Abstract
Toll-like receptors (TLRs) are the germline-coded pattern recognition receptors that sense microbial products. This signaling orchestrates complex signaling pathways that induce expression of inflammatory genes for host defense against invading microorganisms. Recent studies illustrate the role of TLRs on non-infectious inflammatory diseases. Liver has a unique anatomy bridging with intestine by portal vein and bile ducts. This allows delivery of products from intestinal microflora directly into the liver. Subsequently, microbial products cause acute and chronic inflammation through TLR signaling in the liver. Not only exogenous products, endogenous denatured products released from dying cells also facilitate inflammation even in sterile conditions. Consequently, these responses elicit tissue repairing including liver regeneration and fibrogenesis. An aberrant regenerative response may lead to hepatic carcinogenesis. In this review, we highlight the recently accumulated knowledge about TLR signaling in liver regeneration, fibrosis and carcinogenesis.
Keywords: innate immunity, LPS, partial hepatectomy, liver cirrhosis, alcoholic steatohepatitis, non-alcoholic fatty liver disease, hepatocellular carcinoma
Introduction
Inflammation is a biological response against harmful stimuli, such as infection and trauma. This response subsequently removes pathogens and damaged tissues to facilitate regeneration and wound healing response for repairing those tissues. In the liver, regenerative response occurs primarily by the division of liver parenchymal cells, hepatocytes, in response to loss of liver mass following acute inflammation or trauma1. In chronic liver disease, such as chronic hepatitis B and C, alcoholic liver disease and non-alcoholic fatty liver disease, normal liver regeneration is impaired2. In this pathological condition, a wound-healing response is quickly activated for maintaining hepatic functions and organ structures including vascular systems and bile trees in the liver3, 4. This excessive wound healing response induces production and deposition of extracellular matrix (ECM) proteins, resulting in liver fibrosis3, 4. Cirrhosis, the end stage of liver fibrosis, causes portal hypertension and severe liver dysfunctions3. In addition, aberrant regenerative responses in liver cirrhosis may cause the most serious complication, hepatocellular carcinoma, which is an irreversible and fatal liver disease5.
The innate immune system is activated in acute and chronic liver disease6-9. The liver is constantly exposed to the minimum amount of intestine-derived bacterial products through the portal vein by a unique anatomical link between intestine and liver. While liver inflammation does not occur under normal conditions, the breakdown of liver homeostasis and intestinal barrier functions may induce liver inflammation through activation of the innate immune system. Intestinal microbial products include lipopolysaccharide (LPS) and CpG-containing bacterial DNA that contain signature motifs, called pathogen-associated molecular patterns (PAMPs) 10. Germline-encoded pattern recognition receptors, Toll-like receptors (TLRs) recognize PAMPs to facilitate innate immune responses that contribute to acute and chronic liver inflammation6, 9. Recent advances have provided evidence demonstrating that TLR signaling contributes not only to liver inflammation, but also to normal and abnormal repair processes, including liver regeneration, fibrosis and carcinogenesis6, 9. This review highlights the current knowledge of TLR signaling in liver regeneration, fibrosis and carcinogenesis.
TLRs and Ligands
Drosophila Toll was originally discovered as the protein determining the dorsoventral polarity during early embryogenesis10. Later on, its antifungal functions were identified, suggesting the significant contribution of Toll protein to the innate immune system11. In the 1970s, it was recognized that C3H/HeJ mice have a defective response to LPS. At the end of the 1990s, TLRs were identified as the homologs of Drosophila Toll. Subsequently, several groups independently determined the responsible P712H mutation in Tlr4 of C3H/HeJ mice10, 12. Currently, 10 and 12 members of TLRs have been identified in humans and mice, respectively. All TLRs contain the extracellular leucin rich repeats which are responsible for PAMPs recognition, and the conserved cytoplasmic Toll/IL-1 receptor domain which is crucial for intracellular signal-transduction.
Different TLRs recognize their corresponding molecular patterns of pathogens or endogenous molecules (Table. 1). TLR4 recognizes Gram-negative bacterial cell wall component LPS12. This recognition requires the homodimerization of TLR4 and its co-receptor MD-210, 11. TLR2 is a receptor for Gram-positive bacterial cell wall components including lipoproteins. The TLR1/TLR2 heterodimeric complex senses triacyl lipoproteins, and the heterodimer of TLR2/TLR6 recognizes diacyl lipoproteins. TLR5 recognizes bacterial flagellin. In contrast to TLR1, 2, 4, 5 and 6 expressed on the cell surface, TLR3, 7, 8 and 9 are located in the intracellular endosome, and sense microbial-derived nucleic acids10, 11. CpG-containing DNA is a ligand for TLR9. Single and double stranded RNA are recognized by TLR3 and TLR7/8, respectively. Recent advanced studies demonstrated that TLRs also recognize endogenous molecules released from damaged cells, tissues and ECM as danger signals, which have been termed damage-associated molecular patterns (DAMPs). High mobility group protein B-1 (HMGB-1), hyaluronan and saturated fatty acids are recognized by TLR2 and TLR413-15. Oxidized phospholipids activate TLR4 signaling16. Endogenous nucleic acids and mitochondrial DNA activates TLR9 signaling17.
Table 1.
TLRs, ligands, endogenous ligands and localozation.
| TLR | Ligands (pathogen) | endogenous ligands | localization |
|---|---|---|---|
| TLR1 | Triacyl lipoprotein (bacteria) | β-defensin-3 | plasma membrane |
| TLR2 | Lipoprotein (bacteria, viruses, parasites) | HSP60, 70, Gp96 | plasma membrane |
| HMGB1, serum amyloid A | |||
| Hyaluronic acid | |||
| Antiphospholipid antibodies | |||
| TLR3 | dsRNA (bacteria, viruses) | mRNA | Endolysosome |
| TLR4 | LPS (bacteria) | HMGB1, fibronectin EDA, Fibrinogen, HSP60,70,72, Gp96, S100A8, S100A9, Serum amyloid A, Oxidised LDL, Saturated fatty acids Hyaluronic acid fragments Heparan sulfate fragments Antiphospholipid antibodies |
Plasma membrane |
| TLR5 | Flagellin (bacteria) | Plasma membrane | |
| TLR6 | Diacyl lipoprotein (bacteria) | Plasma membrane | |
| TLR7 | ssRNA (virus, bacteria) | ssRNA | Endolysosome |
| Antiphospholipid antibodies | |||
| TLR8 | (human) ssRNA (virus, bacteria) | ssRNA | Endolysosome |
| Antiphospholipid antibodies | |||
| TLR9 | CpG-DNA (bacteria virus, protozoa) | IgG-chromatin complex | Endolysosome |
| mitochondrial DNA | |||
| self denatured DNA | |||
| TLR10 | Unknown | Endolysosome | |
| TLR11 | Profilin-like molecule (protozoa) | Plasma membrane |
TLR Signaling Pathways --- MyD88-Dependent and –Independent Pathways
After the binding of corresponding ligands to TLRs, the intracellular signaling pathways are orchestrated through the adaptor proteins MyD88 and TRIF10, 11 (Figure 1). The MyD88-dependent pathway is activated by all TLRs except for TLR3. TLR2 and TLR4 require another adaptor protein TIRAP for bridging between TLRs and MyD88. Then, MyD88 recruits IL-1R-associated kinase (IRAK)-4, IRAK-1 and IRAK-2, and induces assembly of a multiple protein complex including TRAF6, Ubc13, TAK1, NEMO, cIAP1/2 and TRAF310, 11. The subsequent ubiquitination and degradation of TRAF6 and TRAF3 are required for activating downstream IκB kinase (IKK) complex and MAP kinases18, 19. IKK complex composed of IKKα, IKKβ and NEMO phosphorylates IκBα. Phosphorylated IκBα is ubiquitinated and degraded.
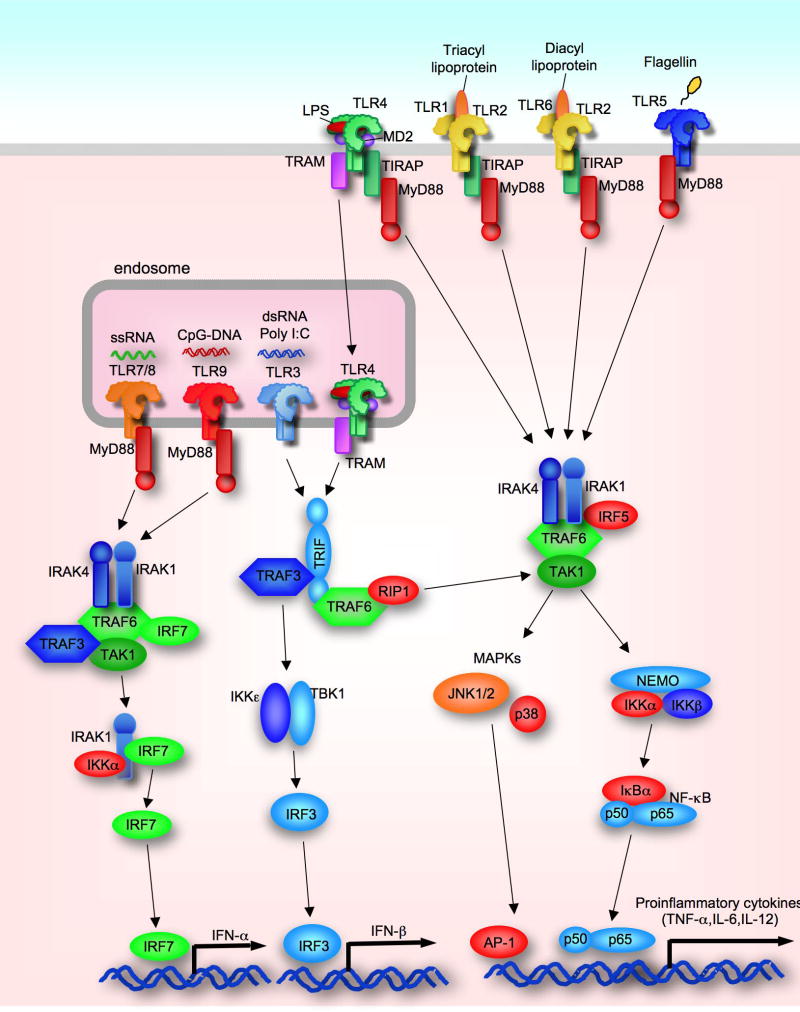
Figure 1. Schematic Overview of TLR signaling pathways.
TLR1/2, TLR2/6, TLR4, and TLR5 are expressed on plasma membrane and recognize triacyl lipopeptides, diacyl lipopeptides, LPS and flagellin, respectively. TLR3, TLR7/8 and TLR9 are located in endosome and sense dsRNA, ssRNA and CpG-DNA, respectively. All TLRs expect for TLR3 activate NF-κB and p38/JNK through MyD88. TIRAP and MyD88 are required for TLR2 and TLR4 signaling. TLR3 activate TBK1/IKKε through TRAF, and TLR4 requires both TRAM and TLR4 internalization for activation of TRIF-dependent pathway. Activated TRIF dependent pathways activate IRF-3 leading to IFN-β production. TLR7/8 and TLR9 require the complex of MyD88/IRAK1/IRF7/IKKα for induction of IFN-α.
Consequently, NF-κB is free from IκBα and translocated into the nucleus. MAP kinases including p38 and c-Jun N-terminal kinases (JNK) activate transcription factor AP-1. These transcription factors are crucial for induction of proinflammatory cytokines, such as TNF-α, IL-6 and IL-1β. On the other hand, TLR7 and TLR9 induce the complex composed of MyD88, IRAK-1, TRAF6, TRAF3, IKK-α and IRF7 that is required for induction of IFN-α10, 11.
The TRIF-dependent pathway is activated by TLR3 and TLR4. Importantly, TLR4 requires another adaptor molecule, TRAM, and its internalization for interacting with TRIF20. Then, TRIF associates with TRAF3 and TRAF621. This complex induces the activation of TANK-binding kinase 1 (TBK1) and IKKi through TRAF3. As a consequence, the pathway activates transcription factor IRF3 to induce the production of IFN-β. In addition, TRAF3 is also required for TLR-mediated IL-10 production, and TRAF6 is needed for TRIF-dependent late phase of NF-κB and MAPK activation through the interaction with RIP1 and RIP3 10, 21. In liver resident macrophages, Kupffer cells, TLR4-mediated activation of caspase-1 and subsequent induction of the active form of IL-1β and IL-18 are TRIF-dependent22, 23.
TLR Expression in the Liver
The liver is composed of hepatocytes, and several types of non-parenchymal cells9. Hepatic non-parenchymal cells are divided into immune cells and non-immune cells. Hepatic immune cells include Kupffer cells, natural killer (NK) cells, NKT cells, dendritic cells (DCs), T cells and B cells. The non-immune cells, sinusoidal endothelial cells (LSECs), hepatic stellate cells (HSCs) and biliary epithelial cells (BECs) are important components constructing the hepatic structure. All types of hepatic cells express TLRs, but their functions and expression patterns are different among the cell types.
Hepatocytes express all TLRs at the transcriptional level, but the expression levels of TLR2, TLR3, TLR4 and TLR5 are very low and their responses are fairly weak in vivo9, 24.
Kupffer cells, liver resident macrophages, reside in hepatic sinusoids and are the primary cell types for inflammatory cytokine production in response to TLR ligands6. Kupffer cells express all TLRs with the exception of TLR5 at mRNA and protein levels25. TNF-α and IL-6 are induced in response to the ligands for TLR1/2, TLR2/6, TLR3, TLR4, TLR7 and TLR9 whereas IFN-β is induced only by the stimulation with TLR3 and TLR4 ligands25. TLR1/2, TLR2/6, TLR3 and TLR4 ligands can secrete IL-1β and IL-18 by the caspase-1-dependent manner in Kupffer cells23.
HSCs are located in the space of Disse in the normal liver3. While quiescent HSCs are the principle cell type storing Vitamin A-containing lipid droplets in the body, activated HSCs are the major source of ECM protein in the fibrotic liver4. After the activation by various fibrogenic stimuli including TGF-β and PDGF, HSCs lose Vitamin A-containing lipid droplets and transdifferentiate into myofibroblasts with a high expression of α-smooth muscle actin (SMA). HSCs also express all TLRs at transcriptional levels in quiescent and activated states26. TLR4 ligand LPS induces the expression of adhesion molecules ICAM-1 and VCAM-1, and chemokines (MCP-1, MIP-1α, MIP-1β, RANTES, KC, MIP-2, and IP-10)27, 28. In addition, TLR4 signaling induces the downregulation of bone morphogenetic protein (BMP) and activin membrane bound inhibitor (Bambi), a transmembrane suppressor of TGF- β signaling28. Thus, there is a crosstalk between TLR4 signaling and TGF-β signaling. IFN-β production is induced through the adaptor TRIF by the activation of both TLR3 and TLR4 signaling in macrophages, but only TLR3 signaling induces IFN-β production in HSCs, suggesting that the TLR3- and TLR4-dependent TRIF pathways are distinct between HSCs and macrophages26.
While the response to TLR2 ligands in HSCs is weak, pretreatment of TNF-α, IL-1β or LPS dramatically upregulates TLR2 expression in HSCs28, 29. This suggests that the priming by inflammatory mediators such as TNF-α, IL-1β and LPS is required for fulfilling TLR2 signaling in HSCs. TLR9 signaling in HSCs is still controversial. Two reports demonstrated that TLR9 signaling induces the upregulation of MCP-1, TGF-β and collagen type I in HSCs30, 31. However, another study did not find CpG-DNA-induced cytokine production and NF-κB activation in HSCs 32. One possible explanation is that the studies tested the different ligands for TLR9; in the initial studies, TLR9 signaling is stimulated with denatured DNA derived from apoptotic hepatocytes, whereas another study used a synthetic CpG-containing DNA30, 32.
LSECs express most of TLRs except for TLR5 and TLR6 at mRNA and protein levels25. The stimulation of TLR3, TLR4 and TLR9 signaling induces inflammatory cytokines, such as TNF-α, IL-6 and IL-1β, but the stimulation of other TLR signaling does not. Only TLR3 signaling induces IFN-β production in LSECs25. A recent study demonstrated that TLR4 signaling-mediated angiogenesis is associated with hepatic fibrogenesis33.
The liver includes high numbers of NK cells compared with other organs34. This suggests an important role of NK cells in liver disease. Liver NK cells synthesize high amounts of IFN-γ in response to IL-12 synergistically with IL-1835. Liver NK cells express TLR1, TLR2, TLR3, TLR4, TLR6, TLR7 and TLR925, 36. These corresponding TLR agonists produce IFN-γ synergistically with IL-1236. Importantly, a TLR3 ligand poly I:C itself induces IFN-γ production and NK cell activation37, 38.
DCs are professional antigen-presenting cells in the liver. Hepatic DCs express all TLRs except for TLR525. The ligands for TLR1/2, TLR2/6, TLR3, TLR4, TLR7 and TLR9 induces the production of TNF-α and IL-6 and up-regulation of co-stimulatory molecules (CD40, CD80 and CD86) in DCs25. IFN-α is induced by stimulation with the ligands for TLR1/2, TLR3, TLR7 and TLR9, while IFN-β production is dependent on the signaling of TLR3, TLR4, TLR7 and TLR925.
BECs express TLR1, TLR2, TLR3, TLR4 and TLR5 at the protein level, and respond to the corresponding ligands39. The luminal side of BECs directly contacts enteric bacteria due to the anatomical association between the biliary system and the intestinal lumen, but inflammation does not occur. This could be regulated by “LPS tolerance”. Similarly, BECs have cross-tolerance between TLR2 and TLR4 signaling. In BECs, initial TLR2 or TLR4 stimulation induces expression of IRAK-M which suppresses secondary stimulation of TLR2 or TLR4 pathway40, 41. Interestingly, TLR3 ligand poly I:C does not induce tolerance against a second stimulation with poly I:C42.
TLR Signaling in Liver Regeneration
MyD88-Dependent Liver Regeneration after Partial Hepatectomy
In the 1990s, TNF-α and IL-6 were reported to be crucial for the initiation of liver regeneration after partial hepatectomy (PHx) 1. TNF-α produced upon PHx binds to TNFR type I to activate NF-κB and JNK/AP-1 pathways. These signals quickly induces the expression of the immediate-early genes, including c-Jun, c-Fos and c-Myc, as well as the production of IL-6 and subsequent STAT3 activation, which then drives the transition of cell cycle from G0 to G1 in hepatocytes1, 43. However, a genetic inactivation of NF-κB in hepatocytes does not reduce hepatocyte proliferation after PHx44. Thus, the TNF-NF-κB axis in hepatocytes is not essential for liver regeneration. In contrast, a quick nuclear-translocation of NF-κBp65 was observed in Kupffer cells, but not hepatocytes, after PHx45 46. In addition, Kupffer cell-depleted mice lacked PHx-induced TNF-α and IL-6 production 47. These findings suggest that Kupffer cells are the initial responders producing TNF-α and IL-6 through NF-κB activation upon PHx.
PHx-mediated elevation of portal LPS levels via bacterial translocation and its contribution to triggering liver regeneration are still being discussed. After the discovery of TLR4 as a receptor for LPS, the hypothesis that TLR signaling is an upstream signal for induction of TNF-α and IL-6 in liver regeneration has been proposed1, 43. Mice deficient in MyD88, a common adaptor molecule for TLRs, lacked activation of NF-κB in Kupffer cells, production of TNF-α, IL-6, and expression of early immediately genes c-myc, c-fos and c-jun in the liver after PHx. Interestingly, mice deficient in TLR2, TLR4 or TLR9 had a normal response after PHx45, 48. MyD88 shares the signaling with IL-1 and IL-18 as well. PHx-induced liver regeneration is normal in caspase-1-deficient mice that lack the secretion of IL-1 and IL-18 45. It is suggested that multiple TLR-MyD88-dependent signaling contributes to the activation of NF-κB, and production of TNF-α and IL-6 in Kupffer cells. Importantly, liver regeneration in MyD88-deficient mice was suppressed until 72 hours after PHx, but their regenerated liver weight at 96 hours after PHx was similar to that of WT mice45. Thus, MyD88-dependent signaling is essential for the initial phase, but not the late phase, in liver regeneration.
TLR3 that utilizes TRIF, but not MyD88, has been reported to regulate liver regeneration. TLR3-deficinet mice show the acceleration of liver regeneration after PHx, suggesting that TLR3 signaling has an inhibitory effect for liver regeneration49. Indeed, injection of TLR3 ligand polyI:C inhibits liver regeneration through the induction of IFN-γ in NK cells50.
Besides the TLR3 ligand, TLR4 ligand LPS treatment also suppresses liver regeneration51, suggesting that the magnitude of TLR signaling is important for regulating liver regeneration positively as well as negatively.
TLR Signaling in Liver Fibrosis
TLR4 Signaling Mediates Liver Fibrosis
In cirrhotic patients, LPS levels in systemic blood and the portal vein have been known to be elevated52. This suggests an important role of LPS-TLR4 signaling in the development of liver fibrosis. In experimental animal models of liver fibrosis induced by bile duct ligation (BDL) and chronic treatment of carbon tetrachloride (CCl4) or thioacetamide, deficiency of functional TLR4 reduces liver inflammation and fibrosis28. Mice deficient in CD14 and LPS-binding protein, TLR4-associated cell surface molecules, show reduced liver fibrosis induced by BDL53. Mice deficient in MyD88 and TRIF, TLR4 adaptor molecules, are also resistant to liver fibrosis28. Thus, LPS-TLR4 signaling is crucial for liver fibrosis.
Because of the specific anatomical link between intestine and liver, intestinal microflora-derived LPS is suggested to be associated with activation of TLR4 signaling in liver fibrosis54. Oral treatment with antibiotics significantly reduced the elevation of LPS levels in plasma and liver fibrosis after BDL 28. These findings suggest that intestinal microflora-derived LPS translocates into the liver via the portal vein and leads to activation of TLR4 in the liver, resulting in liver fibrosis. The contribution of intestinal microflora and TLR4 signaling to fibrogenesis in alcoholic steatohepatitis (ASH) and non-alcoholic steatohepatitis (NASH) has also been proposed54-57. Excessive intake of alcohol or high fat diet facilitates an increase of intestinal permeability that allows bacterial translocation from intestines into the liver58. Thus, TLR4 signaling is strongly associated with ASH and NASH. In fact, mice bearing non-functional TLR4 are protected from hepatic steatosis, inflammation and damage in experimental animal models of ASH as well as NASH55-57. Besides the contribution of molecular mechanisms, recent reports also demonstrated that the composition of intestinal microflora in animals with ASH and NASH was changed59-62. Several studies further tested the modification of altered intestinal flora in ASH and NASH. Treatment with probiotics, prebiotics or antibiotics suppresses the progression of fibrogenesis mediated by ASH or NASH in mice54, 59, 63.
While we do not have strong evidence for the relation between endogenous TLR4 ligands and liver fibrosis, HMGB1, hyaluronan and heat shock protein 60 are the candidates of endogenous ligands for TLR4 (Table 1)52. In both humans and rodents with cirrhosis, these ligands are elevated in the liver and blood (Seki E, unpublished observations) 52.
Interaction Between Kupffer Cells and HSCs in TLR4-Mediated Liver Fibrosis
In the liver, Kupffer cells and HSCs express high levels of TLR464. These two cell types induce the activation of NF-κB and JNK/AP-1 pathways and the production of inflammatory cytokines and chemokines in response to LPS. The relative roles of TLR4 between Kupffer cells and HSCs in liver fibrosis have been studied28. Although both Kupffer cells and HSCs are radio-resistant cells, liposomal clodronate treatment enables Kupffer cells to be depleted. Subsequent bone marrow (BM) transplantation (BMT) with whole body irradiation reconstitutes Kupffer cells, but not HSCs, with BM-derived cells. Using this technique, TLR4-chimeric mice containing different TLR4 genotypes in BM-derived cells including Kupffer cells, and endogenous liver cells including hepatocytes and HSCs were generated28. While the TLR4-chimeric mice with non-functional TLR4 expression on BM-derived cells have significant liver fibrosis similar to the TLR4-intact mice, the TLR4-chimeric mice with non-functional TLR4 expression on endogenous liver cells show a significant reduction of liver fibrosis after BDL28. Furthermore, among resident liver cells, HSCs highly respond to LPS compared with hepatocytes in vivo28. These findings indicate that HSCs are the cell types responsible for TLR4 signaling in liver fibrosis. Several mechanisms mediated by TLR4 signaling in HSCs promote liver fibrogenesis.
Firstly, TLR4 activation induces the production of various chemokines (MCP-1, MIP-1α, MIP-1β, and RANTES) and the expression of adhesion molecules (ICAM-1, VCAM-1, and E-selectin)28. These molecules induce infiltration of Kupffer cells and BM-derived macrophages in the liver. Some chemokines, such as MCP-1 and RANTES directly induce fibrogenic response in HSCs. Indeed, Kupffer cell depletion or genetic knock out in chemokines (RANTES) or chemokine receptors (CCR1, CCR2, CCR5) reduce the grades of liver fibrosis28, 65, 66 (Figure 2).
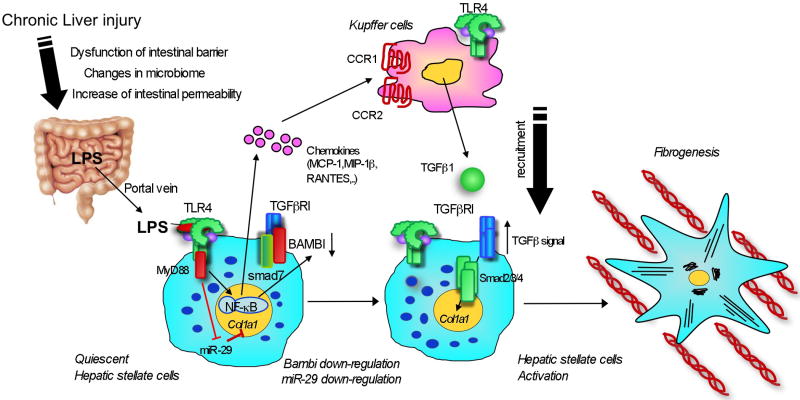
Figure 2. TLR4 regulates Fibrogenic Signal in Hepatic Stellate Cells.
Upon liver injury, intestinal permeability is increased due to the intestinal dysbiosis and tight junction disintegrity. Microflora-derived LPS translocated into the portal vein stimulates TLR4 on hepatic stellate cells (HSCs). High levels of Bambi limits TGF-β signaling in quiescent HSCs. TLR4 signaling induces the production of chemokines (MCP-1, MIP-1β and RANTES) in HSCs, recruiting Kupffer cells through their CCR1 and CCR2. Recruited Kupffer cells then produce TGF-β. Simultaneously, TLR4 signaling induces downregulation of Bambi and miR-29, leading to full-activation of HSCs.
Secondly, there is crosstalk between TLR4 signaling and TGF-b signaling in HSCs. Quiescent HSCs express high levels of Bambi, a transmembrane inhibitor of TGF-b receptor signaling28. High expression of Bambi limits TGF-b receptor signaling in quiescent HSCs. After LPS treatment or activation in vivo, but not in culture, Bambi expression is quickly downregulated in HSCs28. Consequently, TGF-b receptor is free from the restriction by Bambi, allowing induction of a strong TGF-b receptor signaling (Figure 2). TLR4-mediated Bambi regulation is dependent on MyD88, NF-κB and partially JNK, but not TRIF, in HSCs (Seki E, unpublished observation)28. A recent study demonstrated that Bambi interacts with Smad7, which interferes with the complex composed of type I and type II TGF-β receptors and Smad3, resulting in the inhibition of TGF-β signaling67.
Thirdly, LPS signaling regulates microRNA (miR) expression in liver fibrosis. During liver fibrosis, miR-29 expression is downregulated in humans and animals68. Similarly, LPS stimulation suppresses miR-29 expression in HSCs. Overexpression of miR-29 inhibits transcription of collagen a1(I) mRNA68. This suggests that TLR4 signaling suppresses miR-29 expression, and thereby enhances collagen expression in HSCs. Future studies using knockout mice will determine the role of miR-29 in liver fibrosis in vivo.
TLR4 Signaling and Human Liver Fibrosis
Previous studies reported that plasma endotoxin levels are significantly elevated in patients with cirrhosis compared to those with chronic hepatitis and in healthy subjects69. Elevated plasma endotoxin levels were observed in 41% of patients with liver cirrhosis and were correlated with disease severities, suggesting that liver fibrosis progression is closely associated with LPS-TLR4 signaling69. Furthermore, a recent human study of genecentric functional genome scans identified seven single nucleotide polymorphisms (SNPs) that may predict the risk of the progression of liver cirrhosis in patients with chronic hepatitis C. The TLR4 SNPs are included in these seven SNPs70. Among the multiple TLR4 SNPs that were identified, TLR4 SNPs T399I and D299G are the most predictive signatures in protecting the progression of liver cirrhosis. Both TLR4 T399I and D299G SNPs are associated with a blunt response of TLR4 to LPS. This large patient cohort demonstrated the relevance of TLR4 in the progression of liver fibrosis70. Their following study tested the function of TLR4 D299G and T399I SNPs in human and mouse HSCs. LX-2 human stellate cell line and TLR4-/- mouse HSCs expressing either one or both SNPs, had diminished NF-κB activation and proinflammatory cytokine production (MCP-1 and IL-6) after LPS treatment71. These SNPs also suppressed LPS-mediated Bambi downregulation and the growth of HSCs. Moreover, the TLR4 SNPs aggravated starvation-induced HSC apoptosis71. These findings confirmed the mechanistic functions of TLR4 SNPs in HSCs and liver fibrosis.
TLR4 Signaling in Kupffer Ccells and HSCs during ASH and NASH
As we discussed above, HSCs are more important than Kupffer cells in TLR4-mediated fibrogenic response. In contrast, TLR4 signaling in Kupffer cells has been determined to be a major component in alcoholic liver damage72, 73. A recent study investigated the relative roles of TLR4 between Kupffer cells and HSCs in alcohol-induced liver injury and fibrogenesis. Using TLR4-BM-chimeric mice, this study demonstrated that TLR4 on BM-derived cells including Kupffer cells is more important than that on non-BM cells including HSCs for production of inflammatory cytokines TNF-α and IL-6 and chemokines74. In contrast, both Kupffer cells and HSCs contribute to hepatocyte injury, steatosis, inflammatory cell infiltration and fibrogenic responses including upregulation of collagen a1(I), TIMP-1 and TGF-β1 mRNA expression and aSMA protein expression 74. Similar to ASH, the importance of TLR4 signaling in Kupffer cells during NASH was reported56, but the role of TLR4 on HSCs in NASH remains to be studied.
Other TLRs in Liver Fibrosis
TLR3 signaling is a potent inducer of type I interferon. A natural ligand for TLR3 is double stranded RNA which is generated during the replication of virus10. Synthetic polyinosinic-polycytidylic acid (poly I:C) is also a powerful activator of TLR3 signaling. Poly I:C treatment stimulates hepatic NK cells to produce IFN-γ 34. This signaling induces anti-viral and anti-tumor defense activities. Recent studies showed that this signaling also induces anti-fibrogenic activity. Poly I:C or IFN-γ stimulation upregulates TRAIL expression in NK cells to enhance cytotoxic activity of NK cells75. NK cells primed by poly I:C are able to kill activated HSCs, resulting in the attenuation of liver fibrosis. However, this effect is observed only in the early stage of liver fibrosis, not in advanced liver fibrosis76. Similarly, poly I:C treatment did not attenuate CCl4-mediated fibrogenic response in alcoholic liver disease. Poly I:C-NK cell-dependent HSC killing was diminished in ethanol-fed animals37. This suggests that chronic ethanol consumption results in HSCs being resistant against TLR3-dependent NK cell cytotoxicity. Thus, TLR3-mediated NK cell activation is one of the mechanisms by which hepatic fibrogenesis is aggravated in advanced liver fibrosis and alcoholic liver disease.
A major natural ligand for TLR9 is bacterial unmethylated CpG containing DNA. Besides endotoxin, bacterial DNA levels are also elevated in plasma and ascites of patients and animals with cirrhosis77-80. This shows the tight relationship between TLR9 signaling and chronic liver disease. Not only CpG-containing DNA, but denatured host DNA from dying hepatocytes also stimulates TLR9 signaling in the liver30. TLR9 signaling induces MCP-1 and collagen production and inhibits PDGF-mediated chemotaxis in HSCs. Indeed, TLR9 deficiency inhibits liver fibrosis after BDL and chronic CCl4 treatment in mice30, 31. A recent study shows that plasma bacterial DNA is elevated in diet-induced NASH, suggesting that bacterial translocation and intestinal barrier dysfunction are induced in NASH32. In NASH, TLR9 signaling is activated in Kupffer cells, but not HSCs, to produce IL-1β. This IL-1β then induces lipid accumulation and apoptosis in hepatocytes, and increases fibrogenic responses in HSCs32. Thus, both bacterial DNA and host denatured DNA from dying cells contribute to the progression of liver fibrosis through TLR9 on HSCs and Kupffer cells.
Another study shows that TLR9 on DCs is crucial for liver fibrosis. DC-depleted animals exhibited a significant reduction of liver fibrosis. DCs in the fibrotic liver, but not in the normal liver produce TNF-α, IL-6, and chemokines in response to CpG-DNA81. CpG-DNA treatment stimulates DCs of fibrotic livers to produce TNF-α that activates HSCs and enhanced NK cell cytotoxicity81.
TLR Signaling in Liver Cancer
TLRs and HCCs
There are two classification of liver cancer, primary and metastatic. Primary liver cancer includes hepatocellular carcinoma (HCC). Hepatitis B and C viral infections are major risk factors for HCC. Alcoholic and obesity-related non-alcoholic steatohepatitis also increase the risk for HCC. Recent animal studies suggest that TLRs promote hepatocarcinogenesis not only through indirect effects by aforementioned underlying diseases, but also through direct actions5. Diethylnitrosamine (DEN) is a chemical carcinogen that causes inflammation-associated HCC in rodents. Downstream of TLRs, both NF-κB and JNK/AP-1 have been identified as essential components for DEN-induced hepatocarcinogenesis82-84. TLR signaling could be associated with the development of HCC. In fact, loss of a common adaptor MyD88 diminished the incidence, number and size of DEN-induced liver cancer85. IL-6 production and hepatocyte injury and proliferation are blunted in MyD88-deficient mice after DEN treatment. As expected, mice deficient in IL-6 displayed a significant decreased tumor incidence in the liver85. Interestingly, IL-6 production is largely dependent on gender specificity. Only male animals produce IL-6 upon liver injury induced by treatment of DEN and CCl4. Removal of ovaries in female mice increased cancer incidence with high levels of IL-685. In contrast, estradiol treatment suppressed tumor development and IL-6 elevation in male mice85. Given that IL-6 production is largely dependent on MyD88 and gender in DEN models, TLR/MyD88 signaling might be regulated by gender disparity.
A more specific study has been carried out. Depletion of gut microflora by oral administration of antibiotics decreased plasma endotoxin levels and hepatic mRNA levels of TNF-α and IL-6 after DEN injection86. This results in the reduction of the number and the size of DEN-induced liver tumors in rats. Because gut microflora-derived LPS is closely associated with TLR4 signaling in the liver, the significant role of TLR4 in liver cancer was anticipated. Expectedly, TLR4-deficient mice displayed a reduction in number and size of DEN-induced liver cancer, which was associated with reduced hepatic levels of TNF-α and IL-686. The subsequent mouse experiment using TLR4 BM chimera clearly demonstrated that TLR4 on BM-derived cells, rather than endogenous liver cells, is responsible for inflammatory cytokine production after DEN treatment86.
The relation between TLR2 and liver cancer has been shown in infectious models using Listeria monocytogenes which activates TLR2 signaling. Listeria monocytogenes infection aggravated the growth of transplanted liver tumor. This effect was blunted by silencing TLR2 on tumor cells, indicating that TLR2 signaling promotes liver tumor growth87.
In patients, 53% and 85% of HCC express TLR3 and TLR9, respectively88, 89. HCC cell line expresses both TLR3 and TLR9 on cell membranes and in cytoplasm88, 89. Activation of cytoplasmic TLR3 potentiates TRAIL-mediated apoptosis by suppressing anti-apoptotic gene expression88. In contrast, activation of cell surface TLR9 induces cancer cell proliferation and thereby cancer cells become resistance against the cytotoxicity of the anti-cancer drug adriamycin89. TLR9 agonists induce upregulation of anti-apoptosis genes including survivin, Bcl-xL, XIAP and cFLIP, independently of NF-κB and type I interferon89. Although TLR9 agonists are widely accepted as candidates for anti-cancer therapy, this study suggests TLR9 agonists promote cancer.
Chronic alcohol consumption is known to potentiate hepatitis C virus (HCV)-associated hepatocarcinogenesis clinically and epidemiologically. HCV nonstructural protein NS5A transgene upregulates TLR4 expression in hepatocytes90. HCV NS5A transgenic mice are highly sensitive to LPS and ethanol in liver injury and tumor development due to TLR4 overexpression91. Indeed, TLR4 silencing decreased tumor development in HCV NS5A transgenic mice. In addition, TLR4 signaling induces expression of Nanog, a stem/progenitor cell marker91. LPS treatment promotes liver tumor development, but tumor growth was suppressed by Nanog silencing, indicating that Nanog is a crucial target for TLR4-mediated cancer growth. This further suggests that Nanog-associated cancer stem cells are involved in TLR4-mediated carcinogenesis.
TLRs, Metabolic Disease and Liver Cancer
Based on epidemiological studies, HCC incidence is significantly increased in obese patients 92. Inflammatory signaling is suggested to be involved in tumor progression in obese patients. Our research group has shown that DEN-induced hepatocarcinogenesis is enhanced in obese mice. TNF-α and IL-6/STAT3 pathways play a crucial role in tumor progression in obese mice93. IL-6 deficiency suppresses an increase of body weight and tumor development. As demonstrated, TNF receptor knock-out mice did not show any differences in tumor development after DEN injection, and regular chow diet feeding85, 93. Interestingly, however, tumor development was significantly suppressed in TNF receptor knock-out mice when DEN injection and high fat diet feeding were combined. This suggests that there is a synergistic effect between carcinogenesis and obesity, and this synergy is dependent on TNF receptor and IL-6/STAT3 signaling93. Because TNF-α and IL-6 are major downstream targets of TLR signaling, TLR signaling could be important in obesity-associated cancer progression. Instead of primary hepatocarcinogenesis, metastasis in obesity has been tested. In obese mice, colorectal cancer MC38 cells transplanted into the liver grow greater than tumors in lean mice. Silencing of TLR4 in tumor cells, but not in host livers, ameliorated tumor growth94. This demonstrated that TLR signaling is important for tumor growth in the liver.
TAK1 in Hepatocarcinogenesis
TAK1 is a downstream MAP3K activated by TLRs, IL-1 receptor, TNF receptor, and TGF-β receptor. Upon signaling activation, TAK1 activates both IKK/NF-κB and JNK/AP-1 pathways. The IKK/NF-κB pathway induces expression of anti-apoptotic genes, including Bcl-2, Bcl-xL, A20, c-FLIP and IAPs, and prevents cell death induced by death receptor and mitochondrial pathways. In parallel, NF-κB prevents JNK-mediated cell death pathway. The JNK pathway phosphorylates and ubiquitinates the E3 ligase Itch, degrading caspase-8 inhibitor c-FLIP, resulting in enhanced hepatocyte apoptosis. Both NF-κB and JNK regulate liver homeostasis and prevent hepatocyte apoptosis in normal conditions. What is the role of TAK1 on NF-κB and JNK pathways in the liver? To answer this question, hepatocyte-specific TAK1-/- mice were generated. As expected, upon TNF-α stimulation, neither NF-κB nor JNK were activated in TAK1-/- hepatocytes95. Unexpectedly, TAK1-/- hepatocytes were susceptible to TNF-α-mediated cell death, and hepatocyte-specific TAK1-/- mice displayed spontaneous liver injury, inflammation and fibrosis95, 96. Finally, aged TAK1-/- mice developed HCC95, 96. In hepatocyte-specific TAK1-/- mice, compensated hepatocyte proliferation occurs in response to spontaneous hepatocyte death. This might stimulate transformation of hepatocytes to cancer cells. This finding is consistent with the previous studies demonstrating that hepatocyte-specific IKKβ-/- mice are susceptible to DEN-induced HCC and liver-specific NEMO-/- mice develop spontaneous liver cancer82, 97. It is noteworthy that TAK1-/- mice develop HCC along with fibrosis. Thus, hepatocyte specific TAK1-/- mice will be a good tool for determining the contribution of fibrosis to liver cancer development.
Future perspective
TLR signaling pathways play a crucial role in activating innate immunity and subsequent adaptive immunity for invading microorganisms. An accumulation of recent evidence demonstrates that TLR signaling mediates acute and chronic liver inflammation even in the absence of exogenous pathogens7, 9. Although TLR signaling is a key component for initiating regeneration, tissue repair, fibrosis and carcinogenesis in the liver, TLR agonists are also considered to be a new clinical application for hepatitis virus B and C infection and cancer therapy. In fact, poly I:C inhibits liver fibrosis and cell growth of HCC cell line, and a TLR2/4 agonist inhibits transplanted liver cancer in rats88, 98. It is suggested that TLR signaling has a double-edged sword-like function in liver regeneration, fibrosis and cancer. One side could lead to beneficial effects that promote liver regeneration and prevent liver fibrosis and cancer. The other side may lead to harmful effects that inhibit liver generation and host defense, and aggravate fibrosis and cancer development. Further studies for TLR signaling in the liver are essential for developing new approaches for regenerative medicine and therapy of chronic liver disease.
Acknowledgments
This study was supported by a research grant from NIH grant R01AA020172 and R01DK085252, ABMRF and a pilot grant from the UCSD-DDRDC.
Abbreviations
- ASH
alcoholic steatohepatitis
- BDL
bile duct ligation
- BEC
biliary epithelial cells
- BM
bone marrow
- BMT
BM transplantation
- BMP
bone morphogenetic protein
- Bambi
BMP and activin membrane bound inhibitor
- CCl4
carbon tetrachloride
- DAMP
damage associated molecular pattern
- DC
dendritic cell
- DEN
diethylnitrosamine
- ECM
extracellular matrix
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HMGB-1
high mobility group protein B-1
- HSC
hepatic stellate cell
- IKK
IκB kinase
- JNK
c-Jun N-terminal kinase
- LPS
lipopolysaccharide
- LSEC
liver sinusoidal endothelial cell
- NASH
non-alcoholic steatohepatitis
- NK
natural killer
- PAMP
pathogen-associated molecular pattern
- PHx
partial hepatectomy
- poly I:C
polyinosinic-polycytidylic acid
- SMA
smooth muscle actin
- SNP
single nucleotide polymorphism
- TBK
TANK-binding kinase
- TLR
Toll-like receptor
Footnotes
Conflicts of interest: There is no conflict of interest to disclose for all authors.
References
- 1.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006 Feb;43:S45–53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 2.Fausto N. Involvement of the innate immune system in liver regeneration and injury. J Hepatol. 2006 Sep;45:347–9. doi: 10.1016/j.jhep.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008 May;134:1655–69. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005 Feb;115:209–18. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maeda S. NF-kappaB, JNK, and TLR Signaling Pathways in Hepatocarcinogenesis. Gastroenterol Res Pract. 2010:367694. doi: 10.1155/2010/367694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–63. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 7.Szabo G, Dolganiuc A, Mandrekar P. Pattern recognition receptors: a contemporary view on liver diseases. Hepatology. 2006 Aug;44:287–98. doi: 10.1002/hep.21308. [DOI] [PubMed] [Google Scholar]
- 8.Schwabe RF, Seki E, Brenner DA. Toll-like receptor signaling in the liver. Gastroenterology. 2006 May;130:1886–900. doi: 10.1053/j.gastro.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 9.Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. 2008 Jul;48:322–35. doi: 10.1002/hep.22306. [DOI] [PubMed] [Google Scholar]
- 10.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. Mar 19;140:805–20. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto M, Takeda K. Current views of toll-like receptor signaling pathways. Gastroenterol Res Pract. 2010:240365. doi: 10.1155/2010/240365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998 Dec 11;282:2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 13.Tsung A, Sahai R, Tanaka H, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005 Apr 4;201:1135–43. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang D, Liang J, Fan J, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005 Nov;11:1173–9. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 15.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006 Nov;116:3015–25. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imai Y, Kuba K, Neely GG, et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008 Apr 18;133:235–49. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Q, Raoof M, Chen Y, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. Mar 4;464:104–7. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tseng PH, Matsuzawa A, Zhang W, Mino T, Vignali DA, Karin M. Different modes of ubiquitination of the adaptor TRAF3 selectively activate the expression of type I interferons and proinflammatory cytokines. Nat Immunol. Jan;11:70–5. doi: 10.1038/ni.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vallabhapurapu S, Matsuzawa A, Zhang W, et al. Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-kappaB signaling. Nat Immunol. 2008 Dec;9:1364–70. doi: 10.1038/ni.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008 Apr;9:361–8. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hacker H, Redecke V, Blagoev B, et al. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature. 2006 Jan 12;439:204–7. doi: 10.1038/nature04369. [DOI] [PubMed] [Google Scholar]
- 22.Tsutsui H, Imamura M, Fujimoto J, Nakanishi K. The TLR4/TRIF-Mediated Activation of NLRP3 Inflammasome Underlies Endotoxin-Induced Liver Injury in Mice. Gastroenterol Res Pract. 2010:641865. doi: 10.1155/2010/641865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imamura M, Tsutsui H, Yasuda K, et al. Contribution of TIR domain-containing adapter inducing IFN-βeta-mediated IL-18 release to LPS-induced liver injury in mice. J Hepatol. 2009 Aug;51:333–41. doi: 10.1016/j.jhep.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 24.Isogawa M, Robek MD, Furuichi Y, Chisari FV. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J Virol. 2005 Jun;79:7269–72. doi: 10.1128/JVI.79.11.7269-7272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu J, Meng Z, Jiang M, et al. Toll-like receptor-induced innate immune responses in non-parenchymal liver cells are cell type-specific. Immunology. Mar;129:363–74. doi: 10.1111/j.1365-2567.2009.03179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang B, Trippler M, Pei R, et al. Toll-like receptor activated human and murine hepatic stellate cells are potent regulators of hepatitis C virus replication. J Hepatol. 2009 Dec;51:1037–45. doi: 10.1016/j.jhep.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 27.Paik YH, Schwabe RF, Bataller R, Russo MP, Jobin C, Brenner DA. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology. 2003 May;37:1043–55. doi: 10.1053/jhep.2003.50182. [DOI] [PubMed] [Google Scholar]
- 28.Seki E, De Minicis S, Osterreicher CH, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007 Nov;13:1324–32. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 29.Paik YH, Lee KS, Lee HJ, et al. Hepatic stellate cells primed with cytokines upregulate inflammation in response to peptidoglycan or lipoteichoic acid. Lab Invest. 2006 Jul;86:676–86. doi: 10.1038/labinvest.3700422. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe A, Hashmi A, Gomes DA, et al. Apoptotic hepatocyte DNA inhibits hepatic stellate cell chemotaxis via toll-like receptor 9. Hepatology. 2007 Nov;46:1509–18. doi: 10.1002/hep.21867. [DOI] [PubMed] [Google Scholar]
- 31.Gabele E, Muhlbauer M, Dorn C, et al. Role of TLR9 in hepatic stellate cells and experimental liver fibrosis. Biochem Biophys Res Commun. 2008 Nov 14;376:271–6. doi: 10.1016/j.bbrc.2008.08.096. [DOI] [PubMed] [Google Scholar]
- 32.Miura K, Kodama Y, Inokuchi S, et al. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1βeta in mice. Gastroenterology. Jul;139:323–34 e7. doi: 10.1053/j.gastro.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jagavelu K, Routray C, Shergill U, O'Hara SP, Faubion W, Shah VH. Endothelial cell toll-like receptor 4 regulates fibrosis-associated angiogenesis in the liver. Hepatology. Aug;52:590–601. doi: 10.1002/hep.23739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao B, Jeong WI, Tian Z. Liver: An organ with predominant innate immunity. Hepatology. 2008 Feb;47:729–36. doi: 10.1002/hep.22034. [DOI] [PubMed] [Google Scholar]
- 35.Tsutsui H, Adachi K, Seki E, Nakanishi K. Cytokine-induced inflammatory liver injuries. Curr Mol Med. 2003 Sep;3:545–59. doi: 10.2174/1566524033479618. [DOI] [PubMed] [Google Scholar]
- 36.Sawaki J, Tsutsui H, Hayashi N, et al. Type 1 cytokine/chemokine production by mouse NK cells following activation of their TLR/MyD88-mediated pathways. Int Immunol. 2007 Mar;19:311–20. doi: 10.1093/intimm/dxl148. [DOI] [PubMed] [Google Scholar]
- 37.Jeong WI, Park O, Gao B. Abrogation of the antifibrotic effects of natural killer cells/interferon-gamma contributes to alcohol acceleration of liver fibrosis. Gastroenterology. 2008 Jan;134:248–58. doi: 10.1053/j.gastro.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yin S, Gao B. Toll-like receptor 3 in liver diseases. Gastroenterol Res Pract. 2010 doi: 10.1155/2010/750904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harada K, Isse K, Nakanuma Y. Interferon gamma accelerates NF-kappaB activation of biliary epithelial cells induced by Toll-like receptor and ligand interaction. J Clin Pathol. 2006 Feb;59:184–90. doi: 10.1136/jcp.2004.023507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harada K, Nakanuma Y. Biliary innate immunity: function and modulation. Mediators Inflamm. 2010 doi: 10.1155/2010/373878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harada K, Nakanuma Y. Biliary innate immunity in the pathogenesis of biliary diseases. Inflamm Allergy Drug Targets. Jun;9:83–90. doi: 10.2174/187152810791292809. [DOI] [PubMed] [Google Scholar]
- 42.Harada K, Sato Y, Isse K, Ikeda H, Nakanuma Y. Induction of innate immune response and absence of subsequent tolerance to dsRNA in biliary epithelial cells relate to the pathogenesis of biliary atresia. Liver Int. 2008 May;28:614–21. doi: 10.1111/j.1478-3231.2008.01740.x. [DOI] [PubMed] [Google Scholar]
- 43.Iimuro Y, Fujimoto J. TLRs, NF-kappaB, JNK, and Liver Regeneration. Gastroenterol Res Pract. 2010 doi: 10.1155/2010/598109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chaisson ML, Brooling JT, Ladiges W, Tsai S, Fausto N. Hepatocyte-specific inhibition of NF-kappaB leads to apoptosis after TNF treatment, but not after partial hepatectomy. J Clin Invest. 2002 Jul;110:193–202. doi: 10.1172/JCI15295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seki E, Tsutsui H, Iimuro Y, et al. Contribution of Toll-like receptor/myeloid differentiation factor 88 signaling to murine liver regeneration. Hepatology. 2005 Mar;41:443–50. doi: 10.1002/hep.20603. [DOI] [PubMed] [Google Scholar]
- 46.Yang L, Magness ST, Bataller R, Rippe RA, Brenner DA. NF-kappaB activation in Kupffer cells after partial hepatectomy. Am J Physiol Gastrointest Liver Physiol. 2005 Sep;289:G530–8. doi: 10.1152/ajpgi.00526.2004. [DOI] [PubMed] [Google Scholar]
- 47.Abshagen K, Eipel C, Kalff JC, Menger MD, Vollmar B. Loss of NF-kappaB activation in Kupffer cell-depleted mice impairs liver regeneration after partial hepatectomy. Am J Physiol Gastrointest Liver Physiol. 2007 Jun;292:G1570–7. doi: 10.1152/ajpgi.00399.2006. [DOI] [PubMed] [Google Scholar]
- 48.Campbell JS, Riehle KJ, Brooling JT, Bauer RL, Mitchell C, Fausto N. Proinflammatory cytokine production in liver regeneration is Myd88-dependent, but independent of Cd14, Tlr2, and Tlr4. J Immunol. 2006 Feb 15;176:2522–8. doi: 10.4049/jimmunol.176.4.2522. [DOI] [PubMed] [Google Scholar]
- 49.Zorde-Khvalevsky E, Abramovitch R, Barash H, et al. Toll-like receptor 3 signaling attenuates liver regeneration. Hepatology. 2009 Jul;50:198–206. doi: 10.1002/hep.22973. [DOI] [PubMed] [Google Scholar]
- 50.Sun R, Gao B. Negative regulation of liver regeneration by innate immunity (natural killer cells/interferon-gamma) Gastroenterology. 2004 Nov;127:1525–39. doi: 10.1053/j.gastro.2004.08.055. [DOI] [PubMed] [Google Scholar]
- 51.Akita K, Okuno M, Enya M, et al. Impaired liver regeneration in mice by lipopolysaccharide via TNF-αlpha/kallikrein-mediated activation of latent TGF-beta. Gastroenterology. 2002 Jul;123:352–64. doi: 10.1053/gast.2002.34234. [DOI] [PubMed] [Google Scholar]
- 52.Pradere JP, Troeger JS, Dapito DH, Mencin AA, Schwabe RF. Toll-like receptor 4 and hepatic fibrogenesis. Semin Liver Dis. Aug;30:232–44. doi: 10.1055/s-0030-1255353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Isayama F, Hines IN, Kremer M, et al. LPS signaling enhances hepatic fibrogenesis caused by experimental cholestasis in mice. Am J Physiol Gastrointest Liver Physiol. 2006 Jun;290:G1318–28. doi: 10.1152/ajpgi.00405.2005. [DOI] [PubMed] [Google Scholar]
- 54.Son G, Kremer M, Hines IN. Contribution of gut bacteria to liver pathobiology. Gastroenterol Res Pract. 2010 doi: 10.1155/2010/453563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hritz I, Mandrekar P, Velayudham A, et al. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008 Oct;48:1224–31. doi: 10.1002/hep.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rivera CA, Adegboyega P, van Rooijen N, Tagalicud A, Allman M, Wallace M. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol. 2007 Oct;47:571–9. doi: 10.1016/j.jhep.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Csak T, Velayudham A, Hritz I, et al. Deficiency in myeloid differentiation factor-2 and Toll-like receptor 4 expression attenuates non-alcoholic steatohepatitis and fibrosis in mice. Am J Physiol Gastrointest Liver Physiol. Jan 13; doi: 10.1152/ajpgi.00163.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Szabo G, Bala S. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol. Mar 21;16:1321–9. doi: 10.3748/wjg.v16.i11.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yan AW, F DE, Brandl J, et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. Jan;53:96–105. doi: 10.1002/hep.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol. Aug;299:G440–8. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tilg H. Obesity, metabolic syndrome, and microbiota: multiple interactions. J Clin Gastroenterol. Sep;44(Suppl 1):S16–8. doi: 10.1097/MCG.0b013e3181dd8b64. [DOI] [PubMed] [Google Scholar]
- 62.Murphy EF, Cotter PD, Healy S, et al. Composition and energy harvesting capacity of the gut microbiota: relationship to diet, obesity and time in mouse models. Gut. Dec;59:1635–42. doi: 10.1136/gut.2010.215665. [DOI] [PubMed] [Google Scholar]
- 63.Velayudham A, Dolganiuc A, Ellis M, et al. VSL#3 probiotic treatment attenuates fibrosis without changes in steatohepatitis in a diet-induced nonalcoholic steatohepatitis model in mice. Hepatology. 2008 Dec 29; doi: 10.1002/hep.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aoyama T, Paik YH, Seki E. Toll-like receptor signaling and liver fibrosis. Gastroenterol Res Pract. 2010 doi: 10.1155/2010/192543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seki E, De Minicis S, Gwak GY, et al. CCR1 and CCR5 promote hepatic fibrosis in mice. J Clin Invest. 2009 Jul;119:1858–70. doi: 10.1172/JCI37444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seki E, de Minicis S, Inokuchi S, et al. CCR2 promotes hepatic fibrosis in mice. Hepatology. 2009 Jul;50:185–97. doi: 10.1002/hep.22952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yan X, Lin Z, Chen F, et al. Human BAMBI cooperates with Smad7 to inhibit transforming growth factor-beta signaling. J Biol Chem. 2009 Oct 30;284:30097–104. doi: 10.1074/jbc.M109.049304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roderburg C, Urban GW, Bettermann K, et al. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology. Jan;53:209–18. doi: 10.1002/hep.23922. [DOI] [PubMed] [Google Scholar]
- 69.Lin RS, Lee FY, Lee SD, et al. Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J Hepatol. 1995 Feb;22:165–72. doi: 10.1016/0168-8278(95)80424-2. [DOI] [PubMed] [Google Scholar]
- 70.Huang H, Shiffman ML, Friedman S, et al. A 7 gene signature identifies the risk of developing cirrhosis in patients with chronic hepatitis C. Hepatology. 2007 Aug;46:297–306. doi: 10.1002/hep.21695. [DOI] [PubMed] [Google Scholar]
- 71.Li Y, Chang M, Abar O, et al. Multiple variants in toll-like receptor 4 gene modulate risk of liver fibrosis in Caucasians with chronic hepatitis C infection. J Hepatol. 2009 Oct;51:750–7. doi: 10.1016/j.jhep.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001 Jul;34:101–8. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- 73.Adachi Y, Bradford BU, Gao W, Bojes HK, Thurman RG. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology. 1994 Aug;20:453–60. [PubMed] [Google Scholar]
- 74.Inokuchi S, Tsukamoto H, Park EJ, Liu ZX, Brenner DA, Seki E. Toll-like receptor 4 mediates alcohol-induced steatohepatitis through bone marrow-derived and endogenous liver cells in mice. Alcohol Clin Exp Res. 2011;35 doi: 10.1111/j.1530-0277.2011.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Radaeva S, Sun R, Jaruga B, Nguyen VT, Tian Z, Gao B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology. 2006 Feb;130:435–52. doi: 10.1053/j.gastro.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 76.Jeong WI, Park O, Suh YG, et al. Suppression of innate immunity (natural killer cell/interferon-γ) in the advanced stages of liver fibrosis in mice. Hepatology. 2011 doi: 10.1002/hep.24190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Frances R, Benlloch S, Zapater P, et al. A sequential study of serum bacterial DNA in patients with advanced cirrhosis and ascites. Hepatology. 2004 Feb;39:484–91. doi: 10.1002/hep.20055. [DOI] [PubMed] [Google Scholar]
- 78.Frances R, Zapater P, Gonzalez-Navajas JM, et al. Bacterial DNA in patients with cirrhosis and noninfected ascites mimics the soluble immune response established in patients with spontaneous bacterial peritonitis. Hepatology. 2008 Mar;47:978–85. doi: 10.1002/hep.22083. [DOI] [PubMed] [Google Scholar]
- 79.Guarner C, Gonzalez-Navajas JM, Sanchez E, et al. The detection of bacterial DNA in blood of rats with CCl4-induced cirrhosis with ascites represents episodes of bacterial translocation. Hepatology. 2006 Sep;44:633–9. doi: 10.1002/hep.21286. [DOI] [PubMed] [Google Scholar]
- 80.Guarner C, Runyon BA, Young S, Heck M, Sheikh MY. Intestinal bacterial overgrowth and bacterial translocation in cirrhotic rats with ascites. J Hepatol. 1997 Jun;26:1372–8. doi: 10.1016/s0168-8278(97)80474-6. [DOI] [PubMed] [Google Scholar]
- 81.Connolly MK, Bedrosian AS, Mallen-St Clair J, et al. In liver fibrosis, dendritic cells govern hepatic inflammation in mice via TNF-αlpha. J Clin Invest. 2009 Nov;119:3213–25. doi: 10.1172/JCI37581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKβeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005 Jul 1;121:977–90. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 83.Eferl R, Ricci R, Kenner L, et al. Liver tumor development. c-Jun antagonizes the proapoptotic activity of p53. Cell. 2003 Jan 24;112:181–92. doi: 10.1016/s0092-8674(03)00042-4. [DOI] [PubMed] [Google Scholar]
- 84.Sakurai T, Maeda S, Chang L, Karin M. Loss of hepatic NF-kappa B activity enhances chemical hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1 activation. Proc Natl Acad Sci U S A. 2006 Jul 11;103:10544–51. doi: 10.1073/pnas.0603499103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Naugler WE, Sakurai T, Kim S, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007 Jul 6;317:121–4. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 86.Yu LX, Yan HX, Liu Q, et al. Endotoxin accumulation prevents carcinogen-induced apoptosis and promotes liver tumorigenesis in rodents. Hepatology. Oct;52:1322–33. doi: 10.1002/hep.23845. [DOI] [PubMed] [Google Scholar]
- 87.Huang B, Zhao J, Shen S, et al. Listeria monocytogenes promotes tumor growth via tumor cell toll-like receptor 2 signaling. Cancer Res. 2007 May 1;67:4346–52. doi: 10.1158/0008-5472.CAN-06-4067. [DOI] [PubMed] [Google Scholar]
- 88.Yoneda K, Sugimoto K, Shiraki K, et al. Dual topology of functional Toll-like receptor 3 expression in human hepatocellular carcinoma: differential signaling mechanisms of TLR3-induced NF-kappaB activation and apoptosis. Int J Oncol. 2008 Nov;33:929–36. [PubMed] [Google Scholar]
- 89.Tanaka J, Sugimoto K, Shiraki K, et al. Functional cell surface expression of toll-like receptor 9 promotes cell proliferation and survival in human hepatocellular carcinomas. Int J Oncol. Oct;37:805–14. [PubMed] [Google Scholar]
- 90.Machida K, Cheng KT, Sung VM, Levine AM, Foung S, Lai MM. Hepatitis C virus induces toll-like receptor 4 expression, leading to enhanced production of beta interferon and interleukin-6. J Virol. 2006 Jan;80:866–74. doi: 10.1128/JVI.80.2.866-874.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Machida K, Tsukamoto H, Mkrtchyan H, et al. Toll-like receptor 4 mediates synergism between alcohol and HCV in hepatic oncogenesis involving stem cell marker Nanog. Proc Natl Acad Sci U S A. 2009 Feb 3;106:1548–53. doi: 10.1073/pnas.0807390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003 Apr 24;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 93.Park EJ, Lee JH, Yu GY, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. Jan 22;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Earl TM, Nicoud IB, Pierce JM, et al. Silencing of TLR4 decreases liver tumor burden in a murine model of colorectal metastasis and hepatic steatosis. Ann Surg Oncol. 2009 Apr;16:1043–50. doi: 10.1245/s10434-009-0325-8. [DOI] [PubMed] [Google Scholar]
- 95.Inokuchi S, Aoyama T, Miura K, et al. Disruption of TAK1 in hepatocytes causes hepatic injury, inflammation, fibrosis, and carcinogenesis. Proc Natl Acad Sci U S A. 2010 Jan 12;107:844–9. doi: 10.1073/pnas.0909781107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bettermann K, Vucur M, Haybaeck J, et al. TAK1 Suppresses a NEMO-Dependent but NF-kappaB-Independent Pathway to Liver Cancer. Cancer Cell. May 18;17:481–96. doi: 10.1016/j.ccr.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 97.Luedde T, Beraza N, Kotsikoris V, et al. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell. 2007 Feb;11:119–32. doi: 10.1016/j.ccr.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 98.Yu P, Cheng X, Guo J, Wang X. Toll-like receptors 2/4 agonists: a potential strategy for preventing invasion and metastasis of hepatocellular carcinoma. Gut. Oct;59:1447–8. doi: 10.1136/gut.2009.190835. author reply 8-9. [DOI] [PubMed] [Google Scholar]