Abstract
Purpose
Nanoparticles based on stimuli-sensitive drug delivery have been extensively investigated for tumor targeting. Among them, pH-responsive drug targeting using pH-sensitive polymers has attracted attention because solid tumors have an acidic environment. A dextran-b-poly(L-histidine) (DexPHS) copolymer was synthesized and pH-responsive nanoparticles were fabricated for drug targeting.
Methods and results
A DexPHS block copolymer was synthesized by attaching the reductive end of dextran to the amine groups of poly(L-histidine). pH-responsive nanoparticles incorporating doxorubicin were fabricated and studied in HuCC-T1 cholangiocarcinoma cells. Synthesis of DexPHS was confirmed by 1H nuclear magnetic resonance spectroscopy, with specific peaks of dextran and PHS observed at 2–5 ppm and 7.4–9.0 ppm, respectively. DexPHS nanoparticles showed changes in particle size with pH sensitivity, ie, the size of the nanoparticles increased at an acidic pH and decreased at a basic pH. DexPHS block copolymer nanoparticles incorporating doxorubicin were prepared using the nanoprecipitation dialysis method. The doxorubicin release rate was increased at acidic pH compared with basic pH, indicating that DexPHS nanoparticles have pH-sensitive properties and that drug release can be controlled by variations in pH. The antitumor activity of DexPHS nanoparticles incorporating doxorubicin were studied using HuCC-T1 cholangiocarcinoma cells. Viability was decreased in cells treated with nanoparticles at acidic pH, whereas cell viability in response to treatment with doxorubicin did not vary according to changes of pH.
Conclusion
Our results indicated that DexPHS polymeric micelles are promising candidates for antitumor drug targeting.
Keywords: pH-responsive drug targeting, nanoparticles, block copolymer, poly(L-histidine), dextran
Introduction
The microenvironment of solid tumors is quite different from that of normal tissues, and is normally characterized as having poor perfusion, a high metabolic rate, and an acidic pH.1–3 In particular, the highly acidic environment of tumor tissue is related to tumor progression, metastatic potential, increased migration, and local invasion.1–4 Low extracellular pH in the tumor microenvironment also promotes expression of specific enzymes related to tumor cell migration and invasion, such as vascular endothelial growth factor, cathepsin B, matrix metalloproteinases-2/9, and carbonic anhydrase.5–9 Further, the acidic environment of tumor tissue is known to form a physiologic barrier against weak base chemotherapeutic drugs and induce drug resistance.5,10
Targeted drug delivery systems based on stimuli-sensitive pathways such as temperature, pH, and magnetic field have been extensively investigated over the past two decades.11–13 Among these, nanoparticles or colloidal drug carriers based on pH-responsive systems have been highlighted due to their targetability to a specific site of action.13–15 Given that the cellular endosome has an acidic environment, pH-sensitive colloidal carriers are promising vehicles for intracellular delivery of bioactive agents, such as genetic drugs.14,15 Among them, polymeric nanoparticles based on histidine derivatives or poly(L-histidine) (PHS) are a promising vehicle for intracellular drug delivery due to the fact that the imidazole group on histidine is normally ionized at acidic pH and then expresses cationic properties.14–16 pH-responsive nanoparticles are regarded as an ideal carrier for tumor-specific targeting because tumor tissue has a strong acidic environment compared with normal tissues,17,18 ie, pH-sensitive nanoparticles are able to release their cargo in the acidic environment of tumor tissue and induce specific death of tumor cells. Putnam et al reported that polyhistidine-poly(ethylene glycol) (PEG) conjugates have low cytotoxicity against macrophage cells and that their nanocomplexes with DNA have similar transfection efficiency compared with DNA-polylysine complexes.16 Liu et al reported that pH-sensitive nanoparticles containing PEG-PHS-poly(L-lactic acid) block copolymer showed pH-responsive drug release behavior and enhanced anticancer activity at acidic pH.18 In our previous work, we have reported that PHS-tagged photoactivatable drug has pH-sensitive phototoxicity against HCT 116 human colon cancer cells.19 Further, we showed that nanoparticles of poly(2-hydroxyethyl methacrylate)-PHS block copolymer release doxorubicin in a pH-sensitive manner and that uptake of nanoparticles incorporating doxorubicin by tumor cells was enhanced at acidic pH.20
In this study, we synthesized a block copolymer composed of dextran and PHS (DexPHS) to fabricate a pH-sensitive drug delivery system for tumor targeting. Given that dextran and PHS are fully biocompatible, DexPHS block copolymer may have advantages as a biocompatible agent compared with a previously synthesized one.20 Further, dextran has abundant functional groups, including a hydroxyl group, and these functional groups can be used for further modification. DexPHS nanoparticles incorporating doxorubicin were prepared by nanoprecipitation and the dialysis method. The pH-responsive drug targeting capacity of DexPHS nanoparticles was studied in HuCC-T1 human cholangiocarcinoma cells.
Materials and methods
Materials
Dextran (molecular weight 6,000 g/mol), triethylamine, sodium cyanoborohydride, and thiazolyl blue tetrazolium bromide (MTT) were purchased from Sigma Chemical Company (St Louis, MO, USA). Dialysis membranes with molecular weight cutoffs of 8,000 g/mol and 15,000 g/mol were purchased from Spectrum Laboratories Inc (Rancho Dominguez, CA, USA). Dimethylsulfoxide was of high-performance liquid chromatography grade or extra pure grade. Two kinds of PHS was purchased from Sigma Chemical Co and used for synthesis of the block copolymer (molecular weight approximately 5,000 g/mol and 5,000–25,000 g/mol). Doxorubicin HCl was purchased from L C Laboratories (Woburn, MA, USA).
Synthesis and characterization of DexPHS block copolymer
DexPHS block copolymers were synthesized as described previously.21 Dextran 60 mg was dissolved in dimethylsulfoxide, and sodium cyanoborohydride (62.8 mg, 1 mM) was added. These mixtures were stirred for 24 hours at room temperature. Next, 50 mg or 100 mg of PHS was dissolved in solvent mixtures of dimethylsulfoxide/0.1 N HCl solution and then added to the dextran solution. The resulting solution was then stirred for 2 days. The solution was then poured into a dialysis tube (molecular weight cutoff 8,000 g/mol or 15,000 g/mol). The dialysis procedure was used to remove unreacted dextran or PHS, and was performed with 0.01 N HCl solution for the first 6 hours to remove unreacted PHS, and dialysis was then continued for 2 days against deionized water. The deionized water or 0.01 N HCl solution was exchanged at 2-hourly intervals to prevent saturation. The dialyzed solution was then lyophilized for 3 days and kept at −20°C until analysis or nanoparticle fabrication. The yield of DexPHS block copolymer was higher than 82% (w/w) for DexPHS-1 and 73% for DexPHS-2. The synthesized DexPHS block copolymer was analyzed using 1H nuclear magnetic resonance (NMR) spectra (500 mHz superconducting FT-NMR spectrometer, Unity Inova 500 MHz NB high resolution FT NMR, Varian Inc, Santa Clara, CA, USA).
The molecular weight and number-average molecular weight of dextran were measured by gel permeation chromatography as reported prevoiously.21 The molecular weight, number-average molecular weight, and polydispersity index of dextran was 4,800, 4,370, and 1.098, respectively. Based on the molecular weight of dextran, the molecular weight of PHS was evaluated by specific peaks of dextran and PHS from 1H NMR spectroscopy.
Preparation and characterization of DexPHS nanoparticles
First, 50 mg of DexPHS was dissolved in 3 mL of dimethylsulfoxide. Doxorubicin was separately dissolved in 1 mL of dimethylsulfoxide with 10 μL of triethylamine. Doxorubicin in dimethylsulfoxide was mixed with DexPHS solution and then stirred for 3 hours. Next, this solution was slowly dropped into phosphate-buffered saline (pH 7.4, 0.01 M) and dialyzed against deionized water for one day. Water was exchanged at intervals of 3 hours. The dialyzed solution was then used to analyze or lyophilize. To measure drug content and loading efficiency, 5 mg of lyophilized nanoparticles were dispersed into 1 mL of deionized water and then mixed with 9 mL of dimethylsulfoxide. Drug content and loading efficiency were measured using an ultraviolet spectrophotometer (UV-1801, Shimadzu Corporation Kyoto, Japan) at 479 nm.
Characterization of nanoparticles
The morphology of the nanoparticles was observed using a field-emission scanning electron microscope (JEM-2000 FX II, JEOL, Tokyo, Japan). The size of the nanoparticles was measured by dynamic light scattering (DLS-7000, Otsuka Electronics Company, Osaka, Japan). A sample solution (concentration 1 mg/mL) was used to determine particle size.
Drug-release study
Drug release from the DexPHS nanoparticles was carried out in solutions at various pH levels. First, 5 mg of nanoparticles were reconstituted in 5 mL of deionized water and then introduced into a dialysis tube. The dialysis tube was introduced into a 50 mL Falcon tube with 40 mL of various pH buffer solutions. These tubes were placed in a shaking incubator with a stirring speed of 100 rpm at 37°C. At specific time intervals, the medium was sampled for analysis of drug concentration. Afterwards, the entire medium was replaced with fresh medium to prevent drug saturation. The concentration of drug released into the release medium was determined by ultraviolet spectrophotometry (UV-1801, Shimadzu Corporation) at 479 nm.
Cell culture
The HuCC-T1 human cholangiocarcinoma cells used in this study were maintained with Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% fetal bovine serum and 1% antibiotics in a 5% CO2 incubator at 37°C. NIH3T3 mouse fibroblast cells maintained in Dulbecco’s Modified Eagle’s Medium (supplemented with 10% fetal bovine serum and 1% antibiotics) were used to assess the intrinsic toxicity of the DexPHS copolymer.
The anticancer activity of DexPHS nanoparticles incorporating doxorubicin was evaluated in HuCC-T1 cells using an MTT cell proliferation assay. The HuCC-T1 cells were seeded at a density of 3 × 104 cells per well in 96-well plates with 100 μL of medium and incubated overnight in a 5% CO2 incubator at 37°C. Next, the cells were exposed to free doxorubicin or DexPHS nanoparticles incorporating doxorubicin (final concentration of doxorubicin was 5.0 μg/mL) for 6 hours at various pHs. After that, the medium was replaced with fresh serum-free RPMI 1640 and incubated further for 24 hours. Controls were treated with 0.5% v/v dimethylsulfoxide. Next, 30 μL of MTT (5 mg/mL) was added to the 96-well plates and incubated for 4 hours. The formazan crystals formed in living cells were solubilized with dimethylsulfoxide and the absorbance (560 nm test/630 nm reference) was determined using an automated computer-linked microplate reader (Molecular Device Company, Sunnyvale, CA, USA).
The cytotoxicity of DexPHS in HuCC-T1 cholangiocarcinoma cells and NIH3T3 mouse fibroblast cells was assessed. Next, 3 × 104 cells were seeded into each well of a 96-well plate and incubated overnight in a 5% CO2 incubator at 37°C. Empty DexPHS nanoparticles were then reconstituted in serum medium and added to the cells. One day later, cell viability was assessed using the MTT method described above.
Observation of cells
HuCC-T1 cells seeded into a coverglass-embedded six-well plate were treated with doxorubicin or nanoparticles at various pHs for one hour. The free doxorubicin and nanoparticle doxorubicin concentrations were equivalent to 1 μg/mL. Next, the cells were washed with phosphate-buffered saline (pH 7.4, 0.1 M) and treated with 4% paraformaldehyde. The cells were then washed with phosphate-buffered saline once more and fixed with immobilization solution (ImmuMount, Thermo Electron Corporation, Pittsburgh, PA, USA). The cells were observed with a confocal laser scanning microscope (TCS-SP2, Leica, Wetzlar, Germany).
Flow cytometry analysis
HuCC-T1 cells were seeded at a density of 1 × 106 cells in six-well plates and incubated overnight. HuCC-T1 cells were exposed to free doxorubicin or DexPHS nanoparticles incorporating doxorubicin at various pHs for one hour. The cells were then trypsinized and harvested to analyze fluorescence intensity using a flow cytometer.
Results
Characterization of DexPHS block copolymer and its nanoparticles
DexPHS block copolymer was synthesized by treatment of the reductive end of dextran with sodium cyanoborohydride as shown in Figure 1A. Because the PHS has an amine group at the end of the chain, the reductive end of dextran was conjugated with the amine end group of PHS. As shown in Figure 1B, specific peaks of dextran were observed at 2–5 ppm while specific peaks of PHS were observed at 7.4–9.0 ppm. The molecular weight of dextran is known, so the number-average molecular weight of PHS was evaluated from 1H NMR spectroscopy by comparing the specific peaks of PHS (9.0 ppm) and dextran (4.8 ppm). The calculated molecular weights of the DexPHS copolymers were abbreviated as shown in Table 1. Two types of DexPHS block copolymer were synthesized using different molecular weights of PHS as shown in Table 1.
Figure 1.
Synthesis scheme (A) and 1H nuclear magnetic resonance spectra (B) of DexPHS block copolymer.
Abbreviations: DexPHS, dextran-b-poly(L-histidine); TEA, triethylamine.
Table 1.
Characterization of DexPHS block copolymer using 1H nuclear magnetic resonance spectroscopy
| Histidine repeating unit
|
Estimated MW of DexPHS block copolymer | ||
|---|---|---|---|
| Theoreticala | Estimatedb | ||
| DexPHS-1 | 36 | 42 | 10,600 |
| DexPHS-2 | 36–181 | 91 | 17,400 |
Notes: MW of dextran was derived from Chilkoti et al.11 Weight-average MW, number-average MW, and polydispersity index of dextran was 4,800, 4,370, and 1.098, respectively. MW of DexPHS block copolymer was estimated from the known MW of dextran (MW 4,800).
Theoretical number of histidine repeating units of PHS homopolymer was calculated from the manufacturer’s information;
estimated amount of histidine was calculated from DexPHS block copolymer.
Abbreviations: MW, molecular weight; DexPHS, dextran-b-poly(L-histidine).
To fabricate pH-sensitive nanoparticles, DexPHS block copolymer dissolved in dimethylsulfoxide was added to phosphate-buffered saline (pH 7.4) and the solvent was removed by dialysis. As shown in Figure 2, the size of the DexPHS-1 nanoparticles was less than 200 nm at pH 7.4, while their sizes increased to higher than 300 nm at pH 6.0, as shown in Figure 2B. Scanning electron microscopic images of the nanoparticles supported this phenomenon, ie, nanoparticle size was increased or aggregated at acidic pH. Changes in DexPHS nanoparticle size according to pH variation can be explained as shown in Figure 2A, ie, the PHS chain shrinks at a basic pH but swells or becomes partially dissociated at an acidic pH. These results indicate that DexPHS block copolymer can form self-aggregates at basic pHs and PHS block in these self-aggregates can dissociate or swell in acidic pHs. Figure 3 shows the particle size change according to variation in the pH of the solution. As shown in Figure 3, the particle size gradually increased with decreasing pH. The size of the DexPHS-2 nanoparticles increased by at least five-fold at an acidic pH compared with particle size at a basic pH. These results indicate that DexPHS nanoparticles are pH-sensitive and swell or become dissociated at acidic pH. DexPHS-1 showed slightly smaller particle sizes than DexPHS-2 (Figure 3). However, the DexPHS-2 size changes at acidic pHs were greater than for DexPHS-1. These results might be due to the fact that DexPHS-2 has a much longer PHS chain length than DexPHS-1 and that a longer PHS chain forms a stronger inner core than a shorter PHS. Otherwise, the changes in particle size for the longer PHS chain length (DexPHS-2) were greater in an acidic environment than for the shorter PHS chain length (DexPHS-1). DexPHS-1 and DexPHS-2 nanoparticles became completely dissociated at a pH less than 4 (data not shown). These results indicate that the PHS core in the DexPHS nanoparticle swells in an acidic environment and becomes dissociated under extremely high acidic conditions. Figure 3B showed the particle size changes for DexPHS nanoparticles in serum-containing medium. As shown in Figure 3B, neither the DexPHS-1 nanoparticles nor the DexPHS-2 nanoparticles showed significantly different results compared to those of deionized water. Particle sizes in deionized water (Figure 3A) and serum-containing medium (Figure 3B) were not changed significantly 3 days later, indicating that DexPHS nanoparticles were stable in the various aqueous solutions.
Figure 2.
Schematic illustration of changes in particle size by pH variations (A). Typical particle size distribution of DexPHS-1 nanoparticles at pH 6.0 and pH 7.4 (B). Morphologic observation of DexPHS-1 nanoparticles according to pH variation (C).
Abbreviation: DexPHS, dextran-b-poly(L-histidine).
Figure 3.

DexPHS nanoparticle size changes according to changes of pH in deionized water (A) and RPMI 1640 medium (10% fetal bovine serum) (B). For particle size measurement in serum-containing medium, a nanoparticle solution in water was diluted with RPMI medium (10% fetal bovine serum) at various pHs.
Abbreviations: DexPHS, dextran-b-poly(L-histidine); RPMI, Roswell Park Memorial Institute.
Doxorubicin was used as a model drug to investigate pH-sensitive drug release from the nanoparticles. As shown in Table 2, doxorubicin was encapsulated into the nanoparticles with higher than 60% loading efficiency. Although there was no marked difference between DexPHS-1 and DexPHS-2, the particle size of DexPHS-2 was slightly larger than that of DexPHS-1, whereas empty DexPHS-2 nanoparticles were smaller than DexPHS-1 nanoparticles under neutral or basic pH conditions. These results might be due to the fact that the drug content of the DexPHS-2 nanoparticles was slightly higher than that of the DexPHS-1 nanoparticles and that higher drug contents induce larger particle sizes. The doxorubicin release study was performed at various pH conditions as shown in Figure 4. The doxorubicin release rate from both Dex-PHS-1 and DexPHS-2 was increased at acidic pH and delayed at basic pH. Drug release from DexPHS-1 at pH 6.0 was about 1.8-fold faster than that at pH 7.4. These results indicate that DexPHS nanoparticles have a capacity for pH-controlled drug release. The drug release rate from DexPHS-1 was slightly faster than that from DexPHS-2. These results might be due to differences in particle diameter and drug content, ie, the reduced particle size of DexPHS-1 nanoparticles induces more rapid drug release than DexPHS-2 nanoparticles because small nanoparticles have a larger surface area than larger ones and release their drug content more rapidly. The increased drug content could also affect the drug release rate, ie, hydrophobic drugs in nanoparticles frequently aggregate at a higher drug content and the aggregated drug can be released more slowly from nanoparticles.20,21
Table 2.
Characterization of DexPHS nanoparticles incorporating doxorubicin
| Polymer/drug weight ratio (mg/mg)a | Drug content (%, w/w) | Loading efficiency (%, w/w) | Particle size (nm) | |
|---|---|---|---|---|
| DexPHS-1 | 50/5.4 | 6.0 | 64 | 156 |
| DexPHS-2 | 50/5.4 | 6.4 | 68 | 178 |
Notes:
Doxorubicin HCl (5.4 mg) was of the equivalent concentration to doxorubicin when HCl salt was removed. Drug content and loading efficiency were calculated based on doxorubicin.
Abbreviation: DexPHS, dextran-b-poly(L-histidine).
Figure 4.

Effect of pH on drug release from DexPHS nanoparticles. (A) Rate of doxorubicin release from DexPHS-1 nanoparticles. (B) Rate of doxorubicin release from DexPHS-2 nanoparticles.
Abbreviation: DexPHS, dextran-b-poly(L-histidine).
ph sensitivity of DexPHS nanoparticles in tumor cells
HuCC-T1 cholangiocarcinoma cells were used to investigate the pH sensitivity of DexPHS nanoparticles in vitro. Figure 5 shows fluorescence images for HuCC-T1 cells after a one-hour incubation with free doxorubicin and DexPHS-2 nanoparticles incorporating doxorubicin. Because doxorubicin shows a strong red fluorescence color, DexPHS-2 nanoparticles incorporating doxorubicin were used to investigate fluorescence. As shown in Figure 5, tumor cells treated with free doxorubicin showed an increased red color with increasing pH, ie, doxorubicin was taken up more easily at a basic pH than at an acidic pH. However, DexPHS nanoparticles showed a stronger red color at acidic pHs than at basic pHs, indicating that uptake of DexPHS nanoparticles incorporating doxorubicin was greater at acidic pHs. Figure 6 supports these results. Quantitative analysis of uptake of free doxorubicin and nanoparticles incorporating doxorubicin was measured by flow cytometry. As shown in Figure 6, the fluorescence intensity for treatment with DexPHS nanoparticles incorporating doxorubicin was higher at acidic pHs than at basic pHs, while free doxorubicin showed higher fluorescence intensity at basic pHs, indicating that uptake of nanoparticles was higher at acidic pHs than at basic pHs. These results indicate that DexPHS nanoparticles have potential for pH-responsive targeting of tumor cells.
Figure 5.
Fluorescence microscopic images of HuCC-T1 cells after one hour of incubation with free doxorubicin or DexPHS-2 nanoparticles incorporating doxorubicin at various pHs.
Abbreviations: DOX, doxorubicin; DexPHS, dextran-b-poly(L-histidine).
Figure 6.
Flow cytometric analysis of HuCC-T1 cells after one hour of incubation with doxorubicin or DexPHS-2 nanoparticles incorporating doxorubicin at various pHs. In the bar graph, the values are from three different flow cytometry experiments. The y-axis represents the extent of the P2 region.
Abbreviations: DOX, doxorubicin; DexPHS, dextran-b-poly(L-histidine).
Figure 7 showed the pH-sensitive cytotoxicity of free doxorubicin and DexPHS nanoparticles incorporating doxorubicin against HuCC-T1 human cholangiocarcinoma cells. As shown in Figure 7, viability of cells treated with free doxorubicin at acidic pHs was relatively greater at acidic pHs than at basic pHs. However, DexPHS nanoparticles incorporating doxorubicin showed higher cytotoxicity at acidic pHs than at basic pHs, indicating that DexPHS nanoparticles have potential pH-responsive antitumor activity.
Figure 7.

Effect of pH variations on the viability of tumor cells. 3 × 104 hucc-T1 cells were treated with doxorubicin or DexPHS-2 nanoparticles incorporating doxorubicin for 6 hours and then replaced with fresh medium. Twenty-four hours later, viable cells were measured by MTT assay.
Abbreviations: DOX, doxorubicin; DexPHS, dextran-b-poly(L-histidine); MTT, thiazolyl blue tetrazolium bromide.
Intrinsic toxicity of DexPHS nanoparticles in tumor cells and normal cells
The intrinsic toxicity of empty DexPHS nanoparticles was assessed in both tumor cells and normal cells (Figure 8). Figure 8A shows that the viability of HuCC-T1 cells were not significantly affected by treatment with empty DexPHS nanoparticles, ie, more than 84% of cells treated with 100 μg/mL empty DexPHS-1 and DexPHS-2 nanoparticles survived. Further, both empty DexPHS-1 and DexPHS-2 copolymer nanoparticles did not significantly inhibit viability of NIH3T3 cells, which was higher than 87%. These results indicate that the DexPHS copolymer itself did not have an acute toxic effect in tumor cells or normal cells even at a concentration of 100 μg/mL.
Figure 8.
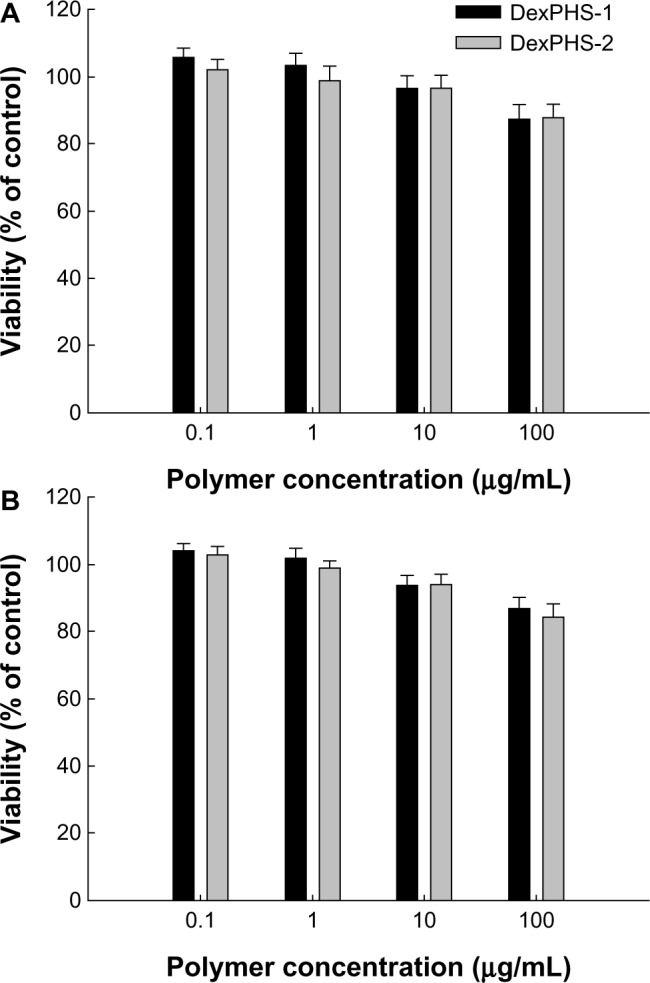
Intrinsic toxicity of DexPHS nanoparticles against HuCC-T1 cells (A) and NIH3T3 cells (B). Cells were treated with empty DexPHS-1 or DexPHS-2 nanoparticles for one day and their viability was evaluated with MTT assay.
Abbreviations: DOX, doxorubicin; DexPHS, dextran-b-poly(L-histidine); MTT, thiazolyl blue tetrazolium bromide.
Discussion
Stimuli-sensitive delivery vehicles using temperature-sensitive or pH-sensitive polymers have been investigated for targeting the tumor cellular endosome and microenvironment.11–20 For example, thermally responsive carriers such as poly(N-isopropylacrylamide-co-acrylamide)-derived copolymers can control drug release in response to temperature changes and deliver an anticancer drug to a specific site in response to heat treatment, such as hyperthermia.11 Otherwise, an external magnetic field can be used to target a specific site of action.12 In particular, superparamagnetic iron oxide nanoparticles coupled with an anticancer drug can be used not only for diagnosis of disease but also for delivering anticancer agents to a specific site with an external magnetic field.12 He et al have described pH-triggered or temperature-triggered drug targeting to the site of action using a stimuli-sensitive polypeptide.13 Stimuli-responsive nanoparticles with sensitivity to pH or oxidative stress can be considered for site-specific treatment of disease.14
Among the various types of carriers, pH-responsive drug carriers have been highlighted in recent decades because the extracellular tumor pH is acidic.17 The acidic environment of tumor tissue offers a significant opportunity to develop pH-sensitive nanoparticles for tumor targeting. Among these, pH-sensitive polymers such as PHS, which contains many imidazole rings, have been extensively investigated because of their superior pH sensitivity. PHS is basically insoluble in physiologic solution with a neutral or basic pH but is soluble in acidic solution. The imidazole rings promote acid-dependent fusion with the cellular membrane and mediate delivery of genes or bioactive agents.22 Pack et al reported enhancement of gene delivery using glycosylated PHS.15 Further, a PEG-PHS conjugate was reported to be a useful vehicle for gene delivery.16 Liu et al reported that PEG-PHS-poly(L-lactide) nanoparticles were nontoxic to both normal cells and tumor cells, and that doxorubicin was released faster at pH 5.0 than at pH 7.4.18 The chain length of PHS also affects the anticancer efficacy of nanoparticles and the pH-responsive drug release rate.23 pH-sensitive nanogels using PHS-based conjugates or copolymers can be used to target the acidic extracellular pH microenvironment of solid tumors.24
In this study, we synthesized block copolymers composed of dextran and PHS for pH-sensitive targeting of cancer cells. Because dextran is biocompatible, biodegradable, and immunoneutral, it is extensively employed for modification of bioactive materials.21,25–28 Further, dextran has the potential to avoid unwanted protein or cellular absorption and to increase the blood circulation time, depending on molecular weight.29–31 Dextran has also been used to deliver anticancer agents in systemic drug delivery systems.32,33 For example, Sugahara et al reported that intravenous injection of a dextran-paclitaxel conjugate had reduced neurotoxicity, a higher maximum tolerated dose, and enhanced antitumor activity against CT26 carcinoma cells.32 Further, in an in vivo study, HT-29 colorectal tumor xenografts regressed completely in response to intravenous administration of a dextran-paclitaxel conjugate.33 Therefore, DexPHS block copolymers can be used as a fully biocompatible material for drug delivery. DexPHS block copolymer nanoparticles showed pH-responsive changes in particle size and drug release, ie, their size and drug release rate was increased at acidic pH. The DexPHS copolymer with a longer PHS chain length showed larger differences in pH-sensitive changes with regard to particle size than shorter PHS chain length.23 These results are in accordance with our previous work and that of others.20,23 As shown in Figure 2, the PHS core in DexPHS nanoparticles can swell in an acidic pH, leading to accelerated drug release compared with that in basic pH. Many reports on PHS describe faster drug release at an acidic pH, and accelerated release of anticancer agents at acidic pH preferentially kills cancer cells.20,22,23,34–36 Because the extracellular pH in tumor tissue is acidic compared with normal tissues and cells, drug release may be faster in the tumor microenvironment, leading to death of tumor cells.. In practical terms, DexPHS nanoparticles were completely solubilized in acidic solutions with a pH less than 4.0 (results not shown). On cytotoxicity testing, DexPHS nanoparticles incorporating doxorubicin showed increased cytotoxicity at an acidic pH while free doxorubicin showed higher cytotoxicity at a basic pH. These findings are demonstrated in Figures 5 and 6, ie, DexPHS nanoparticles incorporating doxorubicin had stronger red fluorescence intensity and a higher doxorubicin content in HuCC-T1 cells at an acidic pH whereas free doxorubicin showed decreased red fluorescence intensity at an acidic pH. These results might be due to the fact that DexPHS nanoparticles have an increased particle size in an acidic environment and these larger nanoparticles can be easily engulfed via the cellular uptake mechanism. In an acidic environment, faster drug release of DexPHS nanoparticles can be also considered for reason of these results, ie, doxorubicin was rapidly released in an acidic environment rather than a basic environment. Then, the liberated drug was also taken up into the tumor cell, together with the nanoparticles. Enhanced cellular uptake of DexPHS nanoparticles incorporating doxorubicin in an acidic environment must be induced by both increased particle size and rapid drug release properties. Our results indicate that DexPHS nanoparticles can be used as a nanomedicine for pH-responsive targeting of cancer cells.
In conclusion, we synthesized novel pH-sensitive polymers composed of dextran and PHS for pH-sensitive drug targeting of cancer cells. DexPHS nanoparticles show pH-responsive changes in particle size and drug release, and DexPHS nanoparticles incorporating doxorubicin show pH-sensitive cytotoxicity in HuCC-T1 cholangiocarcinoma cells. We suggest that DexPHS nanoparticles are a promising vehicle for targeting the acidic tumor microenvironment.
Acknowledgments
This study was supported by a grant from the Korean Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A091047).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Box C, Rogers SJ, Mendiola M, Eccles SA. Tumor-microenvironmental interactions: paths to progression and targets for treatment. Semin Cancer Biol. 2010;20:128–138. doi: 10.1016/j.semcancer.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Sounni NE, Noel A. Targeting the tumor microenvironment for cancer therapy. Clin Chem. 2013;59:85–93. doi: 10.1373/clinchem.2012.185363. [DOI] [PubMed] [Google Scholar]
- 3.Zhao G, Rodriguez BL. Molecular targeting of liposomal nanoparticles to tumor microenvironment. Int J Nanomedicine. 2013;8:61–71. doi: 10.2147/IJN.S37859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashim AI, Zhang X, Wojtkowiak JW, Martinez GV, Gillies RJ. Imaging pH and metastasis. NMR Biomed. 2011;24:582–591. doi: 10.1002/nbm.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wojtkowiak JW, Verduzco D, Schramm KJ, Gillies RJ. Drug resistance and cellular adaptation to tumor acidic pH microenvironment. Mol Pharm. 2011;8:2032–2038. doi: 10.1021/mp200292c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rozhin J, Sameni M, Ziegler G, Sloane BF. Pericellular pH affects distribution and secretion of cathepsin B in malignant cells. Cancer Res. 1994;54:6517–6525. [PubMed] [Google Scholar]
- 7.Shi Q, Le X, Wang B, et al. Regulation of vascular endothelial growth factor expression by acidosis in human cancer cells. Oncogene. 2001;20:3751–3756. doi: 10.1038/sj.onc.1204500. [DOI] [PubMed] [Google Scholar]
- 8.Swietach P, Wigfield S, Cobden P, Supuran CT, Harris AL, Vaughan-Jones RD. Tumor-associated carbonic anhydrase 9 spatially coordinates intracellular pH in three-dimensional multicellular growths. J Biol Chem. 2008;283:20473–20483. doi: 10.1074/jbc.M801330200. [DOI] [PubMed] [Google Scholar]
- 9.Xu L, Fidler IJ. Acidic pH-induced elevation in interleukin 8 expression by human ovarian carcinoma cells. Cancer Res. 2000;60:4610–4616. [PubMed] [Google Scholar]
- 10.Gerweck LE, Vijayappa S, Kozin S. Tumor pH controls the in vivo efficacy of weak acid and base chemotherapeutics. Mol Cancer Ther. 2006;5:1275–1279. doi: 10.1158/1535-7163.MCT-06-0024. [DOI] [PubMed] [Google Scholar]
- 11.Chilkoti A, Dreher MR, Meyer DE, Raucher D. Targeted drug delivery by thermally responsive polymers. Adv Drug Deliv Rev. 2002;54:613–630. doi: 10.1016/s0169-409x(02)00041-8. [DOI] [PubMed] [Google Scholar]
- 12.Wahajuddin, Arora S. Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers. Int J Nanomedicine. 2012;7:3445–3471. doi: 10.2147/IJN.S30320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He C, Zhuang X, Tang Z, Tian H, Chen X. Stimuli-sensitive synthetic polypeptide-based materials for drug and gene delivery. Adv Healthc Mater. 2012;1:48–78. doi: 10.1002/adhm.201100008. [DOI] [PubMed] [Google Scholar]
- 14.Colson YL, Grinstaff MW. Biologically responsive polymeric nanoparticles for drug delivery. Adv Mater. 2012;24:3878–3886. doi: 10.1002/adma.201200420. [DOI] [PubMed] [Google Scholar]
- 15.Pack DW, Putnam D, Langer R. Design of imidazole-containing endosomolytic biopolymers for gene delivery. Biotechnol Bioeng. 2000;67:217–223. [PubMed] [Google Scholar]
- 16.Putnam D, Zelikin AN, Izumrudov VA, Langer R. Polyhistidine-PEG:DNA nanocomposites for gene delivery. Biomaterials. 2003;24:4425–4433. doi: 10.1016/s0142-9612(03)00341-7. [DOI] [PubMed] [Google Scholar]
- 17.Tannock IF, Rotin D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989;49:4373–4384. [PubMed] [Google Scholar]
- 18.Liu R, Li D, He B, et al. Anti-tumor drug delivery of pH-sensitive poly(ethylene glycol)-poly(L-histidine-)-poly(L-lactide) nanoparticles. J Control Release. 2011;152:49–56. doi: 10.1016/j.jconrel.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 19.Johnson RP, Chung CW, Jeong YI, Kang DH, Suh H, Kim I. Poly(L-histidine)-tagged 5-aminolevulinic acid prodrugs: new photosensitizing precursors of protoporphyrin IX for photodynamic colon cancer therapy. Int J Nanomedicine. 2012;7:2497–2512. doi: 10.2147/IJN.S29582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson RP, Jeong YI, Choi E, et al. Biocompatible poly(2-hydroxyethyl methacrylate)-b-poly(L-histidine) hybrid materials for pH-sensitive intracellular anticancer drug delivery. Adv Funct Mater. 2012;22:1058–1068. [Google Scholar]
- 21.Jeong YI, Kim DH, Chung CW, et al. Doxorubicin-incorporated polymeric micelles composed of dextran-b-poly(DL-lactide-coglycolide) copolymer. Int J Nanomedicine. 2011;6:1415–1427. doi: 10.2147/IJN.S19491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asayama S, Sudo M, Nagaoka S, Kawakami H. Carboxymethyl poly(L-histidine) as a new pH-sensitive polypeptide to enhance polyplex gene delivery. Mol Pharm. 2008;5:898–901. doi: 10.1021/mp800094b. [DOI] [PubMed] [Google Scholar]
- 23.Liu R, He B, Li D, et al. Effects of pH-sensitive chain length on release of doxorubicin from mPEG-b-PH-b-PLLA nanoparticles. Int J Nanomedicine. 2012;7:4433–4446. doi: 10.2147/IJN.S32053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee ES, Gao Z, Bae YH. Recent progress in tumor pH targeting nanotechnology. J Control Release. 2008;132:164–170. doi: 10.1016/j.jconrel.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeong YI, Choi KC, Song CE. Doxorubicin release from core-shell type nanoparticles of poly(DL-lactide-co-glycolide)-grafted dextran. Arch Pharm Res. 2006;29:712–719. doi: 10.1007/BF02968257. [DOI] [PubMed] [Google Scholar]
- 26.Ichinose K, Tomiyama N, Nakashima M, et al. Antitumor activity of dextran derivatives immobilizing platinum complex (II) Anticancer Drugs. 2000;11:33–38. doi: 10.1097/00001813-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Kim DH, Kim MD, Choi CW, et al. Antitumor activity of sorafenib-incorporated nanoparticles of dextran/poly(dl-lactide-co-glycolide) block copolymer. Nanoscale Res Lett. 2012;7:91. doi: 10.1186/1556-276X-7-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Manen HJ, van Apeldoorn AA, Verrijk R, van Blitterswijk CA, Otto C. Intracellular degradation of microspheres based on cross-linked dextran hydrogels or amphiphilic block copolymers: a comparative Raman microscopy study. Int J Nanomedicine. 2007;2:241–252. [PMC free article] [PubMed] [Google Scholar]
- 29.Osterberg E, Bergström K, Holmberg K, et al. Protein-rejecting ability of surface-bound dextran in end-on and side-on configurations: comparison to PEG. J Biomed Mater Res. 1995;29:741–747. doi: 10.1002/jbm.820290610. [DOI] [PubMed] [Google Scholar]
- 30.Kaneo Y, Uemura T, Tanaka T, Kanoh S. Polysaccharides as drug carriers: biodisposition of fluorescein-labeled dextrans in mice. Biol Pharm Bull. 1997;20:181–187. doi: 10.1248/bpb.20.181. [DOI] [PubMed] [Google Scholar]
- 31.Mehvar R, Robinson MA, Reynolds JM. Molecular weight dependent tissue accumulation of dextrans: in vivo studies in rats. J Pharm Sci. 1994;83:1495–1499. doi: 10.1002/jps.2600831024. [DOI] [PubMed] [Google Scholar]
- 32.Sugahara S, Kajiki M, Kuriyama H, Kobayashi TR. Carrier effects on antitumor activity and neurotoxicity of AZ10992, a paclitaxel-carboxymethyl dextran conjugate, in a mouse model. Biol Pharm Bull. 2008;31:223–230. doi: 10.1248/bpb.31.223. [DOI] [PubMed] [Google Scholar]
- 33.Sugahara S, Kajiki M, Kuriyama H, Kobayashi TR. Complete regression of xenografted human carcinomas by a paclitaxel-carboxymethyl dextran conjugate (AZ10992) J Control Release. 2007;117:40–50. doi: 10.1016/j.jconrel.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Johnson RP, Jeong YI, John JV, et al. Dual stimuli-responsive poly(N-isopropylacrylamide)-b-poly(L-histidine) chimeric materials for the controlled delivery of doxorubicin into liver carcinoma. Biomacromolecules. 2013;14:1434–1443. doi: 10.1021/bm400089m. [DOI] [PubMed] [Google Scholar]
- 35.Lee ES, Na K, Bae YH. Polymeric micelle for tumor pH and folate-mediated targeting. J Control Release. 2003;91:103–113. doi: 10.1016/s0168-3659(03)00239-6. [DOI] [PubMed] [Google Scholar]
- 36.Yang SR, Lee HJ, Kim JD. Histidine-conjugated poly(amino acid) derivatives for the novel endosomolytic delivery carrier of doxorubicin. J Control Release. 2006;114:60–68. doi: 10.1016/j.jconrel.2006.05.016. [DOI] [PubMed] [Google Scholar]






