Abstract
Previous work from our team and others has shown that manual acupuncture at LI4 (hegu), ST36 (zusanli), and LV3 (taichong) deactivates a limbic-paralimbic-neocortical brain network, and at the same time activates somatosensory regions of the brain. The objective of the present study was to explore the specificity and commonality of the brain response to manual acupuncture at LI4, ST36, and LV3, acupoints that are located on different meridians and are used to treat pain disorders. We used functional magnetic resonance imaging (fMRI) to monitor the brain responses to acupuncture at 3 different acupoints; we examined 46 healthy subjects who, according to their psychophysical responses, experienced deqi sensation during acupuncture. Brain responses to stimulation at each of the acupoints were displayed in conjunction with one another to show the spatial distribution. We found clusters of deactivation in the medial prefrontal, medial parietal and medial temporal lobes showing significant convergence of two or all three of the acupoints. The largest regions showing common responses to all three acupoints were the right subgenual BA25, right subgenual cingulate, right isthmus of the cingulum bundle, and right BA31. We also noted differences in major sections of the medial prefrontal and medial temporal lobes, with LI4 predominating in the pregenual cingulate and hippocampal formation, ST36 predominating in the subgenual cingulate, and LV3 predominating in the posterior hippocampus and posterior cingulate. The results suggest that although these acupoints commonly used for anti-pain and modulatory effects may mobilize the same intrinsic global networks, with substantial overlap of common brain regions to mediate its actions, our findings showing preferential response of certain limbic-paralimbic structures suggests acupoints may also exhibit relative specificity.
Keywords: acupuncture, fMRI, limbic-paralimbic-neocortical network, default mode, acupoint specificity, deqi
INTRODUCTION
Whether specific acupoints elicit responses in specific regions of the brain is a fundamental question in acupuncture research, as well as a point of some controversy within the field. Whereas some investigators have reported results that deny such specificity (Ulett, 1992) others points are evidenced of high specificity, in agreement with traditional Chinese medicine (TCM) meridian theory (Cheng, 2000). While many different acupoints are used for common clinical purposes, empirical experience suggests some acupoints are preferred to others for treatment of specific disorders. Our work during the past decade has focused on the commonality of brain responses to three acupoints: LI4 (Large Intestine 4 or Hegu) on the hand, ST36 (Stomach 36 or Zusanli) on the leg, and LV3 (Liver 3 or Taichong) on the foot. Each of these three acupoints has clinical functions at specific body locations, but they are used to treat pain disorders. LI4 has pain relief function in the head, neck and large intestine, ST36 in the gastrointestinal tract, and LV3 in the pelvic region. These three acupoints are located on different meridians according to the TCM literature.
We and others have shown that manual acupuncture at these three acupoints deactivates a limbic-paralimbic-neocortical brain network (LPNN) and activates somatosensory brain regions (Wu et al., 1999; Wu et al., 2002; Hui et al., 2005; Napadow et al., 2005; Yan et al., 2005; Fang et al., 2006; Wang et al., 2007; Fang et al., 2009). Furthermore, clusters of other deactivated regions in the medial prefrontal, medial parietal and medial temporal regions in response to acupuncture have been shown to be nearly identical to the default mode network (Dhond et al., 2008; Hui et al., 2009; Liu et al., 2009). The integrity of default mode network has been postulated to be central to the balance of global neurological function and the maintenance of health (Buckner et al., 2008). Together these findings suggested that acupuncture mobilizes anti-correlated functional networks (Hui et al., 2009) of the brain to mediate its holistic effects. Such pronounced modulatory brain effects of acupuncture agrees with the diverse physiological and clinical effects of acupuncture treatment (Napadow et al., 2004; Hui et al., 2010).
One of many compelling reasons for using neuroimaging to investigate the commonality and specificity of three different acupoints is the potential to use one acupoint as a control for another. The question of how to select the proper control is an extremely important question in modern acupuncture research. Rong and Zhu (Rong et al., 2002) suggested the interesting approach of using an acupoint on the lung meridian as a control to examine the acupuncture effect of an acupoint on the heart meridian, assuming that clinically distinct acupoints (as defined by TCM) could control for one another. This control paradigm could be used to address expectation (placebo) effects, as subjects do not have knowledge of the anticipated clinical effects particular to each acupoint; furthermore, it avoids some of the controversy associated with using sham (simulated) acupuncture as control. The sham approaches include 1) superficial Streitberger needling (no skin penetration) at verum (real) acupoints, 2) needling of non-classical body points that neighbor verum acupoints, which can include both superficial needling at non-acupoints and shallow needling of non-acupoints, and 3) deep needling of non-acupoints (Langevin et al., 2011). Not only does each of these sham approaches has ardent proponents and opponents, there is also no general agreement on how to identify a sham acupoint. As such, it is not surprising that studies involving sham acupuncture have produced conflicting results. For example, while one German study of acupuncture on back pain (Haake et al., 2007) showed that sham and verum acupoints yielded similar therapeutic effects, another German study of acupuncture effects on shoulder pain (Molsberger et al., 2010) showed that acupuncture at verum acupoints elicited superior treatment results compared to sham acupoints. Therefore, if our three chosen acupoints (LI4, ST36 and LV3) can be shown to demonstrate any specificity based on distinct MRI features, we would have provided new data not only potentially useful for the evaluation of the TCM’s claim of special clinical efficacy for each acupoint, but also for the consideration of a future alternative of the control paradigm.
In our ongoing work to image and map the brain effects of acupuncture at verum acupoints, we, in collaboration with colleagues in the acupuncture imaging community, have rigorously investigated sham acupuncture as a control. In our neuroimaging studies over many years, we employed tactile non-penetrating stimulation using von Frey monofilaments at verum acupoints, as described in several of our previous publications (Napadow et al., 2005; Napadow et al., 2007; Hui et al., 2009). Tactile stimulation is useful for addressing nonspecific acupuncture effects, including placebo effects (Kaptchuk et al., 2008; Langevin et al., 2011), and needle-specific (depth-specific) physiological effects for extraction of the acupuncture-specific brain responses (Ho et al., 2008; Langevin et al., 2011). Together these studies showed that tactile stimulation at verum acupoints elicits reponses significantly different from those associated with acupuncture at the same verum acupoints. The differences were striking. Not only did the blood oxygenation level dependent (BOLD) fMRI signal go in opposite directions for acupuncture and tactile stimulation, but the brain regions prominently modulated by acupuncture were far more extensive than those that showed response to tactile stimulation (Hui et al., 2009). Acupuncture showed significantly reduced BOLD signal in the frontal pole, pregenual cingulate, medial temporal lobe and temporal pole. In contrast, we observed that, compared to tactile stimulation, acupuncture elicited marked increase in the BOLD signal in subcortical and paralimbic structures including the thalamus, right anterior insula, anterior middle cingulate and posterior cingulate BA23 dorsal (Hui et al., 2009). Only some minor overlap was observed in the responses to acupuncture and tactile stimulation in the medial prefrontal cortex and medial parietal cortex. Our findings of the different brain responses to acupuncture and tactile stimulation agree with the recent German Randomized Acupuncture Trial for Chronic Shoulder Pain (GRASP), which reported that verum acupuncture (65% of responders) was more effective than sham acupuncture (24% of responders) for treating chronic shoulder pain. The findings from this large-scale, multi-center, patient-blinded acupuncture clinical trial, which examined 424 chronic shoulder pain patients (Molsberger et al., 2010), differ from those of a 2007 study on back pain (Haake et al., 2007), which suggested little difference in the therapeutic effectiveness of verum and sham acupuncture. Since the findings from tactile stimulation at LI4, ST36 and LV3 as experimental control were already reported in our previous study (Hui et al., 2009), they will be referred to but not repeated here in the manuscript.
We previously reported our fMRI study of the effects of acupuncture at LI4, ST36 and LV3 on the limbic system and anti-correlated networks of the human brain (Hui et al., 2009). Whereas that paper focus on the commonality of the brain response to these three acupoints, we now extend our work to explore specificity as well as the commonality of response to acupuncture stimulation at these acupoints that lie on different meridians and belong to different segmental innervations. We used conjunction analysis to visualize the preferential distributions and regional overlap of the brain responses, hypothesizing that the overall LPNN and its anti-correlated somatosensory network would be mobilized by all three acupoints, but that the response of some brain structures would be specific to individual acupuncture points, hence suggesting relative specificity of acupoint effects. We expect that these findings carry relevance for clinical acupuncture practice, and can offer greater direction for designing control paradigms for future acupuncture studies.
METHODS
Subjects
This study included data from 46 acupuncture-naïve subjects (20–47 years old, mean±SD=29.1±7.63, 19M, 27F) who had participated in the imaging studies at the Athinoula A. Martinos Center for Biomedical Imaging at Massachusetts General Hospital, in compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and the standards established by the Institutional Review Board of the hospital and the National Center of Complementary and Alternative Medicine (NCCAM) of the NIH. Subjects were screened to exclude neurological, mental and medical disorders, drug abuse, history of head trauma with loss of consciousness, and contraindications for exposure to high magnetic field. All experimental procedures were explained to the subjects, and signed informed consent was obtained prior to participation in the study.
Acupuncture
During a single session, we administered acupuncture to classical acupoints on the right side of the body, at LI4 on the hand, LV3 on the foot and ST36 on the lower leg using sterile, single-use acupuncture needles. The order in which we administered acupuncture to these acupoints was randomized for each subject. We used stainless steel needles for LV3 (0.20 mm diameter) and ST36 (0.22 mm diameter) (KINGLI Medical Appliance Co., Wuxi, China), but silver needles (0.23 mm diameter) for LI4 (Matsuka, Tokyo, Japan) because of the proximity of the acupoint to the static magnetic field of the MR scanner. The depth of needle insertion ranged from 0.5–1 cm (for LV3 and LI4) to 2–3 cm (for ST36). Stimulation was enhanced with manipulation of the needle to elicit deqi, the composite of unique sensations related to efficacy according to TCM (Hui et al., 2007). To avoid noxious pain, we tested the subject’s tolerance to needle manipulation after inserting the needle at the acupoint. During the ten-minute scan, the needle was rotated approximately 180° in each direction, with even motion at the rate of 1Hz, for two minutes during the two stimulation periods and left in place during the three rest periods (Fig. 1). To reduce the after effects of acupuncture between 2 consecutive acupoints demonstrated with fMRI (Dhond et al., 2008; Bai et al., 2009), the subjects were asked to rest in the scanner for five to ten minutes. A licensed acupuncturist (JL) with more than 25 years of clinical acupuncture experience administered acupuncture for all subjects. Tactile stimulation over the acupoints was used as a control for expectation and superficial sensory evaluation, as reported previously (Hui et al., 2005; Hui et al., 2007; Hui et al., 2009).
Fig. 1.
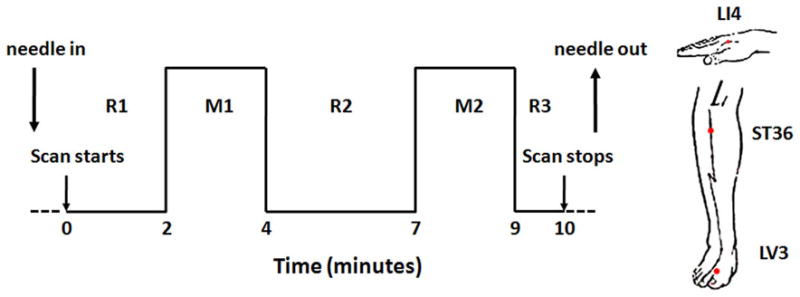
The participants were blinded to the study, and told that acupuncture would be performed at different anatomical points using different techniques; while lying in the supine position in the scanner subjects were not able to see where the acupuncturist was working. At the completion of each scan, the subject was asked to report any sensations of aching, soreness, pressure, heaviness, fullness or distension, warmth or coolness, numbness, tingling, dull pain, sharp pain, and to rate each sensation, if it was experiences, on a scale of 1 to 10. A deqi response was defined as a total score of 3 or higher for the 9 sensations other than sharp pain. Only data from scans in which the subjects reported deqi responses were included in analysis. To avoid the confounding effect of noxious stimulation (Hui et al., 2000; Hui et al., 2005; Hui et al., 2007; White et al., 2008; Hui et al., 2009), scans during which the subjects reported inadvertent sharp pain lasting more than 1 second were excluded from analysis.
fMRI acquisition
fMRI was performed on a 1.5 Tesla scanner (Siemens Sonata, Erlangen, Germany) equipped with a standard quadratic head coil. The subjects lay supine with earplugs to suppress scanner noise and cushions to immobilize the head. We acquired 1) standard high-resolution sagittal images with a T1-weighted 3D-MPRAGE sequence, and 2) whole-brain BOLD fMRI images encompassing the brain stem with a gradient-echo echo planar imaging (EPI) sequence (TR=4000ms, TE=30ms, flip angle=90°, FOV=200mm, matrix=64×64, thickness=3mm, gap=0.6mm) while the subject was administered acupuncture at the LI4, ST36 or LV3 acupoint. Each fMRI run lasted 10 minutes.
Image analysis
All fMRI data were analyzed using the Analysis of Functional NeuroImage (AFNI) software package (Cox, 1996). The first 15 volumes acquired in the first minutes of each functional dataset were discarded to eliminate the drifting of MR signals commonly seen in the beginning of acupuncture fMRI scans. Each functional dataset was motion corrected, registered onto the subject’s anatomical scan, transformed to the standardized space of Talairach and Tournoux (Talairach et al., 1988), spatially smoothed with a Gaussian filter of full-width half-maximum 5.7 mm, and normalized to its mean intensity value across the time series. Multiple regression analysis was performed to identify brain areas showing change in the MR signal as a result of needle manipulation during acupuncture periods, using as reference the needle left in place during the rest periods (REST). Brain volumes with percentage of MR signal change to acupuncture from different subjects were then grouped according to the acupoint and analyzed with a mixed effects model for two-factor Analysis of Variance, where acupoints were treated as fixed effect and participants as a random effect. Activations in 3 contrasts (LI4-REST, LV3-REST and ST36-REST) were obtained. To protect against type I error, we set an individual voxel probability threshold of p<0.003 to correct the overall significance level to α<0.05 using Monte Carlo simulation (Gold et al., 1998). Based on Monte Carlo simulation with 1000 iterations processed with AlphaSim program (Wald, 1997), the overall corrected threshold of the group activation maps for each contrast was p<0.05 with cluster volume of 105 mm3, and each voxel consistently active across the functional datasets at uncorrected p<0.003. Conjunction of activations among 3 contrasts was performed with a step function to examine spatial overlapping and differences in the brain responses to the three different acupoints, with reference to REST in terms of location, size and extent. Conjunction of deactivations among 3 contrasts was performed using the same method as for activations. The conjunction maps were then overlaid on the high-resolution anatomical map of the cohort in the standardized Talairach space. Anatomical localization and masking of the functional data were determined by both Talaraich coordinates and direct inspection. Regions of interest were defined based on published methods (Filipek et al., 1994; Caviness et al., 1996) and standard atlases (Talairach et al., 1988; Mai et al., 2004). The cingulate was subdivided according to Vogt (Vogt et al., 2003; Vogt, 2005; Vogt et al., 2006).
RESULTS
Commonality vs. specificity in the brain activation network
The positive BOLD response of the task-positive network in the limbic and paralimbic structures appears to be dominant in the left hemisphere (Table 1). In both the left and right hemispheres ST36 elicited more regional activations relative to LI4 and LV3 (Fig. 2). Task-positive regions of interest exhibiting increasing signal in response to acupuncture are shown in the activation network (Fig. 2). The activated structures include the secondary somatosensory area, dorsolateral prefrontal cortex, dorsomedial prefrontal cortex, anterior insula and thalamus. The left secondary somatosensory area—contralateral to the sites of stimulation—showed a small region of commonality, although the activation of the surrounding areas suggested differences between acupoints. We observed scattered activations in the left dorsolateral and left dorsomedial prefrontal cortices for all three acupoints. Overlapping activations for all three acupoints were seen in both the left and right thalami.
Table 1.
Summary of the anatomical foci showing positive BOLD responses in all the comparisons (p<0.05 corrected). Gyral descriptions and stereotaxic coordinates refer to the atlas of Talairach and Tournoux (1988).
| Atlas structure at the center of maximum difference | LI4-REST | ST36-REST | LV3-REST | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | t | x | y | z | t | x | y | z | t | |||
| Frontal | Left | Anterior middle cingulate | −10 | 11 | 37 | 4.283 | −6 | 10 | 30 | 3.491 | ||||
| BA46 | −38 | 42 | 9 | 4.122 | −33 | 43 | 6 | 5.343 | ||||||
| Insula | −37 | −3 | −2 | 4.381 | −31 | 10 | 5 | 3.475 | ||||||
|
| ||||||||||||||
| Right | Anterior middle cingulate | 3 | 15 | 21 | 3.571 | 3 | 10 | 23 | 3.534 | |||||
| BA46 | 39 | 40 | 4 | 3.786 | ||||||||||
|
| ||||||||||||||
| Temporal | Left | Temporal pole | −54 | 4 | 1 | 3.593 | ||||||||
| Amygdala | −18 | −4 | −9 | 3.609 | ||||||||||
|
| ||||||||||||||
| Parietal | Left | Secondary somatosensory area | −58 | −19 | 18 | 6.102 | −54 | −19 | 24 | 5.683 | −56 | −19 | 25 | 5.103 |
|
| ||||||||||||||
| Right | Secondary somatosensory area | 54 | −23 | 27 | 3.998 | 51 | −25 | 26 | 4.012 | |||||
Fig. 2.
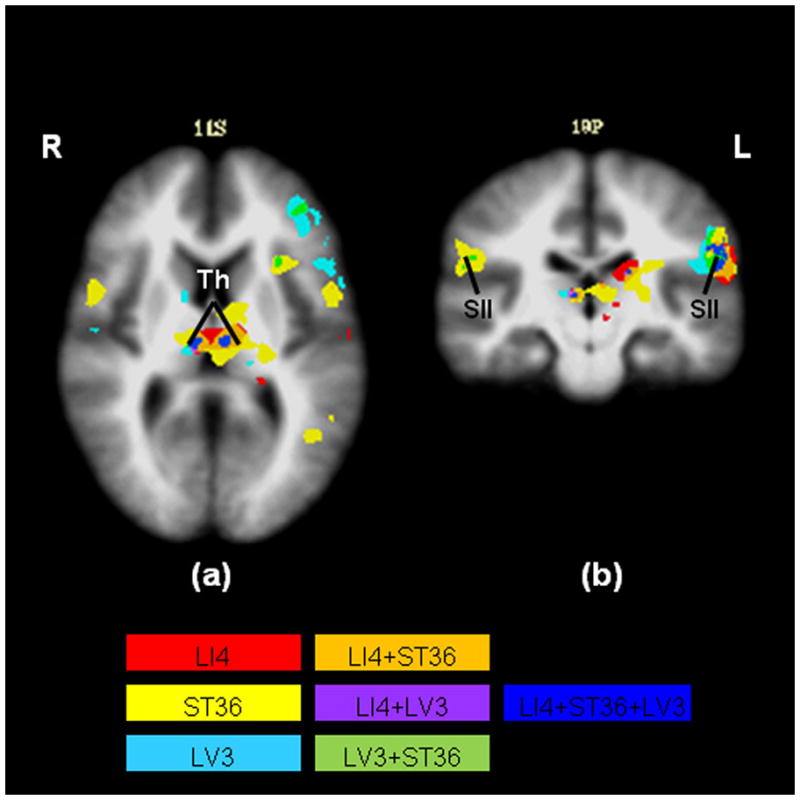
Commonality vs. specificity in the brain deactivation network
The deactivation network showed marked predominance over the activation network in the extent of brain region involvement (Table 2). The negative BOLD response of the limbic and paralimbic structures appeared to be both more widespread and stronger in the right hemisphere (ipsilateral to the sites of stimulation), particularly in the medial prefrontal cortex, medial parietal cortex and medial temporal lobe (Fig. 3). LI4 elicited the greatest regional deactivation in these regions. However, acupuncture at LI4 did not show its own specific regional deactivations without involving deactivations due to one or more other acupoints (Table 2). On the contrary, both LV3 and ST36 showed regional deactivations specific to their own manipulations.
Table 2.
Summary of the anatomical foci showing negative BOLD responses in all the comparisons (p<0.05 corrected). Gyral descriptions and stereotaxic coordinates refer to the atlas of Talairach and Tournoux (1988).
| Atlas structure at the center of maximum difference | LI4-REST | ST36-REST | LV3-REST | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | t | x | y | z | t | x | y | z | t | |||
| Frontal | Left | Subgenual BA25 | −1 | 12 | −8 | −4.181 | −4 | 14 | −8 | −5.103 | −3 | 12 | −7 | −3.543 |
| Subgenual cingulate | −3 | 22 | −10 | −4.504 | −3 | 16 | −8 | −4.404 | −3 | 19 | −8 | −3.524 | ||
| Pregenual cingulate | −1 | 38 | 16 | −3.168 | ||||||||||
|
| ||||||||||||||
| Right | Subgenual BA25 | 5 | 14 | −9 | −5.477 | 4 | 14 | −10 | −5.858 | 4 | 8 | −7 | −4.246 | |
| Subgenual cingulate | 4 | 17 | −8 | −4.986 | 3 | 17 | −9 | −4.979 | 5 | 18 | −8 | −4.360 | ||
| Pregenual cingulate | 8 | 51 | 4 | −5.336 | 8 | 34 | −12 | −3.525 | 10 | 49 | 17 | −4.585 | ||
| Anterior middle cingulate | 8 | 37 | 33 | −4.077 | ||||||||||
| Orbito-frontal cortex | 11 | 32 | −17 | −3.373 | 12 | 31 | −16 | −3.486 | ||||||
| Frontal pole | 6 | 53 | 3 | −6.009 | 22 | 53 | 10 | −3.747 | 6 | 60 | 3 | −4.663 | ||
|
| ||||||||||||||
| Temporal | Left | Temporal pole | −31 | 7 | −33 | −6.746 | −36 | 8 | −33 | −4.346 | ||||
| Amygdala | −21 | −10 | −15 | −3.156 | −17 | −7 | −19 | −3.981 | −13 | −2 | −16 | −4.289 | ||
| Anterior hippocampus | −26 | −18 | −9 | −4.194 | −19 | −8 | −19 | −3.998 | ||||||
| Parahippocampus | −26 | −2 | −30 | −5.491 | −24 | −29 | −10 | −4.636 | ||||||
| Posterior hippocampus | −26 | −24 | −7 | −4.258 | −30 | −24 | −7 | −3.373 | ||||||
|
| ||||||||||||||
| Right | Temporal pole | 35 | 14 | −33 | −7.003 | 32 | 13 | −33 | −5.101 | 29 | 4 | −26 | −5.002 | |
| Amygdala | 26 | −8 | −17 | −4.341 | 19 | −10 | −13 | −4.366 | 13 | 0 | −16 | −4.776 | ||
| Anterior hippocampus | 31 | −12 | −12 | −5.803 | 20 | −15 | −12 | −4.646 | 32 | −18 | −8 | −4.132 | ||
| Parahippocampus | 26 | −1 | −28 | −4.794 | 17 | −43 | 5 | −3.685 | 17 | −31 | −7 | −4.062 | ||
| Posterior hippocampus | 31 | −32 | 2 | −3.887 | 25 | −26 | −4 | −3.719 | ||||||
|
| ||||||||||||||
| Parietal | Left | BA29 and BA30 | −7 | −42 | 11 | −3.513 | ||||||||
| BA31 | −9 | −48 | 46 | −3.902 | −2 | −52 | 39 | −4.318 | −6 | −65 | 23 | −3.564 | ||
| Precuneus | −8 | −45 | 53 | −4.091 | −2 | −64 | 38 | −4.589 | −1 | −72 | 43 | −3.891 | ||
|
| ||||||||||||||
| Right | BA23 ventral | 5 | −53 | 24 | −3.140 | 5 | −50 | 27 | −3.648 | |||||
| BA29 and BA30 | 9 | −41 | 4 | −3.113 | ||||||||||
| BA31 | 5 | −64 | 24 | −4.731 | 2 | −44 | 40 | −4.690 | 12 | −54 | 26 | −4.677 | ||
| Precuneus | 6 | −49 | 60 | −4.787 | 8 | −53 | 46 | −4.771 | 5 | −72 | 41 | −4.260 | ||
| Angular gyrus | 32 | −67 | 41 | −5.251 | 26 | −68 | 46 | −3.967 | 24 | −68 | 42 | −4.146 | ||
|
| ||||||||||||||
| Cerebellum | Cerebellar vermis | −2 | −43 | −28 | −4.402 | −4 | −45 | −25 | −3.719 | |||||
Fig. 3.
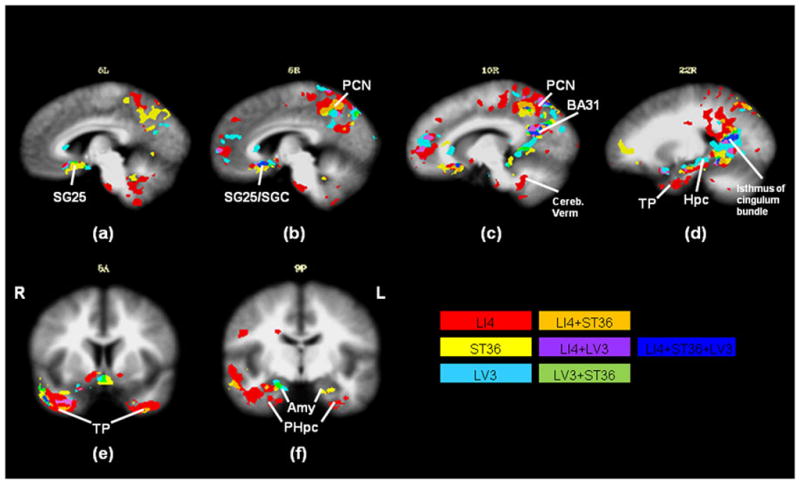
In the medial prefrontal cortex, the right subgenual BA25 and subgenual cingulate showed a large overlap in their responses to all acupoints (Fig. 3b). Other structures within the medial prefrontal cortex showed the greatest spatial variation in response to stimulation at these acupoints (Fig. 3a–d). Whereas the right pregenual cingulate showed greatest deactivation in response to LI4 and LV3 stimulation (Fig. 3c), the left subgenual BA25 showed greatest deactivation for ST36 (Fig. 3a).
In the medial temporal lobe, acupuncture at LI4 created the largest regional deactivation across subjects (Fig. 3d–f). The response was marked on the right, involving the temporal pole, amygdala, hippocampus and parahippocampus. There was a small cluster of deactivation within the amygdala during acupuncture at LV3 but less widespread than at either of the other two acupoints (Fig. 3f). The cluster of deactivation in the right hippocampus was large during LV3 stimulation when compared to the LI4 and ST36 (Fig. 3d). At the far posterior portion of the hippocampal formation, toward the isthmus of the cingulum bundle, there was a sizable region of commonality for all acupoints (Fig. 3d).
In the medial parietal cortex, the right BA31 showed region of commonality for all acupoints (Fig. 3c). Deactivations resulting from both LI4 and ST36 stimulation were predominant in the precuneus (Fig. 3c), with LI4 showing more pronounced effects. LV3 appeared to show more deactivation around the posterior cingulate, and extending to the posterior hippocampus (Fig. 3c,d). By contrast, LI4 showed more deactivation in the anterior hippocampus (Fig. 3d).
The pons and cerebellar vermis showed widespread deactivation with LI4 stimulation (Fig. 3a–c). Minimal deactivation was seen in the cerebellum with ST36 stimulation (Fig. 3a,b).
DISCUSSION
To our knowledge, this is the largest fMRI study to date to compare multiple classical acupoints on the same subjects. fMRI acupuncture studies are challenging because of low signal-to-noise ratios and high inter-subject and inter-session variability—all of which make the inclusion of large sample sizes important for producing reliable results (Kong et al., 2007; Kong et al., 2009). The image data we have acquired on 46 subjects provide significant statistical power.
Our previous work has consistently shown a generalized deactivation of the LPNN as well as activation of a task-positive network across multiple brain levels during acupuncture (Hui et al., 2000; Hui et al., 2005; Napadow et al., 2005; Hui et al., 2009). The conjunction analysis we used in this study has revealed new findings of interest. In addition to demonstrating regions of commonality in the task-positive network (secondary somatosensory area and thalamus) and in the task-negative network (right subgenual BA25, right subgenual cingulate, right isthmus of cingulate bundle and right BA31), this method revealed regions of relative acupoint specificity in the medial prefrontal cortex, medial parietal cortex and medial temporal lobe of the task-negative network.
Identification of commonality and specificity characterized in the task-negative network
Common regions with overlapping deactivation elicited by all three acupoints were observed in the right ventromedial prefrontal cortex (subgenual BA25 and subgenual cingulate) (Fig. 3b), in the right isthmus of the cingulum bundle (Fig. 3d), where the posterior hippocampal formation converged into a narrow path before leading to the temporal lobe, and in the right BA31 (Fig. 3c). Significant areas of overlapping deactivations in response to LI4 and LV3 were found in the right pregenual cingulate (Fig. 3c), while overlapping deactivations from LI4 and ST36 were seen in the right precuneus (Fig. 3c). Interestingly, another recent study (Qin et al., 2011)(Qin et al., 2011)(Qin et al., 2011)(Qin et al., 2011)(Qin et al., 2011)(Qin et al., 2011)(Qin et al., 2011)(Qin et al., 2011)(Qin et al., 2011)(Qin et al., 2011)(Qin et al., 2011)(Qin et al., 2011)(Qin et al., 2011)showed that the precuneus region common to LI4 and ST36 is also a common area for other acupoints including GB37, BL60 and KI8. This study also used electroacupuncture and a different data analysis approach to compare the resting states before and after acupuncture (Qin et al., 2011). Image data, especially fMRI data, on the role of the subgenual BA25 is more limited because of the large concentration of blood vessels in the region. However, a few studies suggest that the subgenual BA25 has extensive projections to the amygdala, as well as to the the posteromedial parietal cortex and medial prefrontal cortex (Vogt, 2005; Buckner et al., 2008). Many studies have demonstrated that the right posteromedial parietal cortex functions as a hub of the default mode network (DMN) (Parvizi et al., 2006; Buckner et al., 2008). Perhaps the most characteristic effect of acupuncture action is deactivation of the medial temporal lobe, which contains the amygdala and hippocampal formation, both major structures for emotional and cognition processing. The finding that these key DMN hubs also comprise common regions of acupuncture action and supports the hypothesis that acupuncture stimulation at different acupoints may use the same global networks for their neurological effects. It is surprising that so many different acupoints have been found to elicit acupuncture responses in the same brain regions. These findings cannot be explained by the effect of sensory input from these different acupoint locations that belong to completely different segmental innervations. Nor can these findings be explained by “expectation” or “attention,” as deactivation in these common brain regions was markedly diminished in response to tactile stimulation (Napadow et al., 2005; Napadow et al., 2007; Hui et al., 2009).
Although broadly speaking these regions showed common responses, we also observed evidence of relative acupoint specificity in the ventral and mid-levels of the medial prefrontal cortex. Deactivation of the pregenual cingulate was most prominent during acupuncture at LI4 (Fig. 3b,c); this region, which is activated by emotions of both negative and positive valence (Vogt et al., 2003; Vogt, 2005), has been reported to be involved in attention deficit hyperactivity disorder (Seidman et al., 2006), major depression (Fu et al., 2004; Anand et al., 2007) and pain (Vogt et al., 1996; Apkarian et al., 2005). Marked deactivations were demonstrated in the subgenual cingulate during acupuncture at ST36 (Fig. 3a). The subgenual cingulate has strong connections to the ventral portions of the posteromedial parietal cortex, and is generally involved in autonomic as well as affective memory creation and recall (George et al., 1995; Vogt, 2005; Vogt et al., 2006; Yoshimura et al., 2009). It is a site used for deep brain stimulation in the treatment of major depression (Lozano et al., 2008).
The temporal lobe shows regions of both commonality and relative acupoint specificity. Acupuncture at LI4, LV3 and ST36 results in deactivation of the amygdala (Fig. 3f), which is associated with generalized fear (Birbaumer et al., 1998; LaBar et al., 1998; Whalen et al., 2001), post-traumatic distress syndrome (PTSD) (Rauch et al., 2000), clinical depression (Siegle et al., 2002; Drevets, 2007) and borderline personality disorder (Herpertz et al., 2001). The body of the hippocampal formation is deactivated during acupuncture at all three points, but most prominently at LI4. It is a key region for memory and affect, with potential involvement in PTSD (Yang et al., 2004; Sakamoto et al., 2005), panic disorders (Maddock et al., 2003; Massana et al., 2003), and generalized anxiety disorder (Bremner, 2004).
While each of these regions in the medial prefrontal cortex and temporal lobe are involved to some degree in the affective dimension of pain as well as many different mood and behavior disorders, their subtle roles in these conditions must be further clarified. Further clinical research must be done to understand how acupuncture at these three acupoints may vary in general potency and specific efficacy to optimize treatment.
Commonality and specificity characterized in the task-positive network of acupuncture
As a form of sensory stimulation, acupuncture is expected to have sensory neurological correlates. Our study did, in fact, show activation of the sensorimotor association cortices, and a few paralimbic regions, although the activation network was markedly less extensive than the deactivation network. The sensorimotor and association cortex effects were found to predominate on the left, contralateral to the site of stimulation. Overlap of core regions of the activation network was less extensive than in the deactivation network. Preferential activation of the right anterior insula with activation at LI4 may indicate relative specificity of acupuncture action in this region. Several studies have shown that pain and pain expectancy, as psychological mechanisms, are closely associated with the thalamus and insula (Davis et al., 1997; Bantick et al., 2002; Wager et al., 2004). The anterior insula plays a sensory role in visceral sensations and pain, as well as a paralimbic role in emotions such as empathy for pain (Singer et al., 2004). Interestingly, LI4 elicited the weakest activation of all three acupoints, but the strongest deactivation in the LPNN. This dichotomy requires further investigation.
Nature of the acupoints
One possible explanation for the observation of discrete regional brain deactivations and activations may be the composition of nerve fibers at the three acupoints (Hui et al., 2007; Hui et al., 2009). One study found that three different components of the deqi sensation (numbness, distention, and soreness) seemed to correlate with activation of three different groups of nerve fibers (Wang et al., 1985). It has been proposed that activation of the sensorimotor cortex and deactivation of limbic structures involve separate afferent tracts (Willis et al., 1997). A different study showed two sets of afferent pathways were involved in the detection of sensory stimuli that ascend by separate pathways; one detects pain and temperature and activates the somatosensory cortices while the other directly activates the insula (Olausson et al., 2002). That study postulated that the latter pathway may be the one involved in the so-called “limbic touch” phenomenon that underlies the emotional response to physical contact in mammals. Although different in meridian origin and segmental innervations, all of the acupoints used were located in the muscle layers, like most acupoints in traditional Chinese acupuncture—these findings may also explain the commonalities observed in our acupuncture studies. Depending on the nature of the stimulus and the histological nature of the acupoint, the proportion of impulses that ascend by different pathways will vary; this in turn may lead to differences in the predominant impulses that reach different brain regions to produce preferential effects and relative specificity.
One potential advantage of the observed relative specificity of brain responses to these acupoints (LI4, ST36 and LV3) is the possibility of using one acupoint to control for another on a different meridian, an important topic that will require future research for confirmation. As noted in the introduction, with this control paradigm, subjects would be unable to relate the acupuncture sensations they experience to the needling of different acupoints.
The technique used by each acupuncturist can vary. This study used manual acupuncture with gentle needle rotation that induced deqi but no noxious pain. Differences such as electroacupuncture, duration of treatment, and number of regions of interest can contribute to both response similarities (Qin et al., 2011) and discrepancies in reports of acupuncture neuroimaging; this is particularly true when examining the effects on the limbic system (Li et al., 2006; Kong et al., 2009). The use of acupoints with other tissue characteristics and other stimulation techniques require further study.
CONCLUSION
Our fMRI results have shown that manual acupuncture at the three classical acupoints of LI4, ST36 and LV3 produced extensive deactivation of the LPNN as well as moderate activation of its anti-correlated activation network. The use of conjunction analysis demonstrates common regions of response for these 3 acupoints, with some overlap in the right subgenual BA25, right subgenual cingulate, right isthmus of the cingulum bundle, and right BA31. However, we also observed differences in the spatial distribution and degree of deactivation in the medial prefrontal cortex, medial parietal cortex and medial temporal lobe in response to these acupoints, which suggests the acupoints elicit brain responses with relative specificity. Whereas the response to LI4 was predominant in the pregenual cingulate and hippocampal formation, ST36 response was predominant in the subgenual cingulate, and LV3 in the posterior hippocampus and posterior cingulate. Although our findings on the commonality and specificity of acupuncture action in the brain have relevance for clinical acupuncture treatment, and suggest it may be possible to use different acupoints as controls in future studies, we concede that the scientific evidence for such fundamental issues is limited and warrants further investigation.
Acknowledgments
The work was supported in part by the National Institutes of Health/National Center for Complementary and Alternative Medicine (R21AT00978, P01AT002048) and the National Center for Research Resources (P41RR14075), and the National Institute of Neurological Disorders and Stroke (R01NS34189).
References
- Anand A, Li Y, Wang Y, Gardner K, Lowe MJ. Reciprocal effects of antidepressant treatment on activity and connectivity of the mood regulating circuit: an FMRI study. J Neuropsych Clin N. 2007;19(3):274–282. doi: 10.1176/appi.neuropsych.19.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9(4):463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Bai L, Qin W, Tian J, Dong M, Pan X, Chen P, Dai J, Yang W, Liu Y. Acupuncture modulates spontaneous activities in the anticorrelated resting brain networks. Brain Res. 2009;1279:37–49. doi: 10.1016/j.brainres.2009.04.056. [DOI] [PubMed] [Google Scholar]
- Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002;125(Pt 2):310–319. doi: 10.1093/brain/awf022. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Grodd W, Diedrich O, Klose U, Erb M, Lotze M, Schneider F, Weiss U, Flor H. fMRI reveals amygdala activation to human faces in social phobics. Neuroreport. 1998;9(6):1223–1226. doi: 10.1097/00001756-199804200-00048. [DOI] [PubMed] [Google Scholar]
- Bremner JD. Brain imaging in anxiety disorders. Expert Rev Neurother. 2004;4(2):275–284. doi: 10.1586/14737175.4.2.275. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Meyer J, Makris N, Kennedy DN. MRI-based topographic parcellation of human neocortex: An anatomically specified method with estimate of reliability. J Cogn Neurosci. 1996;8(6):566–587. doi: 10.1162/jocn.1996.8.6.566. [DOI] [PubMed] [Google Scholar]
- Cheng XN. Chinese Acupuncture and Moxibustion. Beijing: People’s Medical Publishing House; 2000. [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Davis KD, Taylor SJ, Crawley AP, Wood ML, Mikulis DJ. Functional MRI of pain-and attention-related activations in the human cingulate cortex. J Neurophysiol. 1997;77(6):3370–3380. doi: 10.1152/jn.1997.77.6.3370. [DOI] [PubMed] [Google Scholar]
- Dhond RP, Yeh C, Park K, Kettner N, Napadow V. Acupuncture modulates resting state connectivity in default and sensorimotor brain networks. Pain. 2008;136(3):407–418. doi: 10.1016/j.pain.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Orbitofrontal cortex function and structure in depression. Ann N Y Acad Sci. 2007;1121:499–527. doi: 10.1196/annals.1401.029. [DOI] [PubMed] [Google Scholar]
- Fang J, Jin Z, Wang Y, Li K, Kong J, Nixon EE, Zeng Y, Ren Y, Tong H, Wang Y, Wang P, Hui K. The salient characteristics of the central effects of acupuncture needling: Limbic-paralimbic-neocortical network modulation. Hum Brain Mapp. 2009;30(4):1196–1206. doi: 10.1002/hbm.20583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang SH, Zhang SC, Liu HB. Study on brain responses to acupuncture by functional nuclear magnetic resonance -- observations on 10 healthy subjects. Zhongguo Zong Xi Yi Ze He Za Zhi. 2006;26(14):965–968. [PubMed] [Google Scholar]
- Filipek PA, Richelme C, Kennedy DN, Caviness VS., Jr The young adult human brain: an MRI-based morphometric analysis. Cereb Cortex. 1994;4(4):344–360. doi: 10.1093/cercor/4.4.344. [DOI] [PubMed] [Google Scholar]
- Fu CH, Williams SC, Cleare AJ, Brammer MJ, Walsh ND, Kim J, Andrew CM, Pich EM, Williams PM, Reed LJ, Mitterschiffthaler MT, Suckling J, Bullmore ET. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiat. 2004;61(9):877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- George MS, Ketter TA, Parekh PI, Horwitz B, Herscovitch P, Post RM. Brain activity during transient sadness and happiness in healthy women. Am J Psychiat. 1995;152(3):341–351. doi: 10.1176/ajp.152.3.341. [DOI] [PubMed] [Google Scholar]
- Gold S, Christian B, Arndt S, Zeien G, Cizadlo T, Johnson DL, Flaum M, Andreasen NC. Functional MRI statistical software packages: a comparative analysis. Hum Brain Mapp. 1998;6(2):73–84. doi: 10.1002/(SICI)1097-0193(1998)6:2<73::AID-HBM1>3.0.CO;2-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haake M, Muller HH, Schade-Brittinger C, Basler HD, Schafer H, Maier C, Endres HG, Trampisch HJ, Molsberger A. German Acupuncture Trials (GERAC) for chronic low back pain: randomized, multicenter, blinded, parallel-group trial with 3 groups. Arch Intern Med. 2007;167(17):1892–1898. doi: 10.1001/archinte.167.17.1892. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Dietrich TM, Wenning B, Krings T, Erberich SG, Willmes K, Thron A, Sass H. Evidence of abnormal amygdala functioning in borderline personality disorder: a functional MRI study. Biol Psychiat. 2001;50(4):292–298. doi: 10.1016/s0006-3223(01)01075-7. [DOI] [PubMed] [Google Scholar]
- Ho TJ, Duann JR, Chen CM, Chen JH, Shen WC, Lu TW, Liao JR, Lin JG. Carryover effects alter FMRI statistical analysis in an acupuncture study. Am J Chin Med. 2008;36(1):55–70. doi: 10.1142/S0192415X08005588. [DOI] [PubMed] [Google Scholar]
- Hui KK, Liu J, Makris N, Gollub RL, Chen AJ, Moore CI, Kennedy DN, Rosen BR, Kwong KK. Acupuncture modulates the limbic system and subcortical gray structures of the human brain: evidence from fMRI studies in normal subjects. Hum Brain Mapp. 2000;9(1):13–25. doi: 10.1002/(SICI)1097-0193(2000)9:1<13::AID-HBM2>3.0.CO;2-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui KK, Liu J, Marina O, Napadow V, Haselgrove C, Kwong KK, Kennedy DN, Makris N. The integrated response of the human cerebro-cerebellar and limbic systems to acupuncture stimulation at ST 36 as evidenced by fMRI. Neuroimage. 2005;27(3):479–496. doi: 10.1016/j.neuroimage.2005.04.037. [DOI] [PubMed] [Google Scholar]
- Hui KK, Marina O, Claunch JD, Nixon EE, Fang J, Liu J, Li M, Napadow V, Vangel M, Makris N, Chan ST, Kwong KK, Rosen BR. Acupuncture mobilizes the brain’s default mode and its anti-correlated network in healthy subjects. Brain Res. 2009;1287:84–103. doi: 10.1016/j.brainres.2009.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui KK, Marina O, Liu J, Rosen BR, Kwong KK. Acupuncture, the limbic system, and the anticorrelated networks of the brain. Auton Neurosci. 2010;157(1–2):81–90. doi: 10.1016/j.autneu.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui KK, Nixon EE, Vangel MG, Liu J, Marina O, Napadow V, Hodge SM, Rosen BR, Makris N, Kennedy DN. Characterization of the “deqi” response in acupuncture. BMC Complem Altern M. 2007;7:33. doi: 10.1186/1472-6882-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptchuk TJ, Kelley JM, Conboy LA, Davis RB, Kerr CE, Jacobson EE, Kirsch I, Schyner RN, Nam BH, Nguyen LT, Park M, Rivers AL, McManus C, Kokkotou E, Drossman DA, Goldman P, Lembo AJ. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ. 2008;336(7651):999–1003. doi: 10.1136/bmj.39524.439618.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Gollub R, Huang T, Polich G, Napadow V, Hui K, Vangel M, Rosen B, Kaptchuk TJ. Acupuncture de qi, from qualitative history to quantitative measurement. J Altern Complem Med. 2007;13(10):1059–1070. doi: 10.1089/acm.2007.0524. [DOI] [PubMed] [Google Scholar]
- Kong J, Kaptchuk TJ, Webb JM, Kong JT, Sasaki Y, Polich GR, Vangel MG, Kwong K, Rosen B, Gollub RL. Functional neuroanatomical investigation of vision-related acupuncture point specificity--a multisession fMRI study. Hum Brain Mapp. 2009;30(1):38–46. doi: 10.1002/hbm.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20(5):937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Langevin HM, Wayne PM, Macpherson H, Schnyer R, Milley RM, Napadow V, Lao L, Park J, Harris RE, Cohen M, Sherman KJ, Haramati A, Hammerschlag R. Paradoxes in acupuncture research: strategies for moving forward. Evid-Based Compl Alt. 2011;2011:180805. doi: 10.1155/2011/180805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Shan B, Xu J, Liu H, Wang W, Zhi L, Yan B, Tang X. Changes in FMRI in the human brain related to different durations of manual acupuncture needling. J Altern Complem Med. 2006;12(7):615–623. doi: 10.1089/acm.2006.12.615. [DOI] [PubMed] [Google Scholar]
- Liu P, Zhang Y, Zhou G, Yuan K, Qin W, Zhuo L, Liang J, Chen P, Dai J, Liu Y, Tian J. Partial correlation investigation on the default mode network involved in acupuncture: an fMRI study. Neurosci Lett. 2009;462(3):183–187. doi: 10.1016/j.neulet.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiat. 2008;64(6):461–467. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Buonocore MH, Kile SJ, Garrett AS. Brain regions showing increased activation by threat-related words in panic disorder. Neuroreport. 2003;14(3):325–328. doi: 10.1097/00001756-200303030-00006. [DOI] [PubMed] [Google Scholar]
- Mai JK, Assheur J, Paxinos G. Atlas of the Human Brain. London: Elsevier Academic Press; 2004. [Google Scholar]
- Massana G, Serra-Grabulosa JM, Salgado-Pineda P, Gasto C, Junque C, Massana J, Mercader JM. Parahippocampal gray matter density in panic disorder: a voxel-based morphometric study. Am J Psychiat. 2003;160(3):566–568. doi: 10.1176/appi.ajp.160.3.566. [DOI] [PubMed] [Google Scholar]
- Molsberger AF, Schneider T, Gotthardt H, Drabik A. German Randomized Acupuncture Trial for chronic shoulder pain (GRASP) - a pragmatic, controlled, patient-blinded, multi-centre trial in an outpatient care environment. Pain. 2010;151(1):146–154. doi: 10.1016/j.pain.2010.06.036. [DOI] [PubMed] [Google Scholar]
- Napadow V, Kettner N, Liu J, Li M, Kwong KK, Vangel M, Makris N, Audette J, Hui KK. Hypothalamus and amygdala response to acupuncture stimuli in Carpal Tunnel Syndrome. Pain. 2007;130(3):254–266. doi: 10.1016/j.pain.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V, Liu J, Kaptchuk TJ. A systematic study of acupuncture practice: acupoint usage in an outpatient setting in Beijing, China. Complement Ther Med. 2004;12(4):209–216. doi: 10.1016/j.ctim.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Napadow V, Makris N, Liu J, Kettner NW, Kwong KK, Hui KK. Effects of electroacupuncture versus manual acupuncture on the human brain as measured by fMRI. Hum Brain Mapp. 2005;24(3):193–205. doi: 10.1002/hbm.20081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olausson H, Lamarre Y, Backlund H, Morin C, Wallin BG, Starck G, Ekholm S, Strigo I, Worsley K, Vallbo AB, Bushnell MC. Unmyelinated tactile afferents signal touch and project to insular cortex. Nat Neurosci. 2002;5(9):900–904. doi: 10.1038/nn896. [DOI] [PubMed] [Google Scholar]
- Parvizi J, Van Hoesen GW, Buckwalter J, Damasio A. Neural connections of the posteromedial cortex in the macaque. Proc Natl Acad Sci U S A. 2006;103(5):1563–1568. doi: 10.1073/pnas.0507729103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, Bai L, Dai J, Liu P, Dong M, Liu J, Sun J, Yuan K, Chen P, Zhao B, Gong Q, Tian J, Liu Y. The temporal-spatial encoding of acupuncture effects in the brain. Mol Pain. 2011;7(1):19. doi: 10.1186/1744-8069-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiat. 2000;47(9):769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Rong P, Zhu B. Mechanism of relation among heart meridian, referred cardiac pain and heart. Science in China Series C, Life sciences/Chinese Academy of Sciences. 2002;45(5):538–545. doi: 10.1360/02yc9059. [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Fukuda R, Okuaki T, Rogers M, Kasai K, Machida T, Shirouzu I, Yamasue H, Akiyama T, Kato N. Parahippocampal activation evoked by masked traumatic images in posttraumatic stress disorder: a functional MRI study. Neuroimage. 2005;26(3):813–821. doi: 10.1016/j.neuroimage.2005.02.032. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Valera EM, Makris N, Monuteaux MC, Boriel DL, Kelkar K, Kennedy DN, Caviness VS, Bush G, Aleardi M, Faraone SV, Biederman J. Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biol Psychiat. 2006;60(10):1071–1080. doi: 10.1016/j.biopsych.2006.04.031. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can’t shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiat. 2002;51(9):693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303(5661):1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical; 1988. [Google Scholar]
- Ulett GA. The myth of meridian therapy. Biol Psychiat. 1992;31(7):750–751. doi: 10.1016/0006-3223(92)90289-c. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6(7):533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Berger GR, Derbyshire SW. Structural and functional dichotomy of human midcingulate cortex. Eur J Neurosci. 2003;18(11):3134–3144. doi: 10.1111/j.1460-9568.2003.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Derbyshire S, Jones AK. Pain processing in four regions of human cingulate cortex localized with co-registered PET and MR imaging. Eur J Neurosci. 1996;8(7):1461–1473. doi: 10.1111/j.1460-9568.1996.tb01608.x. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Vogt L, Laureys S. Cytology and functionally correlated circuits of human posterior cingulate areas. Neuroimage. 2006;29(2):452–466. doi: 10.1016/j.neuroimage.2005.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Wald LL. Simultaneous inference for fMRI data. 1997. [Google Scholar]
- Wang KM, Yao SM, Xian YL, Hou ZL. A study on the receptive field of acupoints and the relationship between characteristics of needling sensation and groups of afferent fibres. Sci Sin B. 1985;28(9):963–971. [PubMed] [Google Scholar]
- Wang W, Liu L, Zhi X, Huang JB, Liu DX, Wang H, Kong XQ, Xu HB. Study on the regulatory effect of electro-acupuncture on hegu point (LI4) in cerebral response with functional magnetic resonance imaging. Chin J Integr Med. 2007;13(1):10–16. doi: 10.1007/s11655-007-0010-3. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1(1):70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- White P, Bishop F, Hardy H, Abdollahian S, White A, Park J, Kaptchuk TJ, Lewith GT. Southampton needle sensation questionnaire: development and validation of a measure to gauge acupuncture needle sensation. J Altern Complem Med. 2008;14(4):373–379. doi: 10.1089/acm.2007.0714. [DOI] [PubMed] [Google Scholar]
- Willis WD, Westlund KN. Neuroanatomy of the pain system and of the pathways that modulate pain. J Clin Neurophysiol. 1997;14(1):2–31. doi: 10.1097/00004691-199701000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MT, Hsieh JC, Xiong J, Yang CF, Pan HB, Chen YC, Tsai G, Rosen BR, Kwong KK. Central nervous pathway for acupuncture stimulation: localization of processing with functional MR imaging of the brain--preliminary experience. Radiology. 1999;212(1):133–141. doi: 10.1148/radiology.212.1.r99jl04133. [DOI] [PubMed] [Google Scholar]
- Wu MT, Sheen JM, Chuang KH, Yang P, Chin SL, Tsai CY, Chen CJ, Liao JR, Lai PH, Chu KA, Pan HB, Yang CF. Neuronal specificity of acupuncture response: a fMRI study with electroacupuncture. Neuroimage. 2002;16(4):1028–1037. doi: 10.1006/nimg.2002.1145. [DOI] [PubMed] [Google Scholar]
- Yan B, Li K, Xu J, Wang W, Liu H, Shan B, Tang X. Acupoint-specific fMRI patterns in human brain. Neurosci Lett. 2005;383(3):236–240. doi: 10.1016/j.neulet.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Yang P, Wu MT, Hsu CC, Ker JH. Evidence of early neurobiological alternations in adolescents with posttraumatic stress disorder: a functional MRI study. Neurosci Lett. 2004;370(1):13–18. doi: 10.1016/j.neulet.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Ueda K, Suzuki S, Onoda K, Okamoto Y, Yamawaki S. Self-referential processing of negative stimuli within the ventral anterior cingulate gyrus and right amygdala. Brain Cogn. 2009;69(1):218–225. doi: 10.1016/j.bandc.2008.07.010. [DOI] [PubMed] [Google Scholar]


