Abstract
Heat is the most abundant byproduct of cellular metabolism. As such, dynamic exercise in which a significant percentage of muscle mass is engaged generates thermoregulatory demands that are met in part by increases in skin blood flow. Increased skin blood flow during exercise adds to the demands on cardiac output and confers additional circulatory strain beyond that associated with perfusion of active muscle alone. Endurance exercise training results in a number of physiological adaptations which ultimately reduce circulatory strain and shift thermoregulatory control of skin blood flow to higher levels of blood flow for a given core temperature. In addition, exercise training induces peripheral vascular adaptations within the cutaneous microvasculature indicative of enhanced endothelium-dependent vasomotor function. However, it is not currently clear how (or if) these local vascular adaptations contribute to the beneficial changes in thermoregulatory control of skin blood flow following exercise training. The purpose of this Hot Topic review is to synthesize the literature pertaining to exercise training-mediated changes in cutaneous microvascular reactivity and thermoregulatory control of skin blood flow. In addition, we address mechanisms driving changes in cutaneous microvascular reactivity and thermoregulatory control of skin blood flow, and pose the question: what (if any) is the functional role of increased cutaneous microvascular reactivity following exercise training?
Keywords: exercise training, physical activity, vascular adaptations, skin
Introduction
Control of blood flow to the non-acral (hairy) skin is accomplished via two branches of the sympathetic nervous system: an adrenergic vasoconstrictor system and a cholinergic vasodilator system (Kellogg, 2006). Passive thermal stress results in reflex adjustments that decrease vasoconstrictor nerve activity and increase active vasodilatation of the skin circulation in order to increase heat loss. The characteristic skin blood flow response in this setting exhibits a hockey stick shape, with a body core temperature (Tc) threshold for vasodilatation and a subsequent linear increase in blood flow as Tc continues to rise (Johnson & Proppe, 1996) (figure 1). If thermal stress becomes severe, skin blood flow can increase to maximal levels and reach an estimated 7.8 l/min over the entire body surface (Rowell, 1974).
Figure 1.
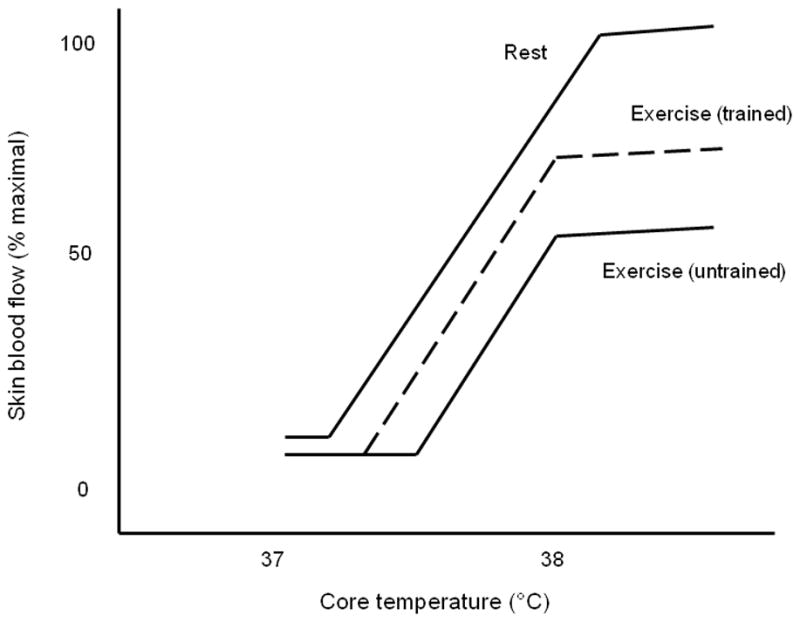
Schematic representing the relation between skin blood flow and core body temperature during thermal stress at rest, during acute exercise in the untrained state, and during acute exercise in the endurance exercise trained condition.
Given that heat is the most abundant byproduct of cellular metabolism, dynamic exercise in which a significant percentage of muscle mass is engaged (~ 50%) generates a thermal load that initiates cutaneous vascular responses similar to those described above. The skin blood flow response to acute dynamic exercise is modified from the response to passive thermal stress in a number of ways. Most notable are a rightward shift in the Tc threshold for vasodilatation and the presence of a plateau that occurs at a Tc of ~ 38°C, beyond which skin blood flow remains at 50–60% of maximal (Kenney & Johnson, 1992) (figure 1). These effects of exercise on the control of skin blood flow by Tc appear to result from the regulation of higher body temperature during exercise and the competing demands of the skeletal muscle and cutaneous circulations for cardiac output (Kenney & Johnson, 1992; Gonzalez-Alonso et al., 2008).
One of the salient adaptations to endurance exercise training is an elevation in skin blood flow relative to core body temperature during exercise (Roberts et al., 1977). This adaptation is achieved in part by earlier initiation of the active vasodilator system during exercise (Thomas et al., 1999). In addition, contemporary studies suggest that the cutaneous vasculature exhibits functional adaptations to exercise training, including increased endothelium-dependent vasodilatation (Kvernmo et al., 1998; Vassalle et al., 2003; Franzoni et al., 2004a; Lenasi & Strucl, 2004; Wang, 2005; Black et al., 2008). These adaptations are reminiscent of those observed in the skeletal muscle circulation following exercise training (Jasperse & Laughlin, 2006). What are not clear are the signals associated with exercise that initiate these cutaneous vascular adaptations, and whether these adaptations play a functional role in the maintenance of higher skin blood flow (and reduced thermoregulatory strain) during exercise in the trained state.
The purpose of this Hot Topic review is to discuss the cutaneous vascular adaptations to exercise training in the context of training-mediated changes in thermoregulatory control during exercise. We conclude by posing the following question: what (if any) is the functional role of increased cutaneous microvascular reactivity following exercise training?
Control of skin blood flow during acute whole body dynamic exercise
The cutaneous vascular response to acute dynamic exercise involves a transient reduction in skin blood flow at the onset of exercise mediated by increased cutaneous sympathetic vasoconstrictor outflow (Blair et al., 1961; Bevegard & Shepherd, 1966; Zelis et al., 1969; Kellogg et al., 1991). As dynamic exercise progresses, core temperature begins to rise while skin blood flow remains unchanged until a Tc threshold is reached beyond which skin blood flow begins to rise. This Tc threshold for vasodilatation is unaffected by peripheral blockade of adrenergic vasoconstrictor activity, suggesting it reflects the onset of active vasodilator activity during dynamic exercise.
As Tc rises beyond the threshold for cutaneous vasodilatation during sustained exercise, skin blood flow increases linearly with increasing core temperature until a point is reached (at a Tc of ~38°C) beyond which further increases in Tc elicit little or no further change in skin blood flow (Roberts et al., 1977; Gonzalez-Alonso et al., 1999) (figure 1). Beyond this “plateau”, Tc continues to rise while skin blood flow remains at approximately 50–60% of maximal (Brengelmann et al., 1977; Kellogg et al., 1993; Johnson & Proppe, 1996). Several lines of evidence suggest this plateau in skin blood flow represents a limit imposed on the magnitude of skin blood flow by cardiopulmonary baroreflexes. First, no plateau in skin blood flow is observed during thermal stress to core temperatures well beyond 38°C in resting conditions (Johnson & Proppe, 1996). This suggests that dynamic exercise, and possibly the demand for cardiac output to active muscle and skin, imposes a limit on how high skin blood flow can increase when Tc is challenged. Second, the presence or absence of this plateau is sensitive to acute manipulations of central blood volume by hypohydration (Nadel et al., 1980), water immersion (Nielsen et al., 1984), or saline infusion (Nose et al., 1990), suggesting that cardiac filling pressure may be an important signal in the reflex that regulates this plateau in skin blood flow. Third, superimposing continuous negative pressure breathing during sustained exercise, which increases atrial transmural pressure and simulates increased cardiac filling, effectively eliminates the plateau in skin blood flow (Nagashima et al., 1998). Taken together, these studies suggest that, during high intensity dynamic exercise, regional vascular conductance in the skeletal muscle and cutaneous circulations increase to the extent that arterial blood pressure regulation is challenged and cannot be regulated by cardiac output owing to a fall in cardiac filling pressure (Rowell, 1974). In this circumstance, it appears that blood flow to the skin is sacrificed at the expense of oxygen delivery to active skeletal muscle (Gonzalez-Alonso et al., 2008).
Impact of endurance exercise training on the control of skin blood flow
Endurance exercise training results in modifications to the cutaneous vascular response during dynamic exercise described above. In the endurance trained state, the Tc threshold for vasodilatation is shifted leftward so that skin blood flow begins to rise at a lower Tc (Roberts et al., 1977). Thomas et al. (1999) determined this leftward shift in the Tc threshold for vasodilatation is not influenced by changes in cutaneous sympathetic vasoconstriction; this suggests active vasodilatation is initiated at a lower Tc after training. Additionally, the magnitude of skin blood flow achieved in the plateau phase is increased after training (Fritzsche & Coyle, 2000; Takeno et al., 2001). In contrast, most (Roberts et al., 1977; Thomas et al., 1999; Takeno et al., 2001; Ichinose et al., 2009) but not all (Hayashi et al., 2009) studies indicate the slope of the relation between Tc and skin blood flow across the linear portion of this relationship is not sensitive to training status. Thus, training-induced modifications in cutaneous vascular control collectively result in higher skin blood flow (and presumably higher active vasodilator activity) for a given Tc during exercise (figure 1).
Endurance exercise training results in characteristic haemodynamic adaptations, including increased cardiac output and blood volume, which ultimately reduce circulatory strain and improve thermoregulatory capacity during exercise (Ekblom et al., 1968; Convertino, 1991). It is thought that these adaptations, which are likely mediated to some extent by chronic exposure to increased Tc, result in an increased availability of blood volume for circulation through the cutaneous vasculature during exercise. Indeed, the expansion of blood and plasma volume after 10 days of heat acclimation also causes a leftward shift in the core temperature – skin blood flow relation (Roberts et al., 1977), suggesting that haemodynamic adaptations alone may be sufficient to modify the control of skin blood flow during exercise. Recently, this hypothesis was directly tested by elimination of the plasma volume expansion associated with exercise training in the heat. Ikegawa et al (2011) trained seven men in the heat for 5 days (30 min/day @ 70% VO2max) and performed both euhydrated and hypohydrated thermoregulatory exercise tests before and after training (measuring forearm skin blood flow, Tc, etc). After training in the heat, plasma volume increased (11%) when comparing euhydrated conditions, while plasma volume was not different pre- vs. post- training in hypohydrated conditions. Comparison of the effect of heat training on euhydrated and hypohydrated thermoregulatory exercise test performance demonstrated that removal of the plasma volume expansion associated with training eliminated both the leftward shift in Tc threshold and the difference in peak skin blood flow during exercise. These results strongly suggest training-induced adaptations in the control of skin blood flow during exercise are critically dependent on expansion of blood/plasma volume and the resultant changes in central blood volume during exercise.
Local cutaneous vascular adaptations to endurance exercise training
The similarity of the slope across the linear portion of the Tc – skin blood flow relationship (before and after training) suggests peripheral adaptations are not responsible for training-induced changes in the control of skin blood flow. That is, the cutaneous vasculature does not appear to adapt with respect to the sensitivity of the active vasodilator response. In contrast, exercise training is associated with increased cutaneous vascular responsiveness to several modes of local stimulation; these adaptations are reminiscent of those observed in the skeletal muscle vasculature following endurance exercise training (for review of skeletal muscle vascular adaptations see Jasperse and Laughlin (2006)). Below we review the evidence for a training effect on three different assays of cutaneous microvascular reactivity and the potential mechanisms responsible for these vascular adaptations.
Young subjects
Acetylcholine Iontophoresis
Most (Kvernmo et al., 1998; Lenasi & Strucl, 2004; Wang, 2005) but not all (Boegli et al., 2003) studies of young healthy subjects suggest responsiveness of the cutaneous circulation to the iontophoresis of Acetylcholine (Ach) is increased in the exercise trained state. For example, Kvernmo et al. (1998) tested cutaneous vascular responses to iontophoresis of Ach and sodium nitroprusside (SNP) in elite endurance athletes (VO2 max = 69.8 ml/kg/min) and young, recreationally active volunteers and found the skin of the endurance athletes to be more responsive to Ach at the lowest dose administered. In contrast, endothelium-independent responses to SNP were not different between groups. Using a longitudinal design, Wang (2005) exercise trained 10 sedentary but otherwise healthy males for 8 weeks (30 minutes/day @ 50% VO2 max, 5 days/week), and tested cutaneous vascular responses to Ach and SNP pre and post training. After completing the exercise training period, cutaneous vascular responsiveness to Ach was increased while responses to SNP were unchanged. These findings suggest endothelium-dependent cutaneous vasodilatation is improved by exercise training. Unfortunately, the endothelium-dependent pathways through which Ach induces vasodilatation in the skin are complex and only partially understood (Morris & Shore, 1996; Kellogg et al., 2003; Boutsiouki et al., 2004; Durand et al., 2004; Holowatz et al., 2005); it remains unclear which of these pathways are affected by exercise training (e.g., prostanoids, nitric oxide, etc).
Local Heating
The cutaneous vascular response to local skin heating is comprised of an initial axon-reflex mediated peak in blood flow followed by a secondary rise to a sustained plateau after roughly 20–30 minutes (Minson, 2010). The secondary plateau phase of the local heating response is largely nitric oxide-mediated (Kellogg et al., 1999; Minson et al., 2001). Skin blood flow during the nitric oxide-dependent plateau phase is higher in endurance trained adolescents compared to matched sedentary controls (Roche et al., 2010). In contrast, the initial peak in skin blood flow appears to be uninfluenced by training status in young healthy subjects (Tew et al., 2011). These results suggest that endurance exercise training promotes increased nitric oxide bioavailability in the cutaneous microvasculature; this mechanism could be responsible for increased Ach-mediated cutaneous vasodilatation as well.
Reactive Hyperaemia
Using cutaneous reactive hyperaemia, Lenasi and Strucl (2004) and Vassalle et al. (2003) compared cutaneous vascular reactivity between endurance trained athletes and age and BMI matched sedentary controls. These studies evaluated different metrics of the blood flow response following a period of total limb occlusion (3 – 8 min); one study assessed the area und the curve (Lenasi & Strucl, 2004) while the other assessed only the peak of the blood flow response (Vassalle et al., 2003). Both studies demonstrated greater reactive hyperemia in the trained subjects. In addition, Franzoni et al. (2004b) demonstrated the magnitude of reactive hyperaemia was directly correlated to total plasma antioxidant capacity, suggesting that greater antioxidant defenses may be a feature of cutaneous vascular adaptation to exercise training. Inasmuch as reactive hyperaemia in the cutaneous circulation is not dependent on nitric oxide (Wong et al., 2003; Zhao et al., 2004) nor cyclooxygenase-derived prostanoids (Lorenzo & Minson, 2007), these combined results suggest that other vasomotor pathways contributing to reactive hyperaemia in the skin may be affected by exercise training (e.g., sensory nerves and BKCa channels (Lorenzo & Minson, 2007)).
Aging
Human aging in the absence of overt pathology is associated with an attenuated reflex vasodilator response during exercise heat stress (Anderson & Kenney, 1987; Kenney, 1988; Ho et al., 1997). This impaired skin blood flow response is apparent even when subjects are matched for fitness level (Ho et al., 1997), acclimation status (Armstrong & Kenney, 1993), and hydration status (Kenney et al., 1990). Peripheral vascular impairments in aged skin include both up- and downstream neurovascular signaling mechanisms mediating reflex vasodilation, including an increased reliance on attenuated NO-dependent vasodilation (Holowatz et al., 2003).
A number of studies have investigated the effects of exercise training (or training status) on cutaneous microvascular reactivity in healthy aging populations. These studies have shown that Ach-mediated vascular responses in the skin are increased by exercise training (Black et al., 2008; Hodges et al., 2010), although this is not a universal finding (Tew et al., 2010). In one well controlled study, Black et al. (2008) blocked nitric oxide production with cutaneous microdialysis of L-NAME (NO synthase inhibitor) during Ach infusions and demonstrated that increased vascular responsiveness following 24 weeks of exercise training was achieved through the increased actions of nitric oxide in the skin. To our knowledge, this study represents the only use of microdialysis and selective pathway inhibitors to investigate the mechanisms of increased cutaneous vasomotor responses following endurance exercise training in any population.
In addition to Ach responses, both the local thermal hyperaemia and reactive hyperemic responses appear to be augmented by exercise training in the aged (Franzoni et al., 2004a; Black et al., 2008; Hodges et al., 2010; Tew et al., 2010). Interestingly, both the initial peak (Tew et al., 2010; Tew et al., 2011) and sustained plateau (Black et al., 2008; Hodges et al., 2010; Tew et al., 2010) portion of the thermal hyperemic response are augmented in trained older subjects. The increased initial peak response during local heating in trained older subjects is normalized following administration of local anesthetic, suggesting sensory nerve-mediated vasomotor responses as well as nitric oxide production are increased by training in this population (Tew et al., 2011).
Proposed signals mediating cutaneous vascular adaptations to endurance exercise training
The signaling events responsible for the cutaneous vascular adaptations to endurance exercise training are unknown. Endurance exercise training typically involves the completion of frequent bouts of whole body dynamic exercise lasting 30 – 60 minutes per session (40 – 80% VO2 max; 3 – 7 days/week). Exercise sessions of this type cause an elevation in Tc that stimulates increases in thermoregulatory skin blood flow, as described above. As a result, the cutaneous circulation is exposed to chronic elevations in perfusion during training, suggesting that haemodynamic forces may provide a key signal for training-induced adaptations in the cutaneous circulation. It is well established that haemodynamic forces associated with increased blood flow during exercise (e.g., shear stress, cyclic strain, etc) are primary signals for the vascular adaptations that occur in the previously trained skeletal muscle (Laughlin et al., 2008). Notwithstanding significant anatomical differences that exist between skeletal muscle and cutaneous vascular beds, some recent findings support the hypothesis that haemodynamic forces are responsible for the transduction of exercise training-mediated adaptations in the cutaneous vasculature. A recent study by Green et al. (2010) examined the hypothesis that repeated forearm heating, which increases skin blood flow in the absence of exercise, improves cutaneous microvascular vasodilator function. In this study, bilateral forearm heating to 42°C (via water immersion) was applied to ten healthy men for 30 min, 3 times per week for 8 weeks. During the immersion sessions, skin blood flow was manipulated in one arm by inflation of a proximal forearm cuff to 100 mmHg, while the other arm remained uncuffed. This manipulation allowed determination of the role of chronic increases in perfusion per se in the cutaneous vascular adaptations to repeated skin heating. After 8 weeks of repeated limb heating, the cutaneous vascular response to acute local heating (thermal hyperaemia) was increased in the uncuffed arm (i.e., the arm with the greatest chronic increase in perfusion) whereas it remained unchanged in the cuffed arm (Green et al., 2010). Because changes in tissue perfusion are often related to changes in vascular wall shear stress, these results support the hypothesis that haemodynamic forces are responsible for the transduction of exercise training-induced vascular adaptations in the skin circulation.
Functional relevance of local cutaneous vascular adaptations to exercise training?
A persistent question in this area is the degree to which exercise training-mediated increases in cutaneous vascular responsiveness bear any functional relevance to the control of skin blood flow during dynamic exercise. That is, training-induced modifications to the control of skin blood flow during exercise appear to be explained by expansions to plasma volume and a subsequent increase in the available cardiac output to meet the competing needs of increasing skeletal muscle and cutaneous vascular conductances (see above). Therefore, although adaptations in cutaneous microvascular reactivity have been demonstrated using a variety of techniques, it does not appear that these adaptations are critical to either the shift in Tc threshold for vasodilatation or the increased skin blood flow during the plateau phase following exercise training. Furthermore, most of the cutaneous vascular adaptations observed in the exercise trained state are suggestive of adaptations in the microvascular endothelium (Kvernmo et al., 1998; Vassalle et al., 2003; Franzoni et al., 2004a; Lenasi & Strucl, 2004; Wang, 2005; Black et al., 2008). Considering the litany of compounds implicated in the active vasodilator response (e.g., vasoactive intestinal polypeptide, histamine, etc), it is not clear that these substances arise from or act through endothelial cells to effect thermoregulatory vasodilatation during exercise (Kellogg et al., 1995; Kellogg et al., 2003; Wong et al., 2004; Wilkins et al., 2005; McCord et al., 2006; Wong & Minson, 2006). Even nitric oxide, responsible for 30–45% of the active vasodilator response, may be generated by the neuronal isoform of nitric oxide synthase (Kellogg et al., 2009). Thus, a synthesis of available literature does not support the hypothesis that local cutaneous vascular adaptations are responsible for training-mediated changes in the control of skin blood flow during exercise, at least in young healthy individuals.
In the context of primary aging, it is possible that training-induced cutaneous vascular adaptations are more critical for changes in the control of skin blood flow during exercise. As mentioned above, primary aging is associated with a reduced ability to increase skin blood flow during exercise thermal stress, due in part to reductions in cardiac output (Kenney, 1988), reductions in the redistribution of blood flow from renal and splanchnic beds (Ho et al., 1997), and decrements in cutaneous microvascular function (Holowatz & Kenney, 2010). Thus, it is possible that exercise training-mediated cutaneous vascular adaptations are required to permit the increased skin blood flow during exercise observed following training in this population (Ho et al., 1997). In support of this concept, some studies indicate the slope of the relation between Tc and skin blood flow is reduced in aged compared to younger populations (Kenney, 1988), while additional (limited) evidence suggests this slope is increased when aging subjects undergo endurance training (Ho et al., 1997). This raises the possibility that, in aging, the cutaneous vasculature can adapt with respect to its sensitivity to active vasodilator activity following training.
Conclusions
In summary, endurance exercise training causes adaptations in the skin blood flow response to exercise, inclusive of changes in cutaneous microvascular reactivity. Increases in skin blood flow at a given exercise core temperature appear to be primarily explained by the expansion of blood volume and increased cardiac output that characterize the trained state. In contrast, adaptations in the cutaneous microvasculature are mediated by changes in the bioavailability or bioactivity of endothelium-derived vasoactive compounds; however, the mechanistic basis for these adaptations remains largely unexplored. Present evidence does not support a causal relation between these two training-mediated adaptations in young healthy persons. That is, it does not appear that skin blood flow during exercise is higher after training because of local cutaneous microvascular adaptations. However, training-mediated vascular adaptations in the skin may be important against a background of preexisting microvascular dysfunction (e.g., in aging).
Acknowledgments
The authors are supported by National Institutes of Health Grants T32-AR48523 (G.H.S.), R01-HL089302 (W.L.K.), R01-AG007004 (W.L.K.), R01-HL093238 (L.A.H.), and R21-HL7770539 (L.A.H.).
References
- Anderson RK, Kenney WL. Effect of age on heat-activated sweat gland density and flow during exercise in dry heat. Journal of Applied Physiology. 1987;63:1089– 1094. doi: 10.1152/jappl.1987.63.3.1089. [DOI] [PubMed] [Google Scholar]
- Armstrong CG, Kenney WL. Effects of age and acclimation on responses to passive heat exposure. Journal of Applied Physiology. 1993;75:2162–2167. doi: 10.1152/jappl.1993.75.5.2162. [DOI] [PubMed] [Google Scholar]
- Bevegard BS, Shepherd JT. Reaction in man of resistance and capacity vessels in forearm and hand to leg exercise. Journal of Applied Physiology. 1966;21:123–132. doi: 10.1152/jappl.1966.21.1.123. [DOI] [PubMed] [Google Scholar]
- Black MA, Green DJ, Cable NT. Exercise prevents age-related decline in nitric-oxide-mediated vasodilator function in cutaneous microvessels. J Physiol. 2008;586:3511–3524. doi: 10.1113/jphysiol.2008.153742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair DA, Glover WE, Roddie IC. Vasomotor Responses in the Human Arm During Leg Exercise. Circulation Research. 1961;9:264–274. [Google Scholar]
- Boegli Y, Gremion G, Golay S, Kubli S, Liaudet L, Leyvraz P-F, Waeber B, Feihl F. Endurance Training Enhances Vasodilation Induced by Nitric Oxide in Human Skin. Journal of Investigative Dermatology. 2003;121:1197–1204. doi: 10.1046/j.1523-1747.2003.12518.x. [DOI] [PubMed] [Google Scholar]
- Boutsiouki P, Georgiou S, Clough GF. Recovery of Nitric Oxide from Acetylcholine-Mediated Vasodilatation in Human Skin In Vivo. Microcirculation. 2004;11:249–259. doi: 10.1080/10739680490425958. [DOI] [PubMed] [Google Scholar]
- Brengelmann GL, Johnson JM, Hermansen L, Rowell LB. Altered control of skin blood flow during exercise at high internal temperatures. Journal of Applied Physiology. 1977;43:790–794. doi: 10.1152/jappl.1977.43.5.790. [DOI] [PubMed] [Google Scholar]
- Convertino VA. Blood volume: its adaptation to endurance training. Medicine and Science in Sports and Exercise. 1991;23:1338–1348. [PubMed] [Google Scholar]
- Durand S, Tartas M, Bouye P, Koitka A, Saumet J, Abraham P. Prostaglandins participate in the late phase of the vascular response to acetylcholine iontophoresis in humans. J Physiol. 2004;561:811–819. doi: 10.1113/jphysiol.2004.069997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblom B, Astrand PO, Saltin B, Stenberg J, Wallstrom B. Effect of training on circulatory response to exercise. Journal of Applied Physiology. 1968;24:518–528. doi: 10.1152/jappl.1968.24.4.518. [DOI] [PubMed] [Google Scholar]
- Franzoni F, Galetta F, Morizzo C, Lubrano V, Palombo C, Santoro G, Ferrannini E, Quinones-Galvan A. Effects of age and physical fitness on microcirculatory function. Clin Sci (Lond) 2004a;106:329–335. doi: 10.1042/CS20030229. [DOI] [PubMed] [Google Scholar]
- Franzoni F, Plantinga Y, Femia FR, Bartolomucci F, Gaudio C, Regoli F, Carpi A, Santoro G, Galetta F. Plasma antioxidant activity and cutaneous microvascular endothelial function in athletes and sedentary controls. Biomedicine and Pharmacotherapy. 2004b;58:432–436. doi: 10.1016/j.biopha.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Fritzsche RG, Coyle EF. Cutaneous blood flow during exercise is higher in endurance-trained humans. Journal of Applied Physiology. 2000;88:738–744. doi: 10.1152/jappl.2000.88.2.738. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Crandall CG, Johnson JM. The cardiovascular challenge of exercising in the heat. J Physiol. 2008;586:45–53. doi: 10.1113/jphysiol.2007.142158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Teller C, Andersen SL, Jensen FB, Hyldig T, Nielsen B. Influence of body temperature on the development of fatigue during prolonged exercise in the heat. Journal of Applied Physiology. 1999;86:1032–1039. doi: 10.1152/jappl.1999.86.3.1032. [DOI] [PubMed] [Google Scholar]
- Green DJ, Carter HH, Fitzsimons MG, Cable NT, Thijssen DHJ, Naylor LH. Obligatory role of hyperaemia and shear stress in microvascular adaptation to repeated heating in humans. J Physiol. 2010;588:1571–1577. doi: 10.1113/jphysiol.2010.186965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Honda Y, Ogawa T, Kondo N, Nishiyasu T. The cross-sectional relationships among hyperthermia-induced hyperventilation, peak oxygen consumption, and the cutaneous vasodilatory response during exercise. European Journal of Applied Physiology. 2009;107:527–534. doi: 10.1007/s00421-009-1152-0. [DOI] [PubMed] [Google Scholar]
- Ho CW, Beard JL, Farrell PA, Minson CT, Kenney WL. Age, fitness, and regional blood flow during exercise in the heat. Journal of Applied Physiology. 1997;82:1126–1135. doi: 10.1152/jappl.1997.82.4.1126. [DOI] [PubMed] [Google Scholar]
- Hodges G, Sharp L, Stephenson C, Patwala A, George K, Goldspink D, Cable NT. The effect of 48 weeks of aerobic exercise training on cutaneous vasodilator function in post-menopausal females. European Journal of Applied Physiology. 2010;108:1259–1267. doi: 10.1007/s00421-009-1330-0. [DOI] [PubMed] [Google Scholar]
- Holowatz LA, Houghton BL, Wong BJ, Wilkins BW, Harding AW, Kenney WL, Minson CT. Nitric oxide and attenuated reflex cutaneous vasodilation in aged skin. American Journal of Physiology - Heart and Circulatory Physiology. 2003;284:H1662–1667. doi: 10.1152/ajpheart.00871.2002. [DOI] [PubMed] [Google Scholar]
- Holowatz LA, Kenney WL. Peripheral mechanisms of thermoregulatory control of skin blood flow in aged humans. Journal of Applied Physiology. 2010;109:1538–1544. doi: 10.1152/japplphysiol.00338.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowatz LA, Thompson CS, Minson CT, Kenney WL. Mechanisms of acetylcholine-mediated vasodilation in young and aged human skin. J Physiol. 2005;563:965–973. doi: 10.1113/jphysiol.2004.080952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose TK, Inoue Y, Hirata M, Shamsuddin AK, Kondo N. Enhanced heat loss responses induced by short-term endurance training in exercising women. Experimental Physiology. 2009;94:90–102. doi: 10.1113/expphysiol.2008.043810. [DOI] [PubMed] [Google Scholar]
- Ikegawa S, Kamijo Y-i, Okazaki K, Masuki S, Okada Y, Nose H. Effects of hypohydration on thermoregulation during exercise before and after 5-day aerobic training in a warm environment in young men. Journal of Applied Physiology. 2011 doi: 10.1152/japplphysiol.01193.2010. in press. [DOI] [PubMed] [Google Scholar]
- Jasperse JL, Laughlin MH. Endothelial Function and Exercise Training: Evidence from Studies Using Animal Models. Medicine and Science in Sports and Exercise. 2006;38:445–454. doi: 10.1249/01.mss.0000191187.24525.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JM, Proppe DW. Cardiovascular adjustments to heat stress. In: Fregly MJ, Blatteis CM, editors. The Handbook of Physiology, section 4: Environmental Physiology. Vol. 1. American Physiological Society; Bethesda, MD: 1996. pp. 215–243. chapter 11. [Google Scholar]
- Kellogg DL., Jr In vivo mechanisms of cutaneous vasodilation and vasoconstriction in humans during thermoregulatory challenges. Journal of Applied Physiology. 2006;100:1709–1718. doi: 10.1152/japplphysiol.01071.2005. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Johnson JM, Kenney WL, Pergola PE, Kosiba WA. Mechanisms of control of skin blood flow during prolonged exercise in humans. American Journal of Physiology. 1993;265:H562–568. doi: 10.1152/ajpheart.1993.265.2.H562. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Johnson JM, Kosiba WA. Competition between cutaneous active vasoconstriction and active vasodilation during exercise in humans. American Journal of Physiology. 1991;261:H1184–1189. doi: 10.1152/ajpheart.1991.261.4.H1184. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Liu Y, Kosiba IF, O’Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. Journal of Applied Physiology. 1999;86:1185–1190. doi: 10.1152/jappl.1999.86.4.1185. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Pergola PE, Piest KL, Kosiba WA, Crandall CG, Grossmann M, Johnson JM. Cutaneous active vasodilation in humans is mediated by cholinergic nerve cotransmission. Circulation Research. 1995;77:1222–1228. doi: 10.1161/01.res.77.6.1222. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Zhao JL, Friel C, Roman LJ. Nitric oxide concentration increases in the cutaneous interstitial space during heat stress in humans. Journal of Applied Physiology. 2003;94:1971–1977. doi: 10.1152/japplphysiol.00826.2002. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Zhao JL, Wu Y. Roles of nitric oxide synthase isoforms in cutaneous vasodilation induced by local warming of the skin and whole body heat stress in humans. Journal of Applied Physiology. 2009;107:1438–1444. doi: 10.1152/japplphysiol.00690.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney WL. Control of heat-induced cutaneous vasodilatation in relation to age. Eur J Appl Physiol Occup Physiol. 1988;57:120–125. doi: 10.1007/BF00691250. [DOI] [PubMed] [Google Scholar]
- Kenney WL, Johnson JM. Control of skin blood flow during exercise. Medicine and Science in Sports and Exercise. 1992;24:303–312. [PubMed] [Google Scholar]
- Kenney WL, Tankersley CG, Newswanger DL, Hyde DE, Puhl SM, Turner NL. Age and hypohydration independently influence the peripheral vascular response to heat stress. Journal of Applied Physiology. 1990;68:1902–1908. doi: 10.1152/jappl.1990.68.5.1902. [DOI] [PubMed] [Google Scholar]
- Kvernmo HD, Stefanovska A, Kirkeboen KA, Osterud B, Kvernebo K. Enhanced endothelium-dependent vasodilatation in human skin vasculature induced by physical conditioning. European Journal of Applied Physiology and Occupational Physiology. 1998;79:30–36. doi: 10.1007/s004210050469. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Newcomer SC, Bender SB. Importance of hemodynamic forces as signals for exercise-induced changes in endothelial cell phenotype. Journal of Applied Physiology. 2008;104:588–600. doi: 10.1152/japplphysiol.01096.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenasi H, Strucl M. Effect of Regular Physical Training on Cutaneous Microvascular Reactivity. Medicine and Science in Sports and Exercise. 2004;36:606–612. doi: 10.1249/01.mss.0000121948.86377.51. [DOI] [PubMed] [Google Scholar]
- Lorenzo S, Minson CT. Human cutaneous reactive hyperaemia: role of BKCa channels and sensory nerves. The Journal of Physiology. 2007;585:295–303. doi: 10.1113/jphysiol.2007.143867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord GR, Cracowski J-L, Minson CT. Prostanoids contribute to cutaneous active vasodilation in humans. Am J Physiol Regul Integr Comp Physiol. 2006;291:R596–602. doi: 10.1152/ajpregu.00710.2005. [DOI] [PubMed] [Google Scholar]
- Minson CT. Thermal provocation to evaluate microvascular reactivity in human skin. Journal of Applied Physiology. 2010;109:1239–1246. doi: 10.1152/japplphysiol.00414.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. Journal of Applied Physiology. 2001;91:1619–1626. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- Morris S, Shore A. Skin blood flow responses to the iontophoresis of acetylcholine and sodium nitroprusside in man: possible mechanisms. J Physiol. 1996;496:531–542. doi: 10.1113/jphysiol.1996.sp021704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel ER, Fortney SM, Wenger CB. Effect of hydration state of circulatory and thermal regulations. Journal of Applied Physiology. 1980;49:715–721. doi: 10.1152/jappl.1980.49.4.715. [DOI] [PubMed] [Google Scholar]
- Nagashima K, Nose H, Takamata A, Morimoto T. Effect of continuous negative-pressure breathing on skin blood flow during exercise in a hot environment. Journal of Applied Physiology. 1998;84:1845–1851. doi: 10.1152/jappl.1998.84.6.1845. [DOI] [PubMed] [Google Scholar]
- Nielsen B, Rowell LB, Bonde-Petersen F. Cardiovascular responses to heat stress and blood volume displacements during exercise in man. Eur J Appl Physiol Occup Physiol. 1984;52:370–374. doi: 10.1007/BF00943365. [DOI] [PubMed] [Google Scholar]
- Nose H, Mack GW, Shi XR, Morimoto K, Nadel ER. Effect of saline infusion during exercise on thermal and circulatory regulations. Journal of Applied Physiology. 1990;69:609–616. doi: 10.1152/jappl.1990.69.2.609. [DOI] [PubMed] [Google Scholar]
- Roberts MF, Wenger CB, Stolwijk JA, Nadel ER. Skin blood flow and sweating changes following exercise training and heat acclimation. Journal of Applied Physiology. 1977;43:133–137. doi: 10.1152/jappl.1977.43.1.133. [DOI] [PubMed] [Google Scholar]
- Roche D, Rowland T, Garrard M, Marwood S, Unnithan V. Skin microvascular reactivity in trained adolescents. European Journal of Applied Physiology. 2010;108:1201–1208. doi: 10.1007/s00421-009-1328-7. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiological Reviews. 1974;54:75–159. doi: 10.1152/physrev.1974.54.1.75. [DOI] [PubMed] [Google Scholar]
- Takeno Y, Kamijo YI, Nose H. Thermoregulatory and aerobic changes after endurance training in a hypobaric hypoxic and warm environment. Journal of Applied Physiology. 2001;91:1520–1528. doi: 10.1152/jappl.2001.91.4.1520. [DOI] [PubMed] [Google Scholar]
- Tew G, Klonizakis M, Saxton J. Effects of ageing and fitness on skin-microvessel vasodilator function in humans. European Journal of Applied Physiology. 2010;109:173–181. doi: 10.1007/s00421-009-1342-9. [DOI] [PubMed] [Google Scholar]
- Tew GA, Klonizakis M, Moss J, Ruddock AD, Saxton JM, Hodges GJ. Role of sensory nerves in the rapid cutaneous vasodilator response to local heating in young and older endurance-trained and untrained men. Experimental Physiology. 2011;96:163–170. doi: 10.1113/expphysiol.2010.055434. [DOI] [PubMed] [Google Scholar]
- Thomas CM, Pierzga JM, Kenney WL. Aerobic training and cutaneous vasodilation in young and older men. Journal of Applied Physiology. 1999;86:1676–1686. doi: 10.1152/jappl.1999.86.5.1676. [DOI] [PubMed] [Google Scholar]
- Vassalle C, Lubrano V, Domenici C, L’Abbate A. Influence of Chronic Aerobic Exercise on Microcirculatory Flow and Nitric Oxide in Humans. International Journal of Sports Medicine. 2003;24:30–35. doi: 10.1055/s-2003-37202. [DOI] [PubMed] [Google Scholar]
- Wang JS. Effects of exercise training and detraining on cutaneous microvascular function in man: the regulatory role of endothelium-dependent dilation in skin vasculature. European Journal of Applied Physiology and Occupational Physiology. 2005;93:429–434. doi: 10.1007/s00421-004-1176-4. [DOI] [PubMed] [Google Scholar]
- Wilkins BW, Wong BJ, Tublitz NJ, McCord GR, Minson CT. Vasoactive intestinal peptide fragment VIP10-28 and active vasodilation in human skin. Journal of Applied Physiology. 2005;99:2294–2301. doi: 10.1152/japplphysiol.00500.2005. [DOI] [PubMed] [Google Scholar]
- Wong BJ, Minson CT. Neurokinin-1 receptor desensitization attenuates cutaneous active vasodilatation in humans. The Journal of Physiology. 2006;577:1043–1051. doi: 10.1113/jphysiol.2006.112508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong BJ, Wilkins BW, Holowatz LA, Minson CT. Nitric oxide synthase inhibition does not alter the reactive hyperemic response in the cutaneous circulation. Journal of Applied Physiology. 2003;95:504–510. doi: 10.1152/japplphysiol.00254.2003. [DOI] [PubMed] [Google Scholar]
- Wong BJ, Wilkins BW, Minson CT. H1 but not H2 histamine receptor activation contributes to the rise in skin blood flow during whole body heating in humans. J Physiol. 2004;560:941–948. doi: 10.1113/jphysiol.2004.071779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelis R, Mason DT, Braunwald E. Partition of blood flow to the cutaneous and muscular beds of the forearm at rest and during leg exercise in normal subjects and in patients with heart failure. Circulation Research. 1969;24:799–806. doi: 10.1161/01.res.24.6.799. [DOI] [PubMed] [Google Scholar]
- Zhao JL, Pergola PE, Roman LJ, Kellogg DL., Jr Bioactive nitric oxide concentration does not increase during reactive hyperemia in human skin. Journal of Applied Physiology. 2004;96:628–632. doi: 10.1152/japplphysiol.00639.2003. [DOI] [PubMed] [Google Scholar]


