Fig. 4B). Treatment of keratinocytes using the bacterial component peptidoglycan elicited no change in TJ protein expression (data not shown).
Fig 4.
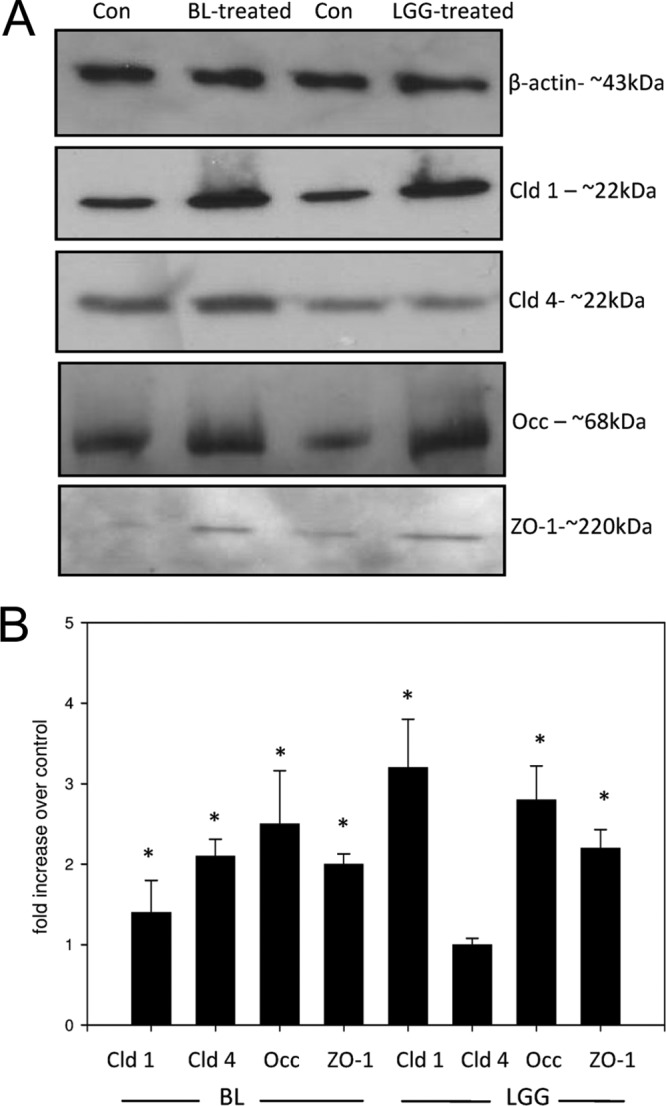
Lysates of probiotic bacteria modulate tight-junction protein expression in human keratinocytes. Human keratinocytes were treated with lysates from 108 CFU/ml of either L. rhamnosus GG (LGG) or B. longum (BL) for 24 h. Subsequently, the keratinocytes were harvested, and the expression of claudin 1, claudin 4, ZO-1, and occludin was investigated using immunoblotting (A) and subsequent densitometry (B). BL increased the expression of all four TJ proteins relative to the control: claudin 1 (cld-1), 3.7× ± 0.08× (P < 0.05); claudin 4 (Cld 4), −2.15× ± 0.02× (P < 0.05); occludin (Occ), 2.53× ± 0.14× (P < 0.005); and ZO-1, 2× ± 0.024× (P < 0.05). However, LGG affected no change in claudin 4 levels but increased the expression of the other proteins: claudin 1, 3.27× ± 0.36× (P < 0.05), occludin, 2.65× ± 0.17× (P < 0.005); ZO-1, 2.22× ± 0.036× (P < 0.05). Con, control. *, statistical significance.
B. longum-induced modulation of TJ function is mediated via TLR2.
Keratinocytes sense the presence of bacteria via pattern recognition receptors such as Toll-like receptors (TLRs). Several lines of evidence in the gut have pointed to a relationship between TLR activation and changes in TJ barrier function. Additionally, recent work by Yuki et al. (23) demonstrated TLR-mediated augmentation of TJ function in keratinocytes in response to bacterial ligands such as peptidoglycan. Therefore, we naturally wondered whether the probiotic-induced increases in TJ function were mediated by TLRs. Of particular interest was TLR2, because it is the major receptor for Gram-positive organisms (24). In order to investigate this, NHEK were incubated in the presence of a TLR2-neutralizing antibody for 1 h prior to the addition of probiotic lysates. The data in Fig. 5A demonstrate that neutralization of TLR2 abolished the rise in TEER elicited by both peptidoglycan (a well-characterized TLR2 agonist) and B. longum. However, L. rhamnosus GG lysates still induced increases in TJ function (Fig. 5A) in the presence of TLR2-neutralizing antibody. Similarly, the increase in TJ protein expression induced by B. longum but not that induced by L. rhamnosus GG was prevented by neutralization of TLR2 (Fig. 5B).
Fig 5.
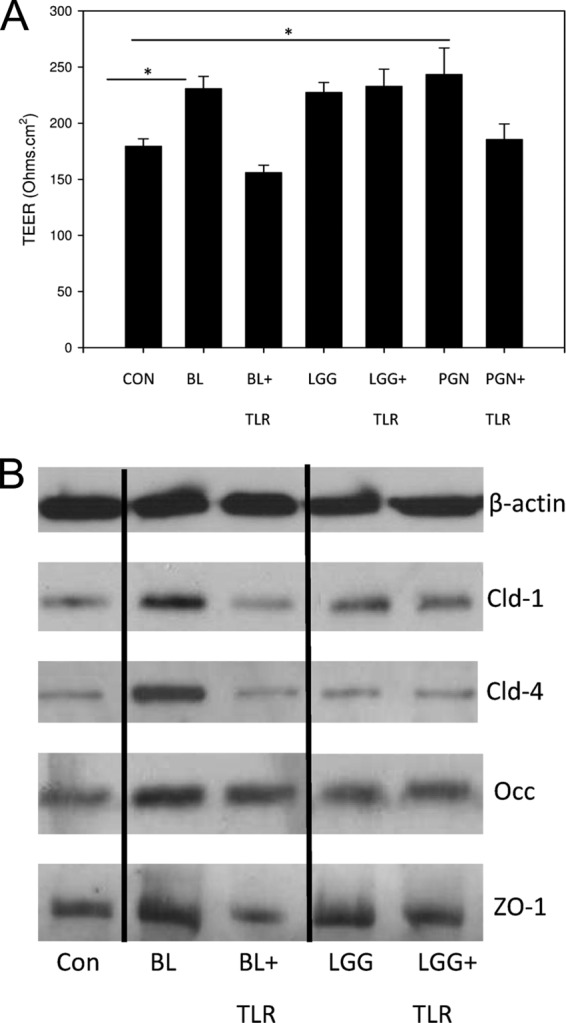
Neutralization of TLR2 abolishes specific probiotic-mediated effects on TJ barrier function and protein expression. Keratinocytes were treated with a TLR2-neutralizing antibody prior to incubation with lysates from B. longum (BL) or L. rhamnosus GG (LGG). CON, control. (A) In cells treated with lysates of BL but not LGG, the probiotic-induced increase in TEER was abolished by incubation with the antibody. Similar results were also obtained using peptidoglycan (PGN) as the ligand. *, statistical significance. (B) Similarly, the increase in TJ protein expression was also abolished by neutralization of TLR-2 in BL-treated but not LGG-treated cells. Con, control.
DISCUSSION
The effects of probiotics on gut barrier function have received extensive research attention. Certain probiotics, e.g., Lactobacillus salivarius, may ameliorate or prevent barrier disruption induced by pathogens or other noxious substances (9–11). Other probiotics can enhance TJ function in gut cells that have not been treated with any stressor (25). The mechanisms by which probiotics exert their effects on the gut barrier may vary, but they include both changes to the expression of individual TJ proteins and activation of signaling pathways involved in barrier formation or regulation. For example, L. rhamnosus GG accelerates intestinal barrier maturation by upregulating claudin 3 expression in the developing mouse (26).
In contrast to the gut, virtually nothing is known regarding the possible roles of the skin microbiome in promoting barrier health and repair. There is currently only one report in the literature relating to the modulation of TJs by the microbiota of the skin, and this demonstrated that S. epidermidis invoked a modest increase in TJ function in the HaCaT cell line (27). Since many species of normal skin bacteria are adventitious pathogens, their use as skin probiotics is complicated by potential safety issues (28). We have therefore been investigating the potential of bifidobacterium and lactobacillus probiotics of intestinal origin to modulate skin health, because these organisms have an excellent safety record. Indeed, many of these bacteria are designated GRAS (generally recognized as safe) for use in the food industry. Although this cannot necessarily be generalized to topical use, the track record of safety of these bacteria encouraged us to investigate their potential for use on the skin. In the present study, lysates were selected in preference to viable bacteria to further minimize the possibility of adverse effects and to eliminate the need to maintain viability (16).
Five strains of bacteria were evaluated for their effect on TJ function. Of these, lysates of all but L. fermentum were able to increase the TEER of keratinocytes. L. fermentum actually reduced the TEER, which is probably related to the observation that this strain also reduced the viability of keratinocytes. The other four strains of lactobacilli all enhanced TJ function, but to different degrees. In this regard, L. rhamnosus GG and B. longum produced greater and more sustained increases in TEER than did L. plantarum or L. reuteri.
In agreement with a single previous study (23), peptidoglycan also induced increased TEER in keratinocytes. However, the changes observed were strikingly different from those seen with whole lysates. TEER increased rapidly when keratinocytes were treated with peptidoglycan, but the increases were not sustained. Nevertheless, this raises the interesting possibility that cell wall components in the lysates may be at least partially responsible for the changes observed in TJ function. However, the significant differences between the effects of peptidoglycan and lysates, and the differential effects of lysates derived from specific strains, suggest that molecules specific to individual bacteria have different efficacies in enhancing barrier function.
Strain-dependent effects of probiotics have been reported in a large number of previous publications. For example, a recent study investigated the ability of 33 different L. salivarius strains to protect Caco-2 cells from the effects of hydrogen peroxide. Of these, only strains that regulated TJ structure prevented the change to TEER induced by hydrogen peroxide (9). We have recently reported the ability of live probiotics to prevent adhesion of S. aureus to keratinocytes. This study also demonstrated significant differences in the ability of strains to inhibit pathogen adherence (17). Such strain-dependent effects are probably due to the expression of different proteins and carbohydrates by individual strains of lactobacilli. Indeed, the increase in TEER induced by L. rhamnosus GG and B. longum exhibited dose-dependent effects, suggesting that a particular molecule(s) and receptive mechanism(s) are involved. However, further work is needed in this area to fully understand how expression of specific molecules relates to function, and to date, very little is known about this relationship, even in the more widely studied context of the gut.
Both L. rhamnosus GG and B. longum increased the expression of TJ proteins in keratinocytes. However, the particular subset of molecules was different in each case. Peptidoglycan-induced increases in TEER were not associated with changes in TJ protein expression. This is in agreement with work by Yuki et al. (23) that showed that activation of protein kinase C is the mechanism by which peptidoglycan increases TJ function. These data again point to the involvement of strain-specific effects of bacterially derived components on the molecular composition of TJs.
The modulation of protein expression by B. longum and L. rhamnosus GG is almost certainly the mechanism by which lysates of increase the TJ barrier function of keratinocytes. Changes in the expression levels of claudins in particular have been shown many times previously to be linked to changes in barrier function. To date, 24 mammalian claudins have been identified, and these generally fall into two classes—those that strengthen the barrier and those that form selective pores (29). Several lines of evidence point to a role for claudins 1 and 4 as barrier-strengthening claudins. In cell lines, overexpression of claudin 1 increased the TEER and decreased the permeability of cells to paracellular markers. Claudin 4 seals the paracellular space against the passage of ions and in doing so increases the TEER of monolayers (30, 31). Increases in ZO-1 expression enhance the TEER of A431 cells (32), and the hormone GLP1 protects and also enhances TJ function in Caco-2 cells by increasing ZO-1 and occludin expression (33). The existence of TJs in skin has been discovered only relatively recently. Therefore, at present the contribution of particular TJ protein species to skin barrier function is largely unknown. However, genetic loss of claudin 1 is known to be lethal in mice (11). Furthermore, the human skin disease atopic dermatitis, where the barrier is aberrant, is known to be associated with reduced claudin 1 expression. Hence, the evidence in skin so far suggests that expression of TJ proteins is associated with barrier function (34).
The mechanism by which B. longum but not L. rhamnosus GG increases TJ protein expression and TEER is almost certainly associated with signaling through TLR2. This is demonstrated by two lines of evidence: (i) neutralization of TLR2 abolishes B. longum-induced increases in TEER, and (ii) the increase in TJ protein expression is also abolished by blocking TLR2. This is perhaps not surprising given that TLR2 is the major pattern recognition receptor for Gram-positive bacteria.
The link between the innate immune system and barrier regulation in the gut has only recently been established. Here, activation of TLRs can either increase or decrease epithelial tight-junction function depending on the particular TLR activated. The only study of its kind in keratinocytes examined the potential of bacterial ligands specific for TLR1, -2, -3, -4, -5, -6, and -9 to increase TJ function (22). The authors of that study demonstrated that activation of most TLRs with purified TLR ligands increased TJ function. The mechanism involved did not appear to be due to increases in TJ protein expression, as observed in the present study. Rather, they demonstrated activation of protein kinase C alpha (PKCα), an enzyme involved in TJ assembly (22). However, their study used ligands rather than whole organism lysates, and they also measured TEER over shorter times than in the present study (23). We also show that use of ligands such as peptidoglycan increases TEER. The peptidoglycan-induced increase in TJ function was TLR dependent but was not associated with changes to TJ protein expression, in agreement with work by Yuki et al. (23). All these data demonstrate the potential for bacterially derived components to augment TJ function in keratinocytes, but the mechanisms used may be different if ligands rather than bacterial lysates are used. Furthermore, whole lysates, in contrast to individual ligands, offer the possibility that multiple signaling pathways may be simultaneously activated, which may alter the downstream effects. This plus the effects of strain-specific molecules may at least in part explain the different effects of individual lactobacilli versus ligands on TJ barrier function.
The L. rhamnosus GG lysate appeared to elicit its effects via a mechanism independent of TLR2 because neutralization of this receptor did not abolish the L. rhamnosus GG-induced increase in TJ function or protein expression. TLR2 is the major receptor for Gram-positive species and is activated by several different ligands from these bacteria, such as lipoteichoic acid. The observation that L. rhamnosus GG-induced increases in TJ function do not appear to be mediated by TLR2 may suggest that this lactobacillus possesses molecules that signal via alternative receptors. The nature of these receptors is under investigation.
In models of the gut epithelium, activation of signaling pathways by such as ERK and P38 by probiotic bacteria has been demonstrated to increase TJ function (35). Additionally, L. rhamnosus GG has been demonstrated to inhibit NF-κB signaling, which led to enhanced barrier integrity in a model of cytokine-induced barrier dysfunction (5). The soluble factor p40, derived from L. rhamnosus GG, has also been implicated in protection of the gut epithelial barrier from peroxide-induced damage. The signaling pathways involved here appeared to be mitogen-activated protein (MAP) kinase and ERK dependent (10). It may be that some of the molecular mechanisms used by probiotic bacteria such as L. rhamnosus GG to increase TJ function are similar between gut and skin. However, currently, virtually nothing is known regarding how TJs are formed or regulated in skin, so much more work is required in the area before firm conclusions can be reached as to how the L. rhamnosus GG lysate enhances keratinocyte TJ barrier function.
Taken together, the data presented here suggest that specific strains of probiotics enhance TJ function in human primary keratinocytes with strain-dependent efficacies and mechanisms. The barrier function of the epidermis is critical in terrestrial organisms, and recent data have highlighted the essential role of TJs in the epidermal permeability barrier to water. If specific bacterial lysates could be suitably formulated, our data suggest that the augmentation of the TJ barrier induced by probiotic bacterial lysates could have a role in enhancing the overall skin barrier to both loss of water and ingress of potential pathogens. Furthermore, lysates of probiotic bacteria could potentially play a role in the treatment of barrier dysfunction under conditions where the TJs are known to be aberrant, such as atopic dermatitis, where loss of expression of claudin 1 is reportedly involved in a subset of patients (34). Since the B. longum and L. rhamnosus GG lysates can increase claudin 1 levels in keratinocytes, it is possible that treatments could be developed using these bacterial lysates to restore the levels of important TJ proteins in TJ-related conditions.
Footnotes
Published ahead of print 14 June 2013
REFERENCES
- 1. Vanderhoof JA, Young RJ. 2001. The role of probiotics in the treatment of intestinal infection and inflammation. Curr. Opin. Gastroenterol. 17:58–62 [DOI] [PubMed] [Google Scholar]
- 2. Meijer BJ, Dielman LA. 2011. Probiotics in the treatment of human inflammatory bowel diseases: update 2011. J. Clin. Gastroenterol. 45:S139–S144 [DOI] [PubMed] [Google Scholar]
- 3. Agostini S, Goubern M, Tondereau V, Salvador-Cartier C, Bezirard V, Lévèque M, Keränen H, Theodorou V, Bourdu-Naturel S, Goupil-Feuillerat N, Legrain-Raspaud S, Eutamene H. 2012. A marketed fermented dairy product containing Bifidobacterium lactis CNCM I-2494 suppresses gut hypersensitivity and colonic barrier disruption induced by acute stress in rats. Neurogastroenterol. Motil. 24:376–e172 [DOI] [PubMed] [Google Scholar]
- 4. Anderson RC, Cookson AL, McNabb C, Park Z, McCann MJ, Kelly WJ, Roy NC. 2010. Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol. 10:316–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Donato KA, Gareau MG, Wang YJ, Sherman PM. 2010. Lactobacillus rhamnosus GG attenuates interferon-{gamma} and tumour necrosis factor-alpha-induced barrier dysfunction and pro-inflammatory signalling. Microbiology 156:3288–3297 [DOI] [PubMed] [Google Scholar]
- 6. Tsukita S, Furuse M. 2002. Claudin based barrier in simple and stratified cellular sheets. Curr. Opin. Cell Biol. 14:531–536 [DOI] [PubMed] [Google Scholar]
- 7. Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Burgel N, Fromm M, Zeitz M, Fuss I, Strober W, Schulzke JD. 2005. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 129:550–564 [DOI] [PubMed] [Google Scholar]
- 8. Zeissig S, Burgel N, Gunzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. 2007. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut 56:61–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miyauchi E, O'Callaghan J, Butto LF, Hurley G, Melgar S, Tanabe S, Shanahan F, Nally K, O'Toole PW. 2012. Mechanism of protection of transepithelial barrier function by Lactobacillus salivarius: strain dependence and attenuation by bacteriocin production. Am. J. Physiol. Gastrointest. Liver Physiol. 303:G1029–G1041 [DOI] [PubMed] [Google Scholar]
- 10. Seth A, Yan F, Polk DB, Rao RK. 2008. Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC- and MAP kinase-dependent mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 294:G1060–G1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mennigen R, Nolte K, Rijcken E, Utech M, Loeffler B, Senninger N, Bruewer M. 2009. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 296:G1140–G1149 [DOI] [PubMed] [Google Scholar]
- 12. Karczewski J, Troost FJ, Konings I, Dekker J, Kleerebezem M, Brummer RJ, Wells JM. 2010. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 298:G851–G859 [DOI] [PubMed] [Google Scholar]
- 13. Peral MC, Huaman Martinez MA, Valdez JC. 2009. Bacteriotherapy with Lactobacillus plantarum in burns. Int. Wound J. 6:73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peral MC, Rachid MM, Gobbato NM, Martinez MAH, Valdez JC. 2010. Interleukin-8 production by polymorphonuclear leukocytes from patients with chronic infected leg ulcers treated with Lactobacillus plantarum. Clin. Microbiol. Infect. 16:281–286 [DOI] [PubMed] [Google Scholar]
- 15. Valdez JC, Peral MC, Rachid M, Santana M, Perdigón G. 2005. Interference of Lactobacillus plantarum with pseudomonas aeruginosa in vitro and in infected burns: the potential use of probiotics in wound treatment. Clin. Microbiol. Infect. 11:472–479 [DOI] [PubMed] [Google Scholar]
- 16. Gueniche A, Bastien P, Ovigne JM, Kermici M, Courchay G, Chevalier V, Breton L, Castiel-Higounenc I. 2010. Bifidobacterium longum lysate, a new ingredient for reactive skin. Exp. Dermatol. 19:e1–e8 [DOI] [PubMed] [Google Scholar]
- 17. Prince T, McBain AJ, O'Neill CA. 2012. Lactobacillus reuteri protects epidermal keratinocytes from Staphylococcus aureus induced cell death by competitive exclusion. Appl. Environ. Microbiol. 78:5119–5126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, Tsukita S. 2002. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J. Cell Biol. 156:1099–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McLaughlin J, Padfield PJ, Burt JP, O'Neill CA. 2004. Ochratoxin A increases permeability through tight junctions by removal of specific claudin isoforms. Am. J. Physiol. Cell Physiol. 287:C1412–C1417 [DOI] [PubMed] [Google Scholar]
- 20. Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 21. Borovac J, Barker RS, Rievaj J, Rasmussen A, Pan W, Wevrick R, Alexander RT. 2012. Claudin-4 forms a paracellular barrier, revealing the interdependence of claudin expression in the loose epithelial cell culture model opossum kidney cells. Am. J. Physiol. Cell Physiol. 303:C1278–C1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Larre I, Lazaro A, Contreras RG, Balda MS, Matter K, Flores-Maldonado C, Ponce A, Flores-Benitez D, Rincon-Heredia R, Padilla-Benavides T, Castillo A, Shoshani L, Cereijido M. 2010. Ouabain modulates epithelial cell tight junction. Proc. Natl. Acad. Sci. U. S. A. 107:11387–11392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yuki T, Yoshida H, Akazawa Y, Komiya A, Sugiyama Y, Inoue S. 2011. Activation of TLR2 enhances tight junction barrier in epidermal keratinocytes. J. Immunol. 187:3230–3237 [DOI] [PubMed] [Google Scholar]
- 24. Fournier B, Philpott DJ. 2005. Recognition of Staphylococcus aureus by the innate immune system. Clin. Microbiol. Rev. 18:521–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Corridoni D, Pastorelli L, Mattioli B, Locovei S, Ishikawa D, Arseneau KO, Chieppa M, Cominelli F, Pizarro TT. 2012. Probiotic bacteria regulate intestinal epithelial permeability in experimental ileitis by a TNF-dependent mechanism. PLoS One 7:e42067. 10.1371/journal.pone.0042067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Patel RM, Myers LS, Kurundkar AR, Maheshwari A, Nusrat A, Lin PW. 2012. Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function. Am. J. Pathol. 180:626–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ohnemus K, Kohrmeyer K, Houdek P, Rohde H, Wladykowski E, Vidal S, Horstkotte MA, Aepfelbacher M, Kirschner N, Behne MJ, Moll I, Brandner JM. 2008. Regulation of epidermal tight-junctions (TJ) during infection with exfoliative toxin-negative Staphylococcus strains. J. Invest. Dermatol. 128(4):906–916 [DOI] [PubMed] [Google Scholar]
- 28. Edmond MB, Wallace SE, McClish DK, Pfaller MA, Jones RN, Wenzel RP. 1999. Nosocomial bloodstream infections in the United States hospitals: a three year analysis. Clin. Infect. Dis. 29:239–244 [DOI] [PubMed] [Google Scholar]
- 29. Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE. 2008. Structure and function of claudins. Biochim. Biophys. Acta 1778:631–645 [DOI] [PubMed] [Google Scholar]
- 30. Coyne CB, Gambling TM, Boucher RC, Carson JL, Johnson LG. 2003. Role of claudin interactions in airway junctional permeability. Am. J. Physiol. Lung Cell Physiol. 285:L1166–L1178 [DOI] [PubMed] [Google Scholar]
- 31. Michikawa H, Fujita-Yoshigaki J, Sugiya H. 2008. Enhancement of barrier function by overexpression of claudin-4 in tight junctions of submandibular gland cells. Cell Tissue Res. 334:255–264 [DOI] [PubMed] [Google Scholar]
- 32. Ko JA, Murata S, Nishida T. 2009. Up-regulation of the tight-junction protein ZO-1 by substance P and IGF-1 in A431 cells. Cell Biochem. Funct. 27:388–394 [DOI] [PubMed] [Google Scholar]
- 33. Moran GW, O'Neill CA, McLaughlin JT. 2012. GLP-2 enhances barrier formation and attenuates TNF-α-induced changes in a Caco-2 cell model of the intestinal barrier. Regul. Pept. 178:95–101 [DOI] [PubMed] [Google Scholar]
- 34. De Benedetto A, Rafaels NM, McGirt LY, Ivanov AI, Georas SN, Cheadle C, Berger AE, Zhang K, Vidyasagar S, Yoishida T, Boguniewicz M, Hata T, Schneider LC, Hanifin JM, Gallo RL, Novak N, Weidinger S, Beaty TH, Leung DY, Barnes KV, Beck LA. 2011. Tight junction defects in patients with atopic dermatitis. J. Allergy Clin. Immunol. 127:773–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dai C, Zhao DH, Jiang M. 2012. VSL#3 probiotics regulate the intestinal epithelial barrier in vivo and in vitro via the p38 and ERK signaling pathways. Int. J. Mol. Med. 29:202–208 [DOI] [PubMed] [Google Scholar]