Abstract
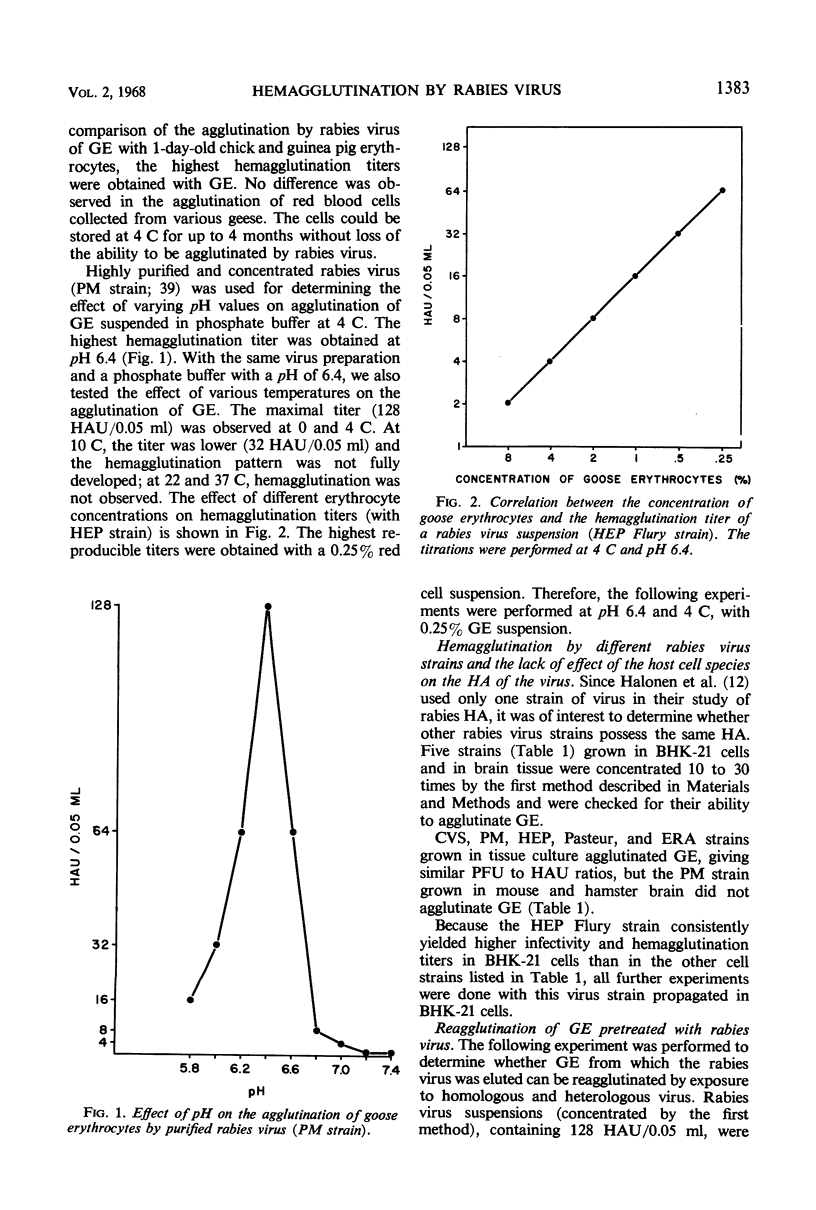
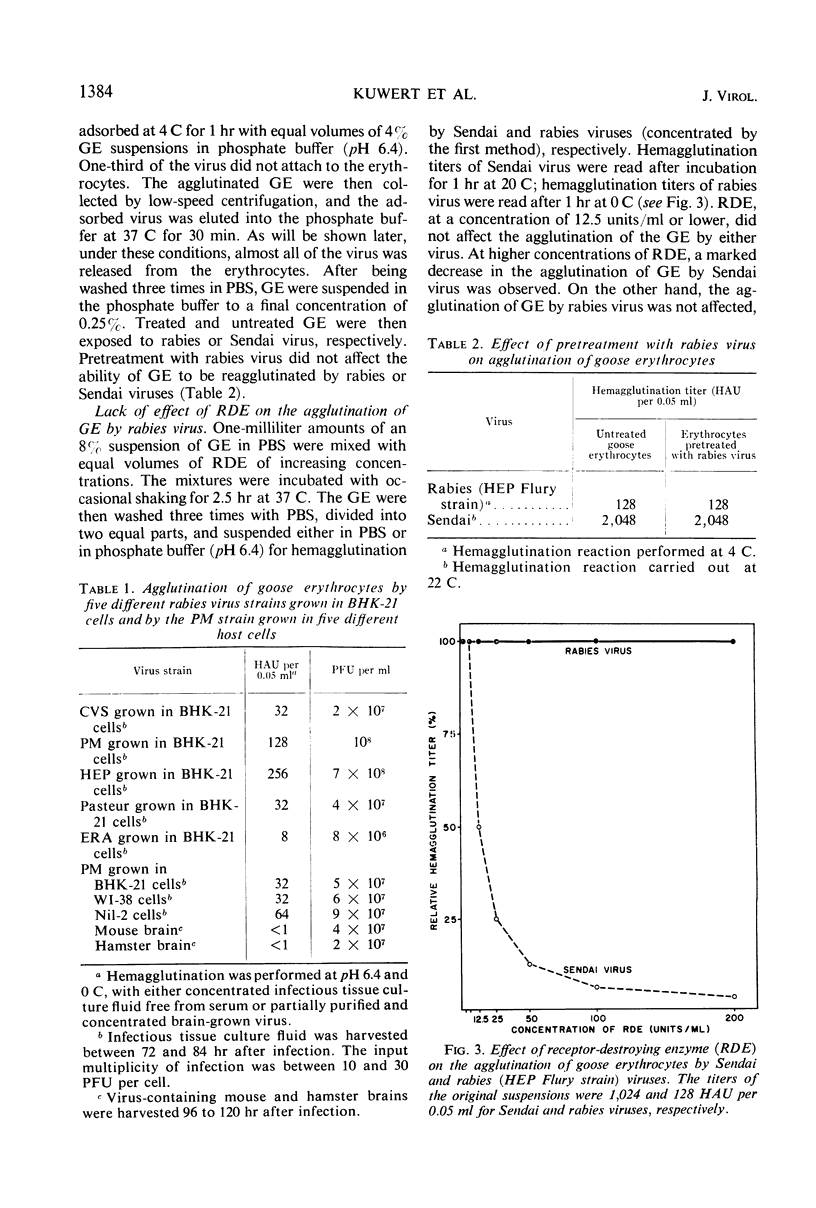
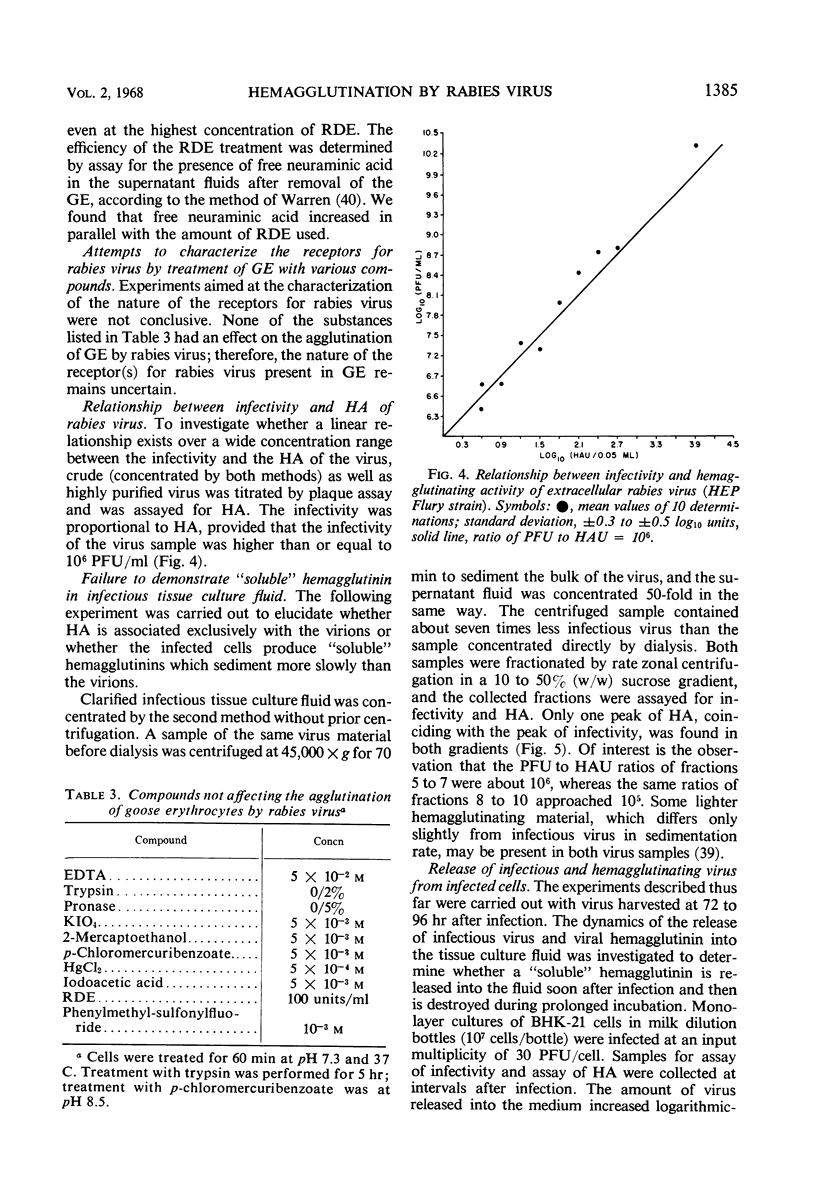
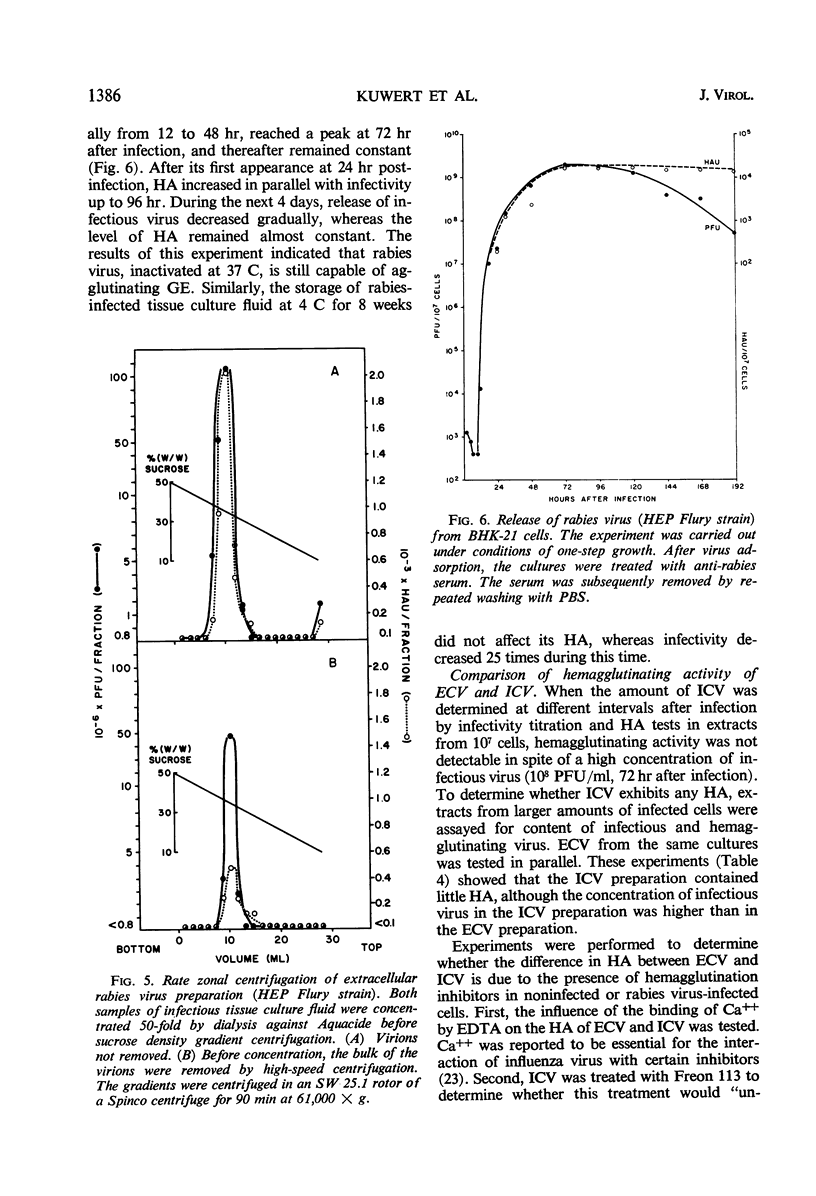
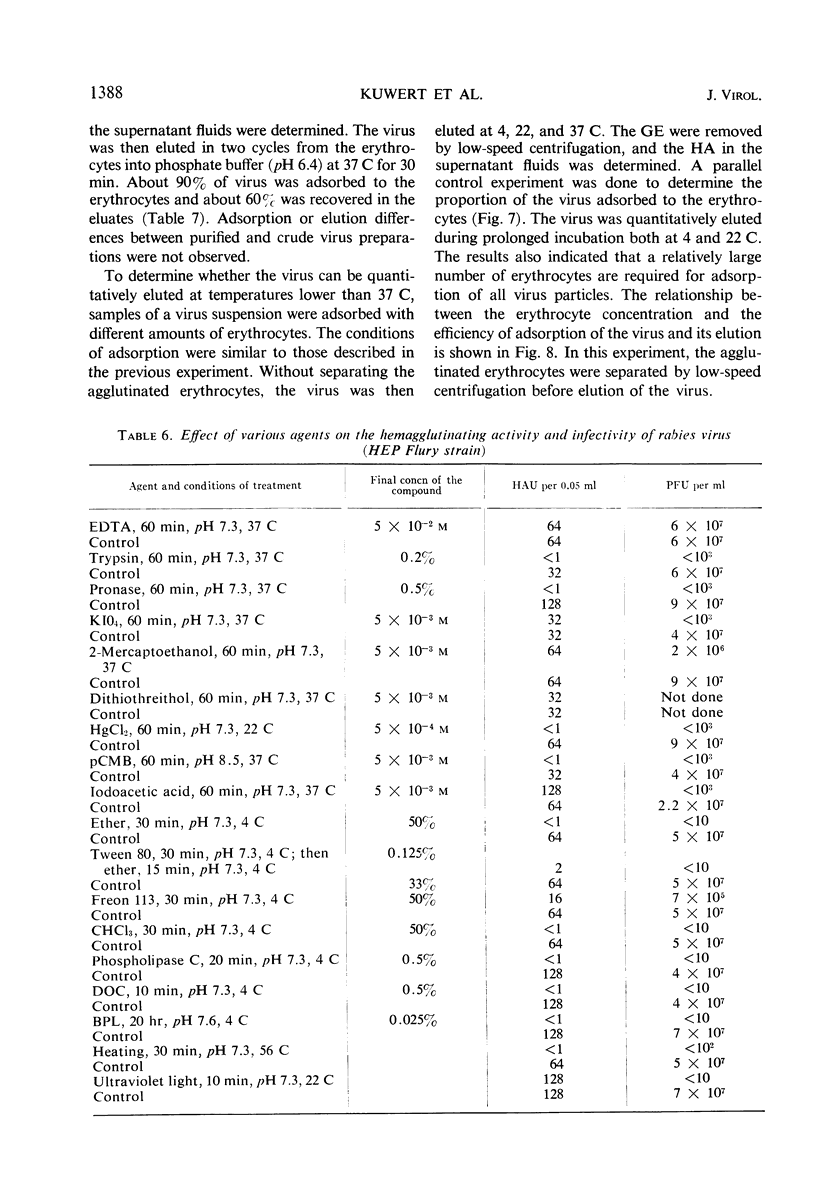
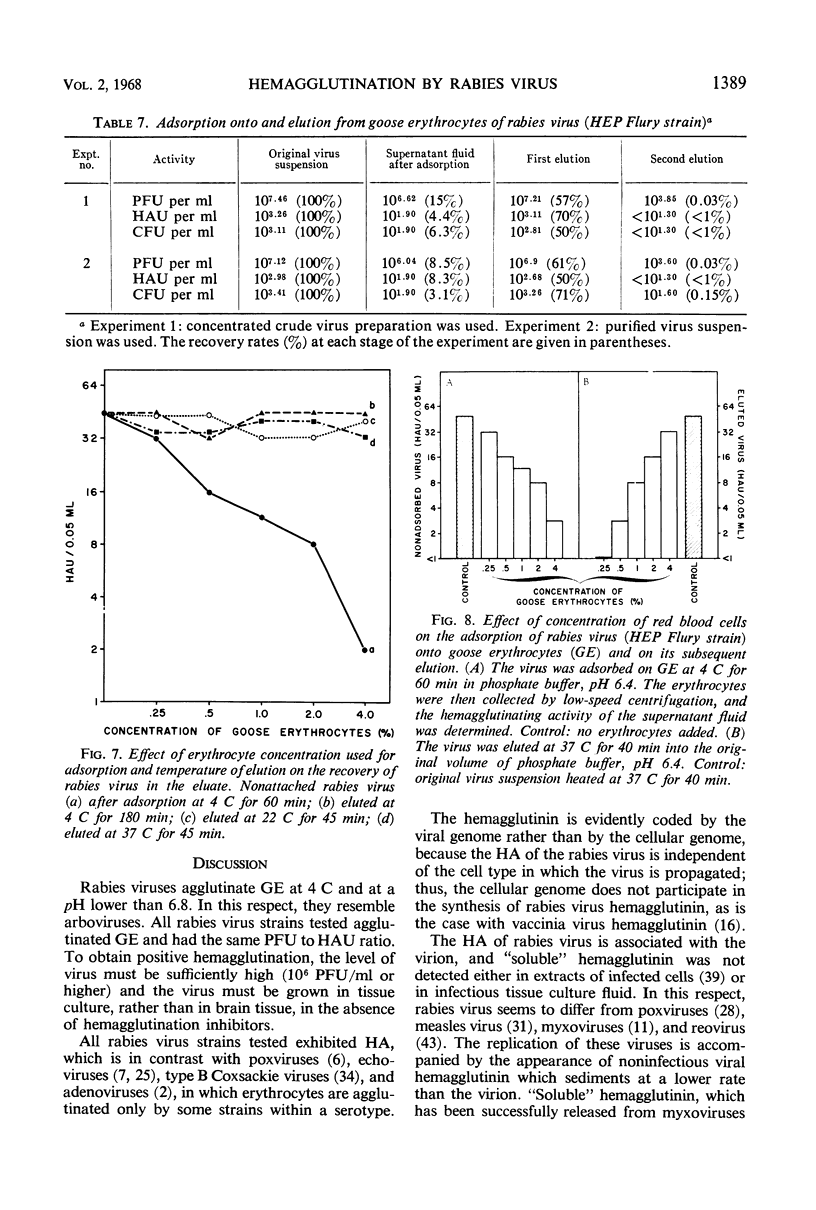
Goose erythrocytes were agglutinated by five strains of rabies virus grown in monolayer cell cultures at pH 6.4 and at 0 to 4 C. Hemagglutination was not affected by the cell type in which the virus was grown. Prerequisites for occurrence of hemagglutination are absence of hemagglutination inhibitors (such as those contained in bovine serum) and a relatively high virus concentration (> 106 plaque-forming units of virus per ml). “Soluble” hemagglutinin was not present in crude preparations of extracellular virus. Treatment of purified preparations of extracellular virus with Tween 80 and ether did not result in release of a “soluble” hemagglutinin. The hemagglutinating property of extracellular virus seemed to be conditioned by the integrity of its coat. Preparations of infectious intracellular virus exhibited about 15 times lower hemagglutinating activity than extracellular virus. This decreased hemagglutinating activity did not seem to be caused by binding of hemagglutination inhibitors to the virus particles. Rabies virus can be quantitatively adsorbed onto and eluted from erythrocytes. Erythrocytes pretreated with rabies virus retained their ability to be agglutinated by the same virus strain. The reaction with rabies virus of erythrocytes treated with the receptor-destroying enzyme or KIO4 was the same as that of nontreated erythrocytes. The hemagglutinating component of rabies virus, therefore, does not exhibit neuraminidase activity. Treatment of extracellular virus by various agents indicated that the hemagglutinating component consists of protein or lipoprotein. Sulfhydryl groups present in the viral hemagglutinin are essential for hemagglutination.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelseth M. K. An Attenuated Rabies Vaccine for Domestic Animals Produced in Tissue Culture. Can Vet J. 1964 Nov;5(11):279–286. [PMC free article] [PubMed] [Google Scholar]
- Bauer W., Wigand R. Vergleichende Untersuchungen an Hämagglutininen von Adenovirusstämmen der Gruppen I und II. Arch Gesamte Virusforsch. 1967;21(1):11–24. [PubMed] [Google Scholar]
- Cherdakov V. V., Voroshilova M. K. On the nature of the haemagglutinating factor in some strains of ECHO 7 and 12 viruses. Acta Virol. 1967 Jul;11(4):297–304. [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond L. Two spontaneously transformed cell lines derived from the same hamster embryo culture. Int J Cancer. 1967 Mar 15;2(2):143–152. doi: 10.1002/ijc.2910020209. [DOI] [PubMed] [Google Scholar]
- FENNER F. The biological characters of several strains of vaccinia, cowpox and rabbitpox viruses. Virology. 1958 Jun;5(3):502–529. doi: 10.1016/0042-6822(58)90042-4. [DOI] [PubMed] [Google Scholar]
- FEORINO P. M., HUMPHREY D. D., GELFAND H. M. Routine HA and HAI tests for identifying enterovirus and reovirus strains. Public Health Rep. 1963 Apr;78:349–354. [PMC free article] [PubMed] [Google Scholar]
- Furukawa T., Vaheri A., Plotkin S. A. Growth of rubella virus in BHK21 cells. 3. Production of complement-fixing antigens. Proc Soc Exp Biol Med. 1967 Aug-Sep;125(4):1098–1102. doi: 10.3181/00379727-125-32285. [DOI] [PubMed] [Google Scholar]
- GIRARDI A. J. The use of fluorocarbon for "unmasking" polyoma virus hemagglutinin. Virology. 1959 Nov;9:488–489. doi: 10.1016/0042-6822(59)90141-2. [DOI] [PubMed] [Google Scholar]
- GOTTSCHALK A. Neuraminidase: the specific enzyme of influenza virus and Vibrio cholerae. Biochim Biophys Acta. 1957 Mar;23(3):645–646. doi: 10.1016/0006-3002(57)90389-x. [DOI] [PubMed] [Google Scholar]
- GRANOFF A. Noninfectious forms of Newcastle disease and influenza viruses; studies on noninfectious virus occurring within cells that are producing fully infectious virus. Virology. 1955 Dec;1(5):516–532. doi: 10.1016/0042-6822(55)90040-4. [DOI] [PubMed] [Google Scholar]
- HALPEREN S., EGGERS H. J., TAMM I. COMPLETE AND CORELESS HEMAGGLUTINATING PARTICLES PRODUCED IN ECHO 12 VIRUS-INFECTED CELLS. Virology. 1964 May;23:81–89. doi: 10.1016/s0042-6822(64)80010-6. [DOI] [PubMed] [Google Scholar]
- HOYLE L. Structure of the influenza virus; the relation between biological activity and chemical structure of virus fractions. J Hyg (Lond) 1952 Jun;50(2):229–245. doi: 10.1017/s0022172400019562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halonen P. E., Murphy F. A., Fields B. N., Reese D. R. Hemagglutinin of rabies and some other bullet-shaped viruses. Proc Soc Exp Biol Med. 1968 Apr;127(4):1037–1042. doi: 10.3181/00379727-127-32864. [DOI] [PubMed] [Google Scholar]
- Halonen P. E., Ryan J. M., Stewart J. A. Rubella hemagglutinin prepared with alkaline extraction of virus grown in suspension culture of BHK-21 cells. Proc Soc Exp Biol Med. 1967 May;125(1):162–167. doi: 10.3181/00379727-125-32038. [DOI] [PubMed] [Google Scholar]
- Hummeler K., Koprowski H., Wiktor T. J. Structure and development of rabies virus in tissue culture. J Virol. 1967 Feb;1(1):152–170. doi: 10.1128/jvi.1.1.152-170.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummeler K., Tomassini N., Sokol F., Kuwert E., Koprowski H. Morphology of the nucleoprotein component of rabies virus. J Virol. 1968 Oct;2(10):1191–1199. doi: 10.1128/jvi.2.10.1191-1199.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John T., Askin P., Fulginiti V. A. The tween-ether treated hemagglutinin of parainfluenza 2 virus. Arch Gesamte Virusforsch. 1967;22(3):381–387. doi: 10.1007/BF01242958. [DOI] [PubMed] [Google Scholar]
- KISSLING R. E., REESE D. R. ANTI-RABIES VACCINE OF TISSUE CULTURE ORIGIN. J Immunol. 1963 Sep;91:362–368. [PubMed] [Google Scholar]
- Kaplan M. M., Wiktor T. J., Maes R. F., Campbell J. B., Koprowski H. Effect of polyions on the infectivity of rabies virus in tissue culture: construction of a single-cycle growth curve. J Virol. 1967 Feb;1(1):145–151. doi: 10.1128/jvi.1.1.145-151.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizanová O., Sokol F. Purification of bovine serum beta-inhibitor by chromatographic methods and its reaction with A1 influenza virus. Acta Virol. 1966 Jan;10(1):35–42. [PubMed] [Google Scholar]
- LAHELLE O. Capacity of certain Echo virus 6 strains to cause hemagglutination. Virology. 1958 Feb;5(1):110–119. doi: 10.1016/0042-6822(58)90008-4. [DOI] [PubMed] [Google Scholar]
- LERNER A. M., CHERRY J. D., FINLAND M. Hemagglutination with reoviruses. Virology. 1963 Jan;19:58–65. doi: 10.1016/0042-6822(63)90024-2. [DOI] [PubMed] [Google Scholar]
- MACPHERSON I., STOKER M. Polyoma transformation of hamster cell clones--an investigation of genetic factors affecting cell competence. Virology. 1962 Feb;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- McCREA J. F., O'LOUGHLIN J. Separation of vaccinia hemagglutinin from infectious virus particles by chromatography on DEAE columns. Virology. 1959 May;8(1):127–129. doi: 10.1016/0042-6822(59)90026-1. [DOI] [PubMed] [Google Scholar]
- NORRBY E. Hemagglutination by measles virus. 4. A simple procedure for production of high potency antigen for hemagglutination-inhibition (HI) tests. Proc Soc Exp Biol Med. 1962 Dec;111:814–818. doi: 10.3181/00379727-111-27930. [DOI] [PubMed] [Google Scholar]
- NORRBY E. Hemagglutination by measles virus. III. Identification of two different hemagglutinins. Virology. 1963 Feb;19:147–157. doi: 10.1016/0042-6822(63)90004-7. [DOI] [PubMed] [Google Scholar]
- ROSEN L., KERN J. Hemagglutination and hemagglutination-inhibition with Coxsackie B viruses. Proc Soc Exp Biol Med. 1961 Jul;107:626–628. doi: 10.3181/00379727-107-26708. [DOI] [PubMed] [Google Scholar]
- SCHMIDT N. J., DENNIS J., HOFFMAN M. N., LENNETTE E. H. INHIBITORS OF ECHOVIRUS AND REOVIRUS HEMAGGLUTINATION. I. INHIBITORS IN TISSUE CULTURE FLUIDS. J Immunol. 1964 Sep;93:367–376. [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- Sedwick W. D., Wiktor T. J. Reproducible plaquing system for rabies, lymphocytic choriomeningitis,k and other ribonucleic acid viruses in BHK-21-13S agarose suspensions. J Virol. 1967 Dec;1(6):1224–1226. doi: 10.1128/jvi.1.6.1224-1226.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol F., Kuwert E., Wiktor T. J., Hummeler K., Koprowski H. Purification of rabies virus grown in tissue culture. J Virol. 1968 Aug;2(8):836–849. doi: 10.1128/jvi.2.8.836-849.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- WIKTOR T. J., FERNANDES M. V., KOPROWSKI H. CULTIVATION OF RABIES VIRUS IN HUMAN DIPLOID CELL STRAIN WI-38. J Immunol. 1964 Sep;93:353–366. [PubMed] [Google Scholar]
- Zalan E., Labzoffsky N. A. The hemagglutinin of reovirus type 3. Arch Gesamte Virusforsch. 1967;21(1):25–30. doi: 10.1007/BF01258473. [DOI] [PubMed] [Google Scholar]



