Abstract
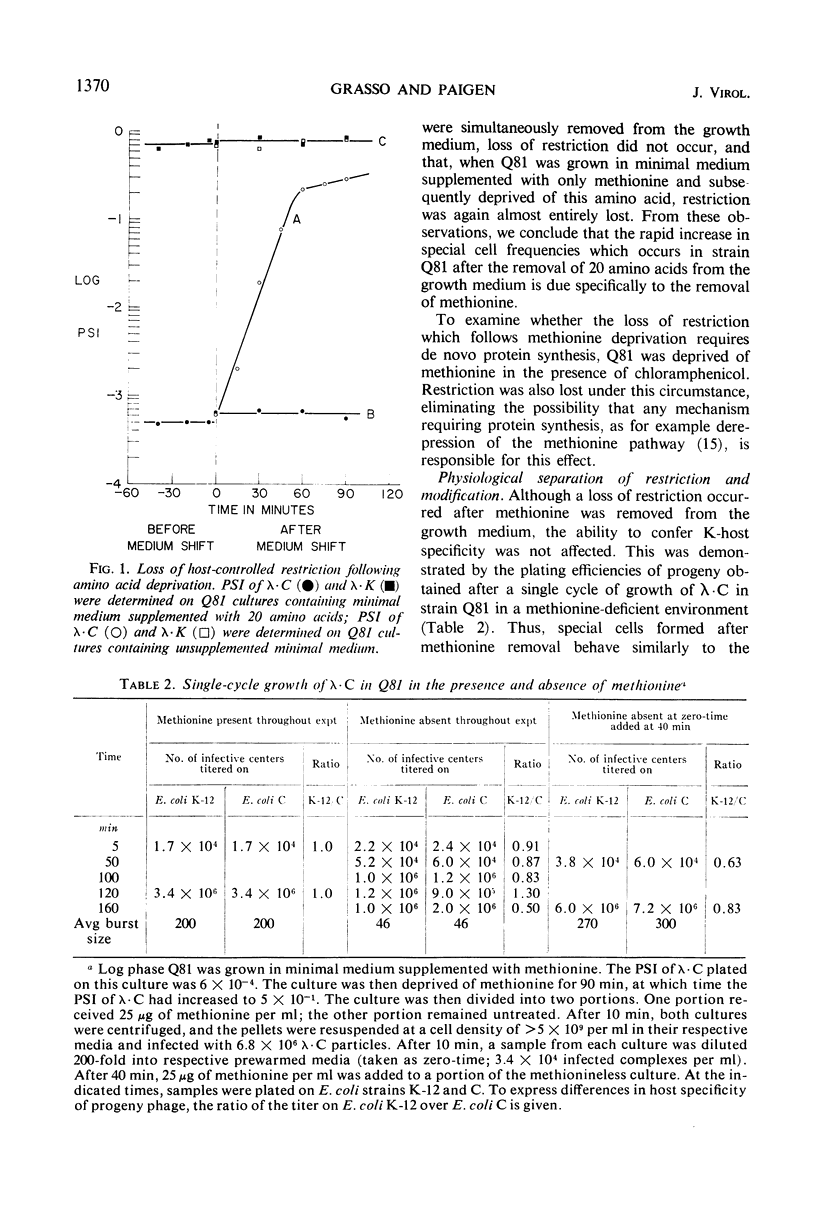
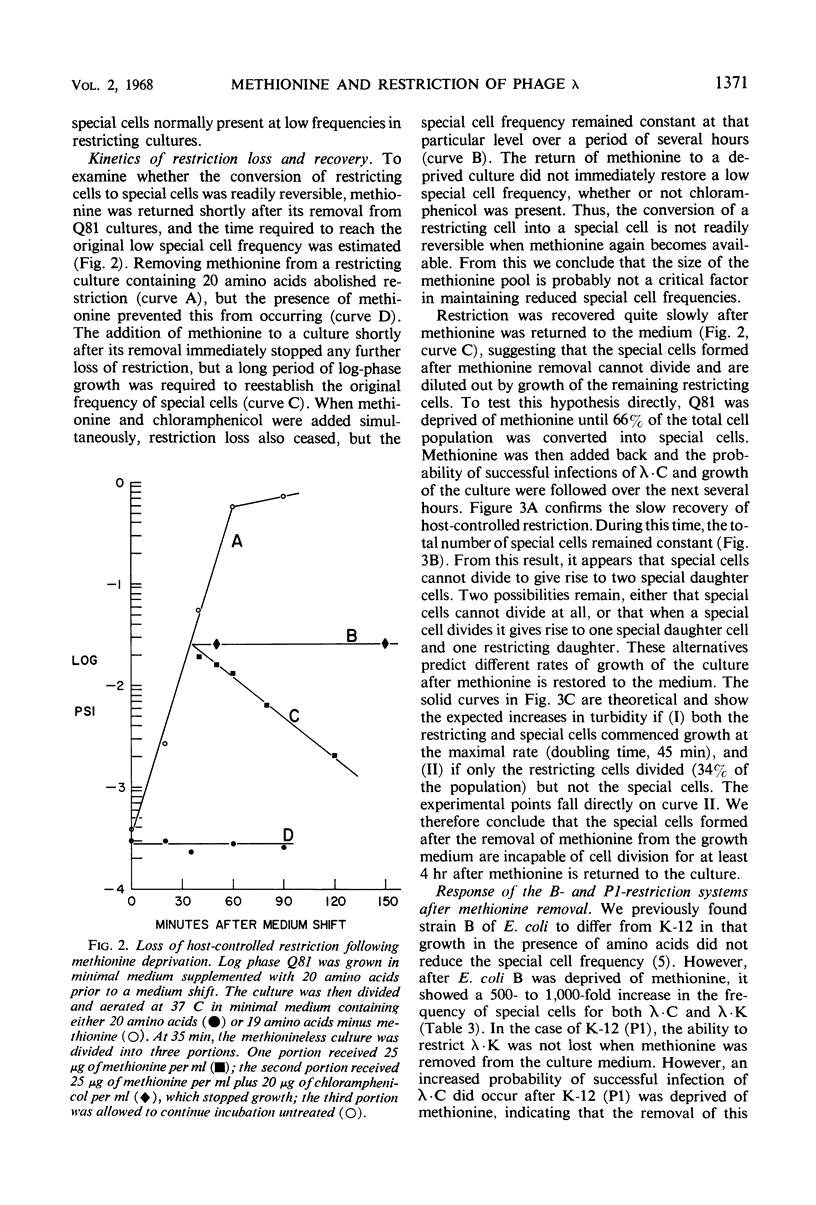
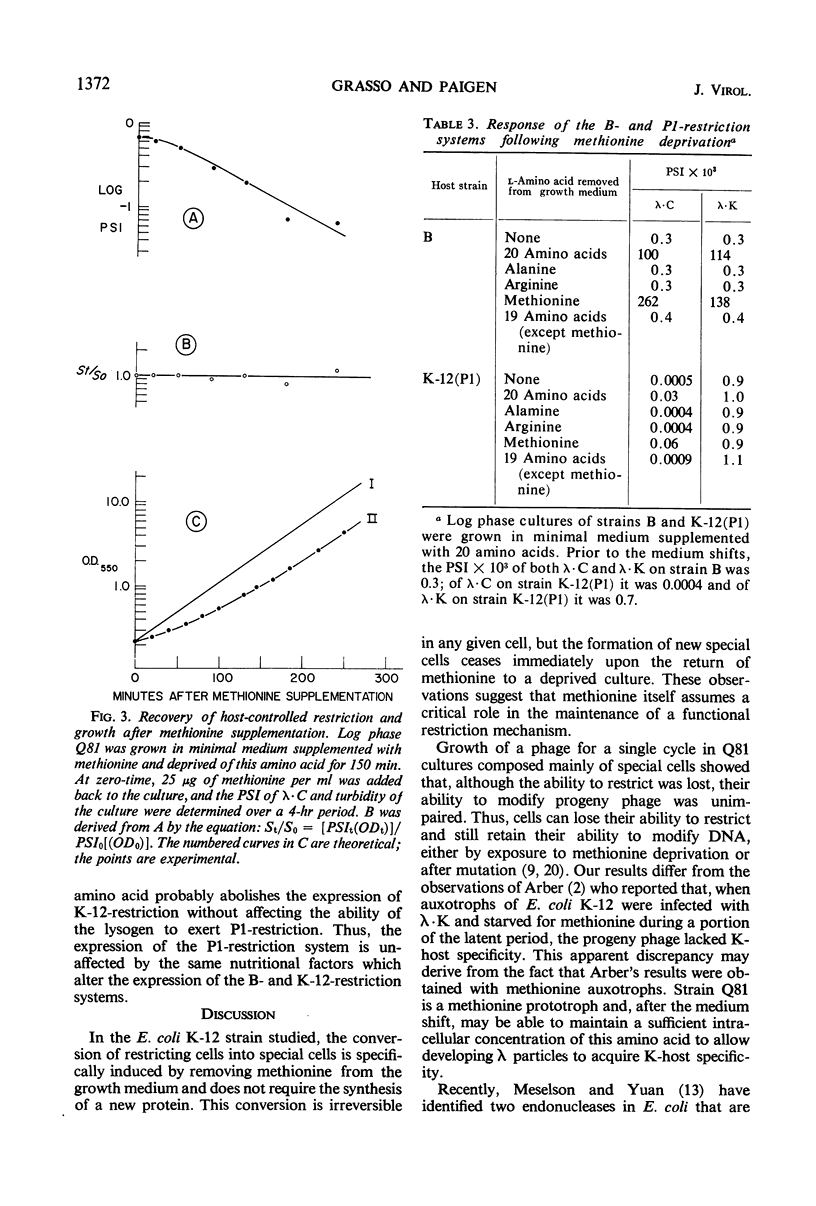
λ Bacteriophages produced in Escherichia coli C (designated as λ · C) are restricted in their ability to grow in E. coli K-12. The rare successful infections that arise in the K-12 population occur in “special” cells which have lost their capacity to restrict λ · C. These infections yield modified progeny phage (designated as λ · K) which, unlike λ · C, plate equally well on E. coli C and E. coli K-12. When methionine, but no other amino acid, was removed from the growth medium of a mutant strain of E. coli K-12, the number of special cells rapidly increased 500- to 3,000-fold. These new special cells retain their capacity to produce modified λ · K progeny. This conversion of restricting cells into special cells does not require the synthesis of new protein. The special cells formed when methionine was removed from the culture did not revert into restricting cells when methionine was restored. Such cells have also lost the ability to divide for at least 4 hr after methionine supplementation. When methionine was restored, the remaining restricting cells, but not the special cells, immediately resumed growth. Removing methionine from cultures of E. coli B caused a similar increase in the number of special cells able to support the growth of λ · C and λ · K. However, when E. coli K-12 (P1) cultures were deprived of methionine, the number of special cells increased for λ · C but not for λ · K. Thus, retention of the P1-restriction system, unlike the B- and the K-12-systems, does not require the presence of methionine.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPLEYARD R. K., MCGREGOR J. F., BAIRD K. M. Mutation to extended host range and the occurrence of phenotypic mixing in the temperate coliphage lambda. Virology. 1956 Aug;2(4):565–574. doi: 10.1016/0042-6822(56)90012-5. [DOI] [PubMed] [Google Scholar]
- ARBER W., DUSSOIX D. Host specificity of DNA produced by Escherichia coli. I. Host controlled modification of bacteriophage lambda. J Mol Biol. 1962 Jul;5:18–36. doi: 10.1016/s0022-2836(62)80058-8. [DOI] [PubMed] [Google Scholar]
- ARBER W. HOST SPECIFICITY OF DNA PRODUCED BY ESCHERICHIA COLI V . THE ROLE OF METHIONINE IN THE PRODUCTION OF HOST SPECIFICITY. J Mol Biol. 1965 Feb;11:247–256. doi: 10.1016/s0022-2836(65)80055-9. [DOI] [PubMed] [Google Scholar]
- BERTANI G., WEIGLE J. J. Host controlled variation in bacterial viruses. J Bacteriol. 1953 Feb;65(2):113–121. doi: 10.1128/jb.65.2.113-121.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso R. J., Paigen K. The effect of amino acids on host-controlled restriction of lambda phage. Virology. 1968 Sep;36(1):1–8. doi: 10.1016/0042-6822(68)90110-4. [DOI] [PubMed] [Google Scholar]
- HOLLOWAY B. W. VARIATIONS IN RESTRICTION AND MODIFICATION OF BACTERIOPHAGE FOLLOWING INCREASE OF GROWTH TEMPERATURE OF PSEUDOMONAS AERUGINOSA. Virology. 1965 Apr;25:634–642. doi: 10.1016/0042-6822(65)90091-7. [DOI] [PubMed] [Google Scholar]
- KAISER A. D. Mutations in a temperate bacteriophage affecting its ability to lysogenize Escherichia coli. Virology. 1957 Feb;3(1):42–61. doi: 10.1016/0042-6822(57)90022-3. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., HUMAN M. L. A nonhereditary, host-induced variation of bacterial viruses. J Bacteriol. 1952 Oct;64(4):557–569. doi: 10.1128/jb.64.4.557-569.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LURIA S. E. Host-induced modifications of viruses. Cold Spring Harb Symp Quant Biol. 1953;18:237–244. doi: 10.1101/sqb.1953.018.01.034. [DOI] [PubMed] [Google Scholar]
- Lederberg S. Genetics of host-controlled restriction and modification of deoxyribonucleic acid in Escherichia coli. J Bacteriol. 1966 Mar;91(3):1029–1036. doi: 10.1128/jb.91.3.1029-1036.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederberg S. Host-controlled restriction and modification of deoxyribonucleic acid in Escherichia coli. Virology. 1965 Nov;27(3):378–387. doi: 10.1016/0042-6822(65)90117-0. [DOI] [PubMed] [Google Scholar]
- Linn S., Arber W. Host specificity of DNA produced by Escherichia coli, X. In vitro restriction of phage fd replicative form. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1300–1306. doi: 10.1073/pnas.59.4.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson M., Yuan R. DNA restriction enzyme from E. coli. Nature. 1968 Mar 23;217(5134):1110–1114. doi: 10.1038/2171110a0. [DOI] [PubMed] [Google Scholar]
- PAIGEN K., WEINFELD H. Cooperative infection by hostmodified lambda phage. Virology. 1963 Apr;19:565–572. doi: 10.1016/0042-6822(63)90052-7. [DOI] [PubMed] [Google Scholar]
- ROWBURY R. J., WOODS D. D. Further studies on the repression of methionine synthesis in Escherichia coli. J Gen Microbiol. 1961 Jan;24:129–144. doi: 10.1099/00221287-24-1-129. [DOI] [PubMed] [Google Scholar]
- Tatum E. L. X-Ray Induced Mutant Strains of Escherichia Coli. Proc Natl Acad Sci U S A. 1945 Aug;31(8):215–219. doi: 10.1073/pnas.31.8.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UETAKE H., TOYAMA S., HAGIWARA S. ON THE MECHANISM OF HOST-INDUCED MODIFICATION. MULTIPLICITY ACTIVATION AND THERMOLABILE FACTOR RESPONSIBLE FOR PHAGE GROWTH RESTRICTION. Virology. 1964 Feb;22:202–213. doi: 10.1016/0042-6822(64)90005-4. [DOI] [PubMed] [Google Scholar]
- Wood W. B. Host specificity of DNA produced by Escherichia coli: bacterial mutations affecting the restriction and modification of DNA. J Mol Biol. 1966 Mar;16(1):118–133. doi: 10.1016/s0022-2836(66)80267-x. [DOI] [PubMed] [Google Scholar]



