Abstract
The mesenchymal elements of the intestinal lamina propria reviewed here are the myofibroblasts, fibroblasts, mural cells (pericytes) of the vasculature, bone marrow–derived stromal stem cells, smooth muscle of the muscularis mucosae, and smooth muscle surrounding the lymphatic lacteals. These cells share similar marker molecules, origins, and coordinated biological functions previously ascribed solely to subepithelial myofibroblasts. We review the functional anatomy of intestinal mesenchymal cells and describe what is known about their origin in the embryo and their replacement in adults. As part of their putative role in intestinal mucosal morphogenesis, we consider the intestinal stem cell niche. Lastly, we review emerging information about myofibroblasts as nonprofessional immune cells that may be important as an alarm system for the gut and as a participant in peripheral immune tolerance.
Keywords: myofibroblast, muscularis mucosae, stem cell niche, mucosal immunology, colorectal cancer
INTRODUCTION
Since our earlier reviews on the biology of intestinal myofibroblasts (1–3), the literature in this area of science has exploded, due in no small part to the work of many productive laboratories whose research for more than 20 years established an important biological role for these mesenchymal cells. Gabbiani and collaborators’ studies of wound healing and fibrosis, Kedinger’s research on intestinal development and morphogenesis, and Furuya & Furuya’s research on the anatomy and morphology of this interconnecting organelle and how it acts as a contractile network through dye-permeable gap junctions and endothelin/purinergic receptors have been particularly important contributions to myofibroblast biology (4–6). In the past decade, the myofibroblast’s role as a paracrine/autocrine mediator of inflammation has also become a productive area of investigation. Arguably, the most important stimulus to the field of myofibroblast research has been recognition of the involvement of mesenchymal cells, particularly fibroblasts and myofibroblasts in the cancer microenvironment, as drivers of carcinogenesis, tumorigenesis, cancer invasion, and metastasis (7). A more recent impetus has been the putative role of myofibroblasts in both the normal (8) and the neoplastic (9) stem cell niche. Space does not allow us to review the role of myofibroblasts in all these important areas; nor can we add much to the excellent reviews referenced above and by others. The focus of this review is on the non-white-blood-cell and nonendothelial mesenchymal elements of the intestinal lamina propria: myofibroblasts and fibroblasts, which together are considered intestinal stromal cells; the mural cells (pericytes) of the vasculature (which may be categorized as part of the intestinal stromal cell compartment); the bone marrow–derived stromal stem cells; the smooth muscle of the muscularis mucosae; and the smooth muscle associated with the lymphatic lacteals. On the basis of similar marker molecules, origins, and coordinated biological functions, we propose that these elements act together to exert functions previously ascribed solely to subepithelial myofibroblasts. We review here their functional anatomy, their origin in the embryo and their replacement in adults, their putative role in intestinal mucosal morphogenesis and the intestinal stem cell niche, and emerging information about myofibroblasts as nonprofessional immune cells.
MESENCHYMAL ELEMENTS OF THE LAMINA PROPRIA
Molecular Markers
Conventionally, myofibroblasts have been identified by their expression of various in-tracellular cytoskeletal proteins—the microfilament α-smooth muscle actin (α-SMA), type 3 intermediate filaments such as vimentin or desmin, and collagen type 1 maturation enzymes such as prolyl-4 hydroxylase—and by the absence of epithelial cytokeratins (3, 10). Although various combinations of these markers have been used to demonstrate the plasticity of myofibroblasts, operationally intestinal myofibroblasts are defined by their expression of α-SMA together with their location and structure and are considered activated fibroblasts. α-SMA remains the best single (but not exclusive or absolutely specific) marker of the subepithelial myofibroblast network and of the other mesenchymal elements of the lamina propria, i.e., mural cells (pericytes; please see related side bar) (1, 11), bone marrow–derived stromal stem cells (11, 12), the muscularis mucosae (13), and the smooth muscle of the small intestinal villus core (14). All express α-SMA. Although the stromal stem cells are difficult to identify by routine histology of tissue sections, the lamina propria smooth muscle fibers of the small intestine are prominent. When histological sections are properly aligned, these smooth muscle fibers of the small intestinal villus are closely associated with the lymphatic lacteals (Figure 1), a conclusion supported by comparing α-SMA-stained histological sections with published scanning electron micrographs of corrosion casts of intestinal lacteals (15) and immunohistochemical sections stained with antibodies against the lymphatic endothelium (LYVE1, a hyaluronan receptor) (16). The idea that these villus core smooth muscle cells surround the lacteal and through piston-like contractions propel lymph to lymph nodes and eventually to the thoracic duct was first proposed in the late 1960s, and the physiology of this system was reviewed in 1989 (17). Intermediate filament stains differentiate the myofibroblasts and mural cells from the muscularis mucosae and the lymphatic lacteal. The muscularis mucosa and the smooth muscle of the villus core stain poorly with antibodies against vimentin but react strongly to desmin antibodies, whereas the myofibroblasts and mural cells stain weakly (if at all) for desmin (13, 14, 18). The intestine of the α-SMA-null mouse has not been studied in detail. These animals have no difficulty feeding or reproducing but have impaired vascular contractility and blood pressure control (19), suggesting that other actin isoforms can substitute for some functions in the digestive and urogenital tracts. Interferon-γ (IFN-γ) downregulates α-SMA (20), suggesting caution when one identifies myofibroblasts in intestinal inflammatory states.
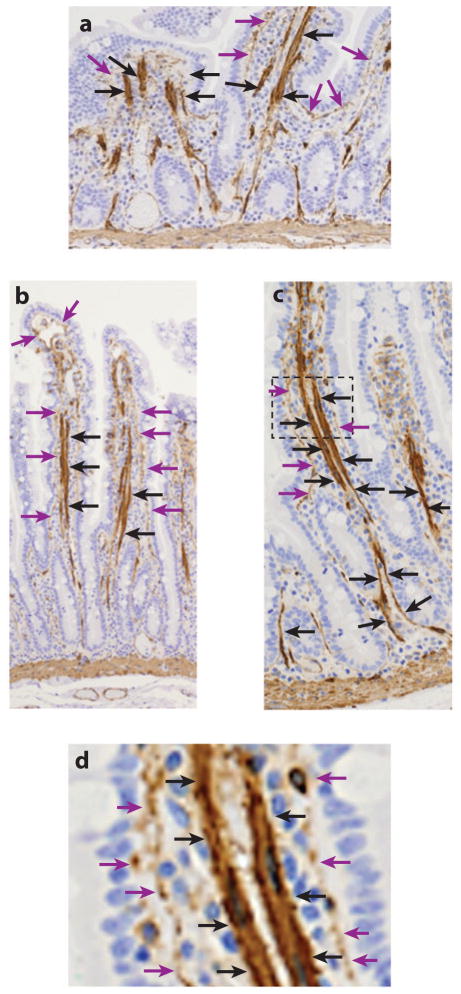
Figure 1.
α-Smooth muscle actin (α-SMA) immunohistology (brown) of the human jejunum. (a) Longitudinal section through a poorly oriented jejunum shows what appears to be random smooth muscle fibers oriented in a longitudinal fashion in villus core. (b,c) Better-oriented sections of (b) the upper aspects and (c) the lower aspects of the villus-crypt axis. In these sections the smooth muscle bundles are closely associated with the lymphatic lacteals, which are covered in their lower aspects after they bifurcate to enter the muscularis by only pericytes rather than organized smooth muscle bundles (black arrows). α-SMA+ subepithelial myofibroblasts and vascular mural cells (pericytes) are located between the epithelium and the lacteal (purple arrows). (d ) An enlarged section of panel c shows that the lacteal and the subepithelial myofibroblasts are separate α-SMA+ elements. Figure courtesy of Patrick Adegboyega, Louisiana State University Health Sciences Center, Shreveport, Louisiana.
To facilitate identification of the mesenchymal elements of the lamina propria, novel markers have been sought. In the human intestine, both fibroblasts and myofibroblasts express CD90 (cluster of differentiation 90), also known as Thy-1 (thymocyte differentiation antigen-1). Thy-1 is a 25–37-kDa, extracellular glycosylphosphatidylinositol–linked glycoprotein that is expressed by subsets of fibroblasts, myofibroblasts, mesangial cells, vascular pericytes, hematopoietic and mesenchymal stem cells (MSCs), and activated endothelial cells. It is also expressed by some murine neurons and murine T cells (21, 22). There is also a soluble form of Thy-1, which is either secreted or cleaved from the surface of producing cells. We used antibodies against this surface protein to identify the human intestinal myofibroblast-fibroblast-pericyte network. Diffuse staining of the matrix associated with the network suggests that the extracellular matrix may capture soluble Thy-1 (23). In both human myometrium and orbit, Thy-1+ and Thy-1− fibroblasts exist. Only Thy-1+ fibroblasts can differentiate into myofibroblasts after treatment with transforming growth factor (TGF)β, whereas only Thy-1− fibroblasts differentiate into lipofibroblasts upon exposure to 15-deoxy-δ-prostaglandin J2 (24). Thy-1 subtypes in other tissues show different abilities to secrete cytokines, eicosanoids, and collagens or to express major histocompatibility complex (MHC) class II molecules upon stimulation (25). We conclude that the human intestinal stromal cell compartment can be identified but not exclusively defined by the reaction to Thy-1 antibodies.
Antibodies against intracellular components of thymus stromal cells (reticular fibroblasts) such as monoclonal antibody clones ER-TR7 (26, 27) and TE-7 (28) have immunoreactivity with fibroblasts, myofibroblasts, pericytes, and lymphatic stromal cells. Preliminary studies in our laboratory show reactivity of Thy-1, ER-TR7, and α-SMA against these mesenchymal elements of the lamina propria, and confocal microscopic merged images give promise that the combination may be useful in demonstrating the villus smooth muscle (lymphatics) and the subepithelial myofibroblast network. Furthermore, an extensive study of 72 different markers suggests that ER-TR7 and TE-7 may be useful in differentiating fibroblasts from tissue macrophages, monocytes, and fibrocytes (stromal stem cells) (29).
Anatomy of the Lamina Propria
On the basis of use of the markers discussed above, Figure 2 illustrates the anatomy of the small intestine and colonic lamina propria. We present the anatomy in the form of diagrammatic representations of both longitudinal and cross sections of the lamina propria of the small intestine in hopes of enhancing the clarity of the relationships and organization of the respective mesenchymal elements. The colonic anatomy is similar to the longitudinal and cross sections of the crypts of the small intestine.
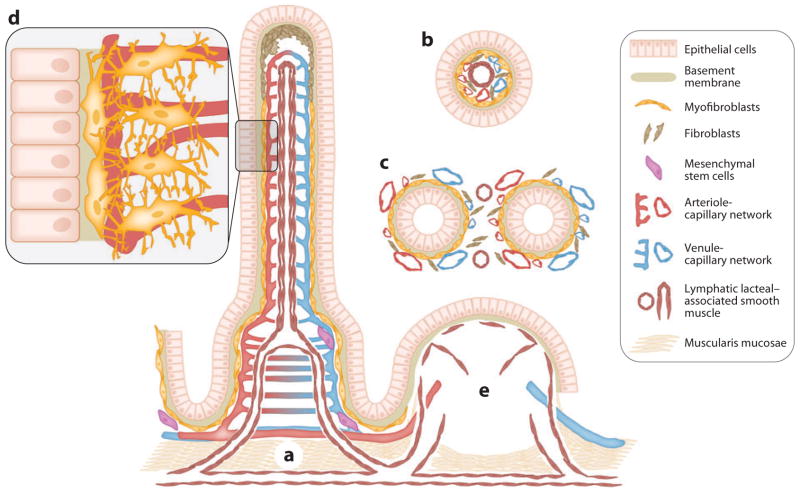
Figure 2.
The spatial relationships of the epithelium and mesenchymal elements of the small intestinal villus-crypt axis. (a) Longitudinal representation of the villus and crypt showing the epithelium and lamina propria containing α-SMA+ subepithelial myofibroblasts and pericytes of the capillaries, mesenchymal stem cells, and smooth muscle associated with the lymphatic lacteal and the muscularis mucosae. Fibroblasts (α-SMA−) are shown, especially in the upper portion of the villus. (b) Cross sections through a villus show the lymphatic lacteal with associated smooth muscle. (c) A cross section through the crypts demonstrates the lymphatic pericytes. Panels b and c also show that the subepithelial myofibroblasts are essentially pericytes in subepithelial locations of the villus. (d ) A higher-power depiction of the myofibroblasts and pericytes with cytoplasmic processes that surround and support the capillaries. (e) A Peyer’s patch with its vascular, lymphatic, and stromal elements. Lymphocytes, macrophages, dendritic cells, and polymorphonuclear leukocytes are not shown.
Ablating Intestinal Myofibroblasts
Mirroring the search for specific markers to identify myofibroblasts, investigators need the identity of specific genes or promoters that would allow ablation (preferably conditionally) of these cells from the intestine to permit study of biological functions or disease. Fibroblast-specific protein 1 (Fsp1) has been used for fibroblast depletion (30). Fsp1 is not highly expressed in α-SMA-expressing stromal cells and thus may not prove useful for myofibroblast depletion (31). Mitogen-activated protein kinase–activated protein kinase 2 (MK2), a key signaling molecule in the TGFβ signaling pathway, plays an important role in the differentiation of fibroblasts to myofibroblasts, and MK2-null mice have reduced numbers of α-SMA+ myofibroblasts in the lung (32). NG2 chondroitin sulfate proteoglycan (NG2) (33), the proline dipeptidyl peptidase IV named fibroblast activation protein (34), and the matrix protein periostin (35) are additional candidate molecules. A completely different strategy for ablation of myofibroblasts would be to couple toxins or antifibrotic drugs to antibodies highly specific for myofibroblasts (36).
ORIGIN OF MYOFIBROBLASTS AND MURAL CELLS
Origin in the Embryo
Myofibroblasts are demonstrable as early as 21 weeks in the human embryo (P. Adegboyega, personal communication) and embryonic day (E)18.5 in the mouse (37). Neural crest cells can be shown through lineage tracing as the origin of a specialized myofibroblast (endoneural fibroblast) and Schwann cells within the peripheral nerve (38) and as pericytes in the vessels of the thymus (39). Thus, although neural crest cells remain a possible source for intestinal myofibroblasts, Bader’s laboratory (40) has presented more compelling evidence that the serosal mesothelium is the source of myofibroblasts and pericytes in the lamina propria. Using Wilm’s tumor protein and Cre recombinase to track serosal mesothelial cells, Bader and colleagues demonstrated that at E11.5 these cells underwent epithelial-to-mesenchymal transition (EMT) and migrated throughout the gut, forming vascular smooth muscle and mural cells as well as other lamina propria mesenchymal cells (probably myofibroblasts). Thus, the subepithelial and perivascular α-SMA+ cells of the lamina propria likely have the same origin, but it is unclear whether the muscularis mucosae and lymphatic smooth muscle originate from these same origins.
Origin of Myofibroblasts and Pericytes in the Adult
Adult myofibroblasts are derived or replenished after injury or in response to neoplastic transformation from several sources: differentiation or activation of resident fibroblasts or resident stellate cells, dedifferentiation from perivascular smooth muscle cells (and perhaps from adipocytes), EMT of epithelial and endothelial cells, and bone marrow–derived stem cells (either MSCs or hematopoietic stem cells via CD14+ monocytes transdifferentiating into circulating CD34+ fibrocytes, which become resident CD34+ fibrocytes) (10). Dedifferentiation of other gut smooth muscle elements (muscularis propria, muscularis mucosae, or lymphatic smooth muscle) has not been demonstrated.
Fibroblast Differentiation
The major, acute source of myofibroblasts in liver and pancreas is stellate cells (41). Fibroblasts serve this function in lung, (42), skin (43), and presumably the intestine. TGFβ is thought to be the key inducer of myofibroblast differentiation, although it requires the presence of the fibronectin ED-A splice variant, and mechanical stress is important as well (discussed in Reference 3). NAD(P)H oxidase 4 mediates TGFβ transdifferentiation in cardiac fibroblasts (44), as does the urokinase plasminogen activator/urokinase plasminogen activator receptor system in corneal stromal cells (45) and NK2 in lung fibroblasts (see above). α-SMA is upregulated in intestinal pericryptal myofibroblasts by low-salt diet and by nonlethal radiation exposure, a phenomenon that is blocked by captopril, an angiotensin-converting enzyme inhibitor (46). Prostaglandin E2 (PGE2) inhibits fibroblast-myofibroblast transition through prostanoid EP2 receptors coupled to cyclic AMP (cAMP) production (47).
Epithelial-to-Mesenchymal Transition
EMT occurs during embryonic development (type 1), during fibrosis (type 2), and during cancer tumorigenesis and metastasis (type 3) (48). Its role in development and cancer is irrefutable (37, 49). In spite of a growing body of evidence incriminating EMT in tissue fibrosis, two recent studies using a more rigorous technique of lineage tracing failed to produce evidence of hepatocye or renal epithelial EMT as a source of liver or kidney fibrosis (50, 51). EMT has been incriminated as a cause of persistent fistulae in Crohn’s disease (52), and recent data from the Kalluri laboratory (53) suggest that the EMT process may play a role in the intestinal fibrosis observed in an animal model of inflammatory bowel disease.
Bone Marrow Stem Cell Origin
Using in situ hybridization to detect the Y chromosome in female humans and mice transplanted with male donor marrow, Brittan & Wright (54) demonstrated that both subepithelial myofibroblasts and lamina propria mural cells are replenished by bone marrow stem cells. Intestinal injury and neoplasia augment the process (54). This process also occurs in liver (55) and lung (56). Which bone marrow stem cell and what chemotactic signals promote this mesenchymal repopulation remain areas of investigation (57).
Both MSCs (58) and fibrocytes (11) are candidate cells of bone marrow origin to be the stromal stem cells of the intestine. MSCs are classically defined as CD45− (a panhematopoietic marker), CD34− (a hematopoietic stem cell/progenitor marker), α-SMA+ cells that do not express antigen-presenting costimulatory molecules such as CD80, CD86, or CD40 (58). This immune tolerance aspect of MSC is a therapeutic advantage, downregulating the immune response, whereas growth factor expression from these cells aids in repairing damage. Other researchers, however, report that MHC class II and negative costimulatory molecule PD-L1 (programmed cell death receptor ligand-1) expression gives these cells the ability to either present antigens or contribute to peripheral tolerance, depending on levels of ambient IFN-γ (59). The fibrocyte is a α-SMA+, CD45+, CD34+ cell with MHC class II and costimulatory molecule (CD80 and CD86) expression and antigen-presenting capability (11, 60). The fibrocyte is derived from a small subset of circulating CD14+ peripheral blood monocytes expressing immunoglobulin G (IgG) receptors. Like MSCs, fibrocytes are found in inflamed tissue throughout the body and in cancers (11). The properties of these two bone marrow–derived cells give promise to stem cell therapeutics for intestinal and liver diseases (57, 61).
Brown et al. (12) reported a cyclooxygenase (COX)-2-expressing stromal cell that moves in response to Toll-like receptor (TLR) signals from a position in the upper aspect of the lamina propria to a position adjacent to the pericryptal myofibroblasts in the base of the crypts, where the epithelial stem cells reside. This relocation and the prostaglandin secretion appear critical to rectal epithelial repair in a dextran sodium sulfate (DSS) colitis model. One possible explanation of this study is that Brown et al. may have caught stromal stem cells in transit from the vasculature of the injured gut to replace either pericytes or subepithelial myofibroblasts at the base of the crypt. Furthermore, the study does not rule out a role for prostaglandin production or TLR responses by the conventional myofibroblasts or pericytes that are present.
MESENCHYMAL-EPITHELIAL INTERACTIONS
During development and into adulthood, intestinal architecture and function are shaped by interactions between the epithelium and underlying mesenchyme. These interactions are important not only for proper morphogenesis but also for appropriate maintenance of the intestinal stem cell niche. Excellent reviews (see Related Resources section following the Literature Cited) of intestinal development/morphogenesis—topics beyond the scope of this article—are available. Here we focus briefly on the role of epithelium-derived hedgehog (Hh) growth factors in the formation of the lamina propria mesenchymal cells and how these cells, in turn, contribute to the supportive microenvironment termed the epithelial stem cell niche (8, 62).
Hedgehog-Directed Mesenchymal Differentiation
Intestinal morphogenesis depends on bidirectional instructive signals (cross-talk) between the endoderm and the mesoderm in the embryo and between the epithelium and mesenchymal cells in the postnatal animal. Hh signaling pays a critical role in the development of the mesenchymal elements of the lamina propria and was reviewed recently in detail by van den Brink (63). Hh signaling is initiated when a Hh binds its receptor Patched (Ptch) on target cells. Ptch normally interacts in an inhibitory fashion with another transmembrane receptor, Smoothened (Smo), and Hh binding to Ptch disrupts this interaction. Active Smo initiates a signaling cascade culminating in the activation and nuclear translocation of the transcription factors Gli1, Gli2, and Gli3. Ptch1 and Gli1 are transcriptional targets of Gli transcription factors. In the gut, Hh signaling is generally epithelial to mesenchymal (14) [although Ptch expression can be observed within hindgut epithelial cells at E10.5 (64)]. Both Indian hedgehog (IHH) and Sonic hedgehog (SHH) are expressed by intestinal epithelial cells at E18.5. IHH knockout mice die at birth. Lesser mutations in SHH, IHH, or downstream signaling molecules result in a host of gross malformations of the foregut, pancreas, and mid- and hindgut in humans. In adults, the Hh pathway dominates the mesenchymal-epithelial interactions because it is critical for the development of the mesenchymal cells of the lamina propria (65) and thus for their ability to modulate proliferation within the epithelium.
To examine the combined role of IHH and SHH in intestinal morphogenesis, the Gumucio laboratory (14) created nonlethal transgenic mice expressing the pan-Hh inhibitor hedgehog-interacting protein (Hhip) in the villus epithelium by coupling it to the villin promoter. Gumucio and colleagues demonstrated a disturbed epithelial-to-mesenchymal paracrine action of Hh. Myofibroblasts were mislocated throughout the villus, and the desmin-positive smooth muscle fibers of the villus core were expanded in a disorganized pattern. One interpretation of these results is that decreased Hh signaling in the embryo results in failure of myofibroblasts to develop in an orderly subepithelial location and failure to form the villus core smooth muscle into elements that will eventually become the smooth muscle of the lymphatic lacteal. Gumucio and colleagues did not report in detail changes in the muscularis mucosae. The mice in this study also demonstrated increased epithelial proliferation with Ki67-positive cells existing at the villus tips instead of being relegated to the villus bases, as in wild-type mice. These investigators concluded that the misdirected myofibroblasts may be responsible for loss of the anchoring properties that keep the normal proliferating epithelium at the villi bases in the area that becomes the crypts.
To study the effects of chronic reductions in Hh signaling, the Gumucio laboratory (66) created a double transgenic villin-Hhip mouse (VFHhip) that shows only 30% of normal Hh signaling by 1 month of age. The Chen laboratory (67) created and studied a similar model (Villin-Cre:IHh flox/flox) with intestinal IHH mRNA levels at 4%, Ptch1 mRNA levels at 19%, and Gli mRNA levels at 9% of controls. In both models early death occurred (6–10 months for the Gumucio animals and less than 1 month for the Chen model) from varying degrees of villous atrophy, diarrhea, malabsorption, and malnutrition. Both models demonstrate mislocation of subepithelial myofibroblasts and progressive loss of villus smooth muscle. The Gumucio laboratory did not report changes in the muscularis mucosae, but the Chen mice showed striking loss of muscularis mucosae smooth muscle. By 6 months, even though there was increased epithelial proliferation, the Gumucio animals developed progressive loss of villi, crypt hyperplasia, and inflammation of the lamina propria. By 10 months these animals were dead from progressive diarrhea and malnutrition. Because the villin promoter is not avidly expressed in the colon, few morphological changes were expected or noted in the Gumucio animals. In the colon, where mosaic Cre expression occurs, the Chen model showed spotty areas of muscularis mucosae loss coinciding with dilated colonic crypts, suggesting an important role for the muscularis mucosae in normal crypt development.
The Gumucio laboratory (66) concluded that its model is phenotypically similar to chronic small intestinal inflammatory diseases, especially celiac disease, where villus loss is a common histology. Moreover, Gumucio and colleagues proposed that the Hh ligand has previously unrecognized anti-inflammatory properties. Although this may be so, the absence of small intestinal villous smooth muscle in this model is also compatible with loss of the lymphatic lacteals, a condition in humans termed lymphangectasia, which can be congenital or acquired, and with a histopathology in the early stages of the disease that is similar to that of this transgenic mouse models (68). The Chen mouse showed spotty areas of intestinal inflammation, possibly due to a weakened epithelial barrier (67). These changes may also be the result of increased interstitial pressure due to poor lymph drainage that would accompany the loss of lymphatics in the villus.
The Intestinal Epithelial Stem Cell Niche
Stem cell dynamics are regulated by environmental factors, the sum of which constitutes the stem cell niche. The intestinal epithelial stem cell niche is composed of neighboring epithelial cells, mesenchymal cells, basement membrane, and soluble and cell- or matrix-associated growth factors (8, 62). Although neighboring epithelial cells in the establishment and maintenance of the intestinal stem cell niche are critical, as exemplified by the necessity of Notch signaling for stem cell maintenance and ephrin interactions for proper trafficking, we focus on the role of mesenchymal cells in this process.
Wnt signaling
The fundamental pathway driving proliferation within the intestinal epithelium is the Wnt pathway (69, 70). Canonical Wnt signaling results in nuclear accumulation of β-catenin, where it binds to T cell factor 4 (TCF4) transcription factor, resulting in enhanced transcription of Wnt/TCF4 target genes. By expressing a dominant-negative TCF4 peptide in human colorectal cancer cell lines, Van der Flier et al. (71) discovered a panel of genes regulated by Wnt signaling within intestinal epithelial cells. Many of these are preferentially expressed within intestinal epithelial stem cells, leading to the conclusion that Wnt signaling is enhanced within the stem cell niche. Of these genes, the leucine-rich repeat–containing G protein–coupled receptor 5 (Lgr5) and olfactomedin 4 (Olfm4) are specifically expressed within intestinal epithelial stem cells or immediate progenitors (71–73). A detailed survey of the cellular source of intestinal Wnts concluded that canonical Wnts (Wnts 3, 6, 9b) are secreted predominantly by epithelial cells, whereas noncanonical Wnts (Wnts 2b, 4, 5a, 5b) and Wnt antagonists (SFRP-1, Dkk-3) emanate from the mesenchyme (74). Microarray analysis of microdissected tissue demonstrated a stromal source for the Wnt antagonists Dkk-2 and SFRP-2 (75). Therefore, one of the key functional roles of mesenchymal cells may be to restrict Wnt/β-catenin signaling in intestinal stem cells or progenitor cells and to prevent the abnormal activity of this critical pathway in mature intestinal epithelial cells. Paneth cells are a rich source of canonical Wnts, an interesting fact given their proximity to stem cells and the importance of Wnts for maintenance of Paneth cell differentiation (74, 76, 77).
Bone morphogenetic protein signaling
One major pathway that antagonizes Wnt signaling along the crypt-villus axis involves the mesenchymally derived bone morphogenetic proteins (Bmps) (78, 79). Bmp signaling inhibits intestinal epithelial stem cell expansion and promotes epithelial differentiation (80). Bmps antagonize Wnt signaling by stabilizing phosphatase and tensin homolog (PTEN), resulting in decreased Akt activity, which culminates in decreased nuclear β-catenin (78). Bmps are members of the TGFβ superfamily of growth and differentiation factors in which ligand binding stimulates phosphorylation of the type 1 receptor subunit by the type 2 receptor subunit. The activated type 1 receptor phosphorylates a receptor-associated SMAD (SMAD1, -5, or -8), which then complexes with SMAD4. The SMAD dimer then translocates to the nucleus, where a specific program of gene transcription is activated (81). SMAD-independent Bmp signaling also occurs whereby a type 2 receptor initiates mitogen-activated protein (MAP) kinase cascades (81). In the intestine, Bmp4 is synthesized by the intervillus stromal cells, whereas epithelial cells contain type 1 Bmp receptors (82). Active Bmp signaling, as evidenced by phosphorylated SMADs 1, 5, and 8, is observed within differentiated epithelial cells and throughout the stroma of the lamina propria (82, 83). Kosinski et al. (84) recently discovered why Bmp signaling is not active within the stem cell zone of intestinal crypts, finding that myofibroblasts and muscularis mucosae cells in proximity to this zone secrete the Bmp-inhibitory molecules gremlin 1, gremlin 2, and chordin-like 1. Kosinski et al. propose a model in which cryptal myofibroblasts and muscularis mucosae cells, via localized secretion of Bmp antagonists, assist in the establishment of a stem cell niche where Wnt signaling proceeds unopposed. As one proceeds lumenally, Bmp tone increases, generating an environment promoting epithelial differentiation (Figure 3) (84). An active role of the epithelium in the establishment of this Bmp gradient is suggested by a recent study by Klapholz-Brown et al. (85), who demonstrated that canonical Wnt signaling within lung fibroblasts leads to a dramatic induction of gremlin 2 synthesis. Thus, in the intestine, canonical Wnts derived from epithelial cells, Paneth cells, or Paneth-like cells within the proliferative zone may also induce localized synthesis of Bmp inhibitors in the underlying mesenchyme. Epithelial synthesis of Hh proteins in adults is generally restricted to differentiated epithelial cells, further ensuring a lower level of Bmps within the proliferative zone (63) (Figure 3).
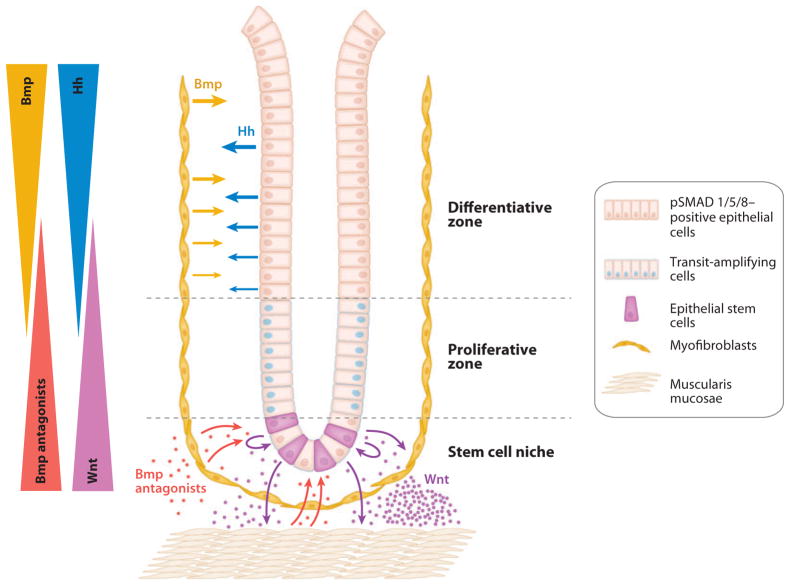
Figure 3.
Epithelial-mesenchymal interactions orchestrate hedgehog (Hh), bone morphogenetic protein (Bmp), and Wnt signaling in the intestinal crypt. Hh proteins produced by differentiated epithelial cells trigger stromal Bmp synthesis. Active Bmp signaling within differentiated epithelial cells, evidenced by phosphorylated SMADs (pSMAD) 1, 5, and 8, contributes to maintenance of the differentiated state and proliferative quiescence. Within the stem cell niche, lack of Hh signaling leads to decreased stromal Bmp synthesis. Secretion of canonical Wnts by crypt-based epithelial and Paneth cells, in addition to promoting self-renewal of intestinal epithelial stem cells, may also induce synthesis and secretion of Bmp antagonists by the underlying myofibroblasts and muscularis mucosae cells (85). Bmp antagonism within the stem cell niche would further enhance Wnt signaling. Not shown in this model are autocrine effects of Bmp signaling upon the stromal cells or numerous other factors, stromal and epithelial, that regulate differentiation and self-renewal within intestinal crypts (64). Adapted from Kosinski et al. (84), with permission.
In addition to regulating myofibroblast and smooth muscle development during ontogenesis, Hh signaling is also the primary determinant of stromal Bmp synthesis and thus contributes to establishment of the epithelial stem cell niche (86). The targeted IHH knockout mice used by the Chen laboratory discussed above display villus branching, aberrant crypt formation, and increased epithelial proliferation (67). At postnatal day (P)30, adenomatous changes with mild dysplasia were seen. Wnt target gene expression (CD44, Sox9, and EphB2) was no longer restricted to the base of crypts but extended along the villi, and Olfm4 expression increased and expanded in the crypts. Gene array analysis showed upregulation of Wnt target genes, including that of Lgr5. Downregulated genes included those involved in smooth muscle development and extracellular matrix synthesis, and similar gene regulatory changes were found in cultured myofibroblasts. Bmp2, -4, and -5 were downregulated, whereas Bmp antagonists such as gremlin 1 and chordin-like 2 were upregulated. Kosinski et al. (84) propose, therefore, that loss of the muscularis mucosae cells leads to deregulation of the epithelial stem cell compartment through loss of Wnt inhibitor Sfrp2 and extracellular matrix proteins that structurally support the stem cell niche. Additional factors favoring epithelial Wnt-driven proliferative responses would be diminished Bmp secretion and increased production of Bmp inhibitors.
Epimorphin
Additional insight into the role of subepithelial myofibroblasts in epithelial regulation comes from a series of elegant studies by the Rubin laboratory (87–89) on the role of epimorphin in crypt-villus morphogenesis. Epimorphin levels increase in the fetal gut mesenchyme during lumen formation and villous morphogenesis. Myofibroblasts engineered to overexpress epimorphin secrete higher levels of Bmp4, indicating that epimorphin regulates Bmp4 synthesis within myofibroblasts (90). Epimorphin−/− mice demonstrate increased crypt epithelial proliferation, increased crypt fission, and increased bowel length. Hh signaling protein expression was decreased in knockouts, suggesting less Hh responsiveness in these animals. These studies reinforce the idea that the myofibroblast plays a central role in maintenance of the epithelial stem cell niche. Epimorphin expression within myofibroblasts functions perhaps via modulating their response to Hh signaling by mechanisms that remain to be determined (88, 89).
Clevers’s group (91) recently demonstrated that intestinal crypt-villus structures can be assembled in vitro from single Lgr5+ stem cells without a mesenchymal niche. However, several factors are required for the growth of the Lgr5+ stem cells. Among them are a laminin-rich matrix and the Bmp antagonist noggin, which are secreted predominantly by mesenchymal cells. However, the recent studies of Hh signaling during gut development mentioned above demonstrate that ablation of Hh signaling leads to disrupted cryptal mesenchymal cells. Such disruption results in enlarged crypts and expansion of the intestinal stem cell compartment, suggesting that mesenchymal cells may also play a role in restricting intestinal stem cell expansion. Furthermore, a true villus did not form from the single epithelial stem cell; only a flat intercrypt region formed. This suggests that lamina propria mesenchymal cells must be present and are necessary to recapitulate the complete villus-crypt axis. Therefore, mesenchymal components likely balance intestinal stem cell self-renewal and differentiation. More studies are needed to fully elucidate the mesenchymal-epithelial interactions involved in maintaining intestinal stem cell fate.
The cancer stem cell niche
It has been postulated that cancer is a disease of stem cells. A substantial body of evidence supports the hypothesis that neoplasms are initiated and maintained by a small population of cells within a tumor that possesses properties similar to those of normal adult stem cells (92). These qualities include the ability to self-renew and generate differentiated progeny. According to this hypothesis, only a small subset of tumor cells should be able to initiate and sustain malignant tumor growth and to give rise to the phenotypic heterogeneity observed in the original tumor; this is true for a wide variety of cancers, including colorectal cancer (93). It should thus not be surprising that the major pathway regulating stem cells in normal colorectal tissue, the Wnt pathway, is the most frequent and earliest pathway mutated in colorectal carcinogenesis. In human familial adenomatous polyposis (FAP) and rodent models of FAP, expansion of the proliferative stem cell compartment occurs prior to the appearance of recognizable lesions (94). A wealth of published information, including excellent reviews, describes mechanisms whereby stromal cells modulate the process of carcinogenesis (7). We briefly highlight below examples of how the principles of epithelial-mesenchymal interactions in the establishment of the intestinal epithelial stem cell niche also apply during colorectal carcinogenesis.
Altered Bmp signaling can lead to colorectal carcinogenesis. Juvenile polyposis syndrome ( JP) is an autosomal-dominant syndrome that increases the patient’s risk for developing colon cancer by approximately 12-fold. SMAD4 and BmpRIA mutations are frequently observed in JP patients (83), and a small percentage of those with JP harbor PTEN mutations (95). Inhibition of Bmp signaling by conditional knockout of BmpRIA (82) or PTEN (90) or transgenic overexpression of the Bmp inhibitor noggin (96) results in the formation of numerous ectopic crypts that mimic the intestinal histopathology of JP. The lack of epithelial responsiveness to Bmps in these cases leads to an expansion of the intestinal epithelial stem cell proliferative compartment similar to what is observed in mice engineered to contain depressed levels of Hh signaling. Elevated stromal expression of the Bmp antagonist gremlin 1 occurs in a wide variety of solid tumor types, including colorectal, which is expected to expand the cancer stem cell pool (80).
Aberrations in Hh signaling have been noted in a wide variety of cancers, but the role of Hh signaling in colorectal cancer remains controversial (63, 97). IHH synthesis is inhibited by active Wnt signaling, and IHH expression is absent in polyps of FAP patients (98), which is expected to result in decreased synthesis of stromal Bmps. Unlike normal epithelium, a number of colorectal epithelial cancer cell lines express Ptch and Gli proteins, yet the significance of this expression is still not understood (63, 97). Arimura et al. (99) recently reported increased expression of Smo within the adenomatous polyps of Apc+/Δ716 mice, a model for FAP. Small interfering RNA–mediated knockdown of Smo expression within colorectal cancer epithelial cell lines suppresses proliferation, and Apc+/Δ716Smo+/− mice harbor smaller polyps that display diminished epithelial proliferation relative to Apc+/Δ716 mice (99). Arimura et al. (99) discovered that within the cancer epithelial cells Smo activates a Gli-independent signal transduction cascade that enhances Wnt signaling, leading to increased levels of nuclear β-catenin.
Wang et al. (100) recently documented the importance of Hh and Bmp signaling during the development of Barrett’s metaplasia, a premalignant condition whereby metaplastic columnar epithelium replaces the normal squamous epithelium lining of the esophagus. SHH expression is dramatically increased within Barrett’s epithelium and is associated with stromal expression of the Hh target genes PTCH1 and Bmp4. Chronic acid or bile exposure is considered to be an etiologic agent for the initiation of Barrett’s esophagus. Acidified medium induces SHH and IHH expression in cultured squamous epithelial cell lines, and Hh expression was also induced in esophageal tissue of mice experiencing experimentally induced reflux esophagitis (esophagojejunostomy). Bmp4 signaling induces expression of SOX9, an intestinal crypt transcription factor, which is highly expressed in Barrett’s epithelium. Additionally, expression of deleted in malignant brain tumors 1, the human homolog of the columnar cell factor hensin, occurs in Barrett’s epithelium and is induced by SOX9. Finally, transgenic expression of SHH in the mouse esophageal epithelium induced the expression of stromal Bmp4, epithelial Sox9, and columnar cytokeratins (100). Thus, inappropriate Hh signaling within esophageal tissue contributes to the development of Barrett’s metaplasia via stromally derived Bmps that trigger reprogramming of the squamous epithelium (100).
STROMAL CELLS IN INNATE AND ADAPTIVE IMMUNITY
This section focuses on the emerging role of the mucosal stromal cells (myofibroblasts, fibroblasts, and probably pericytes) in immune responses and tolerance. The concept that stromal cells of mesenchymal origin participate in the regulation of acute and chronic inflammation in peripheral organs, including secondary lymphoid organs, is now firmly established (101). Aside from their role as structural elements, stromal cells serve as resident sentinels that upon activation produce chemokines, initiating the recruitment of leukocytes to the site of tissue injury and inflammation (102, 103). This topic has been well reviewed (101), and we cannot add to the discussion. Instead, we focus on recent reports from our laboratory and others that mucosal stromal cells interact with professional immune cells to regulate innate and adaptive immune responses in the intestine (Figure 4).
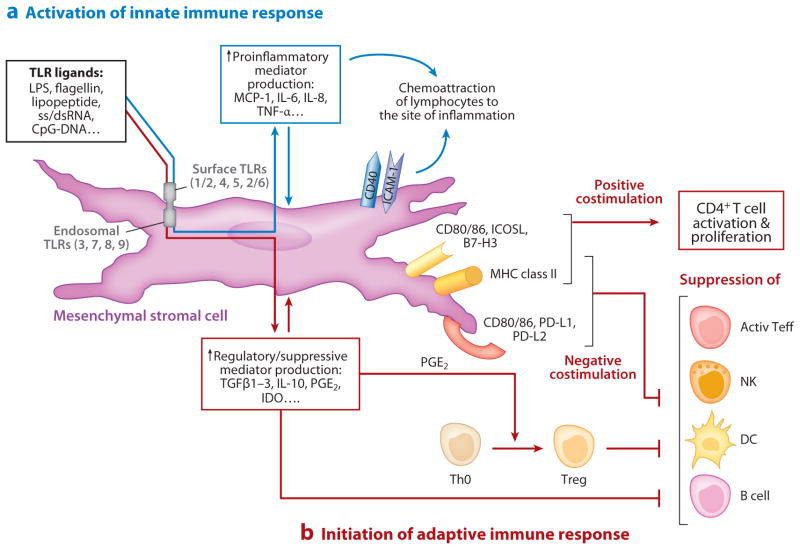
Figure 4.
Mesenchymal stromal cells as links between innate and adaptive immunity. (a) Stromal cells participate in innate immunity via expression of TLRs, whose engagement induces expression of either pro- or anti-inflammatory soluble mediators and surface-associated molecules. The proinflammatory molecules (e.g., MCP-1, IL-8) induce migration of professional immune cells where they may be anchored via interaction with stromal adhesive molecules (e.g., ICAM-1, CD40). (b) Adaptive immunity is initiated via signaling through MHC class II molecules and B7-positive or B7-negative costimulators expressed on mesenchymal stromal cells modulating the activity of CD4+ T cells, NK, DC, and B cells. Depending on the microenvironmental milieu of cytokines, chemokines, growth factors, and pathogen-associated molecular patterns (PAMPs), stromal cells function in either an immunosuppressive (tolerogenic) role or an immunostimulatory role. Abbreviations: active Teff, activated effector T cells; CD, cluster of differentiation; ICAM-1, intercellular adhesion molecule-1; IDO, indoleamine 2,3-dioxygenase; IL, interleukin; NK, natural killer cells; DC, dendritic cells; MCP-1, monocyte chemotactic protein-1; MHC, major histocompatibility complex; PD-L, programmed cell death receptor ligand; PGE2, prostaglandin E2; ss/dsRNA, single-stranded/double-stranded RNA; TGFβ, transforming growth factor β; Th0, naive CD4+ T helper cells; TLR, Toll-like receptor; TNF-α, tumor necrosis factor-α; Treg, CD4+ CD25high FoxP3+ regulatory T cells.
Innate Immunity: Toll-Like Receptor Signaling
The gastrointestinal mucosal immune response of healthy individuals is characterized by a balance between immunity, which protects mucosal surfaces from harmful microbes, and tolerance, which permits the intestinal mucosa to interact in a nonpathological way with the commensal bacteria and dietary antigens to which it is constantly exposed (104). Epithelial cells have a complex interaction with the intestinal microflora, but the epithelial barrier is not sufficient to provide absolute protection from undesired inflammatory antigens. Therefore, the professional and nonprofessional immune cells that reside just beneath the epithelial layer are poised to be among the first participants to react to leaked lumenal antigens by mounting an immune response. The first line of defense to such antigens is the innate immune response composed of the pathogen-associated molecular pattern–sensing TLRs (104, 105).
Binding of TLRs to their ligands results in activation and/or maturation of TLR-expressing cells, changing their surface-associated and soluble immune mediator expression. The end result is regulation of the efficiency of antigen presentation to T cells. TLR regulation of antigen presentation within the lamina propria is attributed mostly to professional antigen-presenting cells (APCs) such as macrophages and dendritic cells (106). However, human CD90+ intestinal myofibroblasts and fibroblasts also express TLRs (107), and we have recently shown that these cells also function as APCs (108). They compose up to 30% of the lamina propria mononuclear cell population (108). TLR expression by murine intestinal stromal cells has also been reported (109). Therefore, by virtue of their location, their number, and their expression of TLRs, stromal fibroblasts and myofibroblasts may play an important and previously unappreciated role in innate immune responses within the gastrointestinal mucosa. For example, TLR4 engagement on these cells leads to upregulation of expression of proinflammatory mediators [including interleukin (IL)-1α, IL-1β, IL-6, and IL-8] and adhesion molecules, such as intercellular adhesion molecule-1 (ICAM-1) (110), a process that results in the attraction of professional immune cells such as lymphocytes, macrophages, and neutrophils. Studies in our laboratory have shown that stimulation of TLR3, a receptor involved in the recognition of double-stranded RNA from necrotic eukaryotic cells and viruses, upregulates expression of the antiviral cytokine interferon-β (IFN-β) and TLR3 by human intestinal myofibroblasts and fibroblasts (111). In human gingival fibroblasts, TLR2 and TLR4 signaling causes the production of the chemokine CCL17, which triggers migration of T helper 2 cells (Th2) cells (112). The stimulation of TLR4 signaling within human rheumatoid synovial fibroblasts induces synthesis of the chemotactic cytokines monocyte chemotactic protein-1 (MCP-1) and IL-8 (113). Thus, depending on which TLRs are expressed under specific physiological or pathological conditions, stromal cells may be important contributors to the innate immune response by producing cytokines, chemokines, and surface molecules in response to TLR ligation.
Adaptive Immunity: Antigen Presentation
Although CD4+ T cells are central to effective adaptive immunity, their response is dictated by their interactions with APCs. The capacity of intestinal stromal cells to regulate lymphocyte activation through MHC class I expression is widely accepted (114, 115), but their ability to express MHC class II and to regulate the activation of CD4+ T cells has only recently become evident. We have shown that fibroblasts/myofibroblasts in the normal human colonic mucosa represent a significant fraction of lamina propria MHC class II+ nonprofessional APCs (108). These stromal cells induce naive and resting allogeneic CD4+ T cell activation in a MHC class II– and B7 molecule–dependent manner in vitro (108). Furthermore, intestinal stromal cells process and present antigen to antigen-specific CD4+ T cells (108). This capacity has also been reported for human and rodent dermal fibroblasts (116), gingival fibroblasts (117), synovial fibroblasts (118), hepatic stellate cells (119), human decidual stromal cells (120), glial cells (121), and MSCs (122).
Most peripheral T cells exist as resting lymphocytes that can be quickly activated by exposure to presented antigens (123, 124). Cytotoxic T lymphocyte antigen-4 (CTLA-4), a homolog of CD28, is upregulated on the surfaces of activated T cells. CTLA-4 and CD28 both bind CD80 and CD86, which are B7 family costimulatory molecules expressed by APCs and are required for efficient activation of CD4+ T cells. When CTLA-4 and CD28 are both expressed, CTLA-4 may outcompete CD28 because of its higher binding affinity for CD80/86. CTLA-4 functions as a negative regulator that attenuates T cell responses by downregulating T cell activation (125, 126). Compared with professional APCs, colonic myofibroblasts/fibroblasts express low levels of CD80 and CD86 (108). Because the preponderance of lamina propria CD4+ T lymphocytes may be quickly activated and express the inhibitory ligand CTLA-4, together these two findings suggest that intestinal stromal cells may normally play a suppressive role. Thus, one homeostatic role of MHC class II–expressing intestinal stromal cells may be to limit antigen-specific immune responses to lumenal antigens.
Adaptive Immunity: Immune Suppression by B7-Negative Costimulators and Soluble Factors
The induction of tolerance to innocuous antigens is a critically important immunological process in the intestinal mucosa. Many mechanisms have been proposed to explain the T cell–dependent immune responses that occur during tolerance, including clonal anergy, clonal deletion, and active regulatory processes (104). The T cell response during tolerance induction is highly orchestrated by interactions with APCs (104). We recently reported that intestinal CD90+ myofibroblasts/fibroblasts can suppress proliferation of CD3/CD28- activated CD4+ T cells (23). Similar observations have been made for dermal (127), liver (128), decidual (129), and tumor-derived lung (130) fibroblasts, as well as the progenitor of these cells, MSCs (137). These stromal cells were capable of inhibiting CD4+ or CD8+ T cell proliferative responses induced by allogeneic or specific antigens, mitogens (phytohemagglutinin and concavalin A), or anti-CD3/anti-CD28 monoclonal antibodies. Moreover, MSCs have both in vitro and in vivo tolerogenic effects on the function of a broad range of other immune cells, including B cells (131), natural killer (NK) cells (132), and dendritic cells (133). Melanoma-derived fibroblasts alter NK cell cytotoxicity and cytokine production (134). Additionally, recent work by Jones et al. (135) suggests that, in contrast to parenchymal cells, a tolerogenic antiproliferative effect is a fundamental characteristic of all mesenchymal stromal cells.
Direct stromal cell–mediated immunosuppression may involve cell contact–mediated interactions and/or production of soluble factors. Cell contact–mediated immunosuppression involves the novel inhibitory B7 ligands PD-L1 (also known as B7-H1 and CD274) and PD-L2 (also known as B7-DC and CD273) and their cognate T cell counter receptor, programmed death receptor 1 (PD-1; also known as CD279) (136). We recently demonstrated that in addition to low-level expression of B7.1 and B7.2 (CD80 and CD86, respectively), colonic myofibroblasts/fibroblasts express PD-L1 and PD-L2 and that both of these molecules participate in the contact-mediated suppressive effect of colonic myofibroblasts on activated CD4+ T cell proliferation (23). The involvement of PD-L1 in stromal cell–mediated immune suppression has also been reported for hepatic stellate cells (128), decidual stromal cells (129), tumor-derived lung fibroblasts (130), and MSCs (137).
Another mechanism by which stromal cells suppress acute immune responses is via the production of soluble suppressive molecules. Upon activation, myofibroblasts and fibroblasts, as well as other peripheral stromal cells, produce TGFβ1–3, IL-10, PGE2, and indoleamine 2,3-dioxygenase (IDO) (138–141). Although the role of these molecules in the immunosuppressive activity of intestinal stromal cells has not been tested, these factors are involved in the suppressive activity of MSCs and other stromal cells against CD4+ and CD8+ T lymphocytes, NK and B cells, macrophages, and dendritic cells (130, 134, 140, 141).
Adaptive Immunity: Suppression by Induction and Support of Regulatory T Cells
Recent studies suggest that functionally distinct subsets of regulatory T (Treg) cells, CD4+ CD25high FoxP3+ cells, are actively involved in the maintenance of immunological tolerance in the gastrointestinal tract (142). Dysregulation of Treg number and function is thought to be involved in IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked) syndrome, inflammatory bowel disease, and cancer (143–145). We found that colonic myofibroblasts/fibroblasts can contribute to the induction and maintenance of Treg cells (146). This adds intestinal myofibroblasts to the list of stromal cells (dermal and synovial fibroblasts, hepatic stellate cells, astrocytes, and MSCs) that may contribute to the generation of Treg cells (127, 147–150).
Microenvironment and Stromal Cell Immune Function
The discussion above suggests that stromal cells may play an immunosuppressive, tolerogenic role (23, 127, 135, 137, 151) or a role in active immunity by promoting T cell activation (108, 115, 122, 152). We propose that stromal cells have the capacity to function in either an immunosuppressive role or an immunostimulatory role, depending on the microenvironmental milieu of cytokines, chemokines, growth factors, and TLR ligands. The antigen-presenting function of mesenchymal stromal cells requires autocrine stimulation by endogenous but low levels of IFN-γ (122). Low levels of IFN-γ (10–100 U ml−1) are required to reestablish MHC class II expression by cultured human intestinal fibroblasts and myofibroblasts (108). In contrast, elevated and/or chronic levels of IFN-γ suppress the APC function of MSCs (122) and upregulate expression of immunosuppressive molecules PD-L1 and IDO by hepatic stellate cells (128), dermal fibroblasts (140), and intestinal myofibroblasts/fibroblasts (153). Thus, high ambient IFN-γ promotes the immunosuppressive function of these stromal cells.
The understanding of the immune function of intestinal stromal cells is far from complete. However, the recent data acquired for intestinal stromal cells and for those cells in other parenchymal organs allow us to suggest that these cells are appropriately licensed to be active participants in the regulation of professional immune cells during immunity and tolerance. We suggest that gastrointestinal mucosal stromal cells are physically connected to and are of the same fundamental structure as the peripheral secondary lymphoid organs. Thus, lamina propria stromal cells are part of a sophisticated immune lymphatic network throughout the mammalian body.
PERICYTES.
Originally named after their discoverer Charles Rouget and also termed mural cells or vascular smooth muscle cells, these are SMA+ myofibroblast-like cells that wrap around capillaries as single cells. Pericytes contribute proteins to the vascular basement membrane, as do the endothelial cells to which pericytes communicate via paracrine signals. Together with the endothelium, pericytes contribute to angiogenesis and revascularization. Paracrine signals from pericytes can control contractility and permeability of the capillary. The anatomy of and communication between pericytes and endothelial cells through an intervening basement membrane are analogous to the anatomy of and communication between subepithelial myofibroblasts and the intestinal epithelium. Indeed, in the intestine and liver, subepithelial myofibroblasts and stellate cells also serve simultaneously as pericytes and may have a common embryonic origin and bone marrow stem cell replacement process. They share molecular markers with intestinal myofibroblasts, respond to the same cytokines and growth factors (e.g., platelet-derived growth factor B), and likely play similar roles in normal biology and disease. Bergers & Song (154) and Krueger & Bechmann (155) recently reviewed the biology of these cells.
SUMMARY POINTS.
The non-white-blood-cell and nonendothelial mesenchymal elements of the intestinal lamina propria—i.e., myofibroblasts, fibroblasts, mural cells (pericytes) of the vasculature, bone marrow–derived stromal stem cells, the smooth muscle of the muscularis mucosae, and the smooth muscle associated with the lymphatic lacteals—share similar marker molecules, origins, and coordinated biological functions.
The mesenchymal cells of the intestinal lamina propria can be derived from multiple sources in the developing embryo and adult. During development, subepithelial and perivascular α-smooth muscle actin–positive (α-SMA+) cells of the lamina propria probably arise from either serosal mesothelial cells or neural crest cells, but it is unclear whether the muscularis mucosa and lymphatic smooth muscle share this origin. Adult myofibroblasts can be derived by activation of resident fibroblasts, dedifferentiation from perivascular smooth muscle cells, epithelial- and endothelial-to-mesenchymal transitions, and bone marrow–derived stem cells.
During development, the stromal compartment is modeled through epithelial-mesenchymal interactions, of which hedgehog (Hh) signaling plays a critical role. Transgenic mouse models demonstrate that reduced production of Hh proteins by the epithelium results in depletion of stromal myofibroblasts, villus smooth muscle, and muscularis mucosae cells accompanied by expansion of the epithelial proliferative compartment.
Stromal myofibroblasts and muscularis mucosae cells work with epithelial cells to construct the stem cell niche in normal tissues and during carcinogenesis through complex interactions involving Wnt, Bmp, and Hh signaling.
Stromal cells of the intestinal lamina propria are part of the mucosal immune system and display receptors and surface markers that are used for regulation by professional immune cells. This repertoire includes expression of MHC class II, accessory costimulatory (CD80, CD86), and inhibitory (PD-L1, PD-L2) molecules.
Mesenchymal stromal cells express a wide array of Toll-like receptors (TLRs) and thus contribute to innate immune responses. Because the stimulation of TLRs on these cells may alter the profile of cytokine and surface receptors during antigen presentation, TLR-mediated microbial recognition by stromal cells may influence the type of adaptive immune response mounted to each antigen.
Mesenchymal stromal cells can exert either an immunosuppressive or an immunostimulatory influence, depending on the microenvironmental context during their interaction with professional immune cells. In the gastrointestinal mucosa, these interactions occur within a dynamic milieu containing cytokines, chemokines, growth factors, and TLR ligands.
FUTURE ISSUES.
Specific markers to identify the various mesenchymal elements of the lamina propria, as well as specific conditional knockout or transgenic animals that will allow studies of their role in the biology of health or disease, are needed.
The bone marrow origin of intestinal myofibroblasts in the adult animal (mesenchymal stem cell, monocyte-derived fibrocyte, or both) and their embryonic origin (mesothelium or neural crest) need definition.
In-depth studies of the embryology, anatomy, and paracrine functions of the lymphatic lacteal–associated smooth muscle or stromal cells may add new concepts to the field of lamina propria biology.
As a clearer identification of the intestinal epithelial stem cell emerges, a clearer understanding of the elements and functions of myofibroblasts, muscularis mucosae, and lymphatic smooth muscle in the stem cell niche will become critical.
Aside from the role of stroma in cancer, we have a poor understanding of the role of lamina propria mesenchymal cells in intestinal diseases such as viral or bacterial (invasive or enterotoxigenic) infections or in inflammatory bowel diseases such as ulcerative colitis, Crohn’s disease, and celiac disease.
Because of their likely role in tolerance, intestinal stromal cells should be explored for ways to enhance mucosal vaccines, including cancer vaccines, or to suppress organ transplant rejection.
Because stromal cells in the lamina propria are derived from stem cells of bone marrow origin, the use of mesenchymal or hematopoietic stem cells to treat intestinal diseases likely involves these gut elements in a fundamental way. Regenerative medicine as it is applied to the gut will require a more complete knowledge of the functions of lamina propria mesenchymal cells.
Acknowledgments
The authors wish to acknowledge useful discussions with David Bader and Michelle Southard-Smith of Vanderbilt University concerning the embryonic origin of intestinal myofibroblasts. We thank Patrick Adegboyega of the Louisiana State University Health Science Center at Shreveport, Louisiana, for providing the micrographs used in Figure 1 and for allowing us to mention unpublished data. The research performed in the authors’ laboratories mentioned in this article was supported by grants from the NIH: DK55783 (D.W.P.), CA127229 (D.W.P.), DK069309 (X.C.), and CA136606 (X.C.).
Glossary
- α-Smooth muscle actin (α-SMA)
one of six isoforms of the contractile cytoskeletal protein actin; also a smooth muscle differentiation antigen
- Vimentin and desmin
two of the type 3 intermediate filaments. Desmin is expressed primarily by muscle cells; vimentin, by mesodermally derived nonmuscle cells
- Transforming growth factor β (TGFβ)
a family of structurally related secreted growth/differentiation factors including TGFβ isoforms I–III. The TGFβsuperfamily also includes activins and bone morphogenetic proteins
- Major histocompatibility complex (MHC)
MHC class I and II molecules are used to present antigens to CD8+ and CD4+ T cells, respectively
- Fibroblast-specific protein 1 (Fsp1)
a member of the S100 family of intracellular calcium–binding proteins
- NG2 chondroitin sulfate proteoglycan (NG2)
also termed high-molecular-weight melanoma-associated antigen (HMWMAA); the mouse derivate is termed AN2
- Epithelial-to-mesenchymal transition (EMT) and endothelial-to-mesenchymal transition
reversible processes whereby epithelial and endothelial cells acquire characteristics of motile mesenchymal cells
- Programmed cell death receptor ligand (PD-L)
two variants, PD-L1 and PD-L2, exist and are associated with delivery of negative costimulatory signals to T cells
- Toll-like receptors (TLRs)
recognize macromolecules displaying pathogen-associated molecular patterns. Humans have 10 TLRs, each responding to a different molecular pattern class
- Hedgehogs (Hh)
compose a key signaling pathway during development. Three Hh proteins—Indian (IHH), Sonic (SHH), and Desert (DHH)—exist in humans
- Leucine-rich repeat–containing G protein–coupled receptor 5 (Lgr5)
an orphan G protein–coupled receptor that appears to be a specific marker for intestinal epithelial stem cells; also known as GPR49
- Olfactomedin 4 (Olfm4)
a precursor that is another specific marker for mammalian intestinal epithelial stem cells. It is a secreted extracellular protein of unknown function
- Wnt
coined as a combination of Wingless (Drosophila) and Int (mouse). Wnt proteins are key paracrine determinants of epithelial proliferation and differentiation
- Bone morphogenetic proteins (Bmps)
part of the TGFβ superfamily of signaling peptides. These mesenchymally derived factors cause maturation/differentiation of epithelial cells along the crypt-villus axis
- SMAD
term derived from combination of mothers against decapentaplegic (Drosophila) and SMA (Caenorhabditis elegans). Transduces the downstream signals of TGFβsuperfamily members
- Epimorphin/syntaxin 2
a membrane-associated myofibroblast protein homologous to the syntaxin family of vesicle-docking proteins in neurons and pancreas. It is a mediator of epithelial-mesenchymal interaction
- Familial adenomatous polyposis (FAP)
truncating mutations in the adenomatosis polyposis coli gene, involved in Wnt signal transduction, result in multiple colonic adenomas and cancers
- Antigen-presenting cells (APCs)
process antigens and display their peptide fragments on the cell surface complexed with MHC molecules for T cell presentation
- Cytotoxic T lymphocyte antigen-4 (CTLA-4; also known as CD152)
binds CD80 and CD86 on APCs and transmits an inhibitory signal to the T cell
- Cluster of differentiation 28 (CD28)
a T cell marker that binds CD80 and CD86 on APCs and transmits a stimulatory signal to the T cell. It is present on naive T cells
- Regulatory T (Treg) cells
a subpopulation of T cells that suppress immune system activation, allowing for immune system homeostasis and tolerance to self-antigens
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
D.W. Powell, Email: dpowell@utmb.edu.
I.V. Pinchuk, Email: ivpinchuc@utmb.edu.
J.I. Saada, Email: jsaada@utmb.edu.
Xin Chen, Email: xin.chen@ucsf.edu.
R.C. Mifflin, Email: rmifflin@utmb.edu.
LITERATURE CITED
- 1.Powell DW, Adegboyega PA, Di Mari JF, Mifflin RC. Epithelial cells and their neighbors. I Role of intestinal myofibroblasts in development, repair, and cancer. Am J Physiol Gastrointest Liver Physiol. 2005;289:2–7. doi: 10.1152/ajpgi.00075.2005. [DOI] [PubMed] [Google Scholar]
- 2.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. II Intestinal subepithelial myofibroblasts. Am J Physiol Cell Physiol. 1999;277:183–201. doi: 10.1152/ajpcell.1999.277.2.C183. [DOI] [PubMed] [Google Scholar]
- 3.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I Paracrine cells important in health and disease. Am J Physiol Cell Physiol. 1999;277:1–9. doi: 10.1152/ajpcell.1999.277.1.C1. [DOI] [PubMed] [Google Scholar]
- 4.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–16. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simon-Assmann P, Bolcato-Bellemin AL, Klein A, Kedinger M. Tissue recombinants to study extracellular matrix targeting to basement membranes. Methods Mol Biol. 2009;522:309–18. doi: 10.1007/978-1-59745-413-1_20. [DOI] [PubMed] [Google Scholar]
- 6.Furuya S, Furuya K. Subepithelial fibroblasts in intestinal villi: roles in intercellular communication. Int Rev Cytol. 2007;264:165–223. doi: 10.1016/S0074-7696(07)64004-2. This excellent, detailed review addresses many aspects of myofibroblast biology but in particular focuses on the coupling of this network of contractile cells in the lamina propria by dye-permeable gap junctions and the endothelin and purinergic receptor responses that give it mechanosensitive properties. [DOI] [PubMed] [Google Scholar]
- 7.Egeblad M, Nakasone ES, Werb Z. Tumors as organs: complex tissues that interface with the entire organism. 2010;18:884–901. doi: 10.1016/j.devcel.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yen TH, Wright NA. The gastrointestinal tract stem cell niche. Stem Cell Rev. 2006;2:203–12. doi: 10.1007/s12015-006-0048-1. [DOI] [PubMed] [Google Scholar]
- 9.Vermeulen L, De Sousa E, Melo F, van der Heijden M, Cameron K, de Jong JH, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–76. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 10.De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer. 2008;123:2229–38. doi: 10.1002/ijc.23925. [DOI] [PubMed] [Google Scholar]
- 11.Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Investig. 2007;87:858–70. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]
- 12.Brown SL, Riehl TE, Walker MR, Geske MJ, Doherty JM, et al. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. J Clin Investig. 2007;117:258–69. doi: 10.1172/JCI29159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adegboyega PA, Mifflin RC, DiMari JF, Saada JI, Powell DW. Immunohistochemical study of myofibroblasts in normal colonic mucosa, hyperplastic polyps, and adenomatous colorectal polyps. Arch Pathol Lab Med. 2002;126:829–36. doi: 10.5858/2002-126-0829-ISOMIN. [DOI] [PubMed] [Google Scholar]
- 14.Madison BB, Braunstein K, Kuizon E, Portman K, Qiao XT, Gumucio DL. Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development. 2005;132:279–89. doi: 10.1242/dev.01576. [DOI] [PubMed] [Google Scholar]
- 15.Ohtani O, Ohtani Y. Organization and developmental aspects of lymphatic vessels. Arch Histol Cytol. 2008;71:1–22. doi: 10.1679/aohc.71.1. [DOI] [PubMed] [Google Scholar]
- 16.Ma B, von Wasielewski R, Lindenmaier W, Dittmar KE. Immmunohistochemical study of the blood and lymphatic vasculature and the innervation of mouse gut and gut-associated lymphoid tissue. Anat Histol Embryol. 2007;36:62–74. doi: 10.1111/j.1439-0264.2006.00741.x. Uses immunofluorescence and confocal microscopy to portray the relationships of the lymphatics and vasculature in the small intestinal villus and Peyer’s patches. [DOI] [PubMed] [Google Scholar]
- 17.Womack WA, Kvietys PR, Granger DN. Villous motility. In: Schultz SG, Wood JD, Rauner BB, editors. Handbook of Physiology: A Critical, Comprehensive Presentation of Physiological Knowledge and Concepts. Bethesda, MD: Am. Physiol. Soc; 1989. pp. 975–86. [Google Scholar]
- 18.Adegboyega PA, Ololade O, Saada J, Mifflin R, Di Mari JF, Powell DW. Subepithelial myofibroblasts express cyclooxygenase-2 in colorectal tubular adenomas. Clin Cancer Res. 2004;10:5870–79. doi: 10.1158/1078-0432.CCR-0431-03. [DOI] [PubMed] [Google Scholar]
- 19.Schildmeyer LA, Braun R, Taffet G, Debiasi M, Burns AE, et al. Impaired vascular contractility and blood pressure homeostasis in the smooth muscle α-actin null mouse. FASEB J. 2000;14:2213–20. doi: 10.1096/fj.99-0927com. [DOI] [PubMed] [Google Scholar]
- 20.Desmouliere A, Rubbia-Brandt L, Abdiu A, Walz T, Macieira-Coelho A, Gabbiani G. α-Smooth muscle actin is expressed in a subpopulation of cultured and cloned fibroblasts and is modulated by γ-interferon. Exp Cell Res. 1992;201:64–73. doi: 10.1016/0014-4827(92)90348-c. [DOI] [PubMed] [Google Scholar]
- 21.Rege TA, Hagood JS. Thy-1 as a regulator of cell-cell and cell-matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. FASEB J. 2006;20:1045–54. doi: 10.1096/fj.05-5460rev. [DOI] [PubMed] [Google Scholar]
- 22.Bradley JE, Ramirez G, Hagood JS. Roles and regulation of Thy-1, a context-dependent modulator of cell phenotype. Biofactors. 2009;35:258–65. doi: 10.1002/biof.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinchuk IV, Saada JI, Beswick EJ, Boya G, Qiu SM, et al. PD-1 ligand expression by human colonic myofibroblasts/fibroblasts regulates CD4+ T-cell activity. Gastroenterology. 2008;135:1228–37. doi: 10.1053/j.gastro.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koumas L, Smith TJ, Feldon S, Blumberg N, Phipps RP. Thy-1 expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes. Am J Pathol. 2003;163:1291–300. doi: 10.1016/S0002-9440(10)63488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koumas L, Smith TJ, Phipps RP. Fibroblast subsets in the human orbit: Thy-1+ and Thy-1−subpopulations exhibit distinct phenotypes. Eur J Immunol. 2002;32:477–85. doi: 10.1002/1521-4141(200202)32:2<477::AID-IMMU477>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 26.Odaka C. Localization of mesenchymal cells in adult mouse thymus: their abnormal distribution in mice with disorganization of thymic medullary epithelium. J Histochem Cytochem. 2009;57:373–82. doi: 10.1369/jhc.2008.952895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Vliet E, Melis M, Foidart JM, Van Ewijk W. Reticular fibroblasts in peripheral lymphoid organs identified by a monoclonal antibody. J Histochem Cytochem. 1986;34:883–90. doi: 10.1177/34.7.3519751. [DOI] [PubMed] [Google Scholar]
- 28.Goodpaster T, Legesse-Miller A, Hameed MR, Aisner SC, Randolph-Habecker J, Coller HA. An immunohistochemical method for identifying fibroblasts in formalin-fixed, paraffin-embedded tissue. J Histochem Cytochem. 2008;56:347–58. doi: 10.1369/jhc.7A7287.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS ONE. 2009;4:e7475. doi: 10.1371/journal.pone.0007475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okada H, Inoue T, Kanno Y, Kobayashi T, Watanabe Y, et al. Selective depletion of fibroblasts preserves morphology and the functional integrity of peritoneum in transgenic mice with peritoneal fibrosing syndrome. Kidney Int. 2003;64:1722–32. doi: 10.1046/j.1523-1755.2003.00290.x. [DOI] [PubMed] [Google Scholar]
- 31.Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther. 2006;5:1640–46. doi: 10.4161/cbt.5.12.3354. [DOI] [PubMed] [Google Scholar]
- 32.Liu T, Warburton RR, Guevara OE, Hill NS, Fanburg BL, et al. Lack of MK2 inhibits myofibroblast formation and exacerbates pulmonary fibrosis. Am J Respir Cell Mol Biol. 2007;37:507–17. doi: 10.1165/rcmb.2007-0077OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terada N, Ohno N, Murata S, Katoh R, Stallcup W, Ohno S. Immunohistochemical study of NG2 chondroitin sulfate proteoglycan expression in the small and large intestines. Histochem Cell Biol. 2006;126:483–90. doi: 10.1007/s00418-006-0184-3. [DOI] [PubMed] [Google Scholar]
- 34.Santos AM, Jung J, Aziz N, Kissil JL, Puré E. Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J Clin Investig. 2009;119:3613–25. doi: 10.1172/JCI38988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kikuchi Y, Kashima TG, Nishiyama T, Shimazu K, Morishita Y, et al. Periostin is expressed in pericryptal fibroblasts and cancer-associated fibroblasts in the colon. J Histochem Cytochem. 2008;56:753–64. doi: 10.1369/jhc.2008.951061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Douglass A, Wallace K, Koruth M, Barelle C, Porter AJ, Wright MC. Targeting liver myofibroblasts: a novel approach in antifibrogenic therapy. Hepatol Int. 2008;2:405–15. doi: 10.1007/s12072-008-9093-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McLin VA, Henning SJ, Jamrich M. The role of the visceral mesoderm in the development of the gastrointestinal tract. Gastroenterology. 2009;136:2074–91. doi: 10.1053/j.gastro.2009.03.001. A detailed review of the embryonic development of the mesenchymal element of the intestinal tract. [DOI] [PubMed] [Google Scholar]
- 38.Joseph NM, Mukouyama YS, Mosher JT, Jaegle M, Crone SA, et al. Neural crest stem cells undergo multilineage differentiation in developing peripheral nerves to generate endoneurial fibroblasts in addition to Schwann cells. Development. 2004;131:5599–612. doi: 10.1242/dev.01429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muller SM, Stolt CC, Terszowski G, Blum C, Amagai T, et al. Neural crest origin of perivascular mesenchyme in the adult thymus. J Immunol. 2008;180:5344–51. doi: 10.4049/jimmunol.180.8.5344. [DOI] [PubMed] [Google Scholar]
- 40.Wilm B, Ipenberg A, Hastie ND, Burch JB, Bader DM. The serosal mesothelium is a major source of smooth muscle cells of the gut vasculature. Development. 2005;132:5317–28. doi: 10.1242/dev.02141. [DOI] [PubMed] [Google Scholar]
- 41.Iredale JP. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Investig. 2007;117:539–48. doi: 10.1172/JCI30542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sousa AM, Liu T, Guevara O, Stevens J, Fanburg BL, et al. Smooth muscle α-actin expression and myofibroblast differentiation by TGFβare dependent upon MK2. J Cell Biochem. 2007;100:1581–92. doi: 10.1002/jcb.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Investig. 2007;117:557–67. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, et al. NAD(P)H oxidase 4 mediates transforming growth factor-β1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res. 2005;97:900–7. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 45.Bernstein AM, Twining SS, Warejcka DJ, Tall E, Masur SK. Urokinase receptor cleavage: a crucial step in fibroblast-to-myofibroblast differentiation. Mol Biol Cell. 2007;18:2716–27. doi: 10.1091/mbc.E06-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thiagarajah JR, Griffiths NM, Pedley KC, Naftalin RJ. Evidence for modulation of pericryptal sheath myofibroblasts in rat descending colon by transforming growth factor βand angiotensin II. BMC Gastroenterol. 2002;2:4. doi: 10.1186/1471-230X-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kolodsick JE, Peters-Golden M, Larios J, Toews GB, Thannickal VJ, Moore BB. Prostaglandin E2 inhibits fibroblast to myofibroblast transition via E-prostanoid receptor 2 signaling and cyclic adenosine monophosphate elevation. Am J Respir Cell Mol Biol. 2003;29:537–44. doi: 10.1165/rcmb.2002-0243OC. [DOI] [PubMed] [Google Scholar]
- 48.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Investig. 2009;119:1429–37. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–73. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 50.Humphreys BD, Lin S-L, Kobayashi A, Hudson TE, Nowlin BT, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taura K, Miura K, Iwaisako K, Osterreicher CH, Kodama Y, et al. Hepatocytes do not undergo epithelial-mesenchymal transition in liver fibrosis in mice. Hepatology. 2010;51:1027–36. doi: 10.1002/hep.23368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rieder F, Fiocchi C. Intestinal fibrosis in IBD: a dynamic, multifactorial process. Nat Rev Gastroenterol Hepatol. 2009;6:228–35. doi: 10.1038/nrgastro.2009.31. [DOI] [PubMed] [Google Scholar]
- 53.Flier S, Tanjore H, Kokkotou E, Sugimoto H, Zeisberg M, Kalluri R. Identification of epithelial to mesenchymal transition as a novel source of fibroblasts in intestinal fibrosis. J Biol Chem. 2010;285:20202–12. doi: 10.1074/jbc.M110.102012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brittan M, Wright NA. Stem cells in gastrointestinal structure and neoplastic development. Gut. 2004;53:899–910. doi: 10.1136/gut.2003.025478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Forbes SJ, Russo FP, Rey V, Burra P, Rugge M, et al. A significant proportion of myofibroblasts are of bone marrow origin in human liver fibrosis. Gastroenterology. 2004;126:955–63. doi: 10.1053/j.gastro.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 56.Murphy J, Summer R, Fine A. Stem cells in airway smooth muscle: state of the art. Proc Am Thorac Soc. 2008;5:11–14. doi: 10.1513/pats.200704-052VS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stappenbeck TS, Miyoshi H. The role of stromal stem cells in tissue regeneration and wound repair. Science. 2009;324:1666–69. doi: 10.1126/science.1172687. [DOI] [PubMed] [Google Scholar]
- 58.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–36. doi: 10.1038/nri2395. A concise review of the origin, fates, interactions, and functions of mesenchymal stem cells. [DOI] [PubMed] [Google Scholar]
- 59.Rameshwar P. IFNγ and B7-H1 in the immunology of mesenchymal stem cells. Cell Res. 2008;18:805–6. doi: 10.1038/cr.2008.90. [DOI] [PubMed] [Google Scholar]
- 60.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–62. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 61.Swenson ES, Theise ND. Stem cell therapeutics: potential in the treatment of inflammatory bowel disease. Clin Exp Gastroenterol. 2010;3:1–10. [PMC free article] [PubMed] [Google Scholar]
- 62.Walker MR, Patel KK, Stappenbeck TS. The stem cell niche. J Pathol. 2009;217:169–80. doi: 10.1002/path.2474. [DOI] [PubMed] [Google Scholar]
- 63.van den Brink GR. Hedgehog signaling in development and homeostasis of the gastrointestinal tract. Physiol Rev. 2007;87:1343–75. doi: 10.1152/physrev.00054.2006. [DOI] [PubMed] [Google Scholar]
- 64.Kolterud A, Grosse AS, Zacharias WJ, Walton KD, Kretovich KE, et al. Paracrine hedgehog signaling in stomach and intestine: new roles for hedgehog in gastrointestinal patterning. Gastroenterology. 2009;137:618–28. doi: 10.1053/j.gastro.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Dop WA, Uhmann A, Wijgerde M, Sleddens-Linkels E, Heijmans J, et al. Depletion of the colonic epithelial precursor cell compartment upon conditional activation of the hedgehog pathway. Gastroenterology. 2009;136:2195–203. doi: 10.1053/j.gastro.2009.02.068. [DOI] [PubMed] [Google Scholar]
- 66.Zacharias WJ, Li X, Madison BB, Kretovich K, Kao JY, et al. Hedgehog is an anti-inflammatory epithelial signal for the intestinal lamina propria. Gastroenterology. 2010;138:2368–77. doi: 10.1053/j.gastro.2010.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kosinski C, Stange DE, Xu C, Chan ASY, Ho C, et al. Indian hedgehog regulates intestinal stem cell fate through epithelial-mesenchymal interactions during development. Gastroenterology. 2010;139:893–903. doi: 10.1053/j.gastro.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vignes S, Bellanger J. Primary intestinal lymphangiectasia (Waldmann’s disease) Orphanet J Rare Dis. 2008;3:5. doi: 10.1186/1750-1172-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nusse R. Wnts and hedgehogs: lipid-modified proteins and similarities in signaling mechanisms at the cell surface. Development. 2003;130:5297–305. doi: 10.1242/dev.00821. [DOI] [PubMed] [Google Scholar]
- 70.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–60. doi: 10.1146/annurev.physiol.010908.163145. A thorough review covering stem cells and self-renewal of the mammalian small intestine. Also discusses intestinal epithelial stem cell markers. [DOI] [PubMed] [Google Scholar]
- 71.Van der Flier LG, Sabates-Bellver J, Oving I, Haegebarth A, De Palo M, et al. The intestinal Wnt/TCF signature. Gastroenterology. 2007;132:628–32. doi: 10.1053/j.gastro.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 72.van der Flier LG, Haegebarth A, Stange DE, van de Wetering M, Clevers H. OLFM4 is a robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. Gastroenterology. 2009;137:15–7. doi: 10.1053/j.gastro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 73.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–7. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 74.Gregorieff A, Pinto D, Begthel H, Destree O, Kielman M, Clevers H. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology. 2005;129:626. doi: 10.1016/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 75.Li X, Madison BB, Zacharias W, Kolterud A, States D, Gumucio DL. Deconvoluting the intestine: molecular evidence for a major role of the mesenchyme in the modulation of signaling cross talk. Physiol Genomics. 2007;29:290–301. doi: 10.1152/physiolgenomics.00269.2006. [DOI] [PubMed] [Google Scholar]
- 76.Garcia MI, Ghiani M, Lefort A, Libert F, Strollo S, Vassart G. LGR5 deficiency deregulates Wnt signaling and leads to precocious Paneth cell differentiation in the fetal intestine. Dev Biol. 2009;331:58–67. doi: 10.1016/j.ydbio.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 77.van Es JH, Jay P, Gregorieff A, van Gijn ME, Jonkheer S, et al. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat Cell Biol. 2005;7:381–86. doi: 10.1038/ncb1240. [DOI] [PubMed] [Google Scholar]
- 78.Waite KA, Eng C. From developmental disorder to heritable cancer: It’s all in the BMP/TGF-β family. Nat Rev Genet. 2003;4:763–73. doi: 10.1038/nrg1178. [DOI] [PubMed] [Google Scholar]
- 79.Zhang J, Li L. BMP signaling and stem cell regulation. Dev Biol. 2005;284:1–11. doi: 10.1016/j.ydbio.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 80.Sneddon JB, Zhen HH, Montgomery K, van de Rijn M, Tward AD, et al. Bone morphogenetic protein antagonist gremlin 1 is widely expressed by cancer-associated stromal cells and can promote tumor cell proliferation. Proc Natl Acad Sci USA. 2006;103:14842–47. doi: 10.1073/pnas.0606857103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147:35–51. doi: 10.1093/jb/mvp148. [DOI] [PubMed] [Google Scholar]
- 82.He XC, Zhang J, Tong WG, Tawfik O, Ross J, et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-β-catenin signaling. Nat Genet. 2004;36:1117–21. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- 83.Hardwick JC, Kodach LL, Offerhaus GJ, van den Brink GR. Bone morphogenetic protein signalling in colorectal cancer. Nat Rev Cancer. 2008;8:806–12. doi: 10.1038/nrc2467. [DOI] [PubMed] [Google Scholar]
- 84.Kosinski C, Li VS, Chan AS, Zhang J, Ho C, et al. Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proc Natl Acad Sci USA. 2007;104:15418–23. doi: 10.1073/pnas.0707210104. Identifies stromal myofibroblasts and muscularis mucosae cells as sources of Bmp inhibitors in colonic crypts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Klapholz-Brown Z, Walmsley GG, Nusse YM, Nusse R, Brown PO. Transcriptional program induced by Wnt protein in human fibroblasts suggests mechanisms for cell cooperativity in defining tissue microenvironments. PLoS ONE. 2007;2:e945. doi: 10.1371/journal.pone.0000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sukegawa A, Narita T, Kameda T, Saitoh K, Nohno T, et al. The concentric structure of the developing gut is regulated by Sonic hedgehog derived from endodermal epithelium. Development. 2000;127:1971–80. doi: 10.1242/dev.127.9.1971. [DOI] [PubMed] [Google Scholar]
- 87.Fritsch C, Swietlicki EA, Lefebvre O, Kedinger M, Iordanov H, et al. Epimorphin expression in intestinal myofibroblasts induces epithelial morphogenesis. J Clin Investig. 2002;110:1629–41. doi: 10.1172/JCI13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Y, Wang L, Iordanov H, Swietlicki EA, Zheng Q, et al. Epimorphin mice have increased intestinal growth, decreased susceptibility to dextran sodium sulfate colitis, and impaired spermatogenesis. J Clin Investig. 2006;116:1535–46. doi: 10.1172/JCI25442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shaker A, Swietlicki EA, Wang L, Jiang S, Onal B, et al. Epimorphin deletion protects mice from inflammation-induced colon carcinogenesis and alters stem cell niche myofibroblast secretion. J Clin Investig. 2010;120:2081–93. doi: 10.1172/JCI40676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.He XC, Yin T, Grindley JC, Tian Q, Sato T, et al. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet. 2007;39:189–98. doi: 10.1038/ng1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–65. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 92.Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–99. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- 93.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–84. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 94.Boman BM, Walters R, Fields JZ, Kovatich AJ, Zhang T, et al. Colonic crypt changes during adenoma development in familial adenomatous polyposis: immunohistochemical evidence for expansion of the crypt base cell population. Am J Pathol. 2004;165:1489–98. doi: 10.1016/S0002-9440(10)63407-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lynch ED, Ostermeyer EA, Lee MK, Arena JF, Ji H, et al. Inherited mutations in PTEN that are associated with breast cancer, Cowden disease, and juvenile polyposis. Am J Hum Genet. 1997;61:1254–60. doi: 10.1086/301639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Haramis A-PG, Begthel H, van den Born M, van Es J, Jonkheer S, et al. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science. 2004;303:1684–86. doi: 10.1126/science.1093587. [DOI] [PubMed] [Google Scholar]
- 97.Yang L, Xie G, Fan Q, Xie J. Activation of the hedgehog-signaling pathway in human cancer and the clinical implications. Oncogene. 2010;29:469–81. doi: 10.1038/onc.2009.392. [DOI] [PubMed] [Google Scholar]
- 98.Gregorieff A, Clevers H. Wnt signaling in the intestinal epithelium: from endoderm to cancer. Genes Dev. 2005;19:877–90. doi: 10.1101/gad.1295405. [DOI] [PubMed] [Google Scholar]
- 99.Arimura S, Matsunaga A, Kitamura T, Aoki K, Aoki M, Taketo MM. Reduced level of smoothened suppresses intestinal tumorigenesis by down-regulation of Wnt signaling. Gastroenterology. 2009;137:629–38. doi: 10.1053/j.gastro.2009.04.059. [DOI] [PubMed] [Google Scholar]
- 100.Wang DH, Clemons NJ, Miyashita T, Dupuy AJ, Zhang W. Aberrant epithelial mesenchymal hedgehog signaling characterizes Barrett’s metaplasia. Gastroenterology. 2010;138:1810–22. doi: 10.1053/j.gastro.2010.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fiocchi C, Ina K, Danese S, Leite A, Vogel J. Alterations of mesenchymal and endothelial cells in inflammatory bowel diseases. Adv Exp Med Biol. 2006;579:168–76. doi: 10.1007/0-387-33778-4_11. [DOI] [PubMed] [Google Scholar]
- 102.Vogel JD, West GA, Danese S, de la Motte C, Phillips MH, et al. CD40-mediated immune-nonimmune cell interactions induce mucosal fibroblast chemokines leading to T-cell transmigration. Gastroenterology. 2004;126:63–80. doi: 10.1053/j.gastro.2003.10.046. [DOI] [PubMed] [Google Scholar]
- 103.Pinchuk IV, Beswick EJ, Saada JI, Suarez G, Winston J, et al. Monocyte chemoattractant protein-1 production by intestinal myofibroblasts in response to staphylococcal enterotoxin A: relevance to staphylococcal enterotoxigenic disease. J Immunol. 2007;178:8097–106. doi: 10.4049/jimmunol.178.12.8097. [DOI] [PubMed] [Google Scholar]
- 104.Brandtzaeg P. “ABC” of mucosal immunology. Nestle Nutr Workshop Ser Pediatr Program. 2009;64:23–38. doi: 10.1159/000235781. [DOI] [PubMed] [Google Scholar]
- 105.Medzhitov R, Janeway C., Jr The Toll receptor family and microbial recognition. Trends Microbiol. 2000;8:452–56. doi: 10.1016/s0966-842x(00)01845-x. [DOI] [PubMed] [Google Scholar]
- 106.Denning TL, Wang Y-C, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–94. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 107.Otte JM, Rosenberg IM, Podolsky DK. Intestinal myofibroblasts in innate immune responses of the intestine. Gastroenterology. 2003;124:1866–78. doi: 10.1016/s0016-5085(03)00403-7. [DOI] [PubMed] [Google Scholar]
- 108.Saada JI, Pinchuk IV, Barrera CA, Adegboyega PA, Suarez G, et al. Subepithelial myofibroblasts are novel nonprofessional APCs in the human colonic mucosa. J Immunol. 2006;177:5968–79. doi: 10.4049/jimmunol.177.9.5968. [DOI] [PubMed] [Google Scholar]
- 109.Walton KL, Holt L, Sartor RB. Lipopolysaccharide activates innate immune responses in murine intestinal myofibroblasts through multiple signaling pathways. Am J Physiol Gastrointest Liver Physiol. 2009;296:601–11. doi: 10.1152/ajpgi.00022.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pang G, Couch L, Batey R, Clancy R, Cripps A. GM-CSF, IL-1α, IL-1β, IL-6, IL-8, IL-10, ICAM-1 and VCAM-1 gene expression and cytokine production in human duodenal fibroblasts stimulated with lipopolysaccharide, IL-1αand TNF-α. Clin Exp Immunol. 1994;96:437–43. doi: 10.1111/j.1365-2249.1994.tb06048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Johnson JR, Pinchuk IV, Saada JI, Mifflin RC, Reyes VE, Powell DW. TLR-3 mediated regulation of B7 negative co-stimulators on stromal cells: a potential mechanism of colonic immune evasion during intestinal viral infection. Gastroenterology. 2010;138:S269. (Abstr.) [Google Scholar]
- 112.Hosokawa Y, Hosokawa I, Ozaki K, Nakae H, Matsuo T. CC chemokine ligand 17 in periodontal diseases: expression in diseased tissues and production by human gingival fibroblasts. J Periodontal Res. 2008;43:471–77. doi: 10.1111/j.1600-0765.2007.01080.x. [DOI] [PubMed] [Google Scholar]
- 113.Gutierrez-Canas I, Juarranz Y, Santiago B, Arranz A, Martinez C, et al. VIP down-regulates TLR4 expression and TLR4-mediated chemokine production in human rheumatoid synovial fibroblasts. Rheumatology. 2006;45:527–32. doi: 10.1093/rheumatology/kei219. [DOI] [PubMed] [Google Scholar]
- 114.Fletcher AL, Lukacs-Kornek V, Reynoso ED, Pinner SE, Bellemare-Pelletier A, et al. Lymph node fibroblastic reticular cells directly present peripheral tissue antigen under steady-state and inflammatory conditions. J Exp Med. 2010;207:689–97. doi: 10.1084/jem.20092642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Roberts AI, Nadler SC, Ebert EC. Mesenchymal cells stimulate human intestinal intraepithelial lymphocytes. Gastroenterology. 1997;113:144–50. doi: 10.1016/s0016-5085(97)70089-1. [DOI] [PubMed] [Google Scholar]
- 116.Laning JC, Deluca JE, Isaacs CM, Hardin-Young J. In vitro analysis of CD40-CD154 and CD28-CD80/86 interactions in the primary T-cell response to allogeneic “nonprofessional” antigen presenting cells. Transplantation. 2001;71:1467–74. doi: 10.1097/00007890-200105270-00019. [DOI] [PubMed] [Google Scholar]
- 117.Wassenaar A, Snijders A, Abraham-Inpijn L, Kapsenberg ML, Kievits F. Antigen-presenting properties of gingival fibroblasts in chronic adult periodontitis. Clin Exp Immunol. 1997;110:277–84. doi: 10.1111/j.1365-2249.1997.tb08328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tran CN, Davis MJ, Tesmer LA, Endres JL, Motyl CD, et al. Presentation of arthritogenic peptide to antigen-specific T cells by fibroblast-like synoviocytes. Arthritis Rheum. 2007;56:1497–506. doi: 10.1002/art.22573. [DOI] [PubMed] [Google Scholar]
- 119.Winau F, Hegasy G, Weiskirchen R, Weber S, Cassan C, et al. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity. 2007;26:117–29. doi: 10.1016/j.immuni.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 120.Olivares EG, Montes MJ, Oliver C, Galindo JA, Ruiz C. Cultured human decidual stromal cells express B7–1 (CD80) and B7–2 (CD86) and stimulate allogeneic T cells. Biol Reprod. 1997;57:609–15. doi: 10.1095/biolreprod57.3.609. [DOI] [PubMed] [Google Scholar]
- 121.Taniguchi Y, Ono K, Yoshida S, Tanaka R. Antigen-presenting capability of glial cells under glioma-harboring conditions and the effect of glioma-derived factors on antigen presentation. J Neuroimmunol. 2000;111:177–85. doi: 10.1016/s0165-5728(00)00361-1. [DOI] [PubMed] [Google Scholar]
- 122.Chan JL, Tang KC, Patel AP, Bonilla LM, Pierobon N, et al. Antigen-presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon-γ. Blood. 2006;107:4817–24. doi: 10.1182/blood-2006-01-0057. Identifies IFN-γ as a key factor in the regulation of the immunopromoting and immunosuppressive function of mesenchymal stem cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Garside P, Millington O, Smith KM. The anatomy of mucosal immune responses. Ann N Y Acad Sci. 2004;1029:9–15. doi: 10.1196/annals.1309.002. [DOI] [PubMed] [Google Scholar]
- 124.O’Farrelly C. Just how inflamed is the normal gut? Gut. 1998;42:603–4. doi: 10.1136/gut.42.5.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Greenfield EA, Nguyen KA, Kuchroo VK. CD28/B7 costimulation: a review. Crit Rev Immunol. 1998;18:389–418. doi: 10.1615/critrevimmunol.v18.i5.10. [DOI] [PubMed] [Google Scholar]
- 126.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–82. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 127.Clark RA, Kupper TS. IL-15 and dermal fibroblasts induce proliferation of natural regulatory T cells isolated from human skin. Blood. 2007;109:194–202. doi: 10.1182/blood-2006-02-002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yang HR, Chou HS, Gu X, Wang L, Brown KE, et al. Mechanistic insights into immunomodulation by hepatic stellate cells in mice: a critical role of interferon-γ signaling. Hepatology. 2009;50:1981–91. doi: 10.1002/hep.23202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nagamatsu T, Schust DJ, Sugimoto J, Barrier BF. Human decidual stromal cells suppress cytokine secretion by allogenic CD4+ T cells via PD-1 ligand interactions. Hum Reprod. 2009;24:3160–71. doi: 10.1093/humrep/dep308. [DOI] [PubMed] [Google Scholar]
- 130.Nazareth MR, Broderick L, Simpson-Abelson MR, Kelleher RJ, Jr, Yokota SJ, Bankert RB. Characterization of human lung tumor-associated fibroblasts and their ability to modulate the activation of tumor-associated T cells. J Immunol. 2007;178:5552–62. doi: 10.4049/jimmunol.178.9.5552. [DOI] [PubMed] [Google Scholar]
- 131.Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–72. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 132.Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327–33. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 133.Spaggiari GM, Abdelrazik H, Becchetti F, Moretta L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood. 2009;113:6576–83. doi: 10.1182/blood-2009-02-203943. [DOI] [PubMed] [Google Scholar]
- 134.Balsamo M, Scordamaglia F, Pietra G, Manzini C, Cantoni C, et al. Melanoma-associated fibroblasts modulate NK cell phenotype and antitumor cytotoxicity. Proc Natl Acad Sci USA. 2009;106:20847–52. doi: 10.1073/pnas.0906481106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jones S, Horwood N, Cope A, Dazzi F. The antiproliferative effect of mesenchymal stem cells is a fundamental property shared by all stromal cells. J Immunol. 2007;179:2824–31. doi: 10.4049/jimmunol.179.5.2824. Presents evidence that suppression of the early activation/proliferation of T cells is a fundamental characteristic of all mesenchymal stromal cells. [DOI] [PubMed] [Google Scholar]
- 136.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–95. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Augello A, Tasso R, Negrini SM, Amateis A, Indiveri F, et al. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 2005;35:1482–90. doi: 10.1002/eji.200425405. [DOI] [PubMed] [Google Scholar]
- 138.Mahida YR, Beltinger J, Makh S, Goke M, Gray T, Podolsky DK. Adult human colonic subepithelial myofibroblasts express extracellular matrix proteins and cyclooxygenase-1 and -2. Am J Physiol Gastrointest Liver Physiol. 1997;273:1341–48. doi: 10.1152/ajpgi.1997.273.6.G1341. [DOI] [PubMed] [Google Scholar]
- 139.McKaig BC, Hughes K, Tighe PJ, Mahida YR. Differential expression of TGF-β isoforms by normal and inflammatory bowel disease intestinal myofibroblasts. Am J Physiol Cell Physiol. 2002;282:172–33. doi: 10.1152/ajpcell.00048.2001. [DOI] [PubMed] [Google Scholar]
- 140.Haniffa MA, Wang XN, Holtick U, Rae M, Isaacs JD, et al. Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. J Immunol. 2007;179:1595–604. doi: 10.4049/jimmunol.179.3.1595. [DOI] [PubMed] [Google Scholar]
- 141.Maby-El Hajjami H, Ame-Thomas P, Pangault C, Tribut O, DeVos J, et al. Functional alteration of the lymphoma stromal cell niche by the cytokine context: role of indoleamine-2,3 dioxygenase. Cancer Res. 2009;69:3228–37. doi: 10.1158/0008-5472.CAN-08-3000. [DOI] [PubMed] [Google Scholar]
- 142.Allez M, Mayer L. Regulatory T cells: peace keepers in the gut. Inflamm Bowel Dis. 2004;10:666–76. doi: 10.1097/00054725-200409000-00027. [DOI] [PubMed] [Google Scholar]
- 143.Ling KL, Pratap SE, Bates GJ, Singh B, Mortensen NJ, et al. Increased frequency of regulatory T cells in peripheral blood and tumor infiltrating lymphocytes in colorectal cancer patients. Cancer Immun. 2007;7:7. [PMC free article] [PubMed] [Google Scholar]
- 144.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–43. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 145.Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked: forkhead box protein 3 mutations and lack of regulatory T cells. J Allergy Clin Immunol. 2007;120:744–50. doi: 10.1016/j.jaci.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 146.Pinchuk IV, Beswick EJ, Saida JI, Reyes VE, Powell DW. Human colonic myofibroblasts promote the expansion of CD4+CD25high Foxp3+ regulatory T cells. Gastroenterology. 2007;132:A397. (Abstr.) [Google Scholar]
- 147.Benito-Miguel M, Garcia-Carmona Y, Balsa A, Perez de Ayala C, Cobo-Ibanez T, et al. A dual action of rheumatoid arthritis synovial fibroblast IL-15 expression on the equilibrium between CD4+CD25+ regulatory T cells and CD4+CD25− responder T cells. J Immunol. 2009;183:8268–79. doi: 10.4049/jimmunol.0900007. [DOI] [PubMed] [Google Scholar]
- 148.Di Ianni M, Del Papa B, De Ioanni M, Moretti L, Bonifacio E, et al. Mesenchymal cells recruit and regulate T regulatory cells. Exp Hematol. 2008;36:309–18. doi: 10.1016/j.exphem.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 149.Jiang G, Yang HR, Wang L, Wildey GM, Fung J, et al. Hepatic stellate cells preferentially expand allogeneic CD4+ CD25+ FoxP3+ regulatory T cells in an IL-2-dependent manner. Transplantation. 2008;86:1492–502. doi: 10.1097/TP.0b013e31818bfd13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Trajkovic V, Vuckovic O, Stosic-Grujicic S, Miljkovic D, Popadic D, et al. Astrocyte-induced regulatory T cells mitigate CNS autoimmunity. Glia. 2004;47:168–79. doi: 10.1002/glia.20046. [DOI] [PubMed] [Google Scholar]
- 151.Yu MC, Chen CH, Liang X, Wang L, Gandhi CR, et al. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology. 2004;40:1312–21. doi: 10.1002/hep.20488. [DOI] [PubMed] [Google Scholar]
- 152.Francois M, Romieu-Mourez R, Stock-Martineau S, Boivin MN, Bramson JL, Galipeau J. Mesenchymal stromal cells cross-present soluble exogenous antigens as part of their antigen-presenting cell properties. Blood. 2009;114:2632–38. doi: 10.1182/blood-2009-02-207795. [DOI] [PubMed] [Google Scholar]
- 153.Saada JI, Barrera CA, Adegboyega PA, Pinchuk IV, Tamerisa RA, et al. Primary isolated human colonic myofibroblasts express B7 family ligands that regulate effector and memory T cells. Gastroenterology. 2004;125:A424. (Abstr.) [Google Scholar]
- 154.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7:452–64. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Krueger M, Bechmann I. CNS pericytes: concepts, misconceptions, and a way out. Glia. 2010;58:1–10. doi: 10.1002/glia.20898. [DOI] [PubMed] [Google Scholar]
RELATED RESOURCES
- 1.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 2.Roberts DJ. Molecular mechanisms of development of the gastrointestinal tract. Dev Dyn. 2000;219:109–20. doi: 10.1002/1097-0177(2000)9999:9999<::aid-dvdy1047>3.3.co;2-y. [DOI] [PubMed] [Google Scholar]
- 3.McLin VA, Henning SJ, Jamrich M. The role of the visceral mesoderm in the development of the gastrointestinal tract. Gastroenterology. 2009;136:2074–91. doi: 10.1053/j.gastro.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Rubin DC. Intestinal morphogenesis. Curr Opin Gastroenterology. 2007;23:111–14. doi: 10.1097/MOG.0b013e3280145082. [DOI] [PubMed] [Google Scholar]
- 5.Mueller SN, Germain RN. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat Rev Immunol. 2009;9:618–29. doi: 10.1038/nri2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hausmann M, Rogler G. Immune-non immune networks in intestinal inflammation. Curr Drug Targets. 2008;9:388–94. doi: 10.2174/138945008784221152. [DOI] [PubMed] [Google Scholar]
- 7.Parsonage G, Filer AD, Haworth O, Nash GB, Rainger GE, et al. A stromal address code defined by fibroblasts. Trends Immunol. 2005;26:150–56. doi: 10.1016/j.it.2004.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]