Abstract
We have previously described the posttranslational modification of pore-forming small proteins of Corynebacterium by mycolic acid, a very-long-chain α-alkyl and β-hydroxy fatty acid. Using a combination of chemical analyses and mass spectrometry, we identified the mycoloyl transferase (Myt) that catalyzes the transfer of the fatty acid residue to yield O-acylated polypeptides. Inactivation of corynomycoloyl transferase C (cg0413 [Corynebacterium glutamicum mytC {CgmytC}]), one of the six Cgmyt genes of C. glutamicum, specifically abolished the O-modification of the pore-forming proteins PorA and PorH, which is critical for their biological activity. Expectedly, complementation of the cg0413 mutant with either the wild-type gene or its orthologues from Corynebacterium diphtheriae and Rhodococcus, but not Nocardia, fully restored the O-acylation of the porins. Consistently, the three-dimensional structure of CgMytC showed the presence of a unique loop that is absent from enzymes that transfer mycoloyl residues onto both trehalose and the cell wall arabinogalactan. These data suggest the implication of this structure in the enzyme specificity for protein instead of carbohydrate.
INTRODUCTION
Members of the Corynebacteriales order, which include many infectious agents such as the etiological agents of tuberculosis and leprosy, are Gram-positive bacteria. They are typified by a unique cell envelope that contains an outer permeability membrane, also called the mycomembrane, resembling that of Gram-negative bacteria. This membrane consists of a bilayer composed of an inner leaflet of mycolic acids, α-branched and β-hydroxylated very-long-chain fatty acids (C30 to C90).
These fatty acids are covalently linked to the cell wall arabinogalactan, which in turn is attached to peptidoglycan. The outer leaflet is believed to contain various noncovalently linked lipids, which also include mycolic acid-containing glycoconjugates such as trehalose mono- and dimycolates. In addition to being biologically active substances involved in the bacterial morphology and virulence of pathogenic species, mycolic acids are the main lipid constituents of the mycobacterial cell envelope. Such an organization may explain why mycolates are indispensable for the survival of mycobacteria. As a consequence, several antituberculosis drugs, e.g., isoniazid, ethionamide, and isoxyl, target enzymes specifically involved in the biosynthesis of mycolates.
In contrast to mycobacteria, mycolates are dispensable in corynebacteria (1, 2), which therefore represent a good model organism for studies of the biosynthesis of these compounds. Interestingly, we have recently described for the latter bacteria a novel form of mycolate-containing substance. This represents the first O-linked acylation onto a hydroxylated amino acid of bacterial polypeptides. Indeed, although both thioester- and amide-linked acylation have been widely reported in many bacterial proteins, this study demonstrated the first direct O-linked acylation of an amino acid residue of proteins in the bacterial world. We have demonstrated that the pore-forming proteins PorA and PorH, as well as a third as-yet-unidentified protein, of Corynebacterium glutamicum are modified by a mycolic acid residue (3). We further showed that this modification is critical for the pore-forming activity of the PorA/PorH heterooligomer (4), a channel localized in the outer membrane of corynebacteria.
The transfer of mycolic acids on the two previously known acceptors, i.e., arabinogalactan and trehalose, has been addressed in several studies showing that the reaction is catalyzed by specific acyltransferases, called mycoloyltransferases (Myts), found in all members of the Corynebacteriales order examined to date, notably those belonging to the Mycobacterium and Corynebacterium genera (5–7). While three paralogous Myts have been functionally characterized in Mycobacterium tuberculosis (also known as antigen 85A [Ag85A], Ag85B, and Ag85C), six orthologues of these proteins (C. glutamicum Myts [CgMyts]) have been identified in C. glutamicum (8, 9). Importantly, despite the occurrence of multiple CgMyt-encoding paralogous genes and the nonessentiality of mycolates in corynebacteria, the inactivation of CgmytA and CgmytB impacted the double mutant strain. This strain was severely impaired in its growth, highlighting the importance of these enzymes and the cell envelope mycolic acids in the physiology of Corynebacteriales (10). Accordingly, in the present study, we show that CgMytC is responsible for the O-acylation of the small proteins PorA and PorH in corynebacteria. We also identify orthologous proteins in related genera of the Corynebacteriales order. We further resolve the crystal structure of CgMytC in order to better understand the structural features that may determine its particular substrate specificity.
MATERIALS AND METHODS
Strains and media.
C. glutamicum RES167, a restriction-deficient strain derived from C. glutamicum ATCC 13032 (11), was grown in brain heart infusion (BHI) broth (3.7%) with shaking (250 rpm) at 30°C. Transformation of C. glutamicum by electroporation was performed as described by Bonamy et al. (12). Antibiotics were added to final concentrations of 25 μg · ml−1 kanamycin (Km) and 6 μg · ml−1 chloramphenicol (Cm) for C. glutamicum and 30 μg · ml−1 Cm for Escherichia coli. E. coli strains TOP10 (Invitrogen) and DH5α were grown on Luria-Bertani medium with shaking (250 rpm) at 37°C.
DNA manipulation.
C. glutamicum RES167 chromosomal DNA was extracted as described by Ausubel et al. (13). Corynebacterium diphtheriae NCTC 13129, Nocardia farcinica IFM 10152, and Rhodococcus erythropolis PR4 chromosomal DNAs were extracted by using a similar protocol. Plasmid DNAs were isolated by using the Promega kit for DNA purification. Oligonucleotide primers were synthesized by Eurogentec. PCR amplifications were performed with a 2720 thermocycler (Applied Biosystems), using GoTaq (Promega) or Phusion (Finnzymes) DNA polymerase. All DNA purifications were done by using the Roche High Pure PCR product purification kit. All DNA sequencing was carried out by MilleGen.
Disruption of the cg0413 gene.
To disrupt the cg0413 (CgmytC) gene, internal fragments of cg0413 were amplified from C. glutamicum RES167 chromosomal DNA using primers 336-1 (5′-AAT TGC GAT GTC CAC CAT TG-3′) and 336-2 (5′-TAC CCA GTC GGT GTA GTA GG-3′) cloned into plasmid pCR2.1-TOPO (TOPO TA cloning kit; Invitrogen). The resulting plasmid was used to electrotransform C. glutamicum strain RES167. Transformants generated by integration of the plasmid were selected on BHI plates containing 25 μg · ml−1 Km. Genomic DNAs from mutants were analyzed by 3 PCR amplifications using 2 primers localized upstream of the 5′ end and downstream of the 3′ end of the cg0413 gene and the M13 forward and reverse primers localized in the pCR2.1-TOPO vector. The PCR products corresponding to the gene disruption borders were systematically sequenced.
Construction of the CgMytC expression vector.
An expression vector encoding CgMytC was constructed by using pCGL482 (14) as the cloning vector. We chose to clone this open reading frame (ORF) under the control of its native promoter by amplifying a DNA fragment starting 432 bp upstream of the cg0413 ATG. The corresponding DNA fragment (1,555 bp) was amplified by PCR from C. glutamicum ATCC 13032 chromosomal DNA using primer pair 336-Bam (5′-TTTGGGCGGATCCTAGCATT-3′)/336-Xho (5′-TTAACTCGAGTTCTAGGCCTCT-3′), digested by BamHI and XhoI, and ligated with pCGL482 to give pCGL482-CgMytC. Transformants were selected on Cm-containing plates. Site-directed mutagenesis in order to replace serine 189 with valine was performed on pCGL482-CgMytC by using the QuikChange site-directed kit from Stratagene and the following primers: 336S189V1 (5′-GGAAGTAGTTGCGGACATGACCATGCCAGCAATTGC-3′) and 336S189V2 (5′-GCAATTGCTGGCATGGTCATGTCCGCAACTACTTCC-3′).
Construction of CgMytC orthologous expression vectors.
An expression vector carrying the promoter, the signal peptide, and the first 7 residues of CgMytC was first constructed (pCGL2418). For this purpose, a PCR fragment containing 340 bp upstream and 105 bp downstream of the ATG of the cg0413 gene was amplified with primers MytCP1 (5′-GGT GAG GAT CCG AAT TTA AAG G-3′) and MytCss2 (5′-TCT CGA GGC CTG CAG GGT GCA ATC GAT TGG GGT TAC TTC GGC-3′). MytCss2 introduces a unique ClaI restriction site overlapping both codons 35 and 36 of the cg0413 gene, allowing the subcloning in frame of the different CgMytC orthologous ORFs at this site. The PCR fragment was then digested with BamHI/StuI and inserted into pCGL482 digested with the same restriction enzymes, to give pCGL2418. Each of the cg0413 orthologous genes was amplified from the corresponding genomic DNA with appropriate primer pairs: Dip365-Cla (5′-ACC TGC ATC GAT ACT GTT CGT GGT CAG G-3′) and Dip365-Xho (5′-ATA ATA CTC GAG CGC TGA TAA CGC ACC-3′) for DIP0365 (C. diphtheriae), Nfa-Cla (5′-CCG ATC ATC GAT TCC AAG GCG-3′) and Nfa-Xho (5′-ATA ATA CTC GAG GCA CAC TCG GGG TG-3′) for nfa7210 (N. farcinica), RER60-Cla (5′-ACC TGC ATC GAT ACG TCC GGA TCG CAC-3′) and RER60-Stu (5′-ATA ATA AGG CCT CCG AAA CAC TGG AGC-3′) for RER_15360 (R. erythropolis), RER70-Cla (5′-ACC TGC ATC GAT CCG TCG ACC ACC ACG-3′) and RER70-Stu (5′-ATA ATA AGG CCT ATT CGA TTG CCT GAC-3′) for RER_15370 (R. erythropolis), and Gbro-Cla (5′-ACC TGC ATC GAT CCC GGC GTC TCG CGG-3′) and Gbro-Pst (5′-TAT TAT CTG CAG GTC GAG TTG TAG ACC C-3′) for Gbro_0876 (Gordonia bronchialis). The amplified regions were as follows: (i) 1,129 bp for DIP0365 with ClaI (overlapping codons 32 and 33) and XhoI artificial sites at each extremity of the fragment, (ii) 1,050 bp for RER_15360 with ClaI (overlapping codons 34 and 35) and StuI artificial sites at each extremity of the fragment, (iii) 1,012 bp for RER_15370 with ClaI (overlapping codons 34 and 35) and StuI artificial sites at each extremity of the fragment, (iv) 1,016 bp for nfa7210 with ClaI (overlapping codons 26 and 27) and XhoI artificial sites at each extremity of the fragment, and (v) 983 bp for Gbro_0876 with ClaI (overlapping codons 28 and 29) and PstI artificial sites at each extremity of the fragment. The resulting inserts were digested and ligated into pCGL482 digested with the appropriate enzymes (ClaI and StuI or XhoI or PstI) to give pCGL2412 (DIP0365), pCGL2413 (RER_15360), pCGL2414 (RER_15370), pCGL2415 (nfa7210), and pCGL2416 (Gbro_0876).
Preparation of proteins for SDS-PAGE and Western blot analysis.
Corynebacterium cells (optical density [OD] at 600 nm of 2) were centrifuged at 3,000 × g for 10 min. The pellet was incubated in 30 μl of Laemmli denaturing buffer at 100°C for 5 min and centrifuged for 5 min at 3,000 × g. The supernatant was recovered, and the proteins were separated by SDS-PAGE (15). After electrophoresis, gels were stained with Coomassie brilliant blue R-250. For Western blotting, gels were blotted onto a nitrocellulose membrane, and the proteins were probed with rabbit polyclonal anti-CgMytC antibodies. Bands were detected by using alkaline phosphatase-conjugated antibodies and 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium (BCIP/NBT; Sigma).
Mass spectrometry analysis.
Bacterial cells were grown to an OD at 600 nm of 10 and harvested by centrifugation. The pellet was then extracted with organic solvents CHCl3 and CH3OH (2:1), and the extract containing PorA, PorH, and protein X was dried and directly solubilized in CHCl3-CH3OH (2:1). One microliter was mixed on target with 1 μl of sinapinic acid matrix solution (5 mg · ml−1 dissolved in H2O–CH3CN 0.1% trifluoroacetic acid [TFA] [1:1]).
Matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) spectra were acquired on a Voyager-DE STR mass spectrometer (PerSeptive Biosystems) equipped with a pulsed nitrogen laser emitting at 337 nm in the linear positive mode, using an extraction delay time set at 100 ns and an accelerating voltage of 25 kV.
Quantification of extractible lipids by radiolabeling [1-14C]palmitic acid.
Bacterial cells (OD at 600 nm of 0.1) were grown at 30°C in BHI medium for 4 h prior to the addition of 10 μCi of [1-14C]palmitic acid (100 μCi · ml−1; PerkinElmer). After incubation (4 h), whole cells were harvested and collected by centrifugation. Lipids were extracted from the cell pellets with CHCl3-CH3OH (1:2) overnight at room temperature. The organic phase was dried, and for each strain, 50 ng dried lipids was loaded onto silica gel-coated thin-layer chromatography (TLC) plates (G-60, 0.25-mm thickness; Merck) developed with CHCl3-CH3OH-H2O (65:25:4, vol/vol/vol) as the migration solvent. The lipid spots were revealed with a phosphorimager (Typhoon Trio; GE Healthcare) after exposition on a Fujifilm screen during 3 days. The relative quantification of different compounds was performed by using Image Quant software (version 5.1; Molecular Dynamics).
Purification of CgMytC-His.
cg0413-his was cloned into pCGL482 under the control of its own promoter, as previously described (16). The 6×His-encoding sequence was designed to be in frame at the C terminus of CgMytC. When this construction was introduced in an arabinosyltransferase mutant (ΔaftB strain) (16), CgMytC-His was highly expressed (5 mg · liter−1) and secreted mainly in the culture supernatant. For large-scale purification, ΔaftB cells (CgMytC-His) were recovered after overnight culture by centrifugation at 7,000 × g at 10°C. The cleared supernatant was further centrifuged at 35,000 × g (45 Ti) for 2 h at 10°C to get rid of membrane fragments that are released by the ΔaftB strain, as reported previously (16). Proteins were then precipitated from the solution by adding ammonium sulfate to 70% saturation. Incubation was carried out for 1 h at room temperature with constant shaking. After centrifugation at 7,000 × g for 15 min at 10°C, the protein-containing pellet was resuspended in 60 ml of 25 mM Tris–200 mM NaCl (pH 7.5) (purification buffer). The solution was dialyzed for 24 h at 4°C against the purification buffer (Spectra/Por, with a molecular mass cutoff of 10 kDa; Spectrum) before loading onto the Ni-nitrilotriacetic acid (NTA) column at 1 ml · min−1. The flowthrough was recovered and loaded onto the column one more time. Proteins weakly associated with the Ni-NTA resin were washed off by running 40 ml of purification buffer. Elution was finally performed with 5 column volumes of purification buffer containing imidazole at 250 mM. Fractions 2 to 4 were pooled and immediately injected onto a Sepharose 75 column at 1 ml · min−1. The protein eluted as a single symmetrical peak, and CgMytC-His-containing fractions were pooled and concentrated to 8 to 12 mg · ml−1 by ultrafiltration on a Vivaspin 20 instrument (molecular mass cutoff of 5 kDa; Sartorius). The whole purification procedure (Ni-NTA, Superose 75, and ultrafiltration) was always done on the same day, and aliquots of CgMytC-His were conserved at −80°C. The purification was controlled by SDS-PAGE gels after ammonium sulfate, Ni-NTA, Superose 75, and ultrafiltration steps. Protein quantification was performed by using the Bio-Rad DC protein assay kit.
Crystallization of CgMytC.
The protein was concentrated up to 16.5 mg · ml−1 for screening of crystallization conditions. Screens at 293 K were set up as sitting drops in Greiner plates by using a Mosquito robot (200 nl of a 1:1 mixture of protein-crystallization liquor) and equilibrated against a 100-μl reservoir.
Optimization of initial conditions was pursued manually in hanging drops by using Qiagen plates (EasyXtal 15-Well Tool X-Seal). The best crystals were obtained by mixing 1 μl of CgMytC at 4 mg · ml−1 with 1 μl of the reservoir solution containing 19% polyethylene glycol 4000, 0.2 M MgCl2, and 0.1 M morpholineethanesulfonic acid (MES) (pH 6.5). Plated crystals appeared within 1 week and had dimensions of 0.1 mm by 0.1 mm by 0.05 mm. Crystals were flash-frozen by liquid nitrogen soaking in two steps, using 15% and 30% glycerol as a cryoprotectant.
Data collection and resolution of the structure.
Diffraction data were collected at 100 K on the PROXIMA 1 beam line at the SOLEIL synchrotron by using an ADSC detector, with a 0.5-s exposure time per frame. Oscillation images (240 at 0.5°) were collected at a wavelength of 0.9788 Å. The images were integrated with the program XDS (17) and processed by using the CCP4 program suite.
The structure was solved by the molecular replacement method using the program Phaser (18), as implemented in the CCP4 package. As a molecular replacement search model, we used the atomic coordinates of Ag85C from Mycobacterium tuberculosis (Protein Data Bank [PDB] accession number 1VA5). The final map, improved by electron density modification, was of very good quality, and most of the protein model (1,190 out of 1,460 residues) was automatically built by ARP/wARP (19–21). The CgMytC crystal structure resolution was finally refined at 1.75 Å to a crystallographic Rfactor/Rfree of 16.3%/20.3% (see statistics in Table 2) by using the phenix program (22).
Table 2.
Data collection and refinement statistics for the CgMytC crystal
| Parameter | Value |
|---|---|
| Crystallographic data collection | |
| X-ray source | PROXIMA 1 |
| Wavelength (Å) | 0.9788 |
| Temp (K) | 100 |
| Unit cell parameter (Å, °) | a = 85.83, b = 190.73, c = 78.52 |
| α = β = γ = 90.0 | |
| Space group | P21212 |
| Resolution limit (Å)a | 42.82–1.72 (1.82–1.72) |
| No. of observationsa | 53,0201 (84,732) |
| No. of unique reflections | 136,509 (21,746) |
| Rmerge (%)a,b | 14.9 (64.7) |
| Completeness (%)a | 99.4 (99.0) |
| I/σ (I)a | 9.51 (2.57) |
| Refinement | |
| No. of nonhydrogen atoms (proteins/water/other) | 9,559/1,033/1 |
| Resolution range (Å) | 42.82–1.72 |
| R/Rfree (%) | 16.92/19.89 |
| RMSD bonds (Å)/angles (°) | 0.006/1.051 |
| Avg temp factor (proteins/water/other) | 17.32/25.41/18.92 |
Values in parentheses refer to the highest-resolution shell (1.86 to 1.75Å).
Rmerge = ΣhΣi|Ihi − 〈Ih〉|/ΣhΣiIhi, where Ihi is the ith observation of reflection h, while 〈Ih〉 is the mean intensity of reflection h.
Protein structure accession numbers.
The three-dimensional (3D) structure of corynomycoloyl transferase C has been deposited under RCBS accession number rcbs074890 and PDB accession number 4H18.
RESULTS AND DISCUSSION
Our previous extensive functional studies on the different CgMyts using C. glutamicum strain CGL2005 have shown that CgMytA, CgMytB, CgMytD, and CgMytF are involved in the mycoloylation of trehalose monocorynomycolate (TMCM) to yield trehalose dicorynomycolate (TDCM) and arabinogalactan. The role of CgMytC in the transfer of mycoloyl residues has remained elusive, since the inactivation of the encoding gene did not impact the lipid profile of C. glutamicum strain CGL2005 (9). Subsequent 3D modeling of CgMyts, based on the crystal structures of the related Myts of M. tuberculosis, suggested two significant structural differences between CgMytC and the other Myts examined. First, CgMytC possesses a large insertion loop that could unfavorably interact with the second trehalose-binding site present in the CgMyts, suggesting that CgMytC may have a different corynomycoloyl transferase substrate specificity. Second, some residues whose side chains are predicted to interact with trehalose in the other CgMyts are different in CgMytC (23, 24). We thus hypothesized that CgMytC is a good candidate for the O-mycoloylation of small proteins.
Inactivation of cg0413 and analysis of the impact of the mutation on the acylation of the small proteins PorA, PorH, and protein X.
To test the hypothesis of the implication of CgMytC in the O-mycoloylation of small proteins, we inactivated the cg0413 (CgmytC) gene in the sequenced C. glutamicum strain ATCC 13032 rather than strain CGL2005. The behavior of the latter strain was shown to be significantly different from that of the type strain in terms of both transformability and lipid and protein profiles (9). To obtain the cg0413 mutant, we inserted a kanamycin resistance cassette into the cg0413 gene, encoding the mycoloyltransferase CgMytC. This resulted in strain CGL2059, which was also complemented with a plasmid (pCGL482-cgO413) carrying the wild-type gene. The different strains were characterized by PCR, and the protein extracts were analyzed by Western blotting with polyclonal anti-CgMytC antibodies. While a single protein band corresponding to CgMytC was observed in the Western blot of the wild-type and CGL2059(pCGL482-cgO413) protein extracts, no such band was seen in the extract of the CGL2059 mutant strain, demonstrating the loss of cg0413 expression (Fig. 1).
Fig 1.

Western blot analysis of protein extracts of C. glutamicum wild-type strain ATCC 13032, the cg0413 mutant (CGL2059), and the complemented cg0413 mutant [CGL2059(pCGL482-cg0413)] with polyclonal anti-CgMytC antibodies.
To determine the role of CgMytC in the O-mycoloylation of small proteins, the lipophilic constituents of both wild-type strain ATCC 13032 and the cg0413 mutant strain were extracted with organic solvents and analyzed directly by MALDI-TOF mass spectrometry. As expected, the signals of the native mycoloylated proteins PorA, PorH, and unknown protein X, observed at m/z 5,207, 6,689, and 4,401, respectively, in the mass spectrum of the extract from the wild-type strain (Fig. 2A), were absent from that obtained for the extract of the CGL2059 mutant strain. The masses of these peaks shifted to m/z 4,703, 6,185, and 3,897, which correspond to the theoretical masses of nonacylated PorA, PorH, and protein X, respectively (Fig. 2B). The mass differences correspond to the loss of C32, C34:1, and C36:2, as previously established (3). Complementation of the CGL2059 mutant strain with the wild-type cg0413 gene, but not with CgmytA or mycobacterial myt genes, fully restored the wild-type phenotype in terms of O-acylation of these proteins (Fig. 2C). It was therefore concluded that CgMytC somehow affects the O-mycoloylation of the small proteins PorA, PorH, and protein X and that its substrate specificity is markedly different from that of the other CgMyts (CgMytA, -B, -D, -E, and -F).
Fig 2.
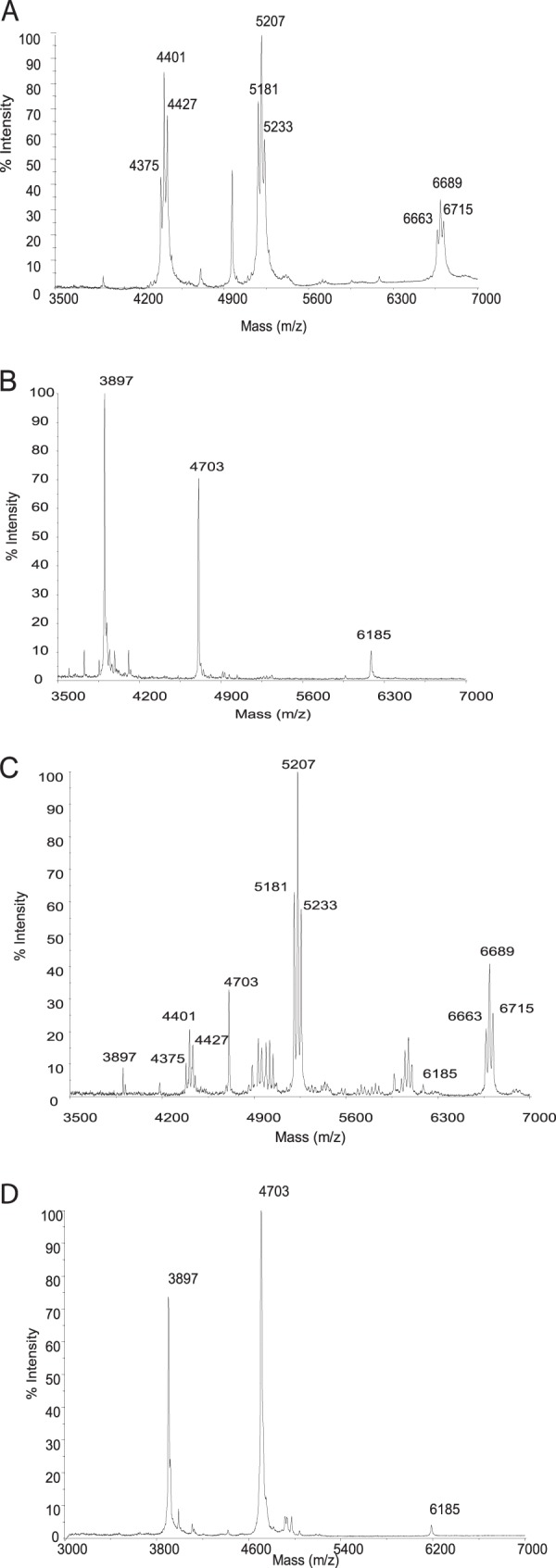
Mass spectra of protein organic extracts. (A) C. glutamicum wild type (ATCC 13032); (B) cg0413 mutant (CGL2059); (C) complemented cg0413 mutant [CGL2059(pCGL482-cg0413)]; (D) cg0413 S189V mutant.
Analysis of the lipid profile of the cg0413 mutant.
The identification of CgMytC as the enzyme that O-acylates small proteins in C. glutamicum prompted us to search for the putative donor of the mycoloyl residue. We reasoned that trehalose monomycolate would be a good candidate because (i) this glycolipid has been shown in an in vitro assay to transfer mycoloyl residues to yield trehalose dimycolate (5), and (ii) an accumulation of TMCM had also been observed in a CgmytA mutant of C. glutamicum CGL 2005. This was due to the decrease of the production of both TDCM and mycoloylated arabinogalactan, indicating that the two mycoloylated compounds use TMCM as their substrate. Accordingly, we comparatively labeled C. glutamicum wild-type strain ATCC 13032 and its cg0413 isogenic mutant strain CGL2059 with [1-14C]palmitate. Their lipids were extracted with organic solvents, and their profiles were examined by TLC to quantify mycoloylated glycolipids, namely, TMCM and TDCM.
As shown in Fig. 3, a marked difference in the TMCM/TDCM ratio between the wild-type strain and cg0413 mutant strain CGL2059 was observed. This ratio was 3-fold higher in the cg0413 mutant than in the wild type (Table 1). The wild-type phenotype was fully restored by complementation. These data are consistent with the hypothesis that TMCM would be the substrate of CgMytC and accumulates in the mutant upon the lack of transfer of mycoloyl residues onto the small proteins.
Fig 3.

TLC of extractable lipids developed in CHCl3-CH3OH-H2O (65:25:4, vol/vol/vol) as the migration solvent after labeling with [14C]palmitate from ATCC 13032 (lane 1), cg0413 mutant strain CGL2059 (lane 2), and complemented strain CGL2059(pCGL482-cg0413) (lane 3).
Table 1.
Lipid quantification of wild-type C. glutamicum and its derivative strainsa
| Strain | Mean % radioactivity ± SD |
Mean TMCM/(TDCM/2) ratio ± SD | ||
|---|---|---|---|---|
| PG | TMCM | TDCM | ||
| C. glutamicum WT | 31.9 ± 1.8 | 3.8 ± 1.3 | 24.1 ± 1.00 | 0.64 ± 0.05 |
| CGL2059 cg0413 mutant | 26.2 ± 2.4 | 17.1 ± 1.3 | 26.3 ± 1.09 | 1.90 ± 0.09 |
| Complemented CGL2059 cg0413 mutant (CgMytC) | 26.6 ± 1.9 | 7.9 ± 0.2 | 20.7 ± 0.9 | 0.77 ± 0.05 |
Cells were labeled with [14C]palmitate for 4 h and then extracted with organic solvents; the extractable lipids were analyzed by radio-TLC, and the radioactivity incorporated into phosphatidylglycerol (PG), trehalose monocorynomycolate (TMCM), and trehalose dicorynomycolate (TDCM) was determined separately. The results are displayed as percentages of the total radioactivity for phosphatidylglycerol, TMCM, and TDCM (one lane for each strain). The molar ratio of TDCM was obtained by dividing the radioactivity measured in TDCM by a factor of 2 (based on the occurrence of 2 molecules of corynomycolates in TDCM, compared to only 1 in TMCM). WT, wild type.
3D structure of CgMytC.
To address the question of the molecular basis of the specificity of CgMytC for polypeptides, we determined the 3D structure of the native form of the protein, which was expressed in the C. glutamicum ΔAftB strain. This strain is derived from ATCC 13032 and has been shown to excrete vesicles containing mycomembrane fragments, including CgMytC (16), a property that facilitates the purification of CgMyt proteins. A summary of the 1.75-Å diffraction data set collected on a native CgMytC crystal is presented in Table 2. A clear molecular replacement solution was obtained by using the structure of Ag85C from M. tuberculosis (PDB accession number 1VA5) as a search model. Four copies of CgMytC are present in the asymmetric unit, but gel filtration chromatography of purified CgMytC showed that the protein is present as a monomer in solution (data not shown).
The overall structure of CgMytC (Fig. 4) is very similar to that of Ag85 (Myt) proteins from M. tuberculosis (with a root mean square deviation [RMSD] of 1.7 Å and a sequence identity of 24.4% for 238 aligned residues between CgMytC and Ag85C). The structure consists of a compact α/β-fold with a central β-sheet consisting of nine mostly parallel strands, with 5 helices being packed on one side and 3 on the opposite side of the central β-sheet. The fold belongs to the α/β-hydrolase superfamily that contains predominantly ester hydrolases. The serine-histidine-glutamate catalytic triad of the active-site pocket of Ag85 is also present in the putative active-site pocket of CgMytC (S189, E310, and H341) (highlighted in Fig. 4). The orientation of the histidine and glutamate of the active site, however, is slightly different in CgMytC (green) compared to that in Ag85 (yellow) (Fig. 4B).
Fig 4.

(A) Superposition of CgMytC (in green) with M. tuberculosis Ag85C (PDB accession number 1VA5) (in yellow). The secondary structures bordering the flexible loop (β-7 and α-10) and the additional helix (η2) in the loop of CgMytC are indicated. The disordered loop contained between residues 276 and 299 is represented by a broken line, and the 61 amino acids at the N-terminal extension compared to the antigen 85 proteins are represented in red. (B) Superposition of catalytic triads of Ser126, Glu230, and His262 from Ag85C (in yellow) and Ser189, Glu310, and His341 from CgMytC (in green).
CgMytC has an extension of 61 amino acids at the N terminus compared to the Ag85 proteins, which forms 2 short helices and a β-strand separated by irregular peptide stretches. The β-strand is integrated into the central β-sheet, which is therefore larger than that found in the Ag85 proteins (Ag85A, Ag85B, and Ag85C) (Fig. 4 and 5). A second significant difference between the structures of CgMytC and Ag85C is the occurrence of an insertion loop of 19 residues in the flexible segment (residues between β-strand 7 and α-helix 10) of CgMytC. Part of this forms an additional helix (η2) in the structure of CgMytC (Fig. 4 and 5). We hypothesized that the presence of this loop in CgMytC and the modified orientations of surrounding secondary structure elements compared to Ag85C may be related to the peptide ligand specificity of the protein.
Fig 5.
Multiple-sequence alignment of CgMytC orthologues. Alignment was made by using the ClustalW (26) and ESPript (27) programs. Stars indicate amino acids from the catalytic triad of Ser189, Glu310, and His341 (numbering from the CgMytC sequence). The secondary structure was deduced from the crystal structures (PDB accession number 1DQY and this work) and is represented above the sequence alignment. The insertion loop is indicated by a box. MytC from C. glutamicum (CgMytC), Myt from C. efficiens (CE0356), Myt from C. diphtheriae (DIP0365), Myts from R. erythropolis (RER_15360 and RER_15370), Myt from N. farcinica (nfa7210), Myt from G. bronchialis (Gbro_0876), Ag85C from M. tuberculosis (sequence obtained from the structure of Ag85C, chain A, under PDB accession number 1DQY), Ag85B from M. tuberculosis (sequence obtained from the structure of Ag85C, chain A, under PDB accession number 1F0P), and FbpC1 (sequence obtained from the structure of MPT51, chain A, under PDB accession number 1R88). The residues from CgMytC not observed in the crystal structure are in cyan.
Directed mutation of a predicted catalytic residue of CgMytC.
To check the functionality of the putative catalytic triad and the implication of CgMytC in the O-acylation of PorA and PorH, the codon of putative catalytic serine 189 was changed into a valine codon. The protein was well expressed but lacked all semblance of mycoloyltransferase activity for the resulting CgMytC, as revealed by MALDI-TOF mass spectrometry analysis (Fig. 2D). These data identified S189 as a catalytic amino acid and established its implication in catalysis.
Search for orthologous proteins of CgMytC.
We searched for CgMytC orthologues by using a NCBI BLASTP program, restricted to the Corynebacteriales. The key features retained for filtering were the insertion sequences and the different amino acids for substrate binding because we considered these to be crucial for mycoloylation of peptide substrates. Figure 5 shows the alignment of the best-scoring putative CgMytC orthologues in other corynebacteria, namely, C. efficiens (protein CE0356) and C. diphtheriae (DIP0365), which possess amino acid identities of 99 and 98%, respectively. This is fully consistent with our previous demonstration of the presence of mycoloylated PorA in both species (3). In addition, orthologous proteins were also found in other Corynebacteriales, which all contain the insertion sequences that typify CgMyt. The bacterial species include Nocardia farcinica, Gordonia bronchialis, and all the sequenced Rhodococcus strains, notably Rhodococcus erythropolis (proteins RER_15360 and RER_15370).
In order to find out whether these in silico-identified orthologous proteins really exhibit an O-acylation activity, the corresponding genes (DIP0365, nfa7210, Gbro_0876, RER_15360, and RER_15370) were cloned into an expression vector and introduced into the CGL2059 CgMytC mutant strain for complementation. In order to help cell envelope localization, the vectors express fusion proteins that possess the signal sequence and the original N-terminal 7-amino-acid sequence of mature CgMytC, except for the 6th (Ala) residue, which is replaced by Ile. Cells of recombinant strains were first checked for the production of the orthologous proteins and then extracted with organic solvents for isolating the lipophilic extracts that were analyzed by MALDI-TOF mass spectrometry. All the recombinant proteins are well expressed in the envelope of CGL2059, except for those from Gordonia (data not shown). The orthologous proteins from both C. diphtheriae (DIP0365) and R. erythropolis (RER_15370) (Fig. 6), but not that from N. farcinica, were able to complement the CgMytC mutant, as judged by the analysis of the mass spectra, which showed mass peaks corresponding to mycoloylated PorA and PorH.
Fig 6.

Mass spectra of protein organic extract. (A) C. glutamicum cg0413 mutant complemented with DIP0365; (B) C. glutamicum cg0413 mutant complemented with RER_15370.
Concluding remarks.
We recently discovered in C. glutamicum a third class of acceptors of mycoloyl residues, namely, polypeptides. Although the role of this modification is still unclear, we have demonstrated that two small proteins of C. glutamicum, PorA and PorH, that combine themselves to form a mycomembrane pore require this modification to be active. This O-mycoloylation was also demonstrated for other corynebacterial species that contain PorA orthologues, i.e., C. diphtheriae and C. efficiens (3). Consequently, it is tempting to postulate that this unprecedented lipid modification of bacterial polypeptides would have at least a role in the structuration and the stability of the mycomembrane of the corynebacterial cell wall. In the present paper, we addressed the questions of both the identity and the specificity of the CgMyt putatively involved in the posttranslational modification of small proteins of corynebacteria.
Inactivation of the cg0413 gene resulted in the total absence of mycoloylation of polypeptides, whereas complementation of the mutant with the wild-type gene fully restored the wild-type phenotype. Furthermore, complementation of the cg0413 mutant with selective orthologous genes from defined bacterial species belonging to the order Corynebacteriales also restored the wild-type phenotype. For the bacterial genera whose orthologous genes do not complement the Corynebacterium mutant, we hypothesize that this may be due to the too-long chain lengths of mycolic acids from Nocardia (C50 to C62) compared to those from Rhodococcus (C30 to C54), which produce chain lengths similar to those of corynebacteria (C30 to C36) (25). Although O-acylated proteins other than PorA, PorH, and the companion unknown small protein may exist in corynebacteria and related genera, they are difficult to predict. We therefore need to develop novel strategies to identify these putative mycoloylated proteins. As expected from their sequences and mycoloyltransferase activities on trehalose and arabinogalactan, the 3D structure of CgMytC is very similar to those of the Ag85 (Myt) proteins from M. tuberculosis. Interestingly, a loop insertion in CgMytC, absent from Ag85A, -B, and -C, may be responsible for the peptide substrate specificity of the protein.
ACKNOWLEDGMENTS
We thank N. Lazar (IBBMC, Orsay, France) for help in CgMytC purification. We thank the PROXIMA 1 staff and especially B. Guimaraes for help with synchrotron data collection.
This work was supported by French Infrastructure for Integrated Structural Biology (FRISBI) grant ANR-10-INSB-05-01.
Footnotes
Published ahead of print 12 July 2013
REFERENCES
- 1.Portevin D, De Sousa-D' Auria C, Houssin C, Grimaldi C, Chami M, Daffe M, Guilhot C. 2004. A polyketide synthase catalyzes the last condensation step of mycolic acid biosynthesis in mycobacteria and related organisms. Proc. Natl. Acad. Sci. U. S. A. 101:314–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tropis M, Meniche X, Wolf A, Gebhardt H, Strelkov S, Chami M, Schomburg D, Kramer R, Morbach S, Daffe M. 2005. The crucial role of trehalose and structurally related oligosaccharides in the biosynthesis and transfer of mycolic acids in Corynebacterineae. J. Biol. Chem. 280:26573–26585 [DOI] [PubMed] [Google Scholar]
- 3.Huc E, Meniche X, Benz R, Bayan N, Ghazi A, Tropis M, Daffe M. 2010. O-mycoloylated proteins from Corynebacterium: an unprecedented post-translational modification in bacteria. J. Biol. Chem. 285:21908–21912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rath P, Demange P, Saurel O, Tropis M, Daffe M, Dotsch V, Ghazi A, Bernhard F, Milon A. 2011. Functional expression of the PorAH channel from Corynebacterium glutamicum in cell-free expression systems: implications for the role of the naturally occurring mycolic acid modification. J. Biol. Chem. 286:32525–32532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belisle JT, Vissa VD, Sievert T, Takayama K, Brennan PJ, Besra GS. 1997. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science 276:1420–1422 [DOI] [PubMed] [Google Scholar]
- 6.Kremer L, Maughan WN, Wilson RA, Dover LG, Besra GS. 2002. The M. tuberculosis antigen 85 complex and mycolyltransferase activity. Lett. Appl. Microbiol. 34:233–237 [DOI] [PubMed] [Google Scholar]
- 7.Puech V, Guilhot C, Perez E, Tropis M, Armitige LY, Gicquel B, Daffe M. 2002. Evidence for a partial redundancy of the fibronectin-binding proteins for the transfer of mycoloyl residues onto the cell wall arabinogalactan termini of Mycobacterium tuberculosis. Mol. Microbiol. 44:1109–1122 [DOI] [PubMed] [Google Scholar]
- 8.Brand S, Niehaus K, Puhler A, Kalinowski J. 2003. Identification and functional analysis of six mycolyltransferase genes of Corynebacterium glutamicum ATCC 13032: the genes cop1, cmt1, and cmt2 can replace each other in the synthesis of trehalose dicorynomycolate, a component of the mycolic acid layer of the cell envelope. Arch. Microbiol. 180:33–44 [DOI] [PubMed] [Google Scholar]
- 9.De Sousa-D'Auria C, Kacem R, Puech V, Tropis M, Leblon G, Houssin C, Daffe M. 2003. New insights into the biogenesis of the cell envelope of corynebacteria: identification and functional characterization of five new mycoloyltransferase genes in Corynebacterium glutamicum. FEMS Microbiol. Lett. 224:35–44 [DOI] [PubMed] [Google Scholar]
- 10.Kacem R, De Sousa-D'Auria C, Tropis M, Chami M, Gounon P, Leblon G, Houssin C, Daffe M. 2004. Importance of mycoloyltransferases on the physiology of Corynebacterium glutamicum. Microbiology 150:73–84 [DOI] [PubMed] [Google Scholar]
- 11.Dusch N, Puhler A, Kalinowski J. 1999. Expression of the Corynebacterium glutamicum panD gene encoding L-aspartate-alpha-decarboxylase leads to pantothenate overproduction in Escherichia coli. Appl. Environ. Microbiol. 65:1530–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonamy C, Guyonvarch A, Reyes O, David F, Leblon G. 1990. Interspecies electro-transformation in Corynebacteria. FEMS Microbiol. Lett. 54:263–269 [DOI] [PubMed] [Google Scholar]
- 13.Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. 1987. Current protocols molecular biology. Wiley, New York, NY [Google Scholar]
- 14.Peyret JL, Bayan N, Joliff G, Gulik-Krzywicki T, Mathieu L, Schechter E, Leblon G. 1993. Characterization of the cspB gene encoding PS2, an ordered surface-layer protein in Corynebacterium glutamicum. Mol. Microbiol. 9:97–109 [DOI] [PubMed] [Google Scholar]
- 15.Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 16.Bou Raad R, Meniche X, de Sousa-d'Auria C, Chami M, Salmeron C, Tropis M, Labarre C, Daffe M, Houssin C, Bayan N. 2010. A deficiency in arabinogalactan biosynthesis affects Corynebacterium glutamicum mycolate outer membrane stability. J. Bacteriol. 192:2691–2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kabsch W. 2010. Xds. Acta Crystallogr. D Biol. Crystallogr. 66:125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. 2007. Phaser crystallographic software. J. Appl. Crystallogr. 40:658–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mooij WT, Cohen SX, Joosten K, Murshudov GN, Perrakis A. 2009. “Conditional restraints”: restraining the free atoms in ARP/wARP. Structure 17:183–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murshudov GN, Vagin AA, Dodson EJ. 1997. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53:240–255 [DOI] [PubMed] [Google Scholar]
- 21.Perrakis A, Harkiolaki M, Wilson KS, Lamzin VS. 2001. ARP/wARP and molecular replacement. Acta Crystallogr. D Biol. Crystallogr. 57:1445–1450 [DOI] [PubMed] [Google Scholar]
- 22.Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. 2010. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66:213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adindla S, Guruprasad K, Guruprasad L. 2004. Three-dimensional models and structure analysis of corynemycolyltransferases in Corynebacterium glutamicum and Corynebacterium efficiens. Int. J. Biol. Macromol. 34:181–189 [DOI] [PubMed] [Google Scholar]
- 24.Ramulu HG, Adindla S, Guruprasad L. 2006. Analysis and modeling of mycolyl-transferases in the CMN group. Bioinformation 1:161–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins MD, Goodfellow M, Minnikin DE. 1982. A survey of the structures of mycolic acids in Corynebacterium and related taxa. J. Gen. Microbiol. 128:129–149 [DOI] [PubMed] [Google Scholar]
- 26.Higgins DG, Thompson JD, Gibson TJ. 1996. Using CLUSTAL for multiple sequence alignments. Methods Enzymol. 266:383–402 [DOI] [PubMed] [Google Scholar]
- 27.Gouet P, Robert X, Courcelle E. 2003. ESPript/ENDscript: extracting and rendering sequence and 3D information from atomic structures of proteins. Nucleic Acids Res. 31:3320–3323 [DOI] [PMC free article] [PubMed] [Google Scholar]



