Abstract
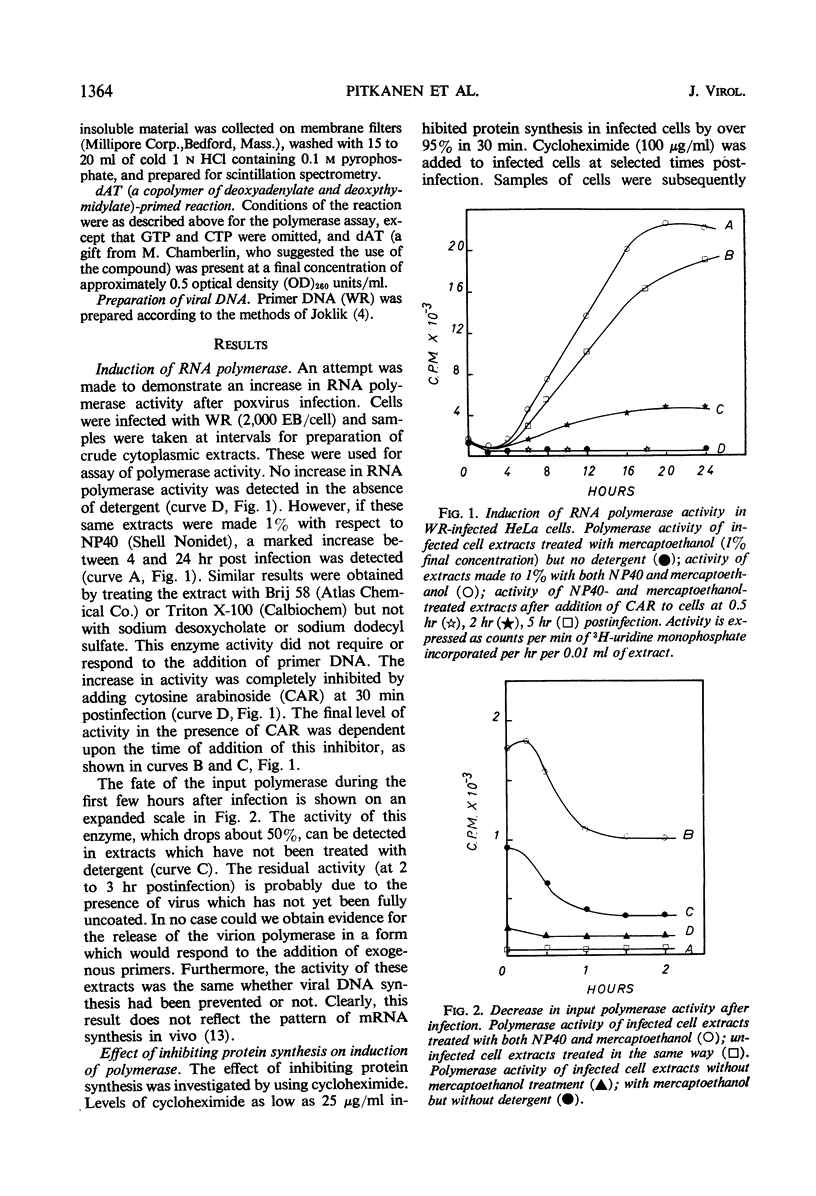
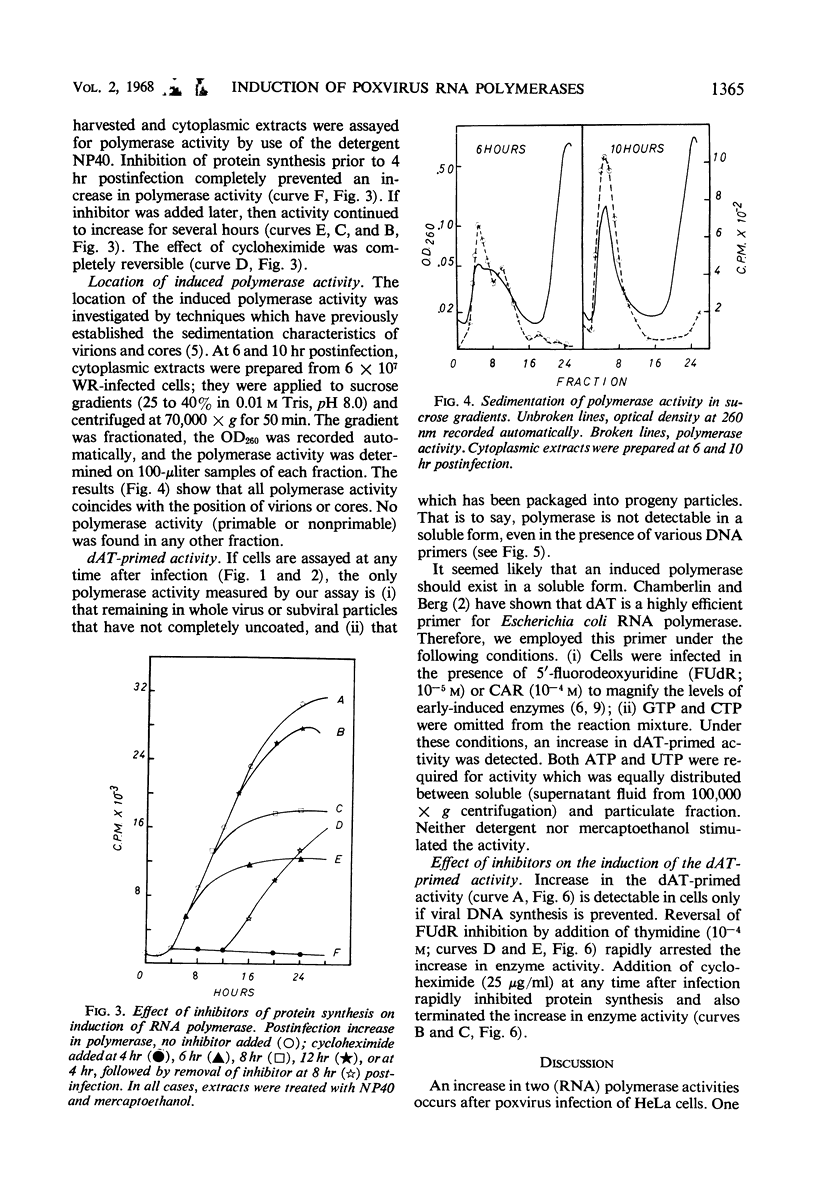
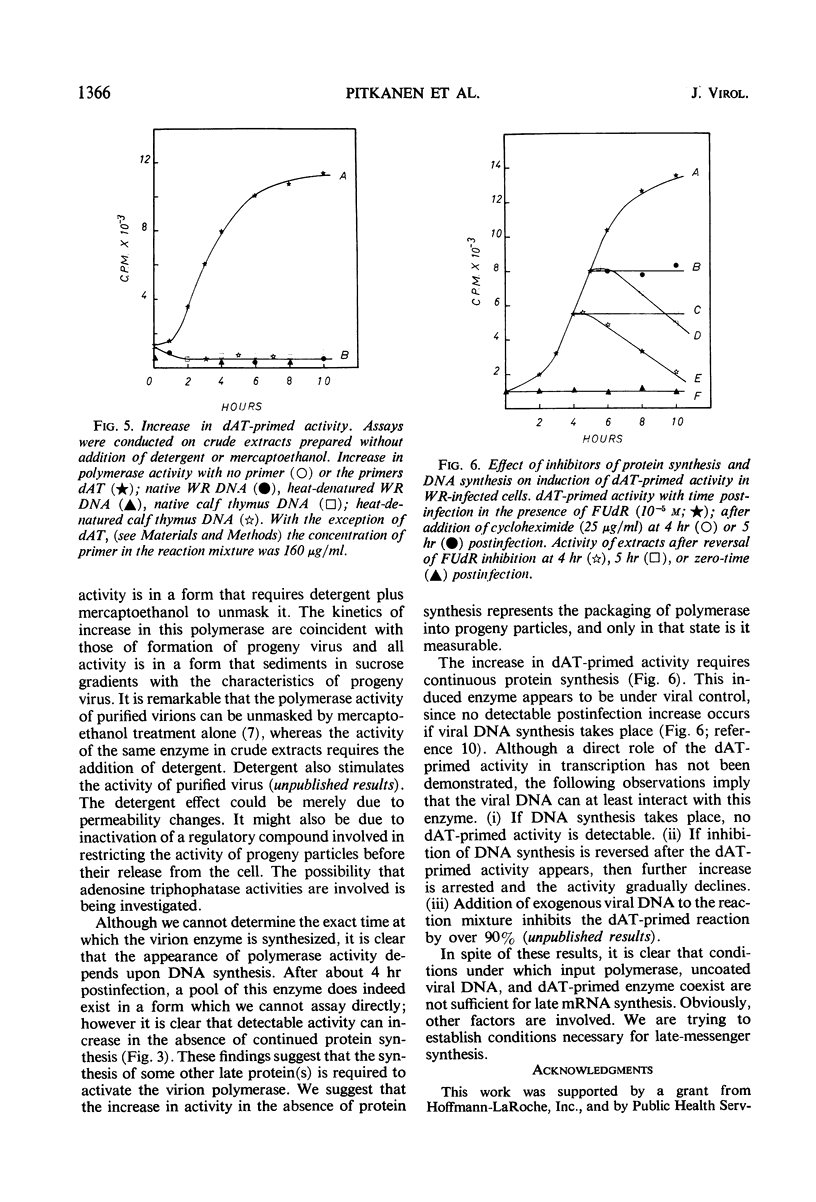
Two distinct ribonucleic acid polymerase activities were induced in HeLa cells by poxvirus infection. These activities differ both in their properties and the time of their appearance after infection. One catalyzes the dAT (copolymer of deoxyadenylate and deoxythymidylate)-primed conversion of adenosine triphosphate and uridine triphosphate into an acid-insoluble product. This enzyme is detectable only if deoxyribonucleic acid synthesis has been blocked. In contrast, the accumulation of progeny genomes is a necessary condition for induction of the second enzyme. The latter activity, which is unmasked by detergent treatment, is found exclusively in maturing virus particles. The possibility that both enzymes are involved in transcribing the viral genome is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKER Y., JOKLIK W. K. MESSENGER RNA IN CELLS INFECTED WITH VACCINIA VIRUS. Proc Natl Acad Sci U S A. 1964 Apr;51:577–585. doi: 10.1073/pnas.51.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAMBERLIN M., BERG P. Deoxyribo ucleic acid-directed synthesis of ribonucleic acid by an enzyme from Escherichia coli. Proc Natl Acad Sci U S A. 1962 Jan 15;48:81–94. doi: 10.1073/pnas.48.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOKLIK W. K., BECKER Y. THE REPLICATION AND COATING OF VACCINIA DNA. J Mol Biol. 1964 Dec;10:452–474. doi: 10.1016/s0022-2836(64)80066-8. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The preparation and characteristics of highly purified radioactively labelled poxvirus. Biochim Biophys Acta. 1962 Aug 20;61:290–301. doi: 10.1016/0926-6550(62)90091-9. [DOI] [PubMed] [Google Scholar]
- Jungwirth C., Joklik W. K. Studies on "early" enzymes in HeLa cells infected with vaccinia virus. Virology. 1965 Sep;27(1):80–93. doi: 10.1016/0042-6822(65)90145-5. [DOI] [PubMed] [Google Scholar]
- Kates J. R., McAuslan B. R. Messenger RNA synthesis by a "coated" viral genome. Proc Natl Acad Sci U S A. 1967 Feb;57(2):314–320. doi: 10.1073/pnas.57.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates J. R., McAuslan B. R. Poxvirus DNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1967 Jul;58(1):134–141. doi: 10.1073/pnas.58.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuslan B. R. Regulation of enzymes induced by animal viruses. Natl Cancer Inst Monogr. 1967 Nov;27:211–219. [PubMed] [Google Scholar]
- Munyon W., Paoletti E., Grace J. T., Jr RNA polymerase activity in purified infectious vaccinia virus. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2280–2287. doi: 10.1073/pnas.58.6.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda K. I., Joklik W. K. Hybridization and sedimentation studies on "early" and "late" vaccinia messenger RNA. J Mol Biol. 1967 Aug 14;27(3):395–419. doi: 10.1016/0022-2836(67)90047-2. [DOI] [PubMed] [Google Scholar]
- Woodson B. Recent progress in poxvirus research. Bacteriol Rev. 1968 Jun;32(2):127–137. doi: 10.1128/br.32.2.127-137.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodson B. Vaccinia mRNA synthesis under conditions which prevent uncoating. Biochem Biophys Res Commun. 1967 Apr 20;27(2):169–175. doi: 10.1016/s0006-291x(67)80057-3. [DOI] [PubMed] [Google Scholar]



