Summary
A number of viral membrane fusion proteins can be expressed alone on the surface of host cells, then triggered to induce cell-to-cell fusion or syncytium formation. Although rapid and easily observed, syncytium formation is not easily quantified and differences in fusion activity are not easily distinguished or measured. To address this problem, we developed a rapid and quantitative cell-to-cell fusion system that is useful for comparative analysis and may be suitable for high throughput screening. In this system, expression of a reporter protein, the enhanced green fluorescent protein (EGFP), is dependent on cell-to-cell fusion. Spodoptera frugiperda (Sf9) insect cells expressing a chimeric Lac Repressor-IE1 protein were fused to Sf9 cells containing an EGFP reporter construct under the control of a responsive lac operator containing promoter. Membrane fusion efficiency was measured from the resulting EGFP fluorescence activity. Sf9 cells expressing the Orgyia pseudotsugata Multicapsid Nucleopolyhedrovirus (OpMNPV) GP64 envelope fusion protein were used as a model to test this fusion assay. Subtle changes in fusion activities of GP64 proteins containing single amino acid substitutions in a putative membrane fusion domain were distinguished, and decreases in EGFP fluorescence corresponded to decreases in the hydrophobicity in the small putative membrane fusion domain.
Introduction
The entry of enveloped animal viruses into host cells usually involves membrane fusion either at the cell surface or within the endosome. Eukaryotic cellular membrane fusion also occurs during a diverse array of processes including endocytosis, exocytosis, organelle trafficking, and cell division (Lindau & Almers, 1995; Rothman & Sollner, 1997; Steinman et al., 1983). Membrane fusion does not occur spontaneously and cells employ complex arrays of proteins to catalyze and regulate the localized fusion of membranes (Gotte & von Mollard, 1998; Hsu et al., 1999). Enveloped viruses also utilize this process to enter host cells although the regulatory mechanisms appear to differ considerably in comparison to cellular membrane fusion (for review see (Hughson, 1995; Stegmann et al., 1989; Weissenhorn et al., 1999)). Some viruses require only a single membrane fusion protein. Examples include the influenza virus hemagglutinin protein (HA) (Doms et al., 1985), the rhabdovirus G protein (Florkiewicz & Rose, 1984), and the baculovirus GP64 envelope fusion protein (Blissard & Wenz, 1992; Volkman & Goldsmith, 1985). Viral fusion proteins can be further subdivided into those from viruses entering cells by fusion at the cell surface, and those from viruses that enter cells through receptor-mediated endocytosis. For proteins that mediate fusion at the cell surface, regulation of membrane fusion may require multiple receptor interactions as in the case of the HIV-1 envelope protein, GP120/41 (Choe et al., 1996; Deng et al., 1996; Feng et al., 1996). For viruses that enter via endocytosis, the acidification of the endosome frequently serves as a trigger to activate the fusion activity of the viral membrane fusion protein. Influenza HA, rhabdovirus G, and baculovirus GP64 are examples of acid triggered fusion proteins.
In many cases, transiently expressed viral membrane fusion proteins are transported to the surfaces of cells. This permits the study of enveloped virus fusion proteins, independently from other viral proteins. When fusion proteins are expressed on the surfaces of cells that are in contact with other cells, triggering of the protein’s activity can result in cell-to-cell fusion. In the case of acid triggered fusion proteins, lowering the extracellular pH is often sufficient to induce cell-to-cell fusion. The multinucleate masses that form after cell-to-cell fusion are referred to as “syncytia.” Although easily observed, syncytium formation is difficult and time consuming to quantify. Quantification of cell-to-cell fusion is desirable as it permits a more sensitive assessment of the molecular and biophysical activities of fusion proteins. In addition, quantitative or semi-quantitative assays for membrane fusion that are suitable for multi-well formats could expedite the discovery of drugs that repress membrane fusion.
A relatively recent strategy for quantifying cell-to-cell fusion involves a vaccinia virus expressed T7 RNA polymerase and a reporter gene under the control of a T7 promoter (Bagai & Lamb, 1995; Fuerst et al., 1986; Nussbaum et al., 1994). The Vaccinia/T7 fusion assay has been extremely useful (Camerini et al., 1994; Nussbaum et al., 1994) although a disadvantage of that system is the use of vaccinia virus to deliver the T7 RNA polymerase. Vaccinia virus expresses its own acid induced membrane fusion protein (Doms et al., 1990; Rodriguez & Esteban, 1987) and cells and membranes may be modified by Vaccinia virus infection. In addition, a vaccinia virus based system can not be utilized in cells that are not permissive for vaccinia virus infection. In many cases, it may be advantageous to examine viral fusion proteins and their receptors in a heterologous (for example, non-mammalian) cell environment. The absence of the background of other mammalian cell surface proteins will permit the study of specific proteins in isolation and will therefore simplify interpretation of results.
In the current studies, we used the baculovirus GP64 envelope fusion protein as a model viral envelope fusion protein for development of a new membrane fusion assay. GP64 is the membrane fusion protein of budded virions (BV) of Autographa californica MNPV (AcMNPV), OpMNPV, and a number of other baculoviruses (Blissard & Wenz, 1992; Monsma et al., 1996). Using site directed mutagenesis, a putative “fusion domain” was identified within the GP64 protein and reductions in the hydrophobicity of the small putative fusion domain appeared to incrementally affect the triggering of GP64 fusion activity (Monsma & Blissard, 1995). In the current study, we developed a quantitative membrane fusion assay based on fusion-dependent promoter activation and EGFP reporter expression. In this system, a LacR-IE1 transcriptional activator (Slack & Blissard, 1997) is transiently expressed in one group of cells, while a second group of cells is transfected with a plasmid containing the enhanced green fluorescent protein (EGFP) gene under the control of a LacR-IE1 regulated promoter. Fusion of the 2 cell groups by a protein such as GP64, results in promoter activation and expression of the EGFP reporter. The capacity of this LacR-IE1-based fusion assay to distinguish differences in fusion activity was examined using the baculovirus GP64 envelope fusion protein and several previously reported fusion domain mutants (Monsma & Blissard, 1995). Using quantitative fluorescent fusion data from GP64 constructs containing mutations in the putative fusion domain, we were able to quantitatively assess the effects of fusion domain mutants.
Methods
Plasmid Constructs
Plasmid pEGFP-92lacO, a plasmid containing the EGFP gene under the control of an inducible promoter containing a lac operator sequence was derived from a previously described plasmid, p64CAT-92lacO (Slack & Blissard, 1997). Using two promoter specific primers (5′-taatacgactcacta t-3′ and 5′-accatcttgtag atcttgtagtgtt-3′), the 225 bp promoter region from p64CAT-92lacO was amplified and a BglII site was engineered into the 3′ end of the promoter (9 bp upstream of the CAT ORF in plasmid p64CAT-92lacO). The promoter-containing PCR product was digested with EcoRI and BglII and cloned into the EcoRI and BamHI sites in plasmid pEGFP-N1 (Clontech) such that the – 92lacO promoter region was 18 bp upstream of the EGFP translation start site and such that the mammalian-specific CMV-IE promoter of pEGFP-N1 was displaced 189 bp upstream from the EGFP translation start site. Preliminary results indicated that the CMV promoter was not functional in Sf9 cells and did not otherwise facilitate expression of EGFP in Sf9 cells.
The plasmid construct NLacR-IE11,2ABCD(M1-G222) (Slack & Blissard, 1997) encodes a Lac Repressor-IE1 chimera under the control of a baculovirus early promoter. The chimeric protein is expressed at high levels in transiently transfected Sf9 cells. The chimeric protein expressed from this construct contains an N-terminal SV-40 nuclear localization signal, the DNA binding domain and dimerization domain from the E.coli Lac Repressor (M1-P333), and the bipartite transactivation domain (M1-G222) from the AcMNPV IE1 transcriptional activator. For simplicity the NLacR-IE11,2ABCD(M1-G222) protein will be referred to here as “LacR-IE1.” The plasmid construct, p64-166 encodes the gp64 gene from OpMNPV and 166 base pairs of that gene’s promoter (Blissard & Rohrmann, 1991). OpMNPV gp64 mutants, L226M, L227M and L226M/L227M were previously generated by site directed mutagenesis (Monsma & Blissard, 1995). The plasmid vector pBS (Stratagene) was used as a control plasmid in transfections.
Cell Transfections
Prior to transfection, all Spodoptera frugiperda (Sf9) cells were grown in spinner flasks in TNM-FH medium (GIBCO BRL) supplemented with 10% (v/v) fetal bovine serum (FBS). For transfections, cells were centrifuged at 1000 × g for 5 min and resuspended at 1×106 cells/ml in Grace’s medium (GIBCO BRL) plus 10% FBS. After plating and attachment in T-flasks, cells were transfected with DNA in transfection buffer (25 mM HEPES, 140 mM NaCl, 125 mM CaCl2, pH 7.1) using the Ca2PO4 precipitation method (Blissard & Rohrmann, 1991). For all transfections, CsCl purified supercoiled DNA was used. Transfections were performed in either 75 cm2 or 150 cm2 T-flasks containing either 1.5×107 or 2.5×107 Sf9 cells, respectively. Cells transfected with the pEGFP-92lacO plasmid were transfected in 150 cm2 T-flasks with 200 μg of plasmid. A second set of cells was transfected simultaneously with plasmids NLacR-IE11,2ABCD(M1-G222) and p64-166 (Blissard & Rohrmann, 1991) (50 μg of each plasmid) in a 75 cm2 T-flask. The second set of cells is referred to as “LacR-IE1/gp64 transfected cells.” LacR-IE1/gp64 DNA was mixed with 8 ml of transfection buffer then slowly added dropwise to 8 ml of Grace’s medium plus 10% FBS in each 75 cm2 flask. Corresponding volumes were 12 ml for 150 cm2 flasks. After 3 h, the transfection mixture was removed and replaced with TNM-FH medium supplemented with 10% FBS.
Cell Fusion Assay
At 24 h post transfection, TNM-FH medium was removed and cells were suspended in Graces medium (pH 6.2) plus 10% FBS at a cell density of 1×106 cells/ml. This was done to remove any residual DNA and to improve adhesion of cells to tissue culture plates. The pEGFP-92lacO transfected cells were mixed 1:1 with the LacR-IE1/gp64 transfected cells. Aliquots of 0.5 ml from each cell mixture (5×105 cells) were deposited into each well of a 24-well plate (Costar) and cells were allowed to attach for 1 h. For pH shift experiments, Graces medium was adjusted to various pH values with sodium citrate and cells were incubated in pH-adjusted Grace’s medium for 20 min at 25 °C, then returned to TNM-FH medium plus 10% FBS (pH 6.0).
EGFP detection and measurement
At 24 h post pH shift (48 h post transfection), the medium from each well (24 well plate) was aspirated and replaced with 400 μl of Graces medium. EGFP-specific fluorescence was read directly from 24-well plates in a Fluorescence plate reader (Dynex FL1000). To maximize detection of EGFP signal strength, Graces medium was aspirated from each well and replaced with 300 μl EGFP lysis buffer (Graces medium, 0.5 % Triton X-100, 0.7 μg/ml Pepstatin, 10 μg/ml Leupeptin, 2.5 mM EDTA and 1 μM E64). After addition of EGFP lysis buffer, cells were lysed by placing the multiwell plates on an orbital shaker at approximately 60 rpm for 1 h at 4 °C. While on ice, aliquots of 150 μl of disrupted cell lysates were transferred from 24-well plates to black 96-well U-bottom plates (Dynex). Samples were read for EGFP-specific fluorescence on a fluorescence microplate reader (480 nm Excitation; 515 nm Emission; 6 Volts).
Western blots
To confirm that cells were expressing similar levels of GP64 prior to induction of fusion activity by pH shift, 0.5 ml cell samples were removed and washed 2× with 1 ml PBS (pH 6.2) followed by resuspension (5×103 cells/μl) in 1 × Laemmli disruption buffer (Laemmli, 1970). Cell proteins (from 5×104 cells per lane) were resolved in 8% SDS-PAGE gels, blotted to Immobilon-P membrane (Millipore), and probed for GP64 using monoclonal antibody AcV5 (Hohmann & Faulkner, 1983) diluted 1:500 in PBS (pH 7.6). An alkaline phosphatase conjugated goat anti-mouse secondary antibody (Pierce) was used with nitroblue tetrazolium/5-bromo-4-chloro-3-indoylphosphate (NBT/BCIP) to detect GP64.
Immunofluorescence microscopy
For immunofluorescence microscopy, cells and syncytia were fixed for 45 min in 2.5% paraformaldehyde in Graces medium. Cells were washed 3× in PBS, then incubated for 45 min with monoclonal antibody AcV5 diluted 1:50 in PBS plus 10% BSA (pH 7.6). Cells were washed 3× in PBS, then incubated with a secondary goat anti-mouse monoclonal antibody conjugated to tetramethyl rhodamine isothiocyanate (TRITC) (Pierce) diluted 1:100 in PBS plus 10% BSA (pH 7.6). Examples of syncytia that were positive for EGFP expression and GP64 expression were photographed under visible light and examined also by epifluorescence microscopy (EGFP, 490 nm excitation filter; and TRITC, 545 nm excitation filter).
Results and Discussion
In previous studies, we used a lac operator - Lac Repressor based system to map a highly active transcriptional activation domain from the AcMNPV IE1 protein (Slack & Blissard, 1997). Lac Repressor - IE1 fusions (LacR-IE1) were used to identify IE1 domains capable of high level (almost 2000 fold) transcriptional activation. In the current studies, we used the most potent LacR-IE1 chimera in combination with the responsive promoter (containing a lac operator) to develop a fusion-dependent promoter activation system, suitable for rapid high throughput screening and analysis of viral fusion proteins. The chimeric LacR-IE1 transcriptional activator protein was expressed in one population of Sf9 cells, and a responsive EGFP reporter gene (under lac operator control) was used to transfect a second cell population (Fig. 1). To detect and quantify membrane fusion mediated by the GP64 membrane fusion protein, GP64 was used to fuse the two populations of cells. When cells that contained the activator protein were fused with cells containing the reporter plasmid, the EGFP reporter gene was activated and EGFP was expressed. We found that expression of EGFP could be used to detect membrane fusion activity (Fig. 2). To determine if EGFP expression could be used to measure differences in membrane fusion protein activity, we compared EGFP expression and membrane fusion mediated by OpMNPV GP64 at a variety of pH values. In addition, we examined GP64 proteins containing point mutations in a small hydrophobic membrane fusion domain that was previously examined (Monsma & Blissard, 1995).
Figure 1. Plasmid transfections and fusion-dependent promoter activation assay.
Relevant features of activator and reporter constructs used for fusion-dependent promoter activation assays are shown in panel (A). Plasmid pNLacR-IE1M1-G222 encodes a chimeric LacR-IE1 protein containing a nuclear localization signal (NLS), the N-terminus of Lac Repressor (LacR), and 222 amino acids from the N-terminus of the AcMNPV IE1 protein (Slack & Blissard, 1997). The LacR-IE1 protein is constitutively expressed under the control of a previously described baculovirus early promoter (Blissard & Rohrmann, 1991). The reporter construct, pEGFP-92lacO, encodes an EGFP protein under the transcriptional control of a promoter responsive to the LacR-IE1 protein. The responsive promoter consists of a previously characterized minimal RNA polymerase II promoter plus 2 upstream lac operator (lacO) sequence elements. LacR-IE1 binding to the lacO sequences activates transcription from the minimal promoter sequence and results in EGFP expression. Panel (B) shows the strategy of the fusion-dependent promoter activation assay. Two groups of Sf9 cells were transfected separately. One group received plasmids pNLacR-IE1M1-G222 and p64-166, which constitutively express LacR-IE1 and GP64, respectively. A second group of Sf9 cells received the responsive pEGFP-92lacO reporter plasmid. Cells were mixed at a 1:1 ratio, plated on multiwell plates (at high densities), and the pH was reduced to activate GP64-mediated membrane fusion. Upon cell-cell fusion LacR-IE1 proteins expressed in the first cell group activate the pEGFP-92lacO reporter plasmid present in the second cell group. Expression of EGFP is detected by fluorescence microscopy or by using a fluorescence microplate reader.
Figure 2. EGFP and GP64 Expression in Syncytia.
Sf9 cells expressing GP64 and LacR-IE1 were mixed with cells that had been transfected with pEGFP-92lacO. The pH was shifted to pH 5.2 for 20 min, then returned to pH 6.0. At 24 h after the pH shift, syncytia were visualized by visible (A) and fluorescence (B) microscopy (490 nm excitation/515 nm emission) to detect EGFP. Cells were then fixed in 2.5 % paraformaldehyde and GP64 was detected on the surface of cells by staining with an anti-GP64 monoclonal antibody and a TRITC-conjugated goat anti-mouse secondary antibody. Cells were examined using visible microscopy (C) and fluorescence excitation wavelengths suitable for detection of EGFP (490 nm, panel D) and GP64 (545 nm, panel E). Syncytia are indicated by open arrowheads. Smaller cells that may represent single unfused cells or two-cell fusions are indicated by filled arrowheads. The white bar represents 100 μM.
To quantify membrane fusion between adjacent cells, three plasmid constructs were used (Fig. 1). Plasmid pEGFP-92lacO contains an EGFP reporter gene under the control of a promoter which was previously shown to be transcriptionally activated by the chimeric Lac Repressor-IE1 protein, NLacR-IE11,2ABCD(M1-G222) (Slack & Blissard, 1997). For simplicity, the activator protein NLacR-IE11,2ABCD(M1-G222), is referred to as LacR-IE1 in the current studies. For initial membrane fusion experiments, a plasmid (p64-166) that encodes and expresses the OpMNPV GP64 envelope fusion protein (Blissard & Wenz, 1992) was used to transfect Sf9 cells. Other GP64 containing constructs used in this study were also based on this construct.
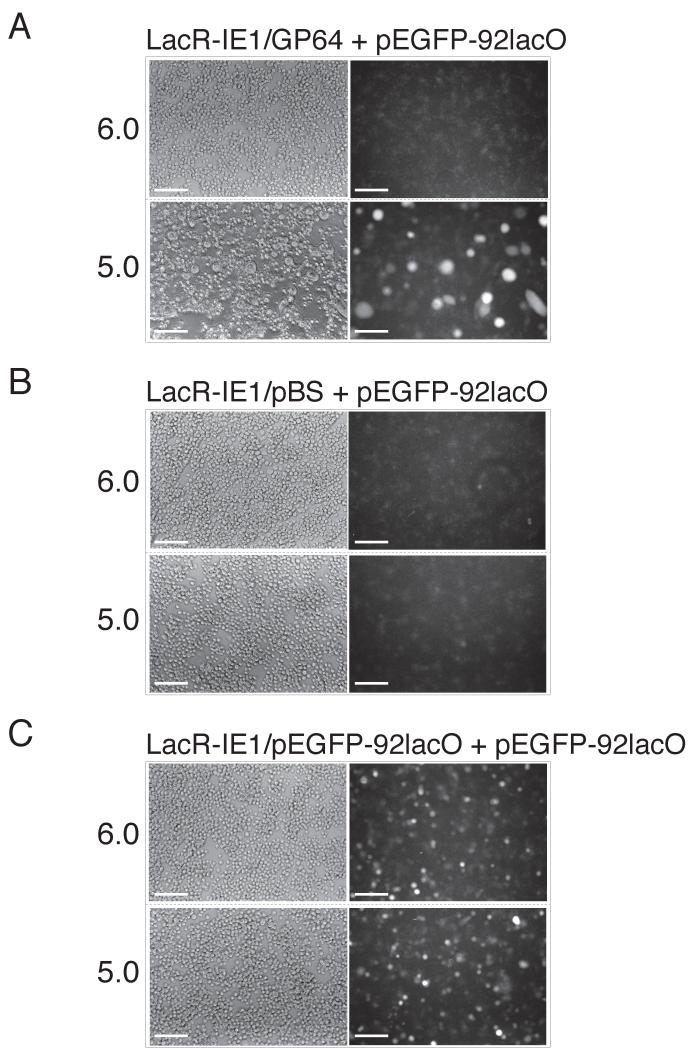
For fusion assays, two groups of Sf9 cells were transfected separately: One group was transfected with plasmid pEGFP-92lacO. Simultaneously, a second group of cells was transfected with two plasmids, pLacR-IE1 and p64-166 (Fig. 1). Cells were incubated for 24 h to permit LacR-IE1 and GP64 expression. Cells that had been transfected with pEGFP-92lacO alone, did not appear to express significant levels of EGFP during that 24 h period. At 24 h post transfection, the cells expressing LacR-IE1 and GP64 were mixed with the pEGFP-92lacO transfected cells at cell ratio of 1:1. Cell mixtures were then deposited into 24-well plates at 5×105 cells/well such that cells were confluent. The pH was then lowered to pH 5.0 for 20 min, to induce GP64 mediated membrane fusion. Syncytia could be observed within 1-3 hours after this pH shift. Cells were incubated at 27° for 24 hours after the pH shift, to permit activated expression of EGFP, then assayed for EGFP expression. When LacR-IE1/GP64 expressing cells fused to pEGFP-92lacO reporter containing cells, the LacR-IE1 transcriptional activator was able to access and activate transcription of the EGFP gene from the pEGFP-92lacO reporter plasmid (Fig. 1B, 2). Syncytia resulting from fusion of the two cell groups were examined by visible light and fluorescence microscopy at 24 h post fusion (Fig. 2, 3A). Syncytia containing EGFP were common whereas single cells showing EGFP fluorescence were only infrequently observed. Because the majority of fluorescence observed was localized to syncytial masses, this indicated that cell-cell fusion was a pre-requisite for EGFP expression. All syncytia did not show EGFP expression, however. This could result from either poor EGFP activation in some fused cells, or a low proportion of cells containing the reporter plasmid (pEGFP-92lacO) in some syncytial masses. (Note: If cells expressing GP64 fuse with each other, no EGFP is expected.) In the absence of GP64, syncytia were not observed and only background fluorescence was observed after low pH treatment (Fig. 3B). However, when the activator (LacR-IE1) and the responsive EGFP reporter plasmid (pEGFP-92lacO) were cotransfected into the same cells, substantial EGFP fluorescence was observed at pH 6.0 and 5.0 (Fig. 3C). Thus, these experiments further demonstrated that LacR-IE1 activated EGFP expression, and that activation was dependent on cell-cell fusion when activator and reporter were transfected into separate cell populations.
Figure 3. Activation of EGFP expression is dependent on cell-cell fusion.
Fusion dependent EGFP expression is illustrated (A) and compared with control experiments in which the GP64 envelope fusion protein was omitted (B), or the activator (LacR-IE1) was co-transfected and expressed in the same cells transfected with the EGFP reporter plasmid (pEGFP-92lacO) (C). In each experiment, cells were examined 24 h after exposure to pH 6.0 or 5.0. Panel A shows cells transfected in two groups with one group receiving the plasmids that expressed LacR-IE1 and GP64, while the other group received the reporter plasmid, pEGFP-92lacO. Panels on the left show visible (phase contrast) micrographs and panels on the right show epifluorescence detection of EGFP. Activated EGFP expression was observed only in syncytia formed from cells that were treated at pH 5.0. Panel B shows a negative control in which an experiment similar to panel A was performed, but the GP64-expressing plasmid was omitted and replaced with an empty vector plasmid (pBS). Panel C shows a positive control in which the first group of cells was transfected with both the EGFP reporter plasmid (pEGFP-92lacO) and the plasmid expressing the activator protein (LacR-IE1). The second group of cells received only the pEGFP-92lacO plasmid. No GP64 was expressed in either cell group. Activated EGFP expression was observed in single cells at both pH 6.0 and 5.0. The white bar represents 200 μM.
To determine whether the expression of GP64 was also colocalized with syncytia and EGFP expression, cells (transfected as diagrammed in Fig. 1B) were fixed in paraformaldehyde at 24 h after the pH shift, and GP64 was detected with an anti-GP64 monoclonal antibody (AcV5) and a TRITC labeled secondary antibody. We observed localization of GP64 on the surfaces of syncytia that were also expressing EGFP (Fig. 2,C-E). An interesting observation was the apparent non-uniform localization of GP64 on the surfaces of these syncytia. This may represent large areas of membrane derived from the GP64 expressing cells, or perhaps aggregation of GP64 in syncytial membranes after fusion.
Quantitative assessment of EGFP expression after triggering membrane fusion at various pH values
The pH dependence of GP64-mediated membrane fusion was previously characterized based on the presence or absence of syncytia in a membrane fusion assay. Although quantitative information can be obtained by counting nuclei in syncytia, the procedure is laborious, prone to variation, and cannot be easily automated. Using Ld652Y cells transfected with OpMNPV GP64 and a visual inspection procedure, the pH required to trigger fusion was determined to be approximately 5.5 (Blissard & Wenz, 1992). Similar data were also obtained using AcMNPV infected cells and a more quantitative assay based on calculating the percentage of nuclei within syncytia (Leikina et al., 1992). To determine whether differences in the response of GP64 to pH can be detected using the promoter activation assay, we examined GP64-mediated membrane fusion and EGFP expression over a range of fusion pH values used to trigger fusion. We transfected two Sf9 cell populations as before with: A) the pEGFP-92lacO plasmid, or B) the pLacR-IE1 and p64-166 plasmids. Cell popultations were then mixed and plated. At 20 hours post transfection, cells were incubated for 20 minutes in medium adjusted to different pH values (ranging from 4.4 to 6.6), then incubated for 24 h at 27° and assayed for EGFP by fluorescence microscopy or by measuring total fluorescence in a multiwell fluorescence plate reader (Fig. 4). Three replicates of each pH treatment were used for the later analysis. Fluorescent syncytia were observed only when cells were exposed to pH values at 5.6 or below (Fig. 4A). This initial observation was consistent with previous visual microscope-based GP64 syncytium assays (Blissard & Wenz, 1992).
FIGURE 4. EGFP fluorescence measurements after induction of GP64-mediated fusion at varying pH values.
Sf9 cells that were transfected with pEGFP-92lacO were mixed with LacR-IE1/GP64 expressing cells, then exposed for 20 min to Graces medium at pH values ranging from pH 5.0 to pH 6.0. Cells were then returned to pH 6.0 and incubated for 24 h. EGFP fluorescence was examined directly by fluorescence microscopy (Panel A) and by fluorescence measurements of cell lysates (Panel B). Numbers in panel A represent the pH values used to trigger membrane fusion. In panel B, pH values used to trigger membrane fusion are indicated on the X-axis and arbitrary fluorescence measurements are indicated on the Y-axis. Various cell treatments are indicated by symbols: Open circles (solid line) represent a group of cells expressing LacR-IE1 and GP64 mixed with a second group of cells containing reporter plasmid pEGFP-92lacO. Positive and negative control experiments are indicated by open and closed triangles, respectively. Open triangles (broad dashed line; positive control) represent a mixture of cells containing one group that was co-transfected with the activator protein (LacR-IE1) and the responsive EGFP reporter plasmid (pEGFP-92lacO) as described in Fig. 3C. Closed triangles (short dashed line) represent a mixture of cells containing one group of cells transfected with the responsive EGFP reporter plasmid, and another group that expressed LacR-IE1 (but no GP64) and were treated as described in Fig. 3B. Error bars represent standard deviations derived from 3 replicate wells of each treatment. The white bar represents 200 μM.
For quantitative analysis of fluorescence resulting from cell-cell fusion, cells were lysed and lysates were used for fluorescence measurements in a fluorescence plate reader as described in the Materials and Methods section. Fluorescence intensity increased significantly in cells that received pH treatments below 5.6 (Fig. 4B). The observed trend in fluorescence intensity was similar to qualitative observations by fluorescence microscopy (Fig. 4A vs. B). To confirm that fluorescence readings reflected EGFP activated after fusion, we also performed control experiments with cell mixtures in which GP64 was omitted (Fig. 4B, filled triangles). In the absence of GP64, cell-cell fusion and syncytium formation was not observed at any pH. As expected, the fluorescence measurements for this control group (no GP64) were low and remained relatively unchanged under all pH treatments. As a positive control, the pLacR-IE1 plasmid (expressing the activator) was transfected into the same cells as the pEGFP-92lacO reporter. These cells were mixed with cells which had been transfected with pEGFP-92lacO alone. Again, no GP64 was present. The cell mixture was exposed to media of varying pH values then fluorescence was measured as described above. As expected, fluorescence levels measured from this cell mixture were significantly higher than the negative control cell mixture since EGFP was expressed in single unfused cells in the positive control. (Fig. 4B, open triangles). These results confirmed that direct fluorescence measurements from fusion experiments represent fusion-dependent EGFP expression.
Fusion profiles of GP64 fusion-domain mutants
To determine if the lacOEGFP/LacR-IE1 fusion assay was sufficiently sensitive to detect more subtle differences in the membrane fusion capacity of a membrane fusion protein, we examined and compared three previously characterized GP64 fusion mutants with wt GP64. The modified forms of GP64, containing amino acid substitutions in a small hydrophobic fusion domain, were previously generated and examined by syncytium formation assays (Monsma & Blissard, 1995). Visual examination of syncytia formed after exposure to different pH values previously suggested that pH required for triggering of fusion by these modified GP64 proteins was altered. The GP64 amino acid substitution mutants, L226M, L227M and L226M/L227M contain leucine to methionine substitutions at positions 226 and 227 in the OpMNPV GP64 protein. The promoter context of all constructs is identical to that of the control plasmid (p64-166) that encodes wt GP64, and expression levels of the wt and modified GP64 proteins were similar (Monsma & Blissard, 1995). In the current study, we used the lacOEGFP/LacR-IE1 fusion assay to compare these modified GP64 proteins with wt GP64, with respect to both pH required for triggering of fusion, and the level of fluorescence observed as a quantitative indicator of the degree of fusion (Fig. 5). Fluorescence profiles indicated that a) the maximal extent of cell-cell fusion decreased with the corresponding reduction in hydrophobicity of the small hydrophobic fusion domain, and b) The pH required for triggering of detectable fusion was lower for GP64L226M, GP64L227M and GP64L226M/L227M than that measured for wt GP64. Western blot analysis of GP64 in each cell group suggests that each construct was expressed at similar levels (Fig. 5A, inset). This confirms that the observed differences in membrane fusion resulted from differences in protein activity and not from substantial differences in quantities of GP64 expressed in transfected cells. It is also important to confirm transfection efficiencies in experiments. In the present study 25% transfection efficiency was achieved by the Ca2PO4/DNA precipitation method (See Methods).
FIGURE 5. Comparisons of EGFP fluorescence profiles for OpMNPV GP64 proteins containing mutations in a putative fusion domain.
The EGFP fluorescence activation profile of wild type OpMNPV GP64 was compared to those from several modified GP64 proteins containing Leucine to Methionine substitutions within a small fusion domain of the OpMNPV GP64 protein. Western blot analysis of wt or mutant GP64 expression in transfected Sf9 cells is shown in the inset of panel A. Lysates for western blots were collected from parallel wells immediately prior to the pH shift (24 h post transfection). Construction of the GP64 mutants (L226M GP64, L227M GP64 and L226M/L227M) was described previously (Monsma & Blissard, 1995). Fusion-dependent fluorescence assays were performed as described in Fig. 4B. For each pH value examined, triplicate samples of cell mixtures were exposed to Graces medium at the indicated pH for 20 min., then returned to pH 6.0 and incubated for 24 h at 27°C. Total fluorescence (480 nm excitation/515 nm emission) was measured from cell lysates. Error bars represent standard deviation calculated from 3 samples. Vertical dashed lines show the calculated pKf value for each construct. To calculate the pKf value, least squares linear regression was used to estimate the trend of data points which lay between background and maximal fluorescence. The pKf value is the calculated pH at which fusion activity was 50% of the maximum. The pH range of changing fusion activity (shaded area) was estimated from the points at which the regression line intersected the maximum and background levels of fluorescence.
Calculation of pKf Values
To develop a more analytical method for comparing fusion profiles, we determined the pH at which fusion was at 50% of the maximal value detected for each construct. For these calculations, the pH at which fusion was 50% of the maximal value is referred to as the pKf value. The calculation of the pKf is detailed in the legend of Figure 5. The pKf values of the wt, L226M, L227M and L226M/L227M GP64 proteins were approximately 5.3, 5.1, 5.2 and 5.0, respectively. The double mutation, GP64L226M/L227M resulted in the greatest reduction in pKf with a value substantially below the wild type protein. The pKf values reflect similar trends to those previously estimated as threshold fusion pH values for triggering fusion. However, the estimated threshold fusion pH values are likely to be less precise as this assumes a very sharp transition from no fusion to a level of fusion visibly detectable by syncytium formation. In contrast, the pKf value represents the midpoint of the transition from background to maximal fusion determined in the fusion/pH profile curve derived from EGFP expression in fused cells. In contrast to standard syncytium formation assays, use of the fluorescent activation assay to derive pKf values is more reliable for detecting and measuring changes in fusion activity since many data points can be used to derive the pKf.
In this study, a membrane fusion assay was developed and used to perform a more detailed examination of fusion parameters, and a more quantitative assessment of membrane fusion by the baculovirus GP64 protein. Specifically, the pH range over which GP64-mediated fusion progressed from the threshold of detectable fusion, to maximum fusion levels was measured. The pH range for wt OpMNPV GP64 expressed in Sf9 cells was approximately 0.6 pH units (Fig. 5). When compared with wt GP64, GP64 fusion domain mutants L226M, L227M and L226M/L227M appeared to have slightly narrower pH ranges over which fusion progressed from threshold levels to maximal levels. In addition, the total level of fusion observed from the L226M/L227M mutant was substantially below that of wt GP64, suggesting both a change in the pH required to trigger fusion and a reduction in the overall efficiency of fusion. Although several studies have examined functional roles of various GP64 domains and GP64 oligomerization (Kingsley et al., 1999; Markovic et al., 1998; Monsma & Blissard, 1995; Oomens et al., 1995), very little is known of the physical structure of GP64. Previous studies suggested that like influenza HA, GP64 may undergo a conformational change upon exposure to low pH (Blissard & Wenz, 1992; Chernomordik et al., 1995; Leikina et al., 1992; Plonsky & Zimmerberg, 1996). Examination of various GP64 mutants in the fusion assay developed for these studies should permit a more objective and detailed comparison of functional domains of GP64. In addition, this assay can be easily applied to membrane proteins from other insect or mammalian viruses.
The membrane fusion assay developed for these studies should be useful for quantitative measurements and comparisons of membrane fusion mediated by viral and other membrane fusion proteins. Although less precise than measurements of conductivity between paired cells, the ease with which this fusion-dependent reporter assay can be used to screen large numbers of cells or conditions, makes this assay ideal for high throughput screening applications. Such applications may include screens for direct agonists or antagonists of membrane fusion. Because fusion in many cases is dependent on prior receptor binding, this insect cell based assay may also be useful for studies examining the interactions between viral membrane fusion proteins and mammalian cellular receptors that are not normally expressed on insect cells.
Acknowledgements
The authors thank Chris Richardson for helpful comments on this work and Elaine Van Etten for editorial assistance. These studies were supported by grants from the NIH (AI33657) and the USDA (99-35302-7952).
References
- Bagai S, Lamb RA. Quantitative measurement of paramyxovirus fusion: Differences in requirements of glycoproteins between simian virus 5 and human parainfluenza virus 3 or Newcastle disease virus. Journal of Virology. 1995;69:6712–6719. doi: 10.1128/jvi.69.11.6712-6719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blissard GW, Rohrmann GF. Baculovirus gp64 gene expression: Analysis of sequences modulating early transcription and transactivation by IE1. Journal of Virology. 1991;65:5820–5827. doi: 10.1128/jvi.65.11.5820-5827.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blissard GW, Wenz JR. Baculovirus GP64 envelope glycoprotein is sufficient to mediate pH dependent membrane fusion. Journal of Virology. 1992;66:6829–6835. doi: 10.1128/jvi.66.11.6829-6835.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerini D, Planelles V, Chen IS. CD26 antigen and HIV fusion? Science. 1994;264:1160–1. doi: 10.1126/science.7909961. 1162-1165. [DOI] [PubMed] [Google Scholar]
- Chernomordik L, Leikina E, Cho M, Zimmerberg J. Control of baculovirus GP64-induced syncytium formation by membrane lipid composition. Journal of Virology. 1995;69:3049–3058. doi: 10.1128/jvi.69.5.3049-3058.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath PD, Wu L, Mackay CR, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–48. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, Davis CB, Peiper SC, Schall TJ, Littman DR, Landau NR. Identification of a major co-receptor for primary isolates of HIV- 1. Nature (London) 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- Doms RW, Blumenthal R, Moss B. Fusion of intracellular and extracellular forms of vaccinia virus with the cell membrane. Journal of Virology. 1990;64:4884–4892. doi: 10.1128/jvi.64.10.4884-4892.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doms RW, Helenius A, White J. Membrane fusion activity of the influenza virus hemagglutinin. The low pH-induced conformational change. Journal of Biological Chemistry. 1985;260:2973–2981. [PubMed] [Google Scholar]
- Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: Functional cDNA cloning of a seven- transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- Florkiewicz RZ, Rose JK. A cell line expressing vesicular stomatitis virus glycoprotein fuses at low pH. Science. 1984;225:721–723. doi: 10.1126/science.6087454. [DOI] [PubMed] [Google Scholar]
- Fuerst TR, Niles EG, Studier FW, Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proceedings of the National Academy of Science U. S. A. 1986;83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotte M, von Mollard GF. A new beat for the SNARE drum. Trends in Cell Biology. 1998;8:215–218. doi: 10.1016/s0962-8924(98)01272-0. [DOI] [PubMed] [Google Scholar]
- Hohmann AW, Faulkner P. Monoclonal antibodies to baculovirus structural proteins: Determination of specificities by Western blot analysis. Virology. 1983;125:432–444. doi: 10.1016/0042-6822(83)90214-3. [DOI] [PubMed] [Google Scholar]
- Hsu SC, Hazuka CD, Foletti DL, Scheller RH. Targeting vesicles to specific sites on the plasma membrane: the role of the sec6/8 complex. Trends in Cell Biology. 1999;9:150–153. doi: 10.1016/s0962-8924(99)01516-0. [DOI] [PubMed] [Google Scholar]
- Hughson FM. Structural characterization of viral fusion proteins. Current Biology. 1995;5:265–274. doi: 10.1016/s0960-9822(95)00057-1. [DOI] [PubMed] [Google Scholar]
- Kingsley DH, Behbahani A, Rashtian A, Blissard GW, Zimmerberg J. A discrete stage of baculovirus GP64-mediated membrane fusion. Molecular Biology of the Cell. 1999;10:4191–4200. doi: 10.1091/mbc.10.12.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leikina E, Onaran HO, Zimmerberg J. Acidic pH induces fusion of cells infected with baculovirus to form syncytia. FEBS Letters. 1992;304:221–224. doi: 10.1016/0014-5793(92)80623-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindau M, Almers W. Structure and function of fusion pores in exocytosis and ectoplasmic membrane fusion. Current Opinion in Cell Biology. 1995;7:509–517. doi: 10.1016/0955-0674(95)80007-7. [DOI] [PubMed] [Google Scholar]
- Markovic I, Pulyaeva H, Sokoloff A, Chernomordik LV. Membrane fusion mediated by baculovirus gp64 involves assembly of stable gp64 trimers into multiprotein aggregates. J Cell Biol. 1998;143:1155–66. doi: 10.1083/jcb.143.5.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsma SA, Blissard GW. Identification of a membrane fusion domain and an oligomerization domain in the baculovirus GP64 Envelope Fusion Protein. Journal of Virology. 1995;69:2583–2595. doi: 10.1128/jvi.69.4.2583-2595.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsma SA, Oomens AGP, Blissard GW. The GP64 Envelope Fusion Protein is an essential baculovirus protein required for cell to cell transmission of infection. Journal of Virology. 1996;70:4607–4616. doi: 10.1128/jvi.70.7.4607-4616.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum O, Broder CC, Berger EA. Fusogenic mechanisms of enveloped-virus glycoproteins analyzed by a novel recombinant vaccinia virus-based assay quantitating cell fusion-dependent reporter gene activation. Journal of Virology. 1994;68:5411–5422. doi: 10.1128/jvi.68.9.5411-5422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomens AGP, Monsma SA, Blissard GW. The baculovirus GP64 Envelope Fusion Protein: synthesis, oligomerization, and processing. Virology. 1995;209:592–603. doi: 10.1006/viro.1995.1291. [DOI] [PubMed] [Google Scholar]
- Plonsky I, Zimmerberg J. The initial fusion pore induced by baculovirus GP64 is large and forms quickly. Journal of Cell Biology. 1996;135:1831–1839. doi: 10.1083/jcb.135.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JF, Esteban M. Mapping and nucleotide sequence of the vaccinia virus gene that encodes a 14-kilodalton fusion protein. Journal of Virology. 1987;61:3550–3554. doi: 10.1128/jvi.61.11.3550-3554.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JE, Sollner TH. Throttles and dampers: controlling the engine of membrane fusion [see comments] Science. 1997;276:1212–1213. doi: 10.1126/science.276.5316.1212. [DOI] [PubMed] [Google Scholar]
- Slack JM, Blissard GW. Identification of two independent transcriptional activation domains in the Autographa californica Multicapsid Nuclear Polyhedrosis Virus IE1 protein. Journal of Virology. 1997;71:9579–9587. doi: 10.1128/jvi.71.12.9579-9587.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmann T, Doms RW, Helenius A. D M, editor. Protein-mediated membrane fusion. Annual Review Of Biophysics And Biophysical Chemistry Engelman. 1989;18:187–212. doi: 10.1146/annurev.bb.18.060189.001155. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Mellman IS, Muller WA, Cohn ZA. Endocytosis and the recycling of plasma membrane. Journal of Cell Biology. 1983;96:1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman LE, Goldsmith PA. Mechanism of neutralization of budded Autographa californica nuclear polyhedrosis virus by a monoclonal antibody: Inhibition of entry by adsorptive endocytosis. Virology. 1985;143:185–195. doi: 10.1016/0042-6822(85)90107-2. [DOI] [PubMed] [Google Scholar]
- Weissenhorn W, Dessen A, Calder LJ, Harrison SC, Skehel JJ, Wiley DC. Structural basis for membrane fusion by enveloped viruses. Molecular Membrane Biology. 1999;16:3–9. doi: 10.1080/096876899294706. [DOI] [PubMed] [Google Scholar]