Abstract
Carbon disulfide (CS2) and carbonyl sulfide (COS) are important in the global sulfur cycle, and CS2 is used as a solvent in the viscose industry. These compounds can be converted by sulfur-oxidizing bacteria, such as Acidithiobacillus thiooxidans species, to carbon dioxide (CO2) and hydrogen sulfide (H2S), a property used in industrial biofiltration of CS2-polluted airstreams. We report on the mechanism of bacterial CS2 conversion in the extremely acidophilic A. thiooxidans strains S1p and G8. The bacterial CS2 hydrolases were highly abundant. They were purified and found to be homologous to the only other described (archaeal) CS2 hydrolase from Acidianus strain A1-3, which forms a catenane of two interlocked rings. The enzymes cluster in a group of β-carbonic anhydrase (β-CA) homologues that may comprise a subclass of CS2 hydrolases within the β-CA family. Unlike CAs, the CS2 hydrolases did not hydrate CO2 but converted CS2 and COS with H2O to H2S and CO2. The CS2 hydrolases of A. thiooxidans strains G8, 2Bp, Sts 4-3, and BBW1, like the CS2 hydrolase of Acidianus strain A1-3, exist as both octamers and hexadecamers in solution. The CS2 hydrolase of A. thiooxidans strain S1p forms only octamers. Structure models of the A. thiooxidans CS2 hydrolases based on the structure of Acidianus strain A1-3 CS2 hydrolase suggest that the A. thiooxidans strain G8 CS2 hydrolase may also form a catenane. In the A. thiooxidans strain S1p enzyme, two insertions (positions 26 and 27 [PD] and positions 56 to 61 [TPAGGG]) and a nine-amino-acid-longer C-terminal tail may prevent catenane formation.
INTRODUCTION
The sulfur compounds carbon disulfide (CS2) and carbonyl sulfide (COS) play an important role in the earth's sulfur cycle. CS2 and COS are released as breakdown products from organic matter, notably S-containing amino acids in soils (1) and dimethyl sulfide (DMS) in marine, mainly coastal and estuarine, environments (2). CS2 is chemically and biologically converted to COS, which is highly stable when not in solution and is the most abundant sulfur species in the atmosphere (3). Anthropogenic CS2 emissions account for approximately one-half of the total global emissions (2). This is due for a great part to the use of CS2 as an organic solvent in the viscose and rayon industry, which brings with it a number of problems. First, CS2 is toxic, causing vascular and coronary heart disease and affecting the central nervous system (4). Second, due to its low boiling point, large CS2-polluted airstreams are created in factory plants and require treatment before release to the atmosphere.
Biofiltration is an effective and sustainable method to remove CS2 from the contaminated airstreams (5–7). Several bacterial species that can grow chemolithoautotrophically on CS2 at neutral pH have been identified from soil, sludge, and freshwater habitats (8–12). At acidic pH, so far only some (Acidi)thiobacillus strains, isolated from hot springs and volcanic areas, were shown to be able to use CS2 (8, 13). Acidophilic Acidithiobacillus thiooxidans strains are currently in use in biotrickling filters (6, 14–16), due to the inherent acidification of the trickling filter upon growth on CS2. The microorganisms convert CS2 via a 2-step hydrolysis reaction (12, 17): CS2 + H2O → COS + H2S and COS + H2O → CO2 + H2S. The H2S is subsequently oxidized, ultimately to sulfuric acid (18), yielding the energy required for growth, but also acidifying the biofilter trickling water. Large quantities of water are used to maintain the pH at levels tolerated by the CS2-removing microorganisms. The use of new, more extreme acidophiles would reduce water usage as well as operational costs, making biofiltration more sustainable and effective (6, 19).
Biofiltration technologies can benefit from more in-depth knowledge about the molecular mechanism of bacterial CS2 conversion. Although several bacterial CS2-converting species are now known (9–13, 17, 20), their CS2-converting enzymes have not been characterized at all. However, we previously purified the CS2 hydrolase from the CS2-converting hyperthermophilic archaeon Acidianus strain A1-3 and showed that it could convert CS2 to COS, H2S, and CO2 via the hydrolysis reaction as described above (21). The CS2 hydrolase appeared to be homologous to β-carbonic anhydrases (β-CAs), which catalyze the reversible hydration of CO2 + H2O ⇆ HCO3− + H+. The crystal structure of the CS2 hydrolase revealed that the enzyme occurs as an octameric ring like the β-CA from the garden pea Pisum sativum (22). However, in the case of Acidianus CS2 hydrolase, two of these rings interlock, forming a highly unusual hexadecameric catenane structure, both in the crystal form and in solution (21, 23). Intriguingly, despite the high homology with CAs, the Acidianus CS2 hydrolase could not use CO2 as a substrate, and CAs have not been found to use CS2 as a substrate, although the conversion is theoretically possible (24–26). Recently, a COS hydrolase enzyme was purified from Thiobacillus thioparus strain THI15, which is also a β-CA homologue. It does not form a catenane structure. Instead, a weakly associated tetrameric ring is formed (27). Its physiological role is COS conversion, not CS2 conversion (27). The COS hydrolase is not closely related to the Acidianus CS2 hydrolase in the β-CA clade D cluster.
With the unusual catenane structure of the archaeal CS2 hydrolase in mind, we set out to study bacterial CS2 hydrolase enzymes from new strains of CS2-degrading Acidithiobacillus thiooxidans isolated from sulfur-rich and highly acidic environments. Here, we report on the purification and characterization of two bacterial CS2 hydrolases. We show that archaeal and bacterial CS2 hydrolases are closely related and that the bacterial homologues also form catenane structures.
MATERIALS AND METHODS
Media and culture conditions.
New Acidithiobacillus thiooxidans strains were isolated from sulfur-rich and highly acidic ecosystems including mixed hot spring samples (strain 2Bp), Solfatara, Naples (strain Sts 4-3), and Solfarata, Rome (strain S1p), or from samples from CS2-removing biotrickling filters (strains G8 and BBW1). The strains were enriched and isolated with CS2 as the sole energy source. Physiological details of the new strains will be described in a future article. Strains were grown in minichemostats, set up using 250-ml Schott bottles, with a culture volume of 150 ml. The medium was a basal salt mineral medium (MM) consisting of (in g · liter−1) KH2PO4 (0.2), NH4Cl (0.5), MgSO4 · 7 H2O (0.75), CaCl2 · 2 H2O (0.1), and 1 ml/liter trace element solution and acidified with 1% (vol/vol) H2SO4 (pH 0.75). Trace elements consisted of the following (in g · liter−1): EDTA-Na · 2 H2O (10.0), ZnSO4 · 7 H2O (2.2), MnCl2 · 4 H2O (1.02), FeSO4 · 7 H2O, (NH4)6Mo7O24 · 4 H2O (0.22), CuSO4 · 5 H2O (0.32), and CoCl2 · 6 H2O (0.32). To maintain the extremely acidophilic strains, the sulfuric acid concentration was kept at 1%. The medium flow was 2.7 ml · h−1 (D = 0.023 h−1). The reactor temperature was 22°C. CS2 gas (8.6 ± 0.05 μM) was bubbled through the reactors with a flow rate of 41.5 ± 0.1 ml · min−1 and mixed into the reactor with a stir bar, stirring at 1,000 rpm. A. thiooxidans strains S1p and G8 were also grown in batch reactors, in MM acidified with sulfuric acid to pH 2. The reactor temperature was 25°C. CS2-containing air was bubbled through the reactor with a flow rate varying between 132 and 140 ml · min−1 and dispersed through the culture by stirring at 500 to 1,000 rpm. The concentration of CS2 gas was varied between 10 nmol · ml−1 for low-density cultures and 125 nmol · ml−1 for high-density cultures. The μmax values were determined by increasing the medium flow and simultaneously increasing the [CS2] supplied to the reactors up to and beyond the point where S0 formation started becoming visible in the reactors.
Methylomicrobium alcaliphilum DSM19304 was grown at 28°C, with shaking at 200 rpm, as 10-ml cultures in 120-ml bottles closed with butyl rubber stoppers, on DSMZ medium 1180, and containing 15 ml CH4. Halopiger xanaduensis DSM18323 was grown in Erlenmeyer flasks at 37°C and shaking at 200 rpm in the medium described in reference 28. The pH of the medium was adjusted to pH 8.0 before and after autoclaving with sterile 1 M NaOH. Mycobacterium marinum strain M was grown statically in 7H9 medium at 30°C.
Preparation of cell extracts.
Batch reactors with a culture optical density at 600 nm (OD600) of 1.7 were harvested by centrifugation at 9,000 × g for 15 min. The pellets (about 4 g, wet weight) were washed with 20 mM Tris, pH 8, to raise the pH to 8.0. Pellets were resuspended in 15 ml 20 mM Tris, pH 8, containing EDTA-free protease inhibitors (Roche Diagnostics). Cells were broken by a French press (21), DNase I was added, and the suspension was centrifuged at 48,000 × g for 60 min. Supernatants were frozen at −20°C. Protein concentrations were determined using the Bio-Rad protein microassay. Using dry weight measurements of purified CS2 hydrolase, we determined that bovine serum albumin (BSA) as a standard rather than IgG produced more accurate protein determinations for CS2 hydrolase. Cell extracts from steady-state reactor-grown bacterial cells were prepared as follows: 30 to 50 ml was removed from the reactors and centrifuged at 4°C for 30 min at 12,000 × g. The cell pellets were washed with 15 ml sterile distilled water (sdH2O) and resuspended in 0.5 ml 20 mM KPi, pH 7. Approximately 350 μl glass beads (size, 80 to 110 μm) were added, and the cells were broken by bead beating for 2 times 2 min at 30 Hz (Retsch, Germany) with intermittent cooling on ice. The broken cell mixtures were centrifuged for 5 min at 16,000 × g, and the supernatants were either used directly for protein assays and kinetic measurements or stored at −20°C with a final concentration of 10% glycerol.
Purification of CS2 hydrolases.
CS2 hydrolases from A. thiooxidans strain G8 and S1p were purified by ammonium sulfate precipitation, followed by hydrophobic interaction chromatography (HIC) and anion-exchange chromatography. Chemicals used for chromatography were of ultrapure grade. Prior to ammonium sulfate precipitation, the Tris concentration was raised from 20 to 50 mM by adding an appropriate volume of 1 M Tris-HCl, pH 8. Extracts were stirred continuously and kept on ice, while finely ground (NH4)2SO4 was added slowly to 30%, wt/vol. The extract was left on ice for 1 h and centrifuged for 45 min at 3,000 × g, and another aliquot of (NH4)2SO4 was added until 60% saturation was reached.
For strain G8, (NH4)2SO4 was added to 60%, wt/vol, followed by incubation and centrifugation as described above. The pellet was dissolved in 3 ml 50 mM Tris-HCl, pH 8, and centrifuged for 5 min at 13,500 × g. The resulting supernatant was filtered through a 0.45-μm filter unit (Millex-HV polyvinylidene difluoride [PVDF] Durapore). (NH4)2SO4 was added from a 3.4 M stock to raise the concentration to 1 M, and the whole fraction was loaded onto a 1-ml HIC Hitrap Phenyl Sepharose 6 Fast Flow (High sub) column (GE Healthcare) equilibrated with 1.7 M (NH4)2SO4 in 50 mM Tris-HCl (pH 8), using the Åkta system (GE Healthcare). Proteins were separated using a linear gradient from 1.7 to 0 M (NH4)2SO4 over 10 column volumes at a flow rate of 2 ml · min−1. Two-milliliter fractions were collected and assayed for CS2 hydrolase activity (see below). The CS2 hydrolase eluted at the very end of the gradient at 122 mM (NH4)2SO4 together with at least 2 other dominant proteins. Active fractions were pooled and concentrated 3 times, and the buffer was exchanged to 20 mM Tris-HCl (pH 8) by centrifugation at room temperature (RT) through a Vivaspin 30-kDa cutoff spin column (Sartorius). Two milliliters was loaded onto a 1-ml Hitrap ANX-Fast Flow (High sub) column (GE Healthcare), and proteins were separated at RT using a linear gradient from 0 to 1 M NaCl over 25 column volumes and a flow rate of 1 ml · min−1. The CS2 hydrolase eluted at 117 mM (11.7%) NaCl.
For strain S1p, the 30% (NH4)2SO4 supernatant fraction was used to separate the proteins on the HIC column equilibrated with 1.3 M (NH4)2SO4 in 50 mM Tris-HCl (pH 8) described above. The S1p CS2 hydrolase eluted in a broad peak between 1.1 M and 179 mM (NH4)2SO4, but the highest activity was seen at the lower salt concentrations, perhaps due to inhibition by (NH4)2SO4. Pooled active fractions were concentrated 13 times, and the buffer was exchanged as described above. The ANX column was loaded with 100-μl samples of the concentrated fractions, and proteins were separated at RT using a linear gradient from 0 to 1 M NaCl over 18 column volumes and a flow rate of 1 ml · min−1. The CS2 hydrolase eluted in a well-separated peak at 202 mM NaCl. Fractions containing the purified enzyme were stored on ice or, if not used within 1 week, frozen at −20°C.
Protein gel electrophoresis.
SDS-PAGE (10 or 12% polyacrylamide, pH 8.3) and native PAGE (6 or 8% polyacrylamide, pH 8.3) was performed using a Mini-Protean III Cell (Bio-Rad) at RT. Fermentas unstained or prestained and the PageRuler Plus prestained molecular weight standards were used for SDS-PAGE. Invitrogen P/N 57030 markers were used for native PAGE. Ten micrograms of protein was loaded per lane. Proteins were visualized by Coomassie brilliant blue (CBB) G 250.
CS2 and COS hydrolase activity measurements.
CS2 hydrolase activity of purified fractions and on native PAGE gels was measured qualitatively as described previously (21) but at 30°C. CS2 and COS hydrolase activities of purified CS2 hydrolases were quantified by gas chromatography (29). To 120-ml bottles, 500 μl 20 mM HEPES (pH 7) was added. As COS was unstable in the buffer and its breakdown rate depended on the buffer volume, the latter was minimized and kept at 500 μl. This small volume simultaneously maximized the gas to liquid transfer of CS2. Experiments were run against abiotic controls to check that substrate conversion was due to enzyme activity rather than chemical breakdown. Bottles were sealed with a gray butyl rubber stopper, and between 0.25 and 10 ml of CS2-saturated air (final concentration in the buffer, between 8 and 800 μM CS2) or between 0.125 and 4 ml COS (final concentrations in the buffer, between 5 and 473 μM COS) was injected. CS2-saturated air was obtained by incubating an airtight 1-liter bottle containing 50 ml CS2 for several hours. COS was injected from a COS gas bottle. The bottles were incubated at 30°C with shaking at 400 rpm for 15 min to ensure good gas transfer into the buffer. Purified CS2 hydrolase (0.3 to 0.7 μg) in 100 μl 20 mM HEPES (pH 7) was injected. The formation of hydrolysis products from CS2 (COS and H2S) and COS (H2S) was measured by gas chromatography, and production rates over the first 3 min were used to calculate enzyme activity. CS2 and COS concentrations in the buffer were determined by measuring the concentration in the headspace of the bottles at the end of each experiment and converting it to liquid concentrations using the solubility of COS and CS2 in 20 mM HEPES buffer (pH 7) at 30°C. The solubility of CS2 or COS was determined by adding known amounts to a 120-ml bottle containing 60 ml buffer, incubating them for 30 min at 30°C with shaking at 400 rpm, and measuring the remaining CS2 or COS in the headspace as well as the liquid phase by gas chromatography. The liquid/gas ratio at 30°C was 0.45:1 for COS and 0.94:1 for CS2.
CS2 hydrolase activity was also quantified using an H2S Clark-type microsensor (Unisense A/S, Denmark), calibrated using an anaerobic Na2S stock diluted in 20 mM HEPES (pH 7). A 6 mM CS2 stock was prepared by mixing 200 μl CS2 with 500 ml dH2O in a 500-ml serum bottle by vigorous shaking for at least 30 min. Up to 0.1 μg cell extract in 20 mM HEPES (pH 7), 0.1 μg purified CS2 hydrolase, or 100 μg bovine carbonic anhydrase was added to a 1-ml glass vessel containing 1 ml of 20 mM HEPES (pH 7) stirred at 500 to 1,000 rpm, in a 22°C water bath. The H2S sensor was lowered into the vessel, CS2 was added from the stock bottle to 600 μM CS2, and the formation of H2S was followed for approximately 30 s. Initial H2S production rates were calculated to determine the Vmax values for each cell extract.
The CS2 hydrolase activity of bacterial strains was also tested by gas chromatography. A CS2 stock bottle was prepared by adding 400 μl CS2 to an empty 500-ml bottle sealed with a rubber seal and incubating it for 30 min at RT for the CS2 to evaporate. Five- or 10-ml samples from late-logarithmic or early-stationary-phase bacterial cultures were added to 120-ml bottles, which were sealed with gray butyl rubber stoppers, and 0.25 ml CS2-containing air was added from the stock bottle to obtain a CS2 concentration of about 10 to 15 nmol · ml−1 CS2. The cultures were incubated at the temperature used for growth and shaken at 300 rpm. Headspace samples were taken at intervals for up to 24 h and analyzed for COS, H2S, and CS2 as described previously (29). The M. marinum strain M cultures were incubated with CS2 statically and at RT. To make sure this strain could adapt to CS2 conversion, fresh medium was added to the grown culture in addition to CS2, and CS2 conversion was followed for 24 h.
Genome sequencing.
Genomic DNA from A. thiooxidans strains G8 and S1p was isolated (30) and sequenced using next-generation sequencing. Strain G8 was sequenced by a combination of 454 titanium technology (31) (about 50 Mb in 450-nucleotide [nt] reads) and Illumina technology (32) (about 1.5 Gb in 75-nt reads) at the genome sequencing facilities of the departments of Human Genetics and Molecular Biology (Radboud University Nijmegen). The reads of both methods were combined using CLC bio software (Aarhus, Denmark) and assembled in about 200 contigs of >1,000 nt, including several large genome fragments of 100 kb. A. thiooxidans strain S1p was sequenced by Illumina sequencing only, resulting in a 1.7-Gb sequence in 75-nt reads. Sequence reads were trimmed to remove low-quality reads and ambiguous nucleotides, selecting those with a minimum read length of 30 nt. The resulting sequences (total, 0.8 Gb) were used for de novo assembly, yielding 265 contigs with an average length of 11.758 nt. Putative G8 and S1p CS2 hydrolase genes were identified in both assemblies using the sequences of the CS2 hydrolases from Acidianus strain A1-3 (GenBank accession number HM805096) and Sulfolobus solfataricus P2 (GenBank accession number AE006641.1) in BLASTP and motif searches.
MALDI-TOF MS.
Purified CS2 hydrolase from pieces excised from SDS-PAGE gels was extracted, digested with trypsin, and analyzed using matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) as described previously (33).
Phylogenetic analysis.
The carbonic anhydrase(-like) protein sequences most homologous to the CS2 hydrolase of Acidianus strain A1-3 and A. thiooxidans strains G8 and S1p were retrieved via Blast. They were aligned using MUSCLE (34), and phylogenetic analysis was performed in MEGA 5.05 (35).
Stopped-flow spectrometry.
Carbonic anhydrase activity was measured by stopped-flow spectrometry (21). Per assay, up to 30 μg purified CS2 hydrolase was used. The positive control consisted of up to 63 ng bovine carbonic anhydrase (Sigma) per assay.
Analytical ultracentrifugation.
The oligomeric state of the CS2 hydrolases in solution was investigated by analytical ultracentrifugation at A2801 cm of 0.2 (S1p) and 0.67 (G8) as described in reference 21.
Structure modeling.
The hexadecameric structure of the Acidianus A1-3 CS2 hydrolase (Protein Data Bank accession number 3TEO) was used as a template to form the hexadecameric Zn-containing CS2 hydrolase from 3TEN (which is an octamer). The YASARA & WHAT IF Twinset (36) was used for homology modeling and subsequent analysis of the A. thiooxidans G8 and S1p CS2 hydrolases. Sequence identity between the models and the Acidianus A1-3 CS2 hydrolase template was 49% for A. thiooxidans G8 CS2 hydrolase and 46% for the CS2 hydrolase from strain S1p. Access routes from outside the enzymes to the active sites were calculated using MOLE 2.0 online (37).
Nucleotide sequence accession numbers.
The sequences of the bacterial CS2 hydrolases were deposited in the GenBank database under accession numbers KC902814 and KC902815.
RESULTS
Purification of the CS2 hydrolases from A. thiooxidans strains G8 and S1p.
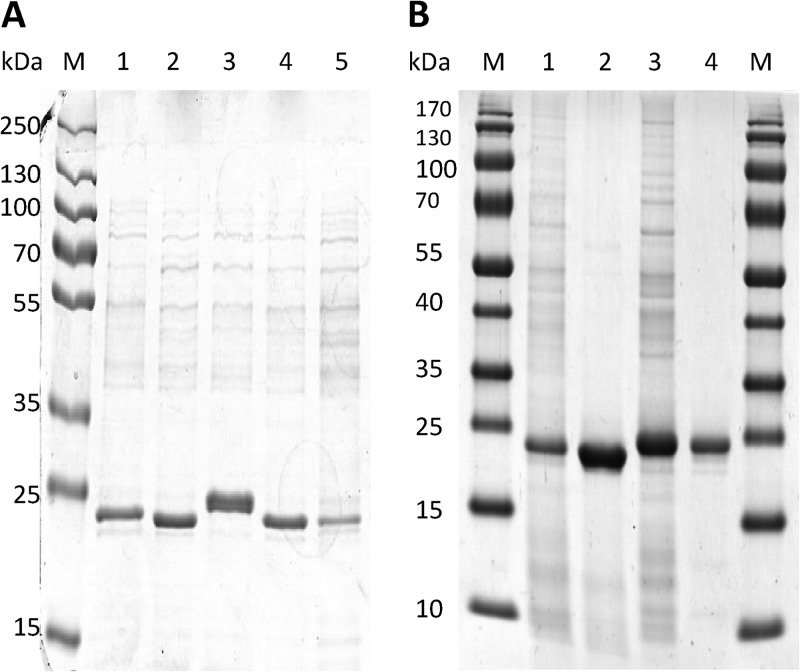
From a variety of sulfur-rich and highly acidic ecosystems, we isolated five new extremely acidophilic CS2-converting A. thiooxidans strains (2Bp, Sts 4-3, S1p, G8, and BBW1). SDS-PAGE analysis of the soluble proteins in cell extracts from cells grown in chemostats with CS2 as the sole energy source indicated that in all strains there was one hugely dominant protein present, of approximately 24 kDa (Fig. 1A). This size corresponds to the size of the monomeric CS2 hydrolase from Acidianus A1-3, which is also present in high abundance in CS2-grown cells (21). Therefore, we postulated that these highly abundant proteins in the A. thiooxidans extracts might be the bacterial CS2 hydrolases.
Fig 1.
Purification of CS2 hydrolases from CS2-converting Acidithiobacillus thiooxidans strains. (A) SDS-PAGE (12% polyacrylamide) of cell extracts from CS2-converting Acidithiobacillus thiooxidans strains 2Bp (lane 1), Sts 4-3 (lane 2), S1p (lane 3), G8 (lane 4), and BBW1 (lane 5) grown in minichemostats, with 10 nmol · ml−1 CS2 as the energy source and at a D of 0.02. (B) Cell extract from A. thiooxidans strains G8 (lane 1) and S1p (lane 3), and purified CS2 hydrolases of strains G8 (lane 2) and S1p (lane 4). Ten micrograms of protein was loaded per lane.
The highly abundant protein of strain S1p was approximately 1 kDa higher in mass than those of the other 4 strains as well as the Acidianus A1-3 CS2 hydrolase. To investigate potential differences between the CS2 hydrolases, we purified the CS2 hydrolase from both strain G8 and strain S1p by ammonium sulfate precipitation followed by HIC and anion-exchange chromatography (Fig. 1B). The purified CS2 hydrolases both corresponded to the highly abundant protein present in the A. thiooxidans cell extracts.
Identification of the CS2 hydrolase genes of A. thiooxidans strains G8 and S1p.
In order to identify the genes encoding the purified CS2 hydrolase enzymes, genomic DNA of A. thiooxidans strain G8 and S1p was sequenced by next-generation sequencing platforms and assembled. Using the sequences of the Acidianus A1-3 and S. solfataricus P2 CS2 hydrolase genes, homologues were identified in the assemblies of both A. thiooxidans strains and aligned with the most homologous genes identified in the NCBI databases. The putative G8 CS2 hydrolase (predicted mass, 22.6 kDa) had 49% amino acid identity and the S1p CS2 hydrolase (predicted mass, 24.3 kDa) 46% amino acid identity to the Acidianus A1-3 enzyme, and they had 50% amino acid identity with each other. The active-site residues that are characteristic of β-CAs are perfectly conserved (Fig. 2). The translated S1p gene was 18 amino acids longer than the translated G8 gene and 13 amino acids longer than the Acidianus A1-3 CS2 hydrolase, corresponding to the larger mass of the S1p enzyme observed on SDS-PAGE (see above). MALDI-TOF MS confirmed that the purified CS2 hydrolases were encoded by the identified Acidianus A1-3 CS2 hydrolase homologous genes: for the purified CS2 hydrolase of strain G8, peptides from the trypsin digest covered 60% of the identified (translated) gene. For strain S1p, the coverage was 90%.
Fig 2.
Amino acid alignment of CS2 hydrolases from Acidianus strain A1-3, Sulfolobus islandicus strains HEV 10/4 and LS 2.15, S. solfataricus P2, A. thiooxidans strain G8, Acidithiobacillus caldus strain SM-1, and A. thiooxidans strain S1p. Underlining indicates residues forming the catalytic center of the enzymes. Boldface indicates perfectly conserved amino acids. Gray box, FF motif.
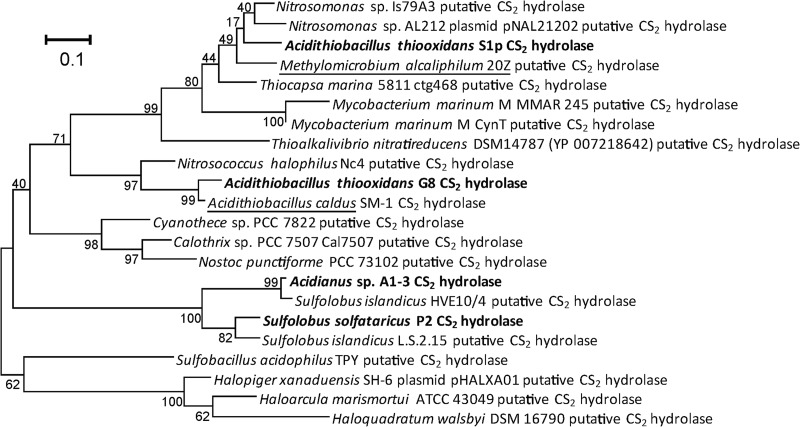
Phylogenetic analysis of CS2 hydrolases and homologous enzymes.
The newly identified CS2 hydrolases cluster within a group of β-CA homologues that contain the two confirmed CS2 hydrolases from Acidianus strain A1-3 and S. solfataricus P2. This group is divided into archaeal and bacterial enzymes, with three cyanobacterial exceptions: three enzymes, from Calothrix sp., Cyanothece sp., and Nostoc punctiforme, fall in the archaeal instead of the bacterial group (Fig. 3). Previously, we identified two phenylalanine residues, F77 and F78, in the Acidianus A1-3 sequence that were present in the other confirmed CS2 hydrolases. These residues form part of a long hydrophobic tunnel, the only access to and from the active center. Amino acid F78 was shown to be crucial for activity of the CS2 hydrolase of Acidianus A1-3 (21). As the F77 and F78 residues were not conserved in β-CA homologues outside the cluster that contained the CS2 hydrolases, we proposed that they may be signature residues for CS2 hydrolase enzymes. In support of this hypothesis, this FF motif was also conserved in the G8 and S1p CS2 hydrolases. In fact, in all the enzymes of both the bacterial and the archaeal branches of the putative CS2 hydrolase cluster within the β-CAs, the FF motif is conserved. There are two exceptions: the enzyme from Nostoc punctiforme has a VF motif, and that of Haloquadratum walsbyi DSM16790 has a YF motif instead, but the F78 residue, important for activity in the Acidianus A1-3 enzyme, is still conserved.
Fig 3.
Phylogenetic analysis of putative and confirmed CS2 hydrolases. Amino acid sequences were retrieved from GenBank and through BLAST and aligned using the MUSCLE aligner as implemented in Mega5.0. All putative CS2 hydrolases are β-CA homologues and are usually annotated as such in GenBank. The evolutionary history was inferred by using the maximum likelihood method based on the Dayhoff matrix-based model. The tree with the highest log likelihood (−3645.9759) is shown. The percentage of trees (500 replicates) in which the associated taxa clustered together is shown next to the branches. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. All positions containing gaps and missing data were eliminated. There were a total of 183 positions in the final data set. A phylogenetic tree based on DNA sequences showed comparable clustering. Underlining indicates putative CS2 hydrolases from strains with confirmed CS2 conversion activity. Boldface indicates confirmed CS2 hydrolases.
CS2 conversion by microorganisms present in the CS2 hydrolase phylogenetic tree.
Three strains present in the putative CS2 hydrolase cluster were grown and tested for CS2 conversion by adding 10 nmol · ml−1 CS2 gas to the headspace of a 10-ml grown culture. Of the three strains, Methylomicrobium alcaliphilum 20Z (DSM19304) converted CS2 immediately to H2S with COS as an intermediate. After 1 h, all CS2 was depleted. Although CS2 conversion was immediate and effective, we did not observe growth of M. alcaliphilum on CS2 instead of CH4, even after incubation periods of more than 1 month. Halopiger xanaduensis SH-6 (DSM18323), on the other hand, was not able to convert CS2 over a period of at least 3 h. A small, nontransient increase in COS was seen over time, but this occurred also in cultures that had been inhibited or killed by heat treatment or addition of sodium azide or potassium cyanide, as well as in Escherichia coli cultures, and did not significantly reduce the concentration of CS2 in the headspace. Mycobacterium marinum strain M was also tested for CS2 conversion activity. However, under the conditions tested, this strain did not show CS2 hydrolase activity, not even after 24 h of incubation of a growing culture with CS2.
Substrate specificity of purified CS2 hydrolases from A. thiooxidans strains G8 and S1p.
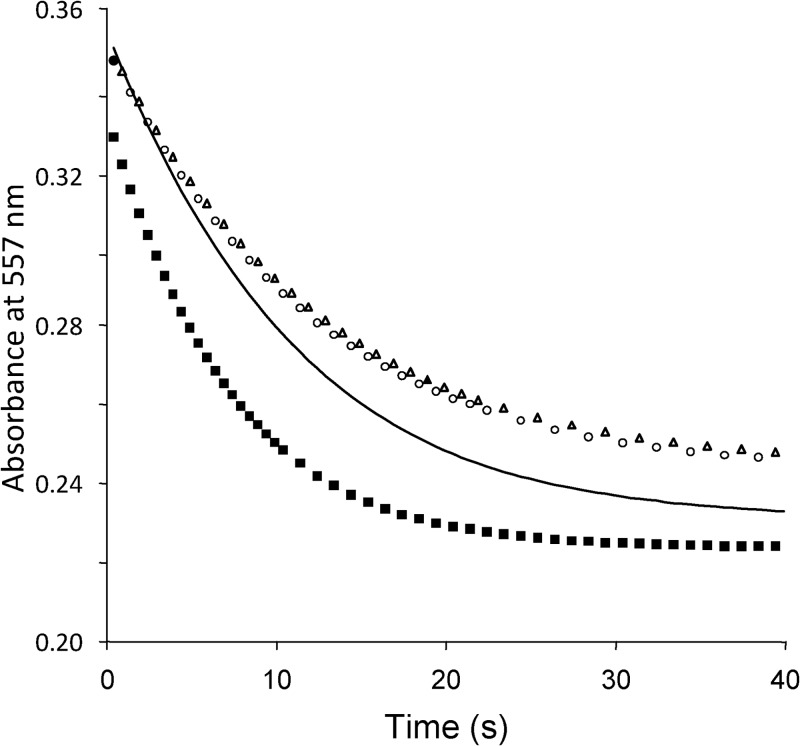
As the CS2 hydrolase enzymes are highly homologous to the β-CAs, it is possible that β-CAs may be able to hydrate CS2 as well as CO2. Although the possibility of this conversion was predicted quantum chemically (24–26), it has never been experimentally confirmed. In addition, we tested potential CS2 hydrolase activity of bovine erythrocyte α-CA, the γ-CAs Cam from Methanosarcina thermophila (38), Cab from Methanobacterium thermoautotrophicum (39), and Cam from Pelobacter carbinolicus (kindly made available by R. Siva Sai Kumar and James G. Ferry) and the β-CA from Streptococcus pneumoniae (40, 41), but none of these could convert CS2. Reversely, CS2 hydrolases may be able to hydrate CO2 instead of CS2. We have already shown that for the CS2 hydrolase from Acidianus strain A1-3 this was not the case (21). Here, we tested the substrate specificity of the A. thiooxidans CS2 hydrolases by stopped-flow spectrophotometry. Like the Acidianus CS2 hydrolase, the A. thiooxidans enzymes could not convert CO2 to HCO3− (Fig. 4).
Fig 4.
Stopped-flow spectrophotometry to measure carbonic anhydrase activity of purified CS2 hydrolases from A. thiooxidans strains G8 and S1p compared with carbonic anhydrase from bovine erythrocytes. Symbols: open circles, strain G8; open triangles, strain S1p; solid squares, bovine carbonic anhydrase; solid line, chemical control.
The newly purified CS2 hydrolases did convert CS2 and COS to H2S (Table 1). With CS2 as the substrate, the A. thiooxidans G8 CS2 hydrolase had a nearly 10-fold-higher catalytic efficiency (Kcat/Km) than the Acidianus A1-3 and A. thiooxidans S1p CS2 hydrolases. This was due to both a higher Vmax (131 versus 32 nmol product · min−1 · μg enzyme) as well as a higher affinity (lower Km of 46 versus 93 μM CS2). The difference in catalytic efficiency between the strain G8 CS2 hydrolase and the strain S1p CS2 hydrolase was even more pronounced with COS as the substrate, due to the much higher affinity of the strain G8 CS2 hydrolase for COS.
Table 1.
Comparison of kinetic constantsa of the conversion of CS2 (to H2S and COS) and COS (to H2S) by purified CS2 hydrolases from Acidithiobacillus thiooxidans strains S1p and G8 with those of Acidianus A1-3 CS2 hydrolase
| Strain | CS2 |
COS |
||||
|---|---|---|---|---|---|---|
| Km | Vmax | Kcat/Km | Km | Vmax | Kcat/Km | |
| A. thiooxidans S1p | 93 ± 21 | 32 ± 2 | 8.4 × 106 | 74 ± 11 | 34 ± 2 | 1.1 × 107 |
| A. thiooxidans G8 | 46 ± 6 | 131 ± 4 | 6.4 × 107 | 14 ± 2 | 97 ± 3 | 1.6 × 108 |
| Acidianus A1-3b | 130 ± 3 | 40 ± 0 | 7.3 × 106 | 22 ± 3 | 74 ± 3 | 8.0 × 107 |
Km values are expressed in μM substrate in the buffer ± standard error of the mean (SEM); Vmax is given in nmol product · min−1 · μg enzyme ± SEM. Km, Vmax, and Kcat/Km values (in S−1 · M−1) were determined by nonlinear regression using GraphPad Prism 5.04 (n ≥ 9).
Data from reference 21.
We compared the kinetic properties of the purified enzymes with those of cell extracts from the five new CS2-converting A. thiooxidans strains growing in chemostats supplied with 10 nmol · ml−1 CS2. Under these conditions, these strains all produced large amounts of CS2 hydrolase (Fig. 1). The Km values varied between 81 μM for strain G8 and 130 μM CS2 for strain 2Bp. The Vmax of cell extracts from strain S1p was higher than those of cell extracts from the 4 other strains tested, which is in contrast to the results obtained with the purified CS2 hydrolases. However, due to the lower affinity of the purified CS2 hydrolase to CS2, strain S1p would require larger amounts of CS2 hydrolase when growing on the low CS2 concentration supplied to the reactor to be able to gain as much energy for growth as strain G8. Indeed, strain S1p had relatively more CS2 hydrolase present in cell extracts than strain G8 (Fig. 1).
Catenane formation in the A. thiooxidans CS2 hydrolases.
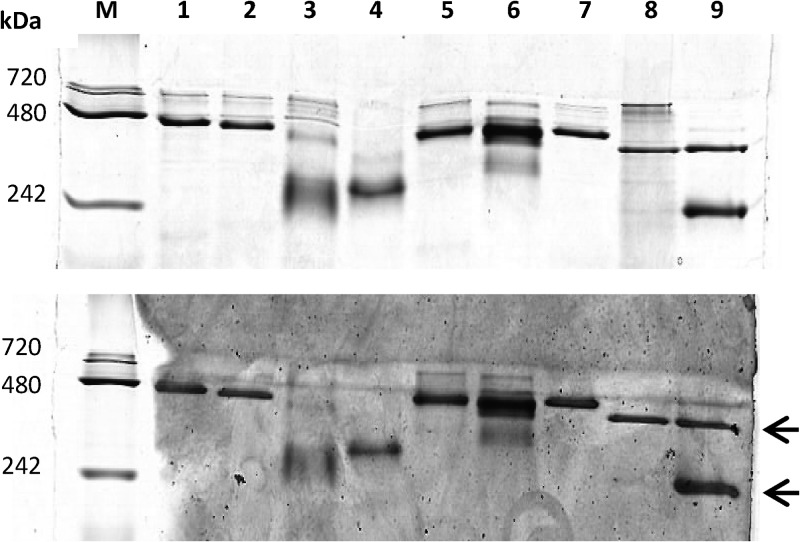
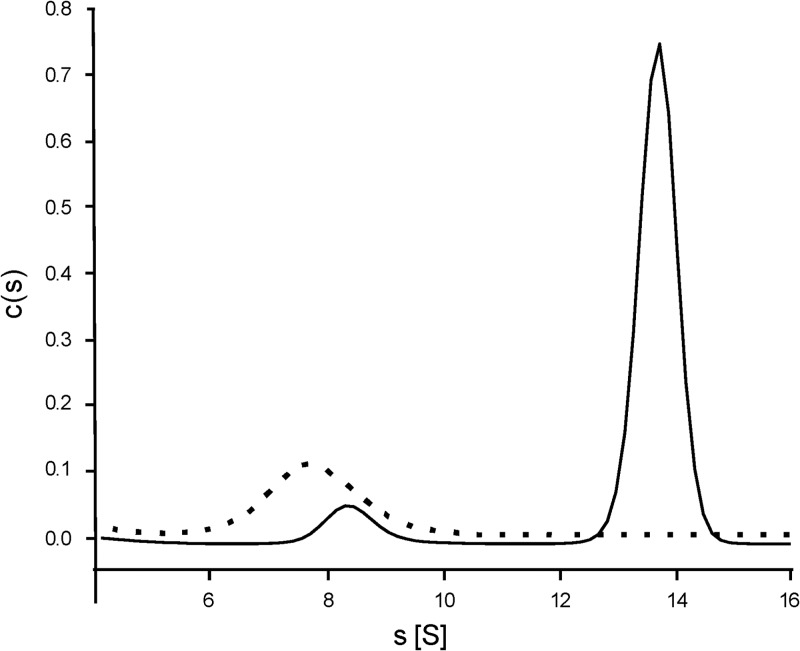
To investigate whether the bacterial CS2 hydrolases form the highly unusual catenanes observed for the Acidianus A1-3 CS2 hydrolase (21), we compared cell extracts from five CS2-converting A. thiooxidans strains and two purified CS2 hydrolases with the Acidianus enzyme by native PAGE. Four of the five A. thiooxidans strains had CS2 hydrolases of a size similar to that of the Acidianus 16-mer form of the native enzyme. This suggests that, like the archaeon Acidianus, these A. thiooxidans strains contain the interlocked, double-ring catenane form of the enzyme rather than the single octameric ring. However, strain S1p CS2 hydrolase migrated faster than the other CS2 hydrolases, both in cell extract and in purified form (Fig. 5), suggesting that the S1p CS2 hydrolase may not form hexadecameric catenanes. To confirm this finding, purified CS2 hydrolases from strains S1p and G8 were analyzed by analytical ultracentrifugation (AUC) (Fig. 6). For the CS2 hydrolase of strain G8, a small peak with sedimentation coefficient at 8.4 S and a large peak at 13.8 S were identified, corresponding to an 8-mer and 16-mer, respectively. The CS2 hydrolase of strain S1p yielded only one peak, at 7.8 S. From these data we conclude that the CS2 hydrolases from A. thiooxidans strains G8, 2Bp, Sts 4-3, and BBW1 share the unique hexadecameric catenane structure with the CS2 hydrolase from Acidianus A1-3. However, the A. thiooxidans S1p CS2 hydrolase forms only the single octameric ring.
Fig 5.
Native PAGE (8% polyacrylamide) of three purified CS2 hydrolases and of cell extracts from five CS2-converting A. thiooxidans strains and of Acidianus A1-3, stained for protein (top) or CS2 hydrolase activity (bottom). Ten micrograms of protein was loaded per lane. Lanes: M, marker; lane 1, cell extracts of strain 2Bp; lane 2, cell extracts of strain Sts 4-3; lane 3, cell extracts of strain S1p; lane 4, purified CS2 hydrolase of strain S1p; lane 5, strain G8 cell extract; lane 6, strain G8 purified CS2 hydrolase; lane 7, strain BBW1 cell extract; lane 8, Acidianus A1-3 cell extract; lane 9, Acidianus A1-3 purified CS2 hydrolase. Arrows indicate the expected positions of 8-mer (about 192-kDa) and 16-mer (about 384-kDa) configurations.
Fig 6.
Analytical ultracentrifugation results for purified CS2 hydrolases from A. thiooxidans strains S1p and G8, fitted as the concentration distribution (c) (arbitrary units) of sedimentation coefficients (s) in Svedberg units (S). For strain G8 (solid line), two species are observed, corresponding to octamers (at 8.3 S) and hexadecamers (13 S). For strain S1p (dashed line), only one peak was observed, corresponding to an octameric form of the enzyme.
Structure models of the A. thiooxidans CS2 hydrolases.
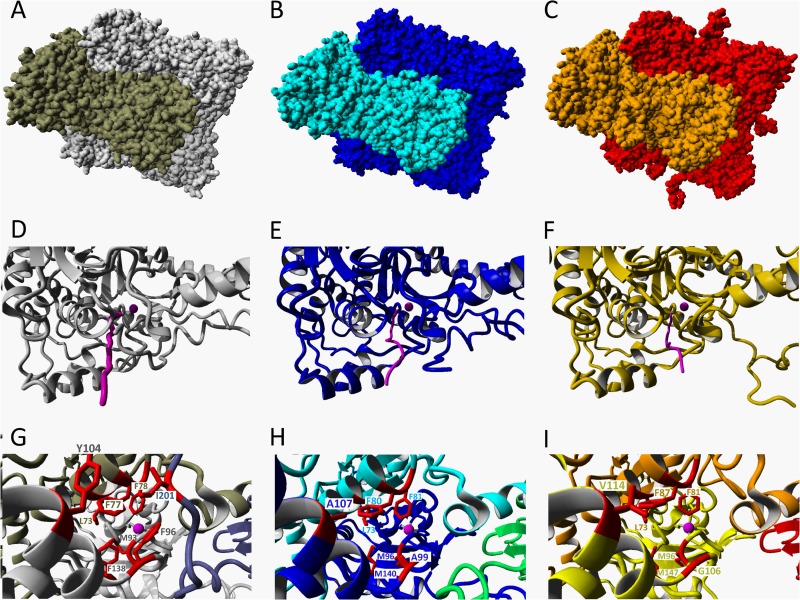
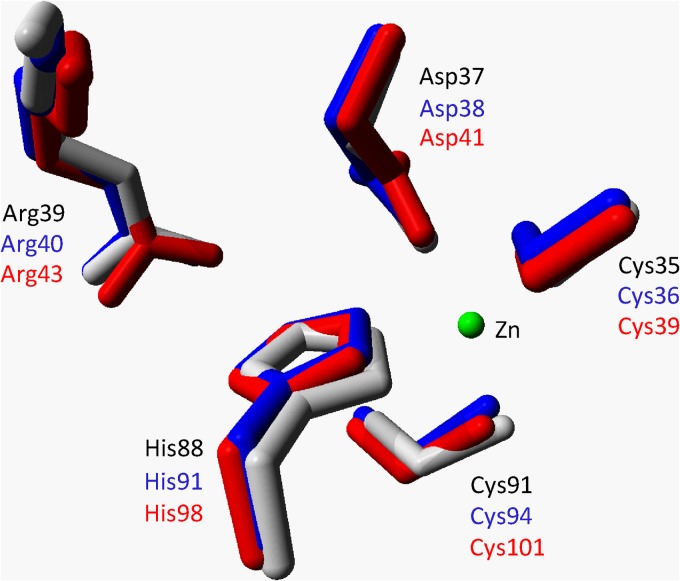
To compare the structural homology between the three CS2 hydrolases and to find a structural basis for the differences in oligomerization between the A. thiooxidans CS2 hydrolases, we used YASARA to create structural models of the A. thiooxidans G8 and S1p enzymes using the hexadecameric Acidianus A1-3 CS2 hydrolase as a template. The CS2 hydrolases of both enzymes could be modeled as catenane hexadecamers (Fig. 7A, B, and C). The models show a highly conserved structural homology in the active sites of the enzymes (Fig. 8). In addition, the long and narrow hydrophobic tunnel that forms the only access to the active site in the Acidianus A1-3 CS2 hydrolase is also predicted to be the only available access route in the modeled structures of A. thiooxidans strains G8 and S1p CS2 hydrolases (Fig. 7D, E, and F). The residues that form these tunnels are partly conserved: the F77F78 motif critical for activity in the Acidianus A1-3 enzyme is perfectly conserved, and some of the residues at the outer entrance of the tunnel are still hydrophobic but smaller, potentially creating a slightly wider tunnel at those positions (Fig. 7G, H, and I). The tunnel in the Acidianus A1-3 enzyme is formed by residues from 3 monomers, with the third monomer contributing one isoleucine residue (I201) from the C-terminal extending arm. However, in both the A. thiooxidans strain G8 and the strain S1p CS2 hydrolase models, the C-terminal domain of the third monomer is positioned away from the tunnel. As the C-terminal domain of the enzyme from strain G8 is shorter and that of the strain S1p enzyme is longer than the C terminus of the Acidianus A1-3 CS2 hydrolase, the model may not be accurate in this position. It therefore remains to be seen if a third monomer contributes to the tunnel formation in the A. thiooxidans CS2 hydrolase.
Fig 7.
Crystal structure and models of CS2 hydrolases. (A) Resolved crystal structure of the CS2 hydrolase from Acidianus strain A1-3 and structural models of the CS2 hydrolases from A. thiooxidans strain G8 (B) and strain S1p (C), modeled using the hexadecameric structure of the Acidianus A1-3 CS2 hydrolase as a template. The two octameric interlocking rings forming the hexadecamer are shown in different colors. (D, E, F) Details of octamers showing the narrow hydrophobic entrance tunnels (pink) to the Zn-containing active site in the Acidianus CS2 hydrolase (D) and in the models of the G8 (E) and S1p (F) CS2 hydrolases. The Zn atoms are indicated by pink spheres. (G, H, I) Views of the tunnels looking in from the entrances to the active sites, showing the three monomers (two shades of gray and blue) and the residues (red) forming the tunnel in the Acidianus CS2 hydrolase (G) and in the models of the G8 hydrolases (blue, cyan, and green monomers) (H) and the S1p CS2 hydrolases (yellow, orange, and red monomers) (I).
Fig 8.
Superposition of active-site residues of the resolved structure of the Acidianus A1-3 CS2 hydrolase (gray) and the models of the A. thiooxidans G8 (blue) and S1p CS2 hydrolases (red). The Zn residue is shown as a green sphere.
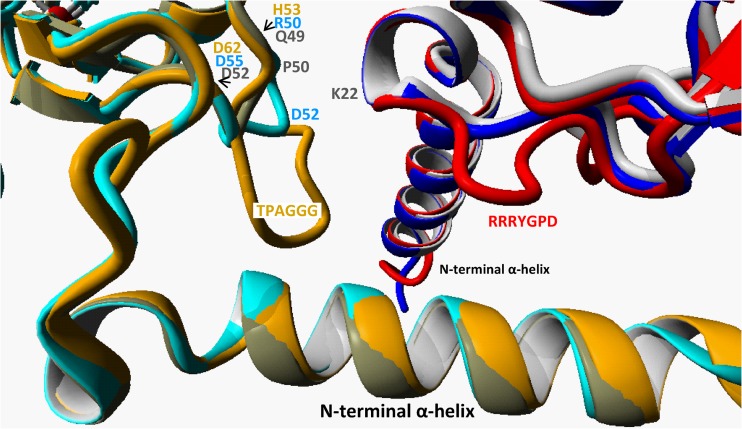
From the structure of the Acidianus A1-3 CS2 hydrolase as well as in the models of the A. thiooxidans strain G8 and strain S1p enzymes, it appears that the N-terminal α-helical extending arms of the monomers are responsible for most of the interaction between the 2 rings in the catenane structure (Fig. 9). In particular, in the Acidianus structure the residue K22 just beyond the N-terminal helix of one octamer is in close proximity to the Q49 and P50 on a small 4-residue loop (residues 49 to 52, QPDD) of the other octamer. In the model of the A. thiooxidans G8 CS2 hydrolase, this small loop is one residue larger (residues 50 to 55, RGDRD) but points in a different direction from that of the Acidianus A1-3 loop. Although the CS2 hydrolase of A. thiooxidans S1p could also be modeled as a catenane double ring, there is a much larger loop corresponding to the sequence insertion at residues 56 to 61, TPAGGG, that is not present in the Acidianus A1-3 or A. thiooxidans G8 CS2 hydrolase (Fig. 2). The A. thiooxidans S1p CS2 hydrolase has a second 2-residue insertion (26P-27D [Fig. 2]) that is part of a loop in the other octamer (residues 21 to 28, RRRYGPD [Fig. 9]). This loop protrudes into the space close to where the TPAGGG loop is positioned. It is therefore possible that these insertions in the A. thiooxidans S1p CS2 hydrolase protein are interfering with catenane formation.
Fig 9.
Structure detail of the proximity of two octamers in the hexadecameric form of the CS2 hydrolase, shown as an overlay of the models of the A. thiooxidans strains G8 (two shades of blue, one for each octamer) and S1p (red and ochre) onto the Acidianus strain A1-3 resolved structure (two shades of gray). In the Acidianus CS2 hydrolase, the smallest distance between the 2 octamers is between a small loop of 4 residues between Gln49 and Asp52 (QPPD) and the Lys22 on the other octamer (residues in gray). In the model of the G8 CS2 hydrolase, the G8 loop is 6 residues long (RGDRGD, in blue). In the model of the S1p, the loop is 10 residues long (HVDTPAGGGD, in ochre). The S1p enzyme is modeled to have an additional loop in the second octamer (RRRYGPD).
In addition, the C-terminal tail of the A. thiooxidans S1p CS2 hydrolase is nine amino acids longer and its sequence differs from that of the Acidianus A1-3 CS2 hydrolase C-terminal tail, making it difficult to model (Fig. 7C). The C-terminal tails of the Acidianus A1-3 CS2 hydrolase as well as the A. thiooxidans G8 modeled CS2 hydrolase contain several residues that make stabilizing interactions with the core of the structure, where potential interactions between the 2 octamers take place, and therefore it seems likely that the C-terminal tail is not very flexible. Whether the C-terminal tail of the A. thiooxidans S1p CS2 hydrolase adopts a similar conformation remains unclear. Because of the differences in length and sequence, it is possible that this tail can also interfere with hexadecameric catenane formation.
DISCUSSION
In this study, we identified the bacterial CS2 hydrolase enzymes from five A. thiooxidans strains, purified the CS2 hydrolases from A. thiooxidans strains G8 and S1p, and identified the encoding genes. The enzymes were highly homologous to each other and to the CS2 hydrolase from Acidianus strain A1-3, both on DNA and on protein levels. All putative CS2 hydrolases enzymes are phylogenetically most related to a group of β-CAs that contain mostly bacterial rather than archaeal enzymes (21). This suggests that the gene evolved in bacteria and spread via lateral gene transfer to archaeal species. Indeed, two of the putative CS2 hydrolases were plasmid encoded. Also, transposon-related sequences surrounding the (putative) CS2 hydrolase were identified in the available genome sequences of both archaeal species (S. solfataricus P2, Sulfolobus islandicus strains L.S.2.15 and HVE10/4, and Haloquadratum walsbyi) and bacterial species (Nitrosomonas sp. AL212 and Mycobacterium marinum M). Acidithiobacillus and the archaeal Sulfolobus and Acidianus species occupy the same sulfur-rich acidic environments such as hot springs and solfataras (42, 43). Gene transfer between these species from the two domains of life is possible and likely (44–46).
In addition to the acidophilic species that are represented in the (putative) CS2 hydrolase phylogenetic tree, most other species were isolated from saline and/or alkaline environments. Saline marshes and estuaries are rich sources of CS2 (47, 48). Also, the haloalkaline soda lakes harbor an active sulfur cycle with a high biodiversity of sulfur-oxidizing bacteria (SOB) that respire mainly on sulfide, thiosulfate (S2O32−), and polysulfide (49). Thioalkalivibrio paradoxus, isolated from a Kenyan soda lake, was shown to be able to respire on CS2 when grown on thiocyanate (SCN−) (50). We could not find a CS2 hydrolase homologue in the available genome sequence of this species (which at the time of writing is still wrongly labeled as the Thioalkalivibrio thiocyanoxidans genome [D. Sorokin, personal communication]). However, the Thioalkalivibrio paradoxus genome does contain a homologue of the recently described COS hydrolase from T. thioparus strain THI 115, one of only three close homologues currently in the databases. T. thioparus strain THI 115 converts SCN− to COS and NH3. COS is subsequently converted to H2S by COS hydrolase. This enzyme can also convert CS2 at a lower rate (27). It is possible that the CS2 hydrolase activity observed for Thioalkalivibrio paradoxus is due to activity of the COS hydrolase homologue. Interestingly, the closely related Thioalkalivibrio nitratireducens, isolated from the Egyptian soda lake Lake Fazda (51), contains both a CS2 hydrolase and a close COS hydrolase homologue. Thioalkalivibrio paradoxus and Thioalkalivibrio nitratireducens both convert SCN−, but they do not produce COS or CS2 as intermediates (D. Sorokin, personal communication). Therefore, the function of the CS2 hydrolase homologues in these strains remains unclear at present.
Of the three species that were tested for CS2 conversion, only M. alcaliphilum was able to convert CS2. This methanotroph was isolated from the highly alkaline soda lake Shara-Nur, in Central Asia (52, 53). CS2 conversion by methane-grown cultures was immediate, suggesting that the enzyme was not induced but already present in the cell. Why this organism requires the presence of CS2 hydrolase activity is not clear at present. However, another Methylomicrobium species, M. kenyense strain AMO1 isolated from a Kenyan soda lake, was also found to have CS2 conversion activity (54). These authors suggested that CS2 could be a suicide substrate for methane monooxygenase (MMO), the enzyme that converts methane to methanol as the first step in the energy-generating pathway of methanotrophs. MMO is mechanistically and evolutionary homologous to the ammonium monoxygenase (AMO) from nitrifiers. Their substrates are often interchangeable, and they share alternative substrates and inhibitors (55). The nitrifier Nitrosomonas europaea was shown to have CS2 conversion activity, but the mechanism of CS2 conversion was not investigated (56). CS2 is one of the oldest known inhibitors of nitrification (57). The inhibitory action has been attributed to the CS2 molecule forming a complex with a nucleophilic amino acid close to the active center of AMO, thereby chelating the Cu cofactor from the active center (58, 59). If MMO is similarly inhibited by CS2, then both methanotrophs and nitrifiers would benefit from an enzyme capable of CS2 removal. Indeed, in addition to the methanotroph M. alcaliphilum, two Nitrosomonas strains and one Nitrosococcus strain were found to contain CS2 hydrolase homologues in their genomes (Fig. 3). We therefore propose that these CS2 hydrolases could function as a detoxification mechanism for environmental CS2 in nitrifiers and methanotrophs.
COS is an ecologically relevant alternative substrate for CS2 hydrolase, as it is the most abundant sulfur species in the atmosphere. The major sinks for COS are vegetation and soil (2). Biological conversion of COS by plants via their carbonic anhydrases has been described (60, 61), and COS-degrading soil microorganisms, including 4 Mycobacterium spp., were readily isolated from Japanese soils (62). It is therefore possible that (some of) the CS2 hydrolase homologues identified in the phylogenetic tree convert mainly COS rather than CS2 in their natural environments. Interestingly, neither the two CS2 hydrolases from A. thiooxidans strains S1p and G8 nor the archaeal CS2 hydrolase (21) or the T. thioparus COS hydrolase (27) converted CO2. This suggests that their function evolved specifically for CS2 and COS conversion and that they are not just broad-specificity CAs.
All (putative) CS2 hydrolase proteins presented in Fig. 3 but two contain the conserved FF residues that are crucial in forming the hydrophobic access tunnel to the active center. As they are not conserved in CAs or in the T. thioparus COS hydrolase, the FF residues were proposed to be a CS2 hydrolase-specific motif (21). However, H. xanaduensis and M. marinum, which have CS2 hydrolase homologues containing the FF motif, do not convert CS2 under the conditions that we tested. It is possible that the amino acids surrounding the FF motif are also important for CS2 hydrolase activity: in all confirmed bacterial and archaeal CS2 hydrolases as well as in M. alcaliphilum, the sequence stretch that is perfectly conserved is NFFGT, but in H. xanaduensis the sequence is NFFDT. In the structural model of the Acidianus CS2 hydrolase, the N76 and G79T80 residues are positioned away from the hydrophobic tunnel that the FF residues are a part of, but it is possible that the larger D79 residue causes a conformational change that affects the positioning of the crucial F78 residue. Although both the CS2 hydrolase homologues present in M. marinum strain M contain the NFFGT motif, they are also the only two CS2 hydrolase homologues with an extended C terminus, 56 amino acids longer than that of the CS2 hydrolase from A. thiooxidans strain S1p. This may have profound effects on oligomerization and therefore enzyme activity (see below).
The Acidianus A1-3 CS2 hydrolase exists as a 16-mer interlocked ring (catenane) structure. Catenanes are extremely rare in biology. We are aware of only 3 reports: the gp5 capsid protein of bacteriophage HK97 (63), the bovine mitochondrial peroxiredoxin III (64), and a Pyrobaculum aerophilum citrate synthase in which the catenanes are formed by disulfide bridging between the N- and C-terminal ends of each of the homodimer chains (65). The molecular mechanism of the catenane assembly in the Acidianus A1-3 enzyme is not understood. The N- and C-terminal domains of the monomers, which are crucial for interlinking dimers into octamers (21), also reside in the field of interaction between the 2 octameric rings (Fig. 9). Interestingly, the bacterial CS2 hydrolases of four of the five isolated A. thiooxidans strains also existed in the 16-mer catenane form. The reason why these enzymes adopt a catenane conformation is unclear at present. However, cells growing on low concentrations of CS2 produce vast amounts of enzyme, probably due to the poor affinity of the CS2 hydrolase to CS2. This results in extremely high intracellular concentrations with very dense packing of the enzyme, similar to what has been reported for the most abundant enzyme on earth, RuBisCo, that can be expressed to about 40% of total protein in cells and is packed in cell structures called carboxysomes (66). Dense packing of CS2 hydrolases can be obtained both with octameric rings and with 16-mer catenanes, but the exclusion volume is a little bit smaller for catenanes than for single rings. Also, highly densely packed Acidianus strain A1-3 CS2 hydrolase in crystals consisted of pure 16-mer catenanes (21).
The A. thiooxidans S1p enzyme was found only in the 8-mer form, both by AUC and by native PAGE analysis. The S1p CS2 hydrolase monomer is 11 amino acids longer at the C terminus than the monomer from strain G8 and 9 amino acids longer than the monomer from Acidianus A1-3. In addition, the S1p enzyme has an insertion at residues 56 to 61 that the other two CS2 hydrolases lack. Modeling the G8 and S1p enzymes on the Acidianus A1-3 CS2 hydrolase structure indicated that in the S1p CS2 hydrolase, both the elongated C-terminal tail and the insertion may interfere with the formation of the 16-mer catenane by filling the space where the other octamer should reside. It is possible that the N- and C-terminal areas of the Acidianus A1-3 and G8 CS2 hydrolases, which are situated in the interaction field between the two octameric rings, may play a role in the formation of the rare catenane structure. Whether this is indeed the case is the subject of future investigations.
ACKNOWLEDGMENTS
We thank Peter Burghout for providing the carbonic anhydrase from Streptococcus pneumoniae and R. Siva Sai Kumar and James Ferry for providing the carbonic anhydrases from M. thermophila, M. thermoautotrophicum, and P. carbinolicus. Huw Williams is thanked for providing M. marinum strain M, and Melanie Wattenberg is thanked for growing this strain. Daan Speth and Sacha van Hijum are thanked for assembly of the genome sequences.
The research was funded by STW project 6353 and ERC 232937.
Footnotes
Published ahead of print 8 July 2013
REFERENCES
- 1.Bremner JM, Bundy LG. 1974. Inhibition of nitrification in soils by volatile sulfur compounds. Soil Biol. Biochem. 6:161–165 [Google Scholar]
- 2.Watts SF. 2000. The mass budgets of carbonyl sulfide, dimethyl sulfide, carbon disulfide and hydrogen sulfide. Atmos. Environ. 34:761–779 [Google Scholar]
- 3.Chin M, Davis DD. 1993. Global sources and sinks of OCS and CS2 and their distributions. Global Biogeochem. Cycles 7:321–337 [Google Scholar]
- 4.Gelbke HP, Goen T, Maurer M, Sulsky SI. 2009. A review of health effects of carbon disulfide in viscose industry and a proposal for an occupational exposure limit. Crit. Rev. Toxicol. 39(Suppl 2):1–126 [DOI] [PubMed] [Google Scholar]
- 5.Estrada JM, Kraakman N, Munoz R, Lebrero R. 2011. A comparative analysis of odour treatment technologies in wastewater treatment plants. Environ. Sci. Technol. 45:1100–1106 [DOI] [PubMed] [Google Scholar]
- 6.Kraakman NJR. 2003. Robustness of a full-scale biological system treating industrial CS2 emissions. Environ. Prog. 22:79–85 [Google Scholar]
- 7.van Groenestijn JW, Kraakman NJR. 2005. Recent developments in biological waste gas purification in Europe. Chem. Eng. J. 113:85–91 [Google Scholar]
- 8.Smith NA, Kelly DP. 1988. Isolation and physiological characterization of autotrophic sulfur bacteria oxidizing dimethyl disulfide as sole source of energy. J. Gen. Microbiol. 134:1407–1417 [Google Scholar]
- 9.Odintsova EV, Wood AP, Kelly DP. 1993. Chemolithoautotrophic growth of Thiothrix ramosa. Arch. Microbiol. 160:152–157 [Google Scholar]
- 10.Plas C, Wimmer K, Holubar P, Mattanovich D, Danner H, Jelinek E, Harant H, Braun R. 1993. Degradation of carbondisulphide by a Thiobacillus isolate. Appl. Microbiol. Biotechnol. 38:820–823 [Google Scholar]
- 11.Jordan SL, Kracziewiczdowjat AJ, Kelly DP, Wood AP. 1995. Novel Eubacteria able to grow on carbon disulfide. Arch. Microbiol. 163:131–137 [Google Scholar]
- 12.Pol A, van der Drift C, Op den Camp HJM. 2007. Isolation of a carbon disulfide utilizing Thiomonas sp. and its application in a biotrickling filter. Appl. Microbiol. Biotechnol. 74:439–446 [DOI] [PubMed] [Google Scholar]
- 13.Hartikainen T, Ruuskanen J, Raty K, von Wright A, Martikainen PJ. 2000. Physiology and taxonomy of Thiobacillus strain TJ330, which oxidizes carbon disulphide (CS2). J. Appl. Microbiol. 89:580–586 [DOI] [PubMed] [Google Scholar]
- 14.Lobo R, Revah S, Viveros-Garcia T. 1999. An analysis of a trickle-bed bioreactor: carbon disulfide removal. Biotechnol. Bioeng. 63:98–109 [PubMed] [Google Scholar]
- 15.Alcantara S, Estrada I, Vasquez MS, Revah S. 1999. Carbon disulfide oxidation by a microbial consortium from a trickling filter. Biotechnol. Lett. 21:815–819 [Google Scholar]
- 16.Hartikainen T, Ruuskanen J, Martikainen PJ. 2001. Carbon disulfide and hydrogen sulfide removal with a peat biofilter. J. Air Waste Manage. Assoc. 51:387–392 [DOI] [PubMed] [Google Scholar]
- 17.Smith NA, Kelly DP. 1988. Oxidation of carbon disulfide as the sole source of energy for the autotrophic growth of Thiobacillus thioparus strain Tk-m. J. Gen. Microbiol. 134:3041–3048 [Google Scholar]
- 18.Barrie Johnson DB, Hallberg KB. 2009. Carbon, iron and sulfur metabolism in acidophilic micro-organisms. Adv. Microb. Physiol. 54:201–255 [DOI] [PubMed] [Google Scholar]
- 19.Hugler W, Acosta C, Revah S. 1999. Biological removal of carbon disulfide from waste air streams. Environ. Prog. 18:173–177 [Google Scholar]
- 20.Jordan SL, McDonald IR, Kraczkiewicz-Dowjat AJ, Kelly DP, Rainey FA, Murrell JC, Wood AP. 1997. Autotrophic growth on carbon disulfide is a property of novel strains of Paracoccus denitrificans. Arch. Microbiol. 168:225. 10.1007/s002030050492 [DOI] [PubMed] [Google Scholar]
- 21.Smeulders MJ, Barends TRM, Pol A, Scherer A, Zandvoort MH, Udvarhelyi A, Khadem AF, Menzel A, Hermans J, Shoeman RL, Wessels H, van den Heuvel LP, Russ L, Schlichting I, Jetten MSM, Op den Camp HJM. 2011. Evolution of a new enzyme for carbon disulphide conversion by an acidothermophilic archaeon. Nature 478:412–416 [DOI] [PubMed] [Google Scholar]
- 22.Kimber MS, Pai EF. 2000. The active site architecture of Pisum sativum β-carbonic anhydrase is a mirror image of that of α-carbonic anhydrases. EMBO J. 19:1407–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Eldijk MB, van Leeuwen I, Mikhailov VA, Neijenhuis L, Harhangi HR, van Hest JCM, Jetten MSM, Op den Camp HJM, Robinson CV, Mecinovic J. 2013. Evidence that the catenane form of CS2 hydrolase is not an artefact. Chem. Commun. (Camb.) 10.1039/c3cc43219 [DOI] [PubMed] [Google Scholar]
- 24.Sinnecker S, Brauer M, Koch W, Anders E. 2001. CS2 fixation by carbonic anhydrase model systems—a new substrate in the catalytic cycle. Inorg. Chem. 40:1006–1013 [DOI] [PubMed] [Google Scholar]
- 25.Notni J, Schenk S, Protoschill-Krebs G, Kesselmeier J, Anders E. 2007. The missing link in COS metabolism: a model study on the reactivation of carbonic anhydrase from its hydrosulfide analogue. ChemBioChem 8:530–536 [DOI] [PubMed] [Google Scholar]
- 26.Schenk S, Kesselmeier J, Anders E. 2004. How does the exchange of one oxygen atom with sulfur affect the catalytic cycle of carbonic anhydrase? Chem. Eur. J. 10:3091–3105 [DOI] [PubMed] [Google Scholar]
- 27.Ogawa T, Noguchi K, Saito M, Nagahata Y, Kato H, Ohtaki A, Nakayama H, Dohmae N, Matsushita Y, Odaka M, Yohda M, Nyunoya H, Katayama Y. 2013. Carbonyl sulfide hydrolase from Thiobacillus thioparus strain THI115 is one of the β-carbonic anhydrase family enzymes. J. Am. Chem. Soc. 135:3818–3825 [DOI] [PubMed] [Google Scholar]
- 28.Gutierrez MC, Castillo AM, Kamekura M, Xue Y, Ma Y, Cowan DA, Jones BE, Grant WD, Ventosa A. 2007. Halopiger xanaduensis gen. nov., sp nov., an extremely halophilic archaeon isolated from saline Lake Shangmatala in Inner Mongolia, China. Int. J. Syst. Evol. Microbiol. 57:1402–1407 [DOI] [PubMed] [Google Scholar]
- 29.Derikx PJL, Op den Camp HJM, van der Drift C, Van Griensven LJLD, Vogels GD. 1990. Odorous sulfur compounds emitted during production of compost used as a substrate in mushroom cultivation. Appl. Environ. Microbiol. 56:176–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kowalchuk GA, de Bruijn FJ, Head IM, Akkermans AD, van Elsas JD. 2004. Molecular microbial ecology manual, 2nd ed. Kluwer Academic Publishing, London, United Kingdom [Google Scholar]
- 31.Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben LA, Berka J, Braverman MS, Chen YJ, Chen ZT, Dewell SB, Du L, Fierro JM, Gomes XV, Godwin BC, He W, Helgesen S, Ho CH, Irzyk GP, Jando SC, Alenquer MLI, Jarvie TP, Jirage KB, Kim JB, Knight JR, Lanza JR, Leamon JH, Lefkowitz SM, Lei M, Li J, Lohman KL, Lu H, Makhijani VB, McDade KE, McKenna MP, Myers EW, Nickerson E, Nobile JR, Plant R, Puc BP, Ronan MT, Roth GT, Sarkis GJ, Simons JF, Simpson JW, Srinivasan M, Tartaro KR, Tomasz A, Vogt KA, Volkmer GA, Wang SH, Wang Y, Weiner MP, Yu PG, Begley RF, Rothberg JM. 2005. Genome sequencing in microfabricated high-density picolitre reactors. Nature 437:376–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bennett S. 2004. Solexa Ltd. Pharmacogenomics 5:433–438 [DOI] [PubMed] [Google Scholar]
- 33.Khadem AF, Pol A, Wieczorek A, Mohammadi SS, Francoijs KJ, Stunnenberg HG, Jetten MSM, Op den Camp HJM. 2011. Autotrophic methanotrophy in Verrucomicrobia: Methylacidiphilum fumariolicum SolV uses the Calvin-Benson-Bassham cycle for carbon dioxide fixation. J. Bacteriol. 193:4438–4446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32:1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krieger E, Koraimann G, Vriend G. 2002. Increasing the precision of comparative models with YASARA NOVA—a self-parameterizing force field. Proteins Struct. Funct. Genet. 47:393–402 [DOI] [PubMed] [Google Scholar]
- 37.Berka K, Hanak O, Sehnal D, Banas P, Navratilova V, Jaiswal D, Ionescu CM, Varekova RS, Koca J, Otyepka M. 2012. MOLEonline 2.0: interactive web-based analysis of biomacromolecular channels. Nucleic Acids Res. 40(W1):W222–W227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alber BE, Colangelo CM, Dong J, Stalhandske CMV, Baird TT, Tu CK, Fierke CA, Silverman DN, Scott RA, Ferry JG. 1999. Kinetic and spectroscopic characterization of the γ-carbonic anhydrase from the methanoarchaeon Methanosarcina thermophila. Biochemistry 38:13119–13128 [DOI] [PubMed] [Google Scholar]
- 39.Smith KS, Ferry JG. 1999. A plant-type (beta-class) carbonic anhydrase in the thermophilic methanoarchaeon Methanobacterium thermoautotrophicum. J. Bacteriol. 181:6247–6253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burghout P, Cron LE, Gradstedt H, Quintero B, Simonetti E, Bijlsma JJE, Bootsma HJ, Hermans PWM. 2010. Carbonic anhydrase is essential for Streptococcus pneumoniae growth in environmental ambient air. J. Bacteriol. 192:4054–4062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burghout P, Vullo D, Scozzafava A, Hermans PWM, Supuran CT. 2011. Inhibition of the β-carbonic anhydrase from Streptococcus pneumoniae by inorganic anions and small molecules: toward innovative drug design of antiinfectives? Bioorg. Med. Chem. 19:243–248 [DOI] [PubMed] [Google Scholar]
- 42.Barns SM, Fundyga RE, Jeffries MW, Pace NR. 1994. Remarkable archaeal diversity detected in a Yellowstone National Park hot-spring environment. Proc. Natl. Acad. Sci. U. S. A. 91:1609–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glamoclija M, Garrel L, Berthon J, Lopez-Garcia P. 2004. Biosignatures and bacterial diversity in hydrothermal deposits of Solfatara Crater, Italy. Geomicrobiol. J. 21:529–541 [Google Scholar]
- 44.Treangen TJ, Rocha EPC. 2011. Horizontal transfer, not duplication, drives the expansion of protein families in prokaryotes. PLoS Genet. 7(1):e1001284. 10.1371/journal.pgen.1001284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reno ML, Held NL, Fields CJ, Burke PV, Whitaker RJ. 2009. Biogeography of the Sulfolobus islandicus pan-genome. Proc. Natl. Acad. Sci. U. S. A. 106:8605–8610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nelson KE, Clayton RA, Gill SR, Gwinn ML, Dodson RJ, Haft DH, Hickey EK, Peterson LD, Nelson WC, Ketchum KA, McDonald L, Utterback TR, Malek JA, Linher KD, Garrett MM, Stewart AM, Cotton MD, Pratt MS, Phillips CA, Richardson D, Heidelberg J, Sutton GG, Fleischmann RD, Eisen JA, White O, Salzberg SL, Smith HO, Venter JC, Fraser CM. 1999. Evidence for lateral gene transfer between Archaea and Bacteria from genome sequence of Thermotoga maritima. Nature 399:323–329 [DOI] [PubMed] [Google Scholar]
- 47.Turner SM, Liss PS. 1985. Measurements of various sulfur gases in a coastal marine-environment. J. Atmos. Chem. 2:223–232 [Google Scholar]
- 48.Steudler PA, Peterson BJ. 1984. Contribution of gaseous sulfur from salt marshes to the global sulfur cycle. Nature 311:455–457 [Google Scholar]
- 49.Sorokin DY, Kuenen JG. 2005. Haloalkaliphilic sulfur-oxidizing bacteria in soda lakes. FEMS Microbiol. Rev. 29:685–702 [DOI] [PubMed] [Google Scholar]
- 50.Sorokin DY, Tourova TP, Lysenko AM, Mityushina LL, Kuenen JG. 2002. Thioalkalivibrio thiocyanoxidans sp. nov. and Thioalkalivibrio paradoxus sp. nov., novel alkaliphilic, obligately autotrophic, sulfur-oxidizing bacteria capable of growth on thiocyanate, from soda lakes. Int. J. Syst. Evol. Microbiol. 52:657–664 [DOI] [PubMed] [Google Scholar]
- 51.Sorokin DY, Tourova TP, Sjollema KA, Kuenen JG. 2003. Thialkalivibrio nitratireducens sp. nov., a nitrate-reducing member of an autotrophic denitrifying consortium from a soda lake. Int. J. Syst. Evol. Microbiol. 53:1779–1783 [DOI] [PubMed] [Google Scholar]
- 52.Khmelenina VN, Kalyuzhnaya MG, Starostina NG, Suzina NE, Trotsenko YA. 1997. Isolation and characterization of halotolerant alkaliphilic methanotrophic bacteria from Tuva soda lakes. Curr. Microbiol. 35:257–261 [Google Scholar]
- 53.Kalyuzhnaya MG, Khmelenina V, Eshinimaev B, Sorokin D, Fuse H, Lidstrom M, Trotsenko Y. 2008. Classification of halo(alkali)philic and halo(alkali)tolerant methanotrophs provisionally assigned to the genera Methylomicrobium and Methylobacter and emended description of the genus Methylomicrobium. Int. J. Syst. Evol. Microbiol. 58:591–596 [DOI] [PubMed] [Google Scholar]
- 54.Sorokin DY, Jones BE, Kuenen JG. 2000. An obligate methylotrophic, methane-oxidizing Methylomicrobium species from a highly alkaline environment. Extremophiles 4:145–155 [DOI] [PubMed] [Google Scholar]
- 55.Bedard C, Knowles R. 1989. Physiology, biochemistry, and specific inhibitors of CH4, NH4+, and co oxidation by methanotrophs and nitrifiers. Microbiol. Rev. 53:68–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Juliette LY, Hyman MR, Arp DJ. 1993. Inhibition of ammonia oxidation in Nitrosomonas europaea by sulfur compounds: thioethers are oxidized to sulfoxides by ammonia monooxygenase. Appl. Environ. Microbiol. 59:3718–3727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Warrington R. 1878. On nitrification. J. Chem. Soc. Trans. 33:44–51 [Google Scholar]
- 58.Hyman MR, Kim CY, Arp DJ. 1990. Inhibition of ammonia monooxygenase in Nitrosomonas europaea by carbon disulfide. J. Bacteriol. 172:4775–4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McCarty GW. 1999. Modes of action of nitrification inhibitors. Biol. Fertil. Soils 29:1–9 [Google Scholar]
- 60.Protoschill-Krebs G, Wilhelm C, Kesselmeier J. 1996. Consumption of carbonyl sulphide (COS) by higher plant carbonic anhydrase (CA). Atmos. Environ. 30:3151–3156 [Google Scholar]
- 61.Blezinger S, Wilhelm C, Kesselmeier J. 2000. Enzymatic consumption of carbonyl sulfide (COS) by marine algae. Biogeochemistry 48:185–197 [Google Scholar]
- 62.Kato H, Saito M, Nagahata Y, Katayama Y. 2008. Degradation of ambient carbonyl sulfide by Mycobacterium spp. in soil. Microbiology 154:249–255 [DOI] [PubMed] [Google Scholar]
- 63.Wikoff WR, Liljas L, Duda RL, Tsuruta H, Hendrix RW, Johnson JE. 2000. Topologically linked protein rings in the bacteriophage HK97 capsid. Science 289:2129–2133 [DOI] [PubMed] [Google Scholar]
- 64.Cao Z, Roszak AW, Gourlay LJ, Lindsay JG, Isaacs NW. 2005. Bovine mitochondrial peroxiredoxin III forms a two-ring catenane. Structure 13:1661–1664 [DOI] [PubMed] [Google Scholar]
- 65.Boutz DR, Cascio D, Whitelegge J, Perry LJ, Yeates TO. 2007. Discovery of a thermophilic protein complex stabilized by topologically interlinked chains. J. Mol. Biol. 368:1332–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ellis RJ. 1979. Most abundant protein in the world. Trends Biochem. Sci. 4:241–244 [Google Scholar]