Abstract
Capsule endoscopy (CE) avoids the ionizing radiation, deep sedation, and general anesthesia required by other imaging modalities, making it particularly valuable in the evaluation of gastrointestinal disease in pediatric patients. In examining the use of CE in pediatric and adult patients through a review of the literature, it was observed that CE is most frequently indicated for the evaluation of Crohn’s disease (CD) in pediatric patients and most frequently indicated for obscure gastrointestinal bleeding (OGIB) in adults, although OGIB is a more frequent indication than CD in pediatric patients younger than 8 years of age. Diagnostic accuracy has been good and comparable to that of magnetic resonance enterography, and capsule retention rates as well as other adverse events appear to be low in pediatric patients. Research is needed to explore broader indications and applications of CE in the diagnosis and monitoring of gastrointestinal disease.
Keywords: Capsule endoscopy, Crohn’s disease, esophageal capsule endoscopy, obscure gastrointestinal bleeding, patency capsule, pediatric gastrointestinal disease, small bowel follow-through
Capsule endoscopy (CE) does not require ionizing radiation and deep sedation or general anesthesia, which are usually needed by other imaging modalities. This makes CE particularly valuable in the diagnosis and treatment of pediatric patients. In light of supportive data, the US Food and Drug Administration (FDA) expanded the role of CE for use in children age 2 years and older, approved the use of a patency capsule (PC) for this age group,1 and recently approved CE use for mucosal monitoring of Crohn’s disease (CD). Data from pooled statistical analysis of 3 recent reports on the use of CE in pediatric patients2-4 suggest that it has utility in the diagnosis and management of pediatric gastrointestinal disease and the potential for broader application. This article compares indications for CE in 1,013 procedures in pediatric patients overall,2-4 83 procedures in pediatric patients younger than 8 years of age,5 and 22,840 procedures in adult patients6 (Table 1).
Table 1.
Clinical Indications by Age
| Adult patients6 | Pediatric patients2-4 | Patients <8 years old5 | |
|---|---|---|---|
| Procedures (n) | 22,840 | 1,013 | 83 |
| OGIB + IDA (%) | 66 | 15 | 36 |
| CD/UC/IC (%) | 10 | 63 | 24 |
| Abdominal pain (%) | 11 | 10 | 14 |
| Polyps/neoplasms (%) | 3 | 8 | - |
| Other (%) | 10 | 4 | 25 |
Reproduced from Cohen S.15
CD=Crohn’s disease; IC=indeterminate colitis; IDA=iron-deficiency anemia; OGIB=obscure gastrointestinal bleeding; UC=ulcerative colitis.
The Small Bowel Capsule
Indications
Guidelines regarding the indications for CE have been developed and disseminated by societies such as the American Society for Gastrointestinal Endoscopy.7 However, the relative frequency of indications in compiled pediatric reports differs from that in data regarding adults. In pediatric patients, 63% of CEs have been performed for CD, 15% for obscure gastrointestinal bleeding (OGIB), 10% for abdominal pain/diarrhea, and 8% for polyposis. In adults, 66% of CEs have been performed for OGIB, including iron-deficiency anemia (IDA), 11% for clinical symptoms only (eg, pain, diarrhea, and weight loss without OGIB), 10% for CD, and the balance (13%) for polyps and other indications.2-5,8-123
In pediatric patients, the suspicion of CD and the evaluation of existing inflammatory bowel disease (IBD) are the most common indications for CE, accounting for 63% of the total indications.15 The presentation of abdominal pain and diarrhea accounts for another 10%.15 More than half of the procedures for IBD indications are related to the evaluation of CD and colitis, with 44% due to the suspicion of CD, 16% related to the evaluation of known CD, 2% to indeterminate colitis (IC), and 1% to ulcerative colitis (UC).15
Even within the pediatric population, clinical indications are age-stratified (Table 1). In a study of 83 procedures in children age 1.5—7.9 years (in whom CD is less prevalent), the most common indication for CE was OGIB, accounting for 30 (36%) procedures, with positive findings in 16 (53%).5 The suspicion of CD was the second most frequent indication, accounting for 20 (24%) procedures, with positive findings in 11 (55%). The indication of abdominal pain accounted for another 12 (14%) procedures, and CD was the indication in 3 patients. CD was found in 14 (31%) of the patients in whom a positive diagnosis was made. Protein loss and malabsorption were the indications for 9 and 12 procedures (11% and 14%), respectively, with positive findings in 6 each. In contrast, OGIB in older children (age, 10—18 years) accounted for only 13—24% of all indications and 40—86% of CD indications.3,5,9,11,16,18 Patients with protein-losing enteropathy and malabsorption were younger than those suspected of having CD or recurrent abdominal pain.5
The approved indications for pediatric and adult populations may expand as the broader utility of CE is recognized. Already, CE is being used to identify eosino-philic enteropathy (with areas of erythematous, denuded mucosa)9 and a newly recognized ulcerative inflammatory enteropathy in cystic fibrosis.24 CE is also being used to evaluate unrecognized causes of abdominal pain. CE has been useful in monitoring medical therapy in CD3,25 and graft-versus-host disease.8 The use of CE to find jejunal lesions in UC and to differentiate it from IC and nonspecific colitis has proven to be useful, especially before a colectomy is performed.4,12 Although CE results may not change the decision to undergo surgery, the results may better guide decisions about the type of surgery needed. Additionally, diagnostic algorithms based on CE results have been used in select intestinal motility disorders.26
Patient Outcomes and Management
In a meta-analysis2 and additional reports from pediatric literature,3,4 995 patients in the pooled studies underwent 1,013 CE procedures. CE yielded positive findings in 511 (61.4%; 95% confidence interval [CI], 52.7—69.7%; Table 2); resulted in a new diagnosis in 162 patients (66.0%; 95% CI, 45.4—83.9%); and directed a change in therapy in 101 patients (71.3%; 95% CI, 45.2—91.5%) where those parameters were quantified. Studies were complete (ie, the capsule reached or passed the ileocecal valve by the end of the recording period) in 846 procedures (86.0%; 95% CI, 81.6—89.9%; P=.0003).2-4 In many other studies, diagnostic findings have been achieved even though the capsule did not enter the colon.5,12,16,17 A total of 824 (88.4%) children in the studies for which ingestion was reported swallowed the capsule uneventfully (95% CI, 86.4—90.3%; P<.0001).15 The youngest patient to swallow the capsule was 4 years old.5 Only 1 patient in the reports could not swallow the capsule and refused endoscopic placement,11 although this is not an infrequent occurrence in clinical practice.
Table 2.
Capsule Endoscopy Outcomes in Pediatric Patients Compared with Adult Patients
| Pediatric patients2-4 | Adult patients6 | |||
|---|---|---|---|---|
| Outcome | Number of studies | N | Pooled rate, % (95% CI) | Pooled rate, % (95% CI) |
| Capsules swallowed | 15 | 824/980 | 88.4 (86.4—90.3) | Not reported |
| Completion rate | 16 | 846/1,013 | 86.0 (81.6—89.9) | 83.5 (82.0—85.0) |
| Positive findings | 16 | 511/1,013 | 61.4 (52.7—69.7) | 59.4 (56.5—62.2) |
| New diagnosis | 12 | 162/334 | 66.0 (45.4—83.9) | Not reported |
| Change in therapy | 8 | 101/164 | 71.3 (45.2—91.5) | Not reported |
Reproduced from Cohen S.15
CI=confidence interval.
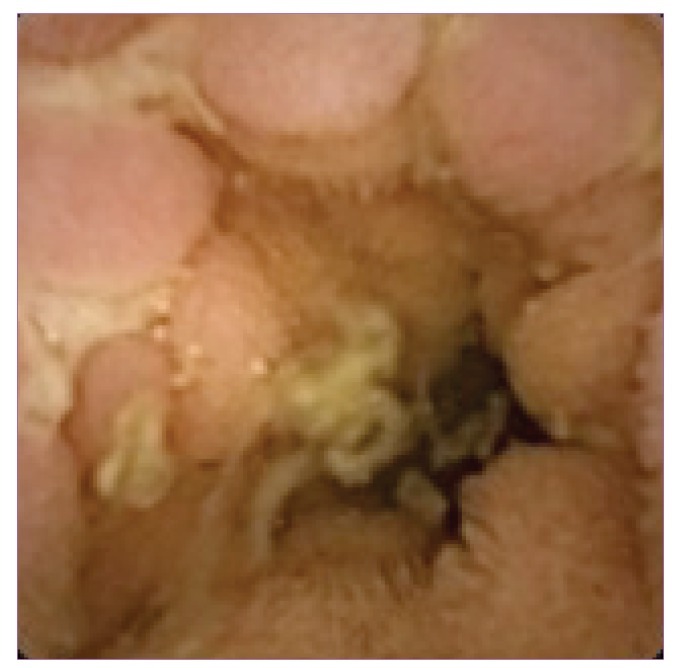
CD (Figure 1) was the most prevalent diagnostic outcome of CE studies performed in the pediatric population, resulting in 234 (53.9%) of 434 positive procedures in studies reporting diagnostic outcomes.15 The diagnosis of CD per CE was based on the criteria of at least 3 mucosal ulcers, as previously reported by Fireman and colleagues27 and Mow and colleagues.28 CE caused a change in medical therapy in 75—92% of patients with known CD in various studies.12,13,16 CE detected a greater extent of CD compared with small bowel follow-through (SBFT), with a relative sensitivity of 100% versus 57% in respective, completed examinations. Thus, medical management was refined for 50% of patients with known CD.23 In addition, CE examination in 1 study demonstrated that 4 of 5 patients with UC and 1 of 2 patients with IC (total, 5 of 7 patients, or 71%) had their disease reclassified to CD due to newly diagnosed small bowel mucosal lesions, resulting in effective therapeutic changes.12
Figure 1.
Jejunal Crohn’s disease in a 12-year-old male with intermittent fevers and abnormal laboratory parameters but normal findings on endoscopy, colonoscopy, and computed tomography scan with minimal jejunal nodularity.
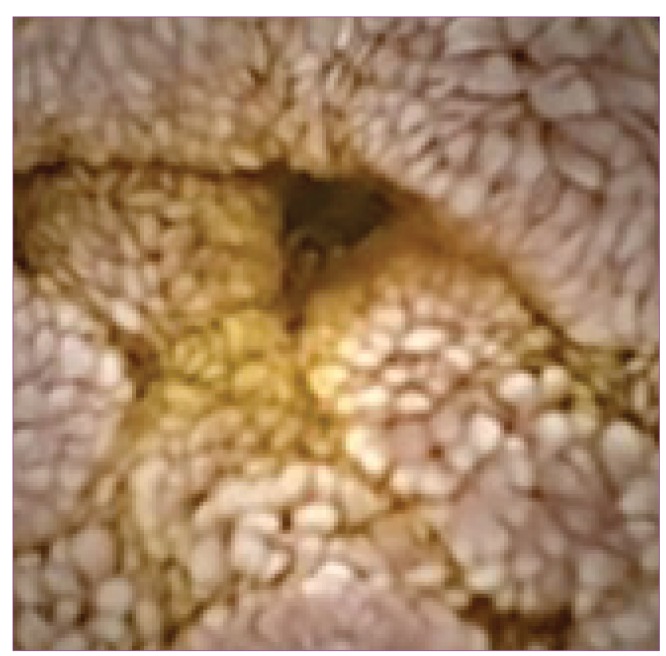
In pediatric patients investigated for OGIB or IDA by CE, 38.4% had confirmed diagnoses.15 In comparison, the positive result rate in adults was 59.4%.6 Of the 46 lesions diagnosed by CE,8-11,13,17 15 involved vascular malformations, 7 involved CD, 14 involved nonspecific enteropathies, 3 involved polyps, 2 involved marked lymphoid hyperplasias, and 1 each involved Meckel diver-ticulum, nonsteroidal anti-inflammatory drug—induced lesions, lymphangiectasia (Figure 2), leukemia-related— disease, and graft-versus-host disease. In patients younger than 8 years of age, there were 4 cases of polyps, 2 cases of angiodysplasias, 2 cases of blue rubber bleb hemangiomas, 2 cases of Meckel diverticulae, 1 case of anastomotic ulcer, and 1 case of intestinal duplication.5 In adult studies, vascular abnormalities also were the most common cause of OGIB (50%), followed by inflammation and ulcers (27%) and neoplasia (9%).6
Figure 2.
Lymphangiectasia in a 12-year-old female with hypoalbuminemia and normal endoscopy findings.
The evaluation of polyposis syndromes, which accounted for 8.0% of the indications in 81 pediatric patients, had the highest ratio of diagnosis to indication by CE: The results were positive in 80.2% of procedures.15,22 This finding compared favorably with findings in adults in which the diagnostic yield of CE for neoplastic lesions was 55.9%.6 Even when pediatric patients were evaluated for chronic, recurrent abdominal pain, CE produced relevant findings in 43%.21 Although the diagnostic yield is relatively low, CE findings may help exclude or identify a cause of abdominal pain, especially when other investigations are equivocal or negative.
Malabsorption remains an infrequent indication for CE, but CE is often helpful in cases such as those of intestinal lymphangiectasia, which can appear beyond the reach of an endoscope.3 Celiac disease is often recognized in the adult population and is distinguished by scalloped, swollen folds and a mosaic pattern similar to the visual findings on endoscopy (Figure 3).29 Its infrequency in pediatric patients may reflect the infrequency of CE use for the evaluation of malabsorption in this population2 or the decreased time of gluten exposure and potentially patchy or very subtle mucosal changes in childhood at histologic levels of Marsh I or II, for which the sensitivity of CE is low.30 CE also has been used to detect small bowel transplant complications and to evaluate the graft’s integ-rity.7,8 Lymphonodular hyperplasia and intussusceptions are often seen. Although they can be clinically significant in certain situations, they are normally nonpathogenic conditions indigenous to the pediatric population.3
Figure 3.
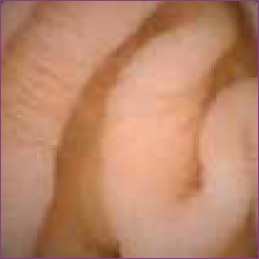
Mosaic celiac scalloping in a 12-year-old female.
Comparative Modalities
In a small study that compared magnetic resonance enterography (MRE; n=60 patients) and small bowel CE (n=37 patients) with clinical examination and colonoscopy in pediatric patients with suspected CD, MRE and CE appeared to be complementary.31 Both had high degrees of accuracy, sensitivity, and specificity at 98.3%, 100%, and 97.6% and 91.9%, 90.9%, and 92.3%, respectively, although some of the findings were discordant. CE was able to detect small bowel ulcers, villous denudation, and proximal disease that was undetectable by MRE, whereas MRE detected extraintestinal disease, some of which was not found at surgery and excluded patients with strictures. Otherwise, the only other comparative data come from a meta-analysis that garnered information from 9 studies in which patients underwent SBFT and CE.2 Of 206 patients screened by SBFT, the pooled diagnostic yield, where measured, was 17.8% (n=31/163; 95% CI, 9.9—27.5%; P=.0892), whereas the rate of positive findings using CE, where measured, was 65.4% (n=413/740; 95% CI, 54.8—75.2%; P<.0001).2
Studies comparing CE with push enteroscopy (PE) have not been performed in pediatric patients. However, in a meta-analysis of 14 studies that included 396 adult patients who were evaluated for occult gastrointestinal bleeding, the diagnostic yield for CE was 63% compared with 28% for PE.32 A randomized study of the 2 modalities as the first-line diagnostic technique found that CE detected the source of bleeding in 50% of patients and PE detected the source of bleeding in 24% of patients.33
Adverse Events
In 1,013 pediatric procedures, capsule retention in the small bowel occurred in 18 procedures and gastric retention occurred in 4 procedures.2-4,15 The overall pooled retention rate was 2.3% (n=22/1,013; 95% CI, 1.5—3.4%; P=.4247). Endoscopy was used to remove 5 (0.5%) capsules, including 4 capsules from the stomach10,16 and 1 capsule from an ileal pouch2; 13 capsules (1.3%) were retrieved surgically while taking appropriate measures to mitigate the cause of the retention.8,10,13,14,16 A retained capsule was successfully evacuated by bowel preparation 22 days postingestion.10 In adults, retention rates for indications of OGIB, CD, and neoplastic lesions were 1.2% (95% CI, 0.9—1.6%; P=.6014), 2.6% (95% CI, 1.6—3.9%), and 2.1% (95% CI, 0.7—4.3%), respectively, with a pooled retention rate of 1.4% (95% CI, 1.2—1.6%).6 On a per-procedure basis, capsule retention in pediatric patients bears a similar risk pattern to that seen in adults, with retention in CE performed for OGIB, CD, and polyps occurring at rates of 1.4%, 2.2%, and 1.2%, respectively (Table 3). Thus, it appears that the risk of retention is dependent on clinical indication and not age.
Table 3.
Retention Rates in Adult Patients and Pediatric Patients by Indication
| Pediatric patients2-4 | Adult patients6 | |||
|---|---|---|---|---|
| Indication | Studies | N | Combined % (95% CI) | Pooled rate % (95% CI) |
| OGIB + IDA | 11 | 2/144 | 1.4 | 1.2 (0.9-1.6) |
| CD | 12 | 13/596 | 2.2 | 2.6 (1.6-3.9) |
| Polyposis | 9 | 1/81 | 1.2 | 2.1 (0.7-4.3) |
| Overall | 16 | 22/1,013 | 2.3 (1.5-3.4) | 1.4 (1.2-1.6) |
Reproduced from Cohen S.15
CD=Crohn’s disease; CI=confidence interval; IDA=iron-deficiency anemia; OGIB=obscure gastrointestinal bleeding.
The highest risk factors for capsule retention include known IBD (5.2% risk), previous SBFT demonstrating small bowel CD (35.7% risk), and a body mass index below the fifth percentile combined with known IBD (43% risk), although retention has occurred despite the absence of strictures on SBFT.14 In 4 patients with capsule passage lasting longer than 5 days (with 3 continuing on to retention), all had CD, with the difference in age being significant (18.8±0.9 vs 14.6±3.5), but not height or weight.16 Rare cases of perforation, aspiration, or small bowel obstruction have been reported in adults, but none have been reported in children, although minor mucosal trauma has occurred in children in which capsules were placed with a Roth net.20 A specific capsule placement device is now available (AdvanCE, US Endoscopy).34
The Patency Capsule: Rationale, Procedure, and Findings in Pediatric Patients
Because the majority of capsule retentions have occurred in patients with normal small bowel radiologic studies and because functional patency may be present in patients with radiologically documented strictures, an identically sized PC was found to be useful in the pediatric population to evaluate functional intestinal patency prior to CE and to reduce the occurrence of retention, particularly when studying patients with known or suspected CD.
A retrospective study reviewed 23 patients with known (n=14) or suspected (n=9) CD who underwent evaluation with a PC prior to using a video capsule.2 Of the 19 who were evaluable, patency was established, and subsequent CE was performed successfully in all but 1 who had a retained capsule from CE the following week.
In a single-center prospective trial that evaluated 18 patients (age, 10-16 years) who ingested a PC, 15 patients excreted an intact PC (mean, 34.5 hours).35 The 18 cases included 5 cases of known CD, 3 cases of IC, 1 case of UC, and 9 cases of suspected CD. CD was eventually diagnosed in all patients who had PC transit that lasted more than 40 hours and in 9 of 12 patients who passed their PC in 40 hours or less. There were no capsule retentions or adverse events. Thus, the PC can serve as a useful guide and may lessen the likelihood of CE retention.
The Esophageal Capsule: Rationale, Procedure, and Findings in Pediatric Patients
In 2004, an esophageal CE (ECE) was approved by the US FDA and introduced for clinical use.36 Even though a second iteration of the capsule, Pillcam ESO 2 (Given Imaging)—which widened the field of view; increased the frame rate to 18 images per second; and improved the image quality with 2 additional lenses, higher spatial resolution, and a wider dynamic range—was approved by the US FDA in 2007,37 its use in pediatrics (or at least clinical trials and the retrospective reporting of that use) has been limited. Only 2 small pediatric trials of the first iteration of the capsule have been reported, and both focused on portal hypertension.38,39 In the first trial, which also included young adults, 27 of the 28 ECEs were complete, each with a total recording time of 20 minutes and a mean esophageal transit of 192 seconds (range, 4-631).38 Esophageal varices were small in 10 (37%), medium to large in 4 (15%), and negative in 13 (48%), with gastric varices in 10 (37%) and other esophageal and duodenal findings also identified. In the other study, the ECE was successful in 10 of 11 patients.39 The mean esophageal transit was 45 seconds (range, 9-171). Varices were small in 4, small and large in 4, multiple/large in 1, and negative in 1, again with other findings present in the esophagus. Although the first study did not report how the varices were graded, the second study appraised the size of the varix as a fraction of the circumference, with a cut-point of 25% distinguishing small versus large varices because the lack of insufflation with CE required a grading system that differed from that used for traditional endoscopy.40
Research and Its Future
Although the quantity of available pediatric data is small compared with the much larger quantity of adult data, the understanding of CE risks and benefits in the pediatric population is still useful, with implications for potential expansion of CE use, particularly in adult IBD. However, the difficulty inherent in combining studies that have disparate inclusion/ exclusion criteria and different objectives is that the interpretation of the data presented is limited. Additionally, the lack of validated criteria for diagnosing mucosal disease, the lack of tissue sampling of pathologies suspected via CE, and the potentially different diagnostic thresholds in different studies impact diagnostic sensitivity and specificity as well as the ability to translate diagnostic yield into diagnostic accuracy.
Certainly, further analyses are warranted as the science of CE advances and as more data on CE in pediatric patients and mucosal monitoring become available. Appropriate analysis of findings requires a fundamental change in how research is conducted. For example, what modality should be the comparator? This first decade of CE is similar to the time when traditional endoscopy was first introduced. An expansion of knowledge occurred. Different visual manifestations of gastroduodenal and colonic diseases that could not be appreciated radiologically or pathologically were able to be recognized, and visual findings could gradually be explained and associated with the conditions that seemed to produce those findings. The same appears true now for CE. Technical advancements and thoughtful research will hopefully allow us to maximize the potential for this modality.
Conclusions
This first decade of small intestinal CE followed by ECE has allowed for visual manifestations of disease that could not be well appreciated radiologically or pathologically. The primary pediatric indication has focused on the evaluation of IBD, with studies demonstrating that CE assists in diagnosis and helps in re-evaluating exacerbations. In doing so, CE has redefined disease for patients with UC and helped to guide and monitor therapy for patients with CD. Moreover, although experience has been limited, PC may help lessen the potential for capsule retention. The use of ECE also may enhance our knowledge of esophageal disease and assist patient care. Further research is needed to determine ways to optimize the use of CE in the pediatric as well as adult populations.
Footnotes
Dr. Cohen serves as a consultant, speaker, and investigator for Given Imaging, Janssen, and UCB; a consultant and investigator for AstraZeneca; an investigator for Abbott; and a speaker for Prometheus.
References
- 1. FDA, Center for Devices and Radiological Health. PC Patency System and Pillcam Platform with Pillcam SB Capsules. 510k number K090557. Approval September 28, 2009.
- 2.Cohen SA, Klevens AI. Use of capsule endoscopy in diagnosis and management of pediatric patients, based on meta-analysis. Clin Gastroenterol Hepatol. 2011;9:490–496. doi: 10.1016/j.cgh.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 3.Cohen SA, Ephrath H, Lewis JD, et al. Pediatric capsule endoscopy: a single center, 5 year retrospective review of small bowel and patency capsules. J Pediatr Gastroenterol Nutr. 2012;54:409–413. doi: 10.1097/MPG.0b013e31822c81fd. [DOI] [PubMed] [Google Scholar]
- 4.Gralnek IM, Cohen SA, Ephrath H, et al. Small bowel capsule endoscopy impacts diagnosis and management of pediatric inflammatory bowel disease: a prospective study. Dig Dis Sci. 2012;57:465–471. doi: 10.1007/s10620-011-1894-5. [DOI] [PubMed] [Google Scholar]
- 5.Fritscher-Ravens A, Scherbakov P, Bufler P, et al. The feasibility of wireless capsule endoscopy in detecting small intestinal pathology in children under the age of 8 years: a multicentre European study. Gut. 2009;58:1467–1472. doi: 10.1136/gut.2009.177774. [DOI] [PubMed] [Google Scholar]
- 6.Liao Z, Gao R, Xu C, Zhao-Shen L. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: a systematic review. Gastro-intest Endosc. 2010;71:280–286. doi: 10.1016/j.gie.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 7.Mishkin DS, Chuttani R, Croffie J, et al. ASGE Technology Status Evaluation Report: wireless capsule endoscopy. Gastrointest Endosc. 2006;63:539–545. doi: 10.1016/j.gie.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Tokuhara D, Watanabe K, Okano Y, et al. Wireless capsule endoscopy in pediatric patients: the first series from Japan. J Gastroenterol. 2010;45:683–691. doi: 10.1007/s00535-010-0209-5. [DOI] [PubMed] [Google Scholar]
- 9.Guilhon de Araujo Sant’, Anna AM, Dubois J, Miron MC, Seldman EG. Wireless capsule endoscopy for obscure small-bowel disorders: final results of the first pediatric controlled trial. Clin Gastroenterol Hepatol. 2005;3:264–270. doi: 10.1016/s1542-3565(04)00715-3. [DOI] [PubMed] [Google Scholar]
- 10.Jensen MK, Tipnis NA, Bajorunaite R, Sheth MK, Sato TT, Noel RJ. Capsule endoscopy performed across the pediatric age range: indications, incomplete studies, and utility in management of inflammatory bowel disease. Gastrointest Endosc. 2010;72:95–102. doi: 10.1016/j.gie.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Antao B, Bishop J, Shawis R, Thomson M. Clinical application and diagnostic yield of wireless capsule endoscopy in children. J Laparoendosc Adv Surg Tech A. 2007;17:364–370. doi: 10.1089/lap.2006.0114. [DOI] [PubMed] [Google Scholar]
- 12.Cohen SA, Gralnek IM, Ephrath H, et al. Capsule endoscopy may reclassify pediatric inflammatory bowel disease: a historical analysis. J Pediatr GastroenterolNutr. 2008;47:31–36. doi: 10.1097/MPG.0b013e318160df85. [DOI] [PubMed] [Google Scholar]
- 13.de’ Angelis GL, Fornaroli F, de’ Angeles N, Magiteri B, Bizzarri B. Wireless capsule endoscopy for pediatric small-bowel diseases. Am J Gastroenterol. 2007;102:1749–1757. doi: 10.1111/j.1572-0241.2007.01209.x. [DOI] [PubMed] [Google Scholar]
- 14.Atay O, Mahajan L, Kay M, Mohr F, Kaplan B, Wyllie R. Risk of capsule endoscope retention in pediatric patients: a large single-center experience and review of the literature. J Pediatr Gastroenterol Nutr. 2009;49:1–6. doi: 10.1097/MPG.0b013e3181926b01. [DOI] [PubMed] [Google Scholar]
- 15.Cohen S. Pediatric capsule endoscopy. Tech Gastrointest Endosc. 2013;15:32–35. [Google Scholar]
- 16.Moy L, Levine J. Wireless capsule endoscopy in the pediatric age group: experience and complications. J Pediatr Gastroenterol Nutr. 2007;44:516–520. doi: 10.1097/MPG.0b013e3180335548. [DOI] [PubMed] [Google Scholar]
- 17.Ge ZZ, Chen HY, Gao YJ, Gu JL, Hu YB, Xiao SD. Clinical application of wireless capsule endoscopy in pediatric patients for suspected small bowel diseases. Eur J Pediatr. 2007;166:825–829. doi: 10.1007/s00431-006-0331-9. [DOI] [PubMed] [Google Scholar]
- 18.Arguelles-Arias F, Caunedo A, Romero J, et al. The value of capsule endoscopy in pediatric patients with a suspicion of Crohn’s disease. Endoscopy. 2004;36:869–873. doi: 10.1055/s-2004-825854. [DOI] [PubMed] [Google Scholar]
- 19.Urbain D, Tresinie M, De Looze D, et al. Capsule endoscopy in paediatrics: multicentric Belgian study. Acta Gastroenterol Belg. 2007;70:11–14. [PubMed] [Google Scholar]
- 20.Barth BA, Donovan K, Fox VL. Endoscopic placement of the capsule endo-scope in children. Gastrointest Endosc. 2004;60:818–821. doi: 10.1016/s0016-5107(04)02052-8. [DOI] [PubMed] [Google Scholar]
- 21.Shamir R, Hino B, Hartman C, Berkowitz D, Eshach-Adiv O, Eliakim R. Wireless video capsule in pediatric patients with functional abdominal pain. J Pediatr Gastroenterol Nutr. 2007;44:45–50. doi: 10.1097/01.mpg.0000239737.64240.72. [DOI] [PubMed] [Google Scholar]
- 22.Postgate A, Hyer W, Phillips R, et al. Feasibility of video capsule endoscopy in the management of children with Peutz-Jeghers syndrome: a blinded comparison with barium enterography for the detection of small bowel polyps. J Pediatr GastroenterolNutr. J Pediatr Gastroenterol Nutr. 2009;2010;4950:417–423. 235. doi: 10.1097/MPG.0b013e31818f0a1f. Erratum in: [DOI] [PubMed] [Google Scholar]
- 23.Thomson M, Fritscher-Ravens A, Mylonaki M, et al. Wireless capsule endos-copy in children: a study to assess diagnostic yield in small bowel disease in pediat-ric patients. J Pediatr Gastroenterol Nutr. 2007;44:192–197. doi: 10.1097/01.mpg.0000252196.91707.ff. [DOI] [PubMed] [Google Scholar]
- 24.Werlin SL, Benuri-Silbiger I, Kerem E, et al. Evidence of intestinal inflammation in patients with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2010;51:304–308. doi: 10.1097/MPG.0b013e3181d1b013. [DOI] [PubMed] [Google Scholar]
- 25.Cohen SA, Lewis J, Stallworth A, et al. Mucosal healing with the specific carbohydrate diet in pediatric Crohn’s disease: preliminary results of a 12 week pilot study. Gastroenterology. 2012;142(supp AB) Sa1992. [Google Scholar]
- 26.Malagelada C, DeIorio F, Azpiroz F, et al. New insight into intestinal motor function via noninvasive endoluminal image analysis. J Gastroenterol. 2008;135:1155–1162. doi: 10.1053/j.gastro.2008.06.084. [DOI] [PubMed] [Google Scholar]
- 27.Fireman Z, Mahajana E, Broide E, et al. Diagnosing small bowel Crohn’s disease with wireless capsule endoscopy. Gut. 2003;52:390–392. doi: 10.1136/gut.52.3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mow WS, Lo SK, Targan SR, et al. Initial experience with wireless capsule enteroscopy in the diagnosis and management of inflammatory bowel disease. Clin Gastroenterol Hepatol. 2004;2:31–40. doi: 10.1016/s1542-3565(03)00289-1. [DOI] [PubMed] [Google Scholar]
- 29.Gay G, Fassler I, Florent C, Delvaux M. Malabsorption. In: Halpern M, Jacob H, editors. Atlas of Capsule Endoscopy. Norcross, Ga: Given Imaging; 2002. pp. 84–90. [Google Scholar]
- 30.Murray JA, Rubio Tapia A, Van Dyke CT, et al. Mucosal atrophy in celiac disease: extent of involvement, correlation with clinical presentation, and response to treatment. Clin Gastroenterol Hepatol. 2008;6:186–193. doi: 10.1016/j.cgh.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casciani E, Masselli G, DiNardo G, et al. MR enterography versus capsule endoscopy in paediatric patients with suspected Crohn’s disease. Eur Radiol. 2011;21:823–831. doi: 10.1007/s00330-010-1976-3. [DOI] [PubMed] [Google Scholar]
- 32.Triester SL, Leighton JA, Leontiadis G, et al. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2005;100:2407–2418. doi: 10.1111/j.1572-0241.2005.00274.x. [DOI] [PubMed] [Google Scholar]
- 33.deLeusse A, Vahedi K, Edery J, et al. Capsule endoscopy or push enteroscopy for the first-line exploration of obscure gastrointestinal bleeding? Gastroenterology. 2007;132:855–862. doi: 10.1053/j.gastro.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Uko V, Atay O, Mashajan L, et al. Endoscopic deployment of the wireless capsule using a capsule delivery device in pediatric patients: a case series. Endoscopy. 2009;41:380–382. doi: 10.1055/s-0029-1214491. [DOI] [PubMed] [Google Scholar]
- 35.Cohen SA, Gralnek IM, Ephrath H, et al. The use of a patency capsule in pediatric Crohn’s disease: a prospective evaluation. Dig Dis Sci. 2011;56:860–865. doi: 10.1007/s10620-010-1330-2. [DOI] [PubMed] [Google Scholar]
- 36. FDA, Center for Devices and Radiological Health. Given Diagnostic System with PillCam ESO Capsule. 510k number K042960. Approval November 24, 2004.
- 37. FDA, Center for Devices and Radiological Health. Given Diagnostic System with PillCam ESO2 Capsule. 510k number K071153. Approval June 11, 2007.
- 38.Fox V. Esophageal capsule endoscopy in children and young adults with portal hypertension. Presented at the 6th International Conference on Capsule Endoscopy (ICCE); June 8-9, 2007; Madrid, Spain. Abstract AB887874. [DOI] [PubMed]
- 39.Schaible TD, Olive AP, Wilson DS, et al. Use of esophageal capsule endoscopy in pediatric patients with portal hypertension. Gastrointest Endosc. 2010;71 M1562. [Google Scholar]
- 40.deFranchis R, Eisen GM, Laine L, et al. Esophageal capsule endoscopy for screening and surveillance of esophageal varices in patients with portal hypertension. Hepatology. 2008;47:1595–1603. doi: 10.1002/hep.22227. [DOI] [PubMed] [Google Scholar]