Abstract
The study of the mechanism of acupuncture action was revolutionized by the use of functional magnetic resonance imaging (fMRI). Over the past decade, our fMRI studies of healthy subjects have contributed substantially to elucidating the central effect of acupuncture on the human brain. These studies have shown that acupuncture stimulation, when associated with sensations comprising deqi, evokes deactivation of a limbic-paralimbic-neocortical network, which encompasses the limbic system, as well as activation of somatosensory brain regions. These networks closely match the default mode network and the anti-correlated task-positive network described in the literature. We have also shown that the effect of acupuncture on the brain is integrated at multiple levels, down to the brainstem and cerebellum. Our studies support the hypothesis that the effect of acupuncture on the brain goes beyond the effect of attention on the default mode network or the somatosensory stimulation of acupuncture needling. The amygdala and hypothalamus, in particular, show decreased activation during acupuncture stimulation that is not commonly associated with default mode network activity. At the same time, our research shows that acupuncture stimulation needs to be done carefully, limiting stimulation when the resulting sensations are very strong or when sharp pain is elicited. When acupuncture induced sharp pain, our studies show that the deactivation was attenuated or reversed in direction. Our results suggest that acupuncture mobilizes the functionally anti-correlated networks of the brain to mediate its actions, and that the effect is dependent on the psychophysical response. In this work we also discuss multiple avenues of future research, including the role of neurotransmitters, the effect of different acupuncture techniques, and the potential clinical application of our research findings to disease states including chronic pain, major depression, schizophrenia, autism, and Alzheimer's disease.
Keywords: acupuncture, fMRI, limbic-paralimbic-neocortical network, default mode network, deqi, amygdala
1. Introduction
The limbic system is a group of limbic, paralimbic and neocortical brain regions that together play a concerted role in the regulation and integration of cognition, affect, sensory perception, biological behavior, and autonomic, immunological and endocrine functions. The advent of functional magnetic resonance imaging (fMRI) enabled in vivo investigation of brain function and definition of additional brain networks. The activity of the resting brain, in particular, when challenged with a task, appears to be organized into two anti-correlated networks that regulate each other to maintain balance (Fransson, 2005; Fox et al., 2005). These networks are termed the task-positive network, which shows activation during a task relative to rest, and the task-negative network, which shows deactivation during a task relative to rest. The regions comprising these networks are task-specific.
The default mode network is an instance of a task-negative network showing extensive deactivation when an attention-demanding task is engaged. It is described in the literature as comprising of clusters of regions in the medial prefrontal cortex, posterior medial parietal cortex and medial temporal lobe that are highly active in the awake and conscious resting state but become deactivated when exposed to external stimuli such as cognition and conceptual tasks (Binder et al., 1999; Buckner et al., 2008; Fransson, 2005; Golland et al., 2008; Gusnard and Raichle, 2001; Shulman et al., 1997). The task-positive network is comprised of the sensorimotor and attention-related cortices that become activated during goal-directed tasks (Corbetta and Shulman, 2002). Although not as extensively engaged as in the task-negative system, a few paralimbic structures such as the anterior middle cingulate, right insula and dorsal division of the posterior cingulate Brodmann area 23 constitute core regions in the anticorrelated system.
Our fMRI studies of the effect of acupuncture on the brain in normal human subjects have led us to define a task-negative network for acupuncture that is centered on the limbic system. We have named this network the limbic-paralimbic-neocortical network (Hui et al., 2005; Fang et al., 2008; Wang et al., 2007). Inspection of the patterns of response of this network during acupuncture stimulation reveals a striking similarity to the default mode network during attention-demanding tasks (Hui et al., 2009). The task-positive network that is anticorrelated to the default mode network in the resting brain shares a few common regions with the activation network in acupuncture, such as the sensorimotor cortex and paralimbic structures.
In this review we summarize our research, discuss the brain networks involved in acupuncture, and describe their relationships. We then discuss future research directions, including the role of neurotransmitters in acupuncture action, the difference between acupuncture methods, and the selection of and differences among acupuncture control stimulation. We then conclude with a summary of the potential clinical application of our research findings given what is currently known about several common disease states that affect the brain.
2. Our research
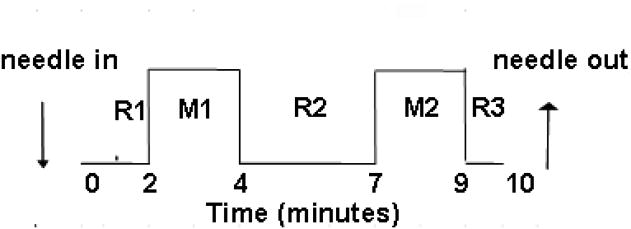
Over the past decade, we have built a database of fMRI scans of the brain response to traditional Chinese acupuncture at multiple acupoints in healthy adults. We have focused on three classical acupoints that are commonly used clinically for their analgesic and regulatory clinical effects, namely LI4 (hegu) on the hand, ST36 (zusanli) on the leg, and LV3 (taichong) on the foot (Hui et al., 2000, 2005, 2009). In our studies we have compared four minutes of acupuncture needling to five minutes of the acupuncture needle at rest, in a standard ten-minute paradigm, excluding the first minute of scanning during the first rest period to allow for baseline equilibration of the fMRI machine, (Fig. 1).
Figure 1.
Time course of acupuncture needling. The acupuncture needle was inserted and the sensitivity of the subject to manipulation was pre-tested and adjusted to tolerance prior to starting functional MRI scanning. The needle remained at rest for 2 min after the start of MRI scanning before bidirectional rotation at 1 Hz for 2 min. The needle was not manipulated for 3 min, then manipulation was repeated for 2 min, followed by a third period of rest for 1min. The needle was removed after MRI scanning was complete. Data analysis compared the blood oxygenation level–dependent (BOLD) MRI signal intensity of the two needling periods with the three rest periods, with the first minute of scanning excluded from analysis to allow for MRI signal equilibration.
We first described the coordinated signal decreases in the limbic system in fMRI of the brain during acupuncture at LI4 in Hui et al. (2000). This partial brain imaging encompassed cortico-limbic and sensorimotor regions of special interest to the study. Our observation of decreased signal represented decreased blood flow during acupuncture needling in brain areas including the nucleus accumbens, amygdala, hippocampus, parahippocampus, hypothalamus, ventral tegmental area, anterior cingulate gyrus, caudate, putamen, temporal pole, and insula. These decreases were found in patients experiencing the constellation of sensations termed deqi, but were absent or markedly attenuated in patients experiencing sharp pain (Fig. 2 and 3). In contrast, signal increases in the somatosensory cortices were present during both acupuncture and sensory control. In this early paper we first showed evidence for the coordinated signal decreases during BOLD fMRI, representing decreased cerebral blood flow due to suppressed neuronal metabolic activity within the limbic and paralimbic systems.
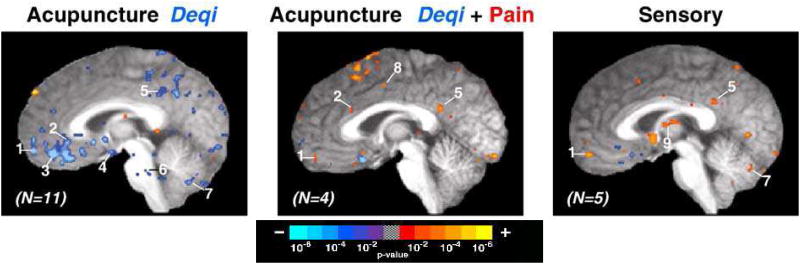
Figure 2.
The influence of sensations on brain fMRI signal changes during acupuncture and sensory control at ST36. Group average functional results showing signal decreases (blue) and increases (red), thresholded at p<0.001 and a 3-voxel cluster size. All slices are 2mm parasagittal in the right hemisphere in Talairach space (Talairach and Tournoux, 1988). (Left) Acupuncture with deqi sensations without sharp pain (n=11) resulted in widespread signal decreases. (Center) Acupuncture with mixed deqi and sharp pain sensations (n=4) resulted primarily in signal increases. (Right) Sensory control (n=5) also resulted in signal increases beyond dedicated sensorimotor areas. Regions: (1) frontal pole, (2) subgenual cingulate, (3) ventromedial prefrontal cortex, (4) hypothalamus, (5) posterior cingulate, (6) reticular formation, (7) cerebellar vermis, (8) middle cingulate, and (9) thalamus.
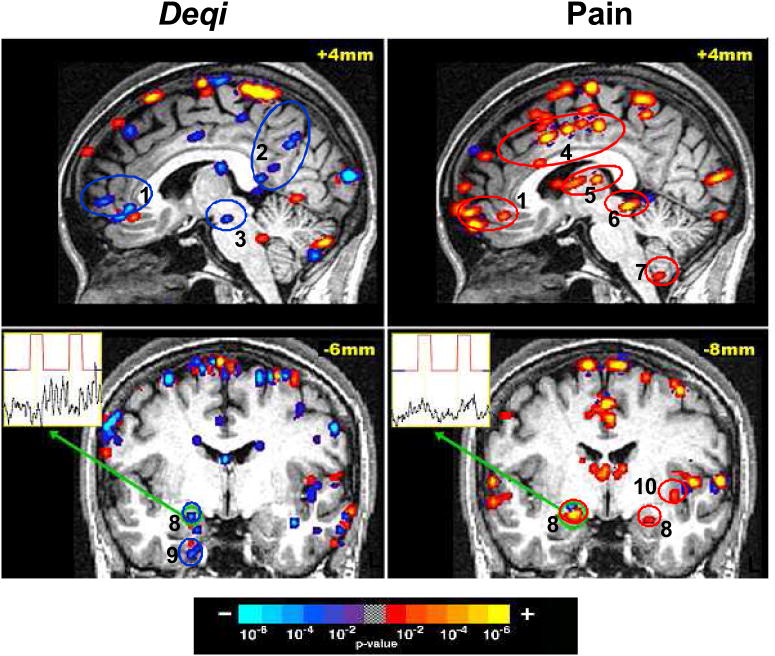
Figure 3.
Brain activity with acupuncture at ST36 in a single subject who reported deqi sensations without sharp pain during one experimental run and only sharp pain without additional sensations during a subsequent experimental run. The broad degree of deactivation (blue) during acupuncture with deqi is in contrast to the general activation (red) noted during acupuncture with sharp pain alone. Time-course for the voxel with peak signal change within the right amygdala is shown for each run. Regions: (1) pregenual cingulate / frontal pole, (2) posterior cingulate Brodmann area 31 / precuneus, (3) substantia nigra, (4) middle cingulate, (5) thalamus, (6) periaqueductal gray, (7) cerebellar tonsil, (8) amygdala, (9) parahippocampus, (10) insula.
In Hui et al. (2005) we extended our fMRI findings to ST36, showing with whole brain imaging that manual acupuncture at this point also led to coordinated decreased signal throughout the limbic system. We also described the central effect of acupuncture on the cerebellum, showing that the cerebro-cerebellar system also demonstrated signal decreases in concert with the limbic system. Again, the pattern of hemodynamic response depended on the psychophysical response to needle manipulation, with deqi sensations leading to signal decrease, while inadvertent sharp pain led to signal increase. Tactile stimulation as control elicited signal increases, predominantly in the somatosensory areas. Based on these findings, we argued that the cerebro-cerebellar and limbic system responds in an integrated manner in correlation with the psychophysical response to acupuncture stimulation at ST36.
In our most recent study (Hui et al., 2009), we described our analysis of 201 acupuncture runs and 74 tactile stimulation control runs in 48 healthy subjects at all three acupuncture points. The large size of this data set provides a foundation for comparison of neuroimaging findings in health and disease and between acupuncture and tactile stimulation controls. We again described clusters of decreased activity in limbic and paralimbic regions including the medial prefrontal, medial parietal and medial temporal lobes, along with increased activity in the sensorimotor cortices and select paralimbic structures. To better analyze and characterize this large fMRI dataset, we used a general linear model and cross-correlation analysis to identify the activation and deactivation networks and their functional connectivity during acupuncture administration (Fig. 4).
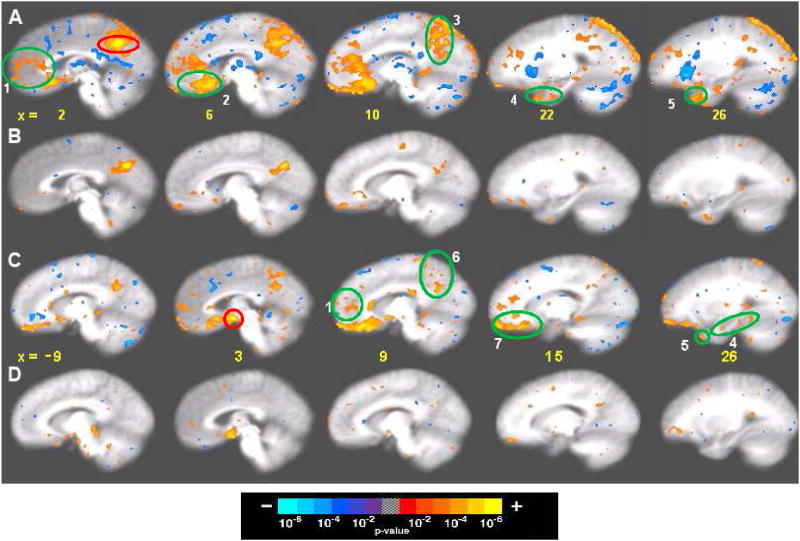
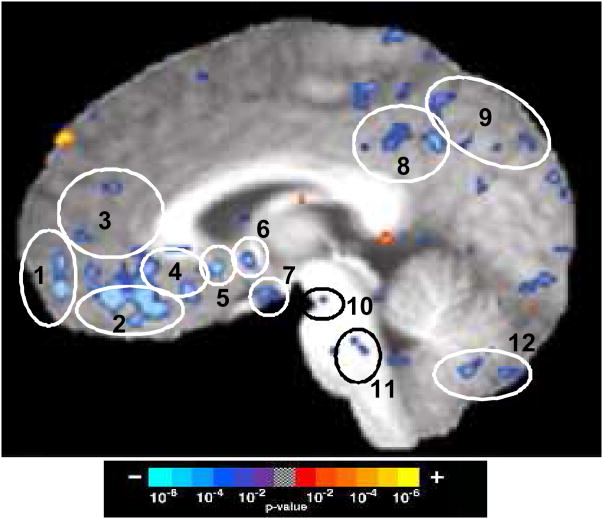
Figure 4.
Seed-based cross-correlation analysis of deactivation network in 48 matched subjects in 201 acupuncture runs with deqi and 74 tactile stimulation runs with and without deqi. Reference regions are circled in red, and regions with positive correlation activity are circled in green. Correlations with p<0.001 are shown. (A, B) Correlations for the posterior cingulate Brodmann area 31: (A) Acupuncture: reference voxel (2, −53, 36), showing correlations in the medial prefrontal, posterior medial parietal, medial temporal lobe and temporal pole. (B) Tactile stimulation: reference voxel (3, −63, 31), showing correlations that partially overlap those in acupuncture, but correlations are markedly weaker. (C,D) Correlations for the hypothalamus: (C) Acupuncture: reference voxel (3, −1, −10), showing correlations with regions similar to (A), but more limited in extent. (D) Tactile stimulation: reference voxel (3, −1, −10), showing no regions with significant signal change. Numbered areas: (1) frontal pole and pregenual cingulate, (2) subgenual cingulate, and subgenual area 25 in the ventral route of the medial prefrontal cortex, (3) precuneus, posterior cingulate and retrosplenial cortex of the posterior medial parietal cortex, (4) amygdala, hippocampus, and parahippocampus of the medial temporal lobe, (5) temporal pole, (6) precuneus and posterior cingulate cortex Brodmann area 31, and (7) orbitofrontal cortex, subgenual cingulate, subgenual area SG25 and ventromedial prefrontal cortex.
The temporal and spatial features of the activation and deactivation networks were compared with descriptions in the resting brain literature to explore their relationships as well as additional effects that acupuncture might evoke. We found that the extensive regions of deactivation and activation observed during acupuncture showed substantial overlap with both the default mode network and the anti-correlated task-positive network in response to stimulation (Fig. 5). However, during acupuncture, the amygdala and hypothalamus—structures not commonly reported as involved in the default mode literature—were found to be a central part of the activation response of the brain to acupuncture.
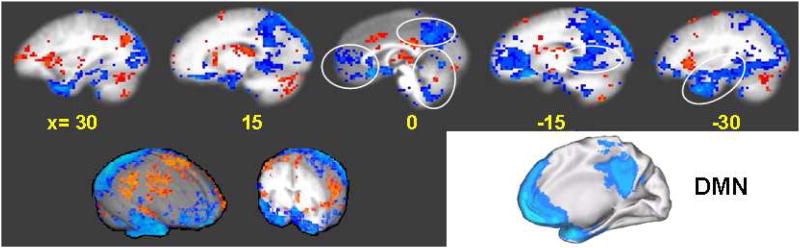
Figure 5.
Comparison of the limbic-paralimbic-neocortical network (LPNN) during acupuncture deqi and the default mode network (DMN) as described in the literature (adapted from Shulman et al., 1997). Multiple sagittal slices through an averaged brain in Talairach space (Talairach and Tournoux, 1988) are shown for the LPNN, spanning both the right hemisphere (left) and the left hemisphere (right), with distance from midline shown in mm. Two brain surface renderings (Cox, 1996) are also shown in the lower left. An oblique view of the DMN is shown in the lower right.
A second reproducible finding of our research has been that the signal increase observed in the somatosensory regions during acupuncture needling, an invasive procedure, is less than the signal increase seen during the control superficial tactile stimulation. Tactile stimulation, as has been previously described, induced greater activation in the somatosensory regions but less extensive involvement of the default mode network and limbic-associated regions of the anti-correlated task-positive network. Moreover, even short periods of inadvertent pain can attenuate or reverse the deactivation of the default mode network that occurs during acupuncture. Such effects indicate that deactivation of the default mode network during acupuncture cannot be completely explained by the demand of attention, as is commonly proposed in the literature. Together, these results strongly suggest that acupuncture engages extensive functionally organized intrinsic systems of the brain as mediators of its diverse effects.
To provide a single terminology for the network of brain regions involved in the acupuncture response, we have defined the limbic-paralimbic-neocortical network, comprised of the default mode network, amygdala and hypothalamus (Fig. 6 and 7). Our findings show that acupuncture mobilizes these anti-correlated functional networks of the brain to mediate its actions, and that the effect is dependent on the psychophysical response to acupuncture stimulation. Functional MRI studies from other groups have also shown generalized deactivation of the limbic-paralimbic-neocortical network across multiple levels of the brain, along with activation of the sensorimotor system, during acupuncture stimulation (Fang et al., 2006; Fang et al., 2008; Napadow et al., 2005a; Wang et al., 2007; Wu et al., 1999). The relevance of our findings to the clinical effect of acupuncture is supported by a recent study showing deactivation of the default mode network and activation of the somatosensory cortex persisting for more than 10 min after the completion of electroacupuncture stimulation (Dhond et al., 2008; Bai et al., 2009).
Figure 6.
Limbic-Paralimbic-Neocortical Network activity during acupuncture deqi. The averaged BOLD signal changes are shown for 11 subjects during acupuncture at point ST36, showing activation (red) and deactivation (blue). A parasagittal image is shown at 2mm from midline within the right hemisphere in Talairach space (Talairach and Tournoux, 1988). Regions: (1) frontal pole, (2) ventromedial prefrontal cortex, (3) pregenual cingulate, (4) subgenual cingulate, (5) subgenual Brodmann area 25, (6) septal nuclei, (7) hypothalamus, (8) posterior cingulate, 9 precuneus, (10) substantia nigra, (11) reticular formation, and (12) cerebellar vermis.
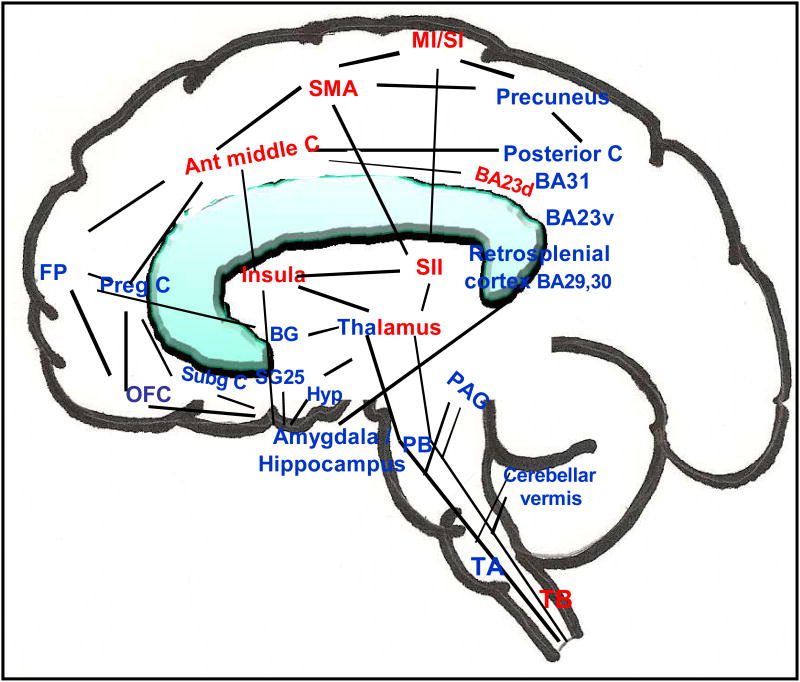
Figure 7.
Functionally anti-correlated task-positive (activation, red) and negative (deactivation, blue) networks involved in acupuncture action shown on a brain sketch, with an overlapping of medial and lateral regions. Regions of deactivation in the limbic–paralimbic–neocortical network aggregate in the medial frontal, parietal and temporal lobes. The basal ganglia, the cerebellar vermis, tonsil and brainstem also show regional deactivation. The cingulate and thalamus have regions in both networks, while the sensorimotor areas, insula, middle cingulate and the dorsal division of posterior cingulate Brodmann area 23 are in the task-positive network. The figure is adapted from Hui et al. (2009). Abbreviations: ant middle C, anterior middle cingulate; BA Brodmann area; BA23d, Brodmann area 23 dorsal; BA23v, Brodmann area 23 ventral; BG, basal ganglia; FP, frontal pole; Hyp, hypothalamus; M1/S1, primary motor/primary sensory cortex; OFC, orbitofrontal cortex; PAG, periaqueductal gray; PB, parabrachial nucleus; preg C, pregenual cingulate; SII, secondary somatosensory cortex; SMA, supplementary motor area; SG25, subgenual area 25; Subg C, subgenual cingulate, TA, tract A; TB, tract B.
2.1 Sensations and deqi
Throughout our studies, subjects have rated the sensations they experienced during acupuncture stimulation, and these sensation ratings have been central to our analyses. Subjects rated 10 sensations, namely, aching, soreness, pressure, fullness, heaviness, numbness, tingling, warmth, coolness, and dull pain, on a scale from 0 (none) to 10 (unbearably strong). According to traditional Chinese Medicine teaching, the clinical efficacy of acupuncture is related to the unique psychophysical response of deqi, which consists of these sensations. Subjects also were asked to report during the acupuncture stimulating runs the occurrence of sharp pain. When sharp pain was reported, the therapist reduced the rate of stimulation until the pain subsided, which took place within seconds. We characterized and detailed subjects' experience of these sensations during acupuncture in Hui et al. (2007). The characterization and definition of deqi is critical to discriminating between the effects of acupuncture stimulation, as we have repeatedly found that the hemodynamic response to acupuncture is dependent on the psychosomatic response. This is further supported by the link of the limbic system to both emotions and acupuncture.
Throughout our studies we have found that 71% of acupuncture and 24% of tactile stimulation control runs elicited deqi, using the sum score of 3 across the 10 tested sensations as a minimal cutoff value for defining deqi. Patient sensations can be categorized as deqi, mixed deqi with sharp pain, or, very rarely, sharp pain alone. The sensations most associated with deqi were aching, soreness and pressure; these findings were consistent with the literature. Differences were found among acupuncture points, with LI4 showing the most prominent response. The data provides scientific support for the general belief that LI4 is the most potent acupuncture point in traditional Chinese acupuncture, and is therefore most often employed in clinical practice (Napadow et al., 2004).
Moreover, we have noted a novel relationship between dull pain and sharp pain. In neurophysiology, dull pain is commonly described as a slow or secondary pain that follows sharp pain (Snell, 2006). The order is reversed in our acupuncture studies, with dull pain often occurring independently of sharp pain and, at times, as incipient sharp pain. The reason for the discrepancy between our findings on pain and those in the literature is unclear; it may be related to the nature of the stimulus. While most acute pain studies employ heat or a sharp device that targets the skin surface, the gentle motion of a smooth fine needle in deeper tissue layers may elicit a more diffuse stimulation of the A delta or c fibers, without reaching the threshold of overt noxious simulation (Fig. 2 and 3).
2.2 Deactivated brain networks in acupuncture
The brain regions that preferentially show a coherent decrease in activity during acupuncture constitute the limbic-paralimbic-neocortical network. Coherent deactivation of a subset of these task-negative regions was also noted, to a lesser degree, during tactile stimulation. This significantly weaker change in signal intensity of tactile stimulation was also more limited in spatial distribution. The difference between the brain response to acupuncture and tactile stimulation was especially marked in the pregenual cingulate, posterior cingulate, and precuneus on both hemispheres, suggesting the presence of subsystems that differ in their sensitivity to the two types of sensory stimuli. Statistical significance was reached with our large sample size.
The deactivation of the amygdala and hypothalamus during acupuncture but not during tactile stimulation suggests a specific role for these major limbic structures in acupuncture action. These limbic structures are central to the regulation and control of emotion, cognition, bio-behavior, endocrine and autonomic nervous functions, and are activated by stress, pain and emotions of negative valence. Major depression and other mood disorders are characterized by amygdala hypersensitivity to activation by negative emotional stimuli. To our knowledge, the amygdala was included in only two reports (Lowe et al., 1998; Shulman et al., 1997) and the hypothalamus in only one report (Greicius et al., 2003) among the many publications on the response of the default mode network to cognitive tasks without emotional components.
Other groups have reported the effects of acupuncture on the default mode network during the resting state more than 10 min after acupuncture (Bai et al., 2009; Dhond J et al., 2008). A substantial overlap was found with the response pattern described for the default mode network in the literature. The amygdala showed deactivation in both studies in agreement with our results, and the DMN literature. The hypothalamus showed activation in Bai's study, as opposed to the deactivation seen in our data base and in a DMN report (Greicius et al., 2003).
The coherent deactivation of the temporal pole described in our most recent study (Hui et al., 2009) also appears to be more extensive than in the current literature on the default mode network. The region has close anatomical interconnections with other medial temporal lobe and prefrontal cortical regions, and is thought to play an important role in memory and social emotional processing (Kahn et al., 2008; Olson et al., 2007). Taken together, the involvement of the amygdala, hypothalamus, and temporal pole in acupuncture may represent a task-negative network subsystem, either within or outside the default mode network, which is mobilized by acupuncture.
The functional connectivity of the default mode network and the additional limbic and paralimbic regions observed during acupuncture is supported by anatomical connectivity demonstrated by multiple approaches (Buckner et al., 2008; Honey et al., 2009). A recent study provided a large scale map of the interconnections between the core components of the default mode network in the macaque (Parvizi et al., 2006), adding to prior anterograde and retrograde tracer studies in animals, including primates (Schmahmann and Pandya, 2006). Additionally, modern techniques in diffusion tensor and diffusion spectral imaging have enabled the demonstration in vivo of the major white fiber paths of these circuits in human subjects (Fujiwara et al., 2008; Hagmann et al., 2007; Honey et al., 2009; Wedeen et al., 2009).
2.3 Activated brain networks during acupuncture
The activation of sensorimotor and heteromodal association cortices, the secondary somatosensory cortex, the supplementary motor area, the dorsolateral prefrontal cortex, the right anterior insula, the anterior middle cingulate and the dorsal divisions of the posterior cingulate Brodmann area 23 observed during acupuncture (Hui et al., 2009) overlaps with that activation of the task-positive network described for the resting brain (Fox et al., 2005; Fransson, 2005). The task-positive system includes the dorsal attention system (Corbetta and Shulman, 2002) and the executive control system (Seeley et al., 2007). Contrary to conventional expectation, our functional MRI studies indicate that activation of the sensorimotor cortex by invasive acupuncture needling is weaker than the activation elicited by gentle tapping with a flexible nylon monofilament (Hui et al., 2000, 2005, 2009). Sensorimotor cortex activation by acupuncture becomes stronger than tactile control only when acupuncture provokes sharp pain. We hypothesize that such a dichotomy may be due to the different pathways taken by different neural impulses arising from peripheral sensory receptors (see tracts A and B in Fig. 7). Activation of the sensorimotor cortex is primarily mediated by impulses ascending via the dorsal column medial lemniscal system, while modulation of the limbic system is primarily mediated by impulses ascending via the spinocervical, spinoreticular and spinomesencephalic tracts (Willis and Westlund, 1997). Studies of animal models demonstrate that axons in these latter fiber tracts send collaterals to synapse directly with neurons in the dorsal thalamus, dorsal midbrain, medullary and pontine reticular formation, hypothalamus, amygdala, septum and nucleus accumbens of the limbic system (Willis, 1989; Willis and Westlund, 1997). We postulate that a greater proportion of impulses generated by acupuncture stimulation results in signals that reach the limbic system to exert its modulatory effects, whereas a greater proportion of impulses generated by tactile stimulation results in signals that reach the sensorimotor cortex to exert its excitatory effects. Such differentiation by pathway would explain the overlap of activation and deactivation, as well as the differences in the predominance of effects between these two stimulations.
2.4 Relationship between deactivation and activation networks
It is suggested that the anti-correlation between the default mode network and the task-positive networks may reflect the dichotomy between increased brain activity in regions supporting execution of a task and decreased brain activity in regions involved in unrelated processes (Fox et al., 2005). The partition of paralimbic structures such as the cingulate into both the anti-correlated deactivation and activation networks can be explained by their differences in cytoarchitecture and function (Vogt, 2005; Vogt et al., 2003, 2006). For example, the middle cingulate, which is involved in attention and pain perception, is activated, whereas both the rostral divisions of the cingulate, which are involved in emotion, autonomic and salience processing, and the caudal divisions of the cingulate, which are involved in self-reference assessment, are deactivated.
The presence of subsystems has been proposed for the anti-correlated networks of the resting brain (Golland et al., 2008). Comparisons of acupuncture with conventional tactile stimulation and noxious stimulation suggests that there may be multiple variables and sub-systems involved. A subset of regions within the task-positive network, including the right anterior insula, thalamus, anterior middle cingulate and dorsal division of the posterior cingulate Brodmann area 23 (all limbic-related structures), appear to be more strongly connected and anti-correlated with the task-negative network than the subset of regions comprising sensorimotor and association cortices such as the second somatosensory cortex and the dorsolateral prefrontal cortex. These limbic-related task-positive structures show strong responses to acupuncture, with and without noxious pain, but markedly less response during tactile stimulation. Meanwhile, the sensorimotor and hetereomodal association cortices may form another sub-system that responds more to tactile stimulation and noxious pain than to acupuncture (see Fig. 7).
3. Future directions and experimental considerations
3.1 Involvement of neurotransmitters: beyond endorphins
Although brain function can be modeled and discussed based on brain regions and interconnections, the underlying mechanism is also based on a complex interplay of neurotransmitters and neuromodulators. The endogenous opioid peptides and serotonin are known for their role in analgesia (Han 2004), but may not account fully for the affective dimension of pain and the diverse modulatory effects of acupuncture. Instead, excitatory and inhibitory mediators of smaller size may play a central role, at least in the early phase.
Of the monoamines, dopamine is the neurotransmitter of highest concentration in the limbic system (Cooper et al., 2002). Animal data acquired by different experimental approaches indicate that acupuncture suppresses the synthesis or release of dopamine induced by pain (Shi et al., 1986), cocaine (Yang et al., 2001) or alcohol (Yoon et al., 2004). Our study in rats demonstrated that electroacupuncture on the forepaw reversed activation of the limbic system induced by amphetamine, accompanied by a marked rise of GABA and return of elevated glutamate and dopamine levels to baseline levels in striatal microdialysates (Chen et al., 2008). The signal increase of the median raphe nuclear groups observed in the present study as well as in an earlier study (Napadow et al., 2005a) is consistent with serotonergic activation. The deactivation of the subgenual cingulate and the reticular formation (Hui et al., 2005, 2009) is consistent with reports of sympathetic tone down-regulation by acupuncture (Cao et al., 1983; Haker and Bjerring, 2000). Thus, the patterns of hemodynamic response observed in fMRI are in accord with the current knowledge of acupuncture effects on the monoaminergic mediators and autonomic system function.
The important role of GABAergic inhibition in the maintenance of brain function is receiving increasing attention. Many studies point to a relation between a decrease of GABAergic tone and interneuron dysfunction in the cortico-limbic system in schizophrenia, depression and anxiety disorders (Benes, 2009). Another amino acid, glycine, is being explored for its inhibitory action (Delaney et al., 2009). The neuronal communication of neural circuits is complex and must be viewed in an integrative way. Much more work is needed to define the components that play major roles at different stages and at different levels of the cortico-limbic system during acupuncture treatment. The complex interplay in the neural circuits is unlikely to be adequately explained by one or two transmitters or modulators or by a few brain structures.
3.2 Acupuncture methods
In practice, acupuncture is performed in a variety of ways. Traditional acupuncture is performed with needle manipulation, and is most commonly used in clinical practice (Napadow et al., 2004). Most practitioners employ gentle stimulation in routine settings, aiming to generate deqi without provoking sharp pain. However, the technique and intensity of manipulation varies by individual, ranging from little or no manipulation to rapid, painful needling. Modern methods of acupuncture include electroacupuncture, where a small electric current is applied to needles inserted into acupoints, or transcutaneous electric neural stimulation, where a small electric current is applied through electrodes placed on the skin. The extent to which different methodology alters the effects of acupuncture in the clinical domain remains to be studied.
In vivo studies of the mechanism of acupuncture action in humans was first enabled by the advent of functional MRI. For our human subjects research program we have rigorously controlled the technique of acupuncture delivery. We used a block design (Fig. 1) with gentle bidirectional needle rotation at 1 Hz for the acupuncture stimulation periods. The needling frequency was reduced when subjects signaled very strong sensations or sharp pain during needling by raising one or two fingers, respectively. As a result, only rarely did a subject have more than a brief episode of sharp pain during our experiments. Moreover, in our data analysis, we separated subjects who experienced sharp pain from the major cohort who experienced deqi without sharp pain to avoid the confounding effect of the noxious stimulus. To our knowledge, most studies currently in the literature fail to differentiate subjects in this way. A confounding effect may be particularly marked in limbic regions associated with emotional processing and the affective dimensions of pain. These or other differences in methodology may contribute to discrepancies in the hemodynamic response in the acupuncture neuroimaging literature, for instance, the report activation versus deactivation of cortico-limbic structures (Bai et al., 2009; Wu et al., 1999; Liu et al., 2007).
Stimulation by other modalities, such as electro-acupuncture, often at different frequencies, adds another dimension of complexity to the comparison. Variation in the effect of different frequencies of electroacupuncture has been previously reported in a study of transcutaneous electrical nerve stimulation (TENS) for primary dysmenorrhea (Lundeberg et al., 1985), where high-frequency TENS was found more effective than low-frequency TENS at reducing symptoms, and where the effect of low- but not high-frequency TENS was found to be reversed by naloxone, a relatively pure opiate antagonist. An imaging study from our group (Napadow et al., 2005b) also showed a difference, with low frequency electro-acupuncture at ST36 found to be generally similar to manual acupuncture in down-regulating corticolimbic activity as determined by functional MRI, while high-frequency electroacupuncture was not.
Finally, the site of acupuncture stimulation may also be a factor. We have chosen acupuncture points located in muscle or in tendino-muscular layers that are widely used for their analgesic and modulatory effects. Acupuncture points with different histological and nerve supply characteristics remain to be explored. Multiple lines of research must be pursued in order to characterize the full spectra of stimulations that are commonly referred to as acupuncture.
3.3 Experimental design – appropriate Controls for Acupuncture studies
The choice of an appropriate control condition for acupuncture research is highly controversial. Based on the ubiquity of sensory receptors and nerve fibers, sensory stimulation generates impulses that target the brain, regardless of the site of stimulation or degree of invasiveness, Multiple approaches to control stimulation for acupuncture have been suggested. These include acupuncture needling at points not located on acupuncture meridians, minimized acupuncture stimulation with the needle inserted into an acupuncture point only superficially, or superficial tactile stimulation over an acupuncture point. Although the pattern and degree of response to these stimulations may vary, no skin location can be considered inert (Lund and Lundeberg, 2006).
We opted to use tactile stimulation of the same acupuncture points as were subsequently stimulated using traditional acupuncture by tapping gently with a von Frey monofilament of standard force. Although in some experimental paradigms the subjects may visually observe the difference in stimulation method, in our studies subjects were in the MRI machine during stimulation and could not observe this difference. Consistent results have been observed for multiple acupoints, and administered by different acupuncturists in our studies over the years.
We believe that acupuncture performed at the 3 acupoints used in our studies primarily stimulates the receptors and nerve fibers in the deeper tendino-muscular tissue layers, while tactile stimulation primarily stimulates those in the skin. Thus, our data suggests that stimulation of the tendino-muscular tissue may predominantly account for the extensive effects on the limbic-paralimbic-neocortical network that we observed acutely during acupuncture, and that stimulation of the sensory elements on the skin may predominantly account for the stimulatory effect on the sensorimotor cortical regions. One possible mechanism of the central effect of acupuncture may be through the low-threshold mechanoreceptors found in hairy skin which are innervated by unmyelinated C tactile afferents. These activate the insular cortex in a manner well-suited to encoding slow, gentle touch, but only poorly encoding discriminative aspects (Olausson et al., 2010). Whether the mild deactivation of the limbic-paralimbic-neocortical network observed during tactile stimulation reflects a mild stimulation of the same pathway as acupuncture action, or whether tactile stimulation simply represents a salient stimulus that competes with the intrinsic networks of the brain, leading to apparent deactivation during the task, remains an open and important question.
3.4 Potential clinical benefits of Acupuncture therapy
Acupuncture has been shown to be promising in the treatment of several disease states. A study from our group on longstanding carpal tunnel syndrome suggests that acupuncture promotes the recovery of the alterations in synaptic plasticity found in this disorder (Napadow et al., 2007). Preliminary results of randomized controlled trials suggest that acupuncture may be effective in the treatment of major depression (Leo and Ligot, 2007; Roschke et al., 2000; Wang H et al., 2008) and post -traumatic stress disorder (Hollifield et al., 2007). The potential for the application of acupuncture is found in the extensive literature on alterations in the functioning of the default mode network as described in multiple disease states, including Alzheimer's disease, with impaired connectivity between the medial prefrontal lobe and the posterior medial parietal cortex (Greicius and Menon, 2004; He et al., 2007; Lustig et al., 2003; Rombouts et al., 2005; Wang et al., 2006); autism, with almost complete loss of connectivity between major core regions (Kennedy and Courchesne, 2008; Kennedy et al., 2006); chronic low back pain, with atrophy of the prefrontal cortex (Baliki et al., 2008); schizophrenia (Bluhm et al., 2007; Liang et al., 2006; Zhou et al., 2007); major depression (Anand et al., 2007; Drevets, 2007; Greicius et al., 2007); attention deficit hyperactivity disorder (Cao et al., 2006; Castellanos et al., 2008; Zang et al., 2007); multiple sclerosis (Lowe et al., 2002); and Parkinson's disease (Stoffers et al., 2007). Additional therapeutic effects for acupuncture have been shown for disorders involving affective states such as anxiety, depression and substance abuse (Edzard et al., 1998; Margolin et al., 1993), where the acupuncture effects are similar to those of deep brain stimulation (Mayberg et al., 2005; Lozano et al., 2008).
Interestingly, two psychostimulant drugs, cocaine and nicotine, induced bilateral fMRI signal increases in many brain regions (Breiter et al., 1997; Stein et al., 1998), a subset of which demonstrated signal decreases during acupuncture needle manipulation. The opposite effects of acupuncture needle manipulation and psychostimulants on these limbic and paralimbic regions suggest that acupuncture treatments could be effective for drug craving and detoxification. This is supported by a meta-analysis of trials of opioid receptor agonists with and without acupuncture for the treatment of opioid detoxification (Liu et al., 2009), which argues that acupuncture use decreases side effects and the dose of opioid agonist needed during detoxification. We can further speculate that acupuncture analgesia for surgical procedures may work by decreasing neural activity in the thalamus, amygdala, and brainstem, structures that are known to modulate the conscious experience of pain (Becerra et al., 1999; Casey et al., 1994; Coghill et al., 1994; Craig et al., 1996; Davis et al., 1997; Jones et al., 1991; Talbot et al., 1991). Future research on the effect of acupuncture on these and other disorders is necessary to evaluate whether our findings in healthy subjects translates to the treatment of disease.
3.5 Acupuncture and traditional Chinese medicine
Acupuncture is deeply rooted in the yin-yang theory of traditional Chinese medicine. The Chinese refer to yin and yang as the natural polarity in all things in the universe, for example dark and light, heat and cold, negative and positive, or inhibitory and excitatory. Traditional Chinese medicine holds that that acupuncture heals by reestablishing the balance between yin and yang and restoring the normal flow of vital energy, called qi, which has become impaired in disease states. Although the anatomic and physiologic substrate of qi is not completely clear, clinical and experimental data indicate that many of the effects are mediated via identifiable components of the nervous system (Lin and Wang, 1994; Cheng, 2000). With the demonstration of the naturally occurring anti-correlated systems in the brain that interact to maintain normal functions and a state of health, it may be possible to draw analogies between yin and the deactivation of the task-negative default mode network, yang and the activation of the task-positive network, and the flow of qi and functional connectivity. This is speculative, but is nonetheless an intriguing relationship that may guide future investigations.
4. Conclusion
We have explored the effect of acupuncture on the brain in healthy subjects through several studies. Together these studies show that the sensations constituting deqi are associated with decreased brain activity in the limbic system and in the default mode network, while sharp pain is generally associated with signal increases in these same regions. To better group the set of regions involved in the response to acupuncture, we have defined the limbic-paralimbic-neocortical network, consisting of the amygdala, hypothalamus, and default mode network, thereby encompassing the limbic system. At the same time, we have shown that tactile sensory stimulation as control primarily affects somatosensory areas. Given our findings, we propose that the observed effect on the limbic system by acupuncture is central to its mechanism of action, and goes beyond the effect of attention on the default mode network, as described in the literature.
There are multiple avenues of future research to be pursued. To what extent neurotransmitters play a role in the response to acupuncture is little understood, and may be elucidated through animal models or by developing avenues such as magnetic resonance spectroscopy or positron emission tomography. The extent to which differences in techniques affect the central effects and its clinical relevance needs further exploration. Finally, the clinical use of acupuncture needs further testing through well-designed clinical trials. We believe our findings of the central effect of acupuncture on the limbic system supports the testing of acupuncture for therapeutic use in affective and cognitive disease states, as well as its traditional role in the treatment of chronic pain.
Acknowledgments
The work was supported in part by the NIH/National Center for Complementary and Alternative Medicine (R21-AT00978) (1-P01-AT002048-01) (2-P01-AT002048-06) (K01-AT-002166-01), (F05-AT003770), the National Center for Research Resources (P41RR14075), the Mental Illness and Neuroscience Discovery Institute and the Brain Project Grant NS 34189. We wish to thank Nikos Makris for consultations on neuroanatomy, and Randy Buckner for useful discussions on the default mode system. There are no conflicts of interest for any author.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anand A, Li Y, Wang Y, Gardner K, Lowe MJ. Reciprocal effects of antidepressant treatment on activity and connectivity of the mood regulating circuit: an FMRI study. J Neuropsychiatry Clin Neurosci. 2007;19:274–282. doi: 10.1176/appi.neuropsych.19.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L, Qin W, Tian J, Dong M, Pan X, Chen P, Dai J, Yang W, Liu Y. Acupuncture modulates spontaneous activities in the anti-correlated resting brain networks. Brain Res. 2009;1279:37–49. doi: 10.1016/j.brainres.2009.04.056. [DOI] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. 2008;28:1398–1403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra LR, Breiter HC, Stojanovic M, Fishman S, Edwards A, Comite A, Gonzalez RG, Borsook D. Human brain activation under controlled thermal stimulation and habituation to noxious heat: an fMRI study. Magn Reson Med. 1999;41:1044–1057. doi: 10.1002/(sici)1522-2594(199905)41:5<1044::aid-mrm25>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Benes FM. Neural circuitry models of schizophrenia: is it dopamine, GABA, glutamate, or something else? Biol Psychiatry. 2009;65:1003–1005. doi: 10.1016/j.biopsych.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state. A functional MRI study J Cogn Neurosci. 1999;11:80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld RW, Theberge J, Schaefer B, Williamson P. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007;33:1004–1012. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schachter DL. The brain's default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cao XD, Xu SF, Lu WX. Inhibition of sympathetic nervous system by acupuncture. Acupunct Electro-Ther Res. 1983;8:25–35. doi: 10.3727/036012983816715028. [DOI] [PubMed] [Google Scholar]
- Cao Q, Zang Y, Sun L, Sui M, Long X, Zou Q, Wang Y. Abnormal neural activity in children with attention deficit hyperactivity disorder: a resting-state functional magnetic resonance imaging study. Neuroreport. 2006;17:1033–1036. doi: 10.1097/01.wnr.0000224769.92454.5d. [DOI] [PubMed] [Google Scholar]
- Casey KL, Minoshima S, Berger KL, Koeppe RA, Morrow TJ, Frey KA. Positron emission tomographic analysis of cerebral structures activated specifically by repetitive noxious heat stimuli. J Neurophysiol. 1994;71:802–807. doi: 10.1152/jn.1994.71.2.802. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, Shaw D, Shehzad Z, Di Martino A, Biswal B, Sonuga-Barke EJ, Rotrosen J, Adler LA, Milham MP. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YI, Ren JQ, Wang FN, Xu HB, Mandeville JB, Kim Y, Rosen BR, Jenkins BG, Hui KKS, Kwong KK. Inhibition of stimulated dopamine release and hemodynamic response in the brain through electrical stimulation of rat forepaw. Neurosci Lett. 2008;431:231–235. doi: 10.1016/j.neulet.2007.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng XN. Chinese Acupuncture and Moxibustion. People's Medical Publishing House; Beijing: 2000. p. 590. [Google Scholar]
- Coghill RC, Talbot JD, Evans AC, Meyer E, Gjedde A, Bushnell MC, Duncan GH. Distributed processing of pain and vibration by the human brain. J Neurosci. 1994;14:4095–4108. doi: 10.1523/JNEUROSCI.14-07-04095.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper TR, Bloom FE, Roth RH. The Biochemical Basis of Neuropharmacology. Oxford Academic Press; New York: 2002. p. 528. [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig AD, Reiman EM, Evans A, Bushnell MC. Functional imaging of an illusion of pain. Nature. 1996;384:258–260. doi: 10.1038/384258a0. [DOI] [PubMed] [Google Scholar]
- Davis KD, Taylor S, Crawley A, Wood M. Functional MRI of pain and attention related activations in the human cingulate cortex. J Neurophysiol. 1997;77:3370–3380. doi: 10.1152/jn.1997.77.6.3370. [DOI] [PubMed] [Google Scholar]
- Delaney AJ, Esmaeili A, Sedlak PL, Lynch JW, Sah P. Differential expression of glycine receptor subunits in the rat basolateral and central amygdala. Neurosci Lett. 2009;469:237–242. doi: 10.1016/j.neulet.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Dhond RP, Yeh C, Park K, Kettner N, Napadow V. Acupuncture modulates resting state connectivity in default and sensorimotor brain networks. Pain. 2008;136:407–418. doi: 10.1016/j.pain.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Orbito-frontal structure and function in depression. Ann N Y Acad Sci. 2007;1121:499–527. doi: 10.1196/annals.1401.029. [DOI] [PubMed] [Google Scholar]
- Edzard EE, Rand JI, Stevison C. Complementary therapies for depression. Arch Gen Psychiatry. 1998;55:1026–1032. doi: 10.1001/archpsyc.55.11.1026. [DOI] [PubMed] [Google Scholar]
- Fang SH, Zhang SC, Liu HB. Study on brain responses to acupuncture by functional nuclear magnetic resonance - observations on 10 healthy subjects. Zhongguo Zhong xi yi ze he za zhi. 2006;26:965–968. [PubMed] [Google Scholar]
- Fang J, Zhen J, Li K, Kong J, Nixon E, Zeng Y, Tong H, Wang P, Hui KKS. The salient characteristics of the central effects of acupuncture needling: limbic-paralimbic-neocortical network modulation. Hum Brain Mapp. 2008;30:1196–1206. doi: 10.1002/hbm.20583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anti-correlated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara S, Sasaki M, Kanbara Y, Inoue T, Hirooka R, Ogawa A. Feasibility of 1.6-mm isotropic voxel diffusion tensor tractography in depicting limbic fibers. Neuroradiology. 2008;50:131–136. doi: 10.1007/s00234-007-0317-y. [DOI] [PubMed] [Google Scholar]
- Golland Y, Golland P, Bentin S, Malach R. Data-driven clustering reveals a fundamental subdivision of the human cortex into two global systems. Neuropsychologia. 2008;46:540–53. doi: 10.1016/j.neuropsychologia.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Menon V. Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J Cogn Neurosci. 2004;16:1484–92. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-State Functional Connectivity in Major Depression: Abnormally Increased Contributions from Subgenual Cingulate Cortex and Thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hagmann P, Kurant M, Gigandet X, Thiran P, Wedeen VJ, Meuli R, Thiran JP. Mapping human whole-brain structural networks with diffusion MRI. PLoS ONE. 2007;2:e597. doi: 10.1371/journal.pone.0000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haker EHE, Bjerring P. Effect of sensory stimulation (acupuncture) on sympathetic and parasympathetic activities in healthy subjects. J Auton Nerv Syst. 2000;79:52–59. doi: 10.1016/s0165-1838(99)00090-9. [DOI] [PubMed] [Google Scholar]
- Han JS. Mini-review. Acupuncture and Endorphins Neurosci Lett. 2004;361:258–261. doi: 10.1016/j.neulet.2003.12.019. [DOI] [PubMed] [Google Scholar]
- He Y, Wang L, Zang Y, Tian L, Zhang X, Li K, Jiang T. Regional coherencechanges in the early stages of Alzheimer's disease: a combined structural and resting-state functional MRI study. Neuroimage. 2007;35:488–500. doi: 10.1016/j.neuroimage.2006.11.042. [DOI] [PubMed] [Google Scholar]
- Hollifield M, Sinclair-Lian N, Warner TD, Hammerschlag R. Acupuncture for post-traumatic stress disorder: a randomized controlled pilot trial. J Nerv Ment Dis. 2007;195:504–513. doi: 10.1097/NMD.0b013e31803044f8. [DOI] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui KK, Liu J, Makris N, Gollub RL, Chen AJ, Moore CI, Kennedy DN, Rosen BR, Kwong KK. Acupuncture modulates the limbic system and subcortical gray structures of the human brain: evidence from fMRI studies in normal subjects. Hum Brain Mapp. 2000;9:13–25. doi: 10.1002/(SICI)1097-0193(2000)9:1<13::AID-HBM2>3.0.CO;2-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui KK, Liu J, Marina O, Napadow V, Haselgrove C, Kwong KK, Kennedy DN, Makris N. The integrated response of the human cerebro-cerebellar and limbic systems to acupuncture stimulation at ST 36 as evidenced by fMRI. Neuroimage. 2005;27:479–496. doi: 10.1016/j.neuroimage.2005.04.037. [DOI] [PubMed] [Google Scholar]
- Hui KK, Nixon EE, Vangel MG, Liu J, Marina O, Napadow V, Hodge SM, Rosen BR, Makris N, Kennedy DN. Characterization of the “Deqi” Response in Acupuncture. BMC Complement Altern Med. 2007;7:33. doi: 10.1186/1472-6882-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui KKS, Marina O, Claunch JD, Nixon EE, Fang JL, Li M, Napadow V, Vangel M, Makris N, Chan ST, Kwong K, Rosen BR. Acupuncture mobilizes the brain's default mode and the anti-correlated network in healthy subjects. Brain Res. 2009;1287:84–103. doi: 10.1016/j.brainres.2009.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AK, Brown WD, Friston KJ, Qi LY, Frackowiak RS. Cortical and subcortical localization of response to pain in man using positron emission tomography. Proc Biol Sci. 1991;244:39–44. doi: 10.1098/rspb.1991.0048. [DOI] [PubMed] [Google Scholar]
- Kahn I, Andrews-Hanna JR, Vincent JL, Snyder AZ, Buckner RL. Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:129–139. doi: 10.1152/jn.00077.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. The intrinsic functional organization of the brain is altered in autism. Neuroimage. 2008;39:1877–1885. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Redcay E, Courchesne E. Failing to deactivate:resting functional abnormalities in autism. Proc Natl Acad Sci U S A. 2006;103:8275–8280. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo RJ, Ligot JS., Jr A systematic review of randomized controlled trials of acupuncture in the treatment of depression. J Affect Disord. 2007;97:3–22. doi: 10.1016/j.jad.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Liang M, Zhou Y, Jiang T, Liu Z, Tian L, Liu H, Hao Y. Widespread functional disconnectivity in schizophrenia with resting-state functional magnetic resonance imaging. Neuroreport. 2006;17:209–213. doi: 10.1097/01.wnr.0000198434.06518.b8. [DOI] [PubMed] [Google Scholar]
- Lin WZ, Wang P. Experimental Acupuncture. Shanghai Science and Technology Press; Shanghai: 1994. pp. 43–44. [Google Scholar]
- Liu S, Zhou W, Ruan X, Li R, Lee T, Weng X, Hu J, Yang G. Activation of the hypothalamus characterizes the response to acupuncture stimulation in heroin addicts. Neurosci Lett. 2007;421:203–208. doi: 10.1016/j.neulet.2007.04.078. [DOI] [PubMed] [Google Scholar]
- Liu TT, Shi J, Epstein DH, Bao YP, Lu L. A meta-analysis of acupuncture combined with opioid receptor agonists for treatment of opiate-withdrawal symptoms. Cell Mol Neurobiol. 2009;29:449–454. doi: 10.1007/s10571-008-9336-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano A, Mayberg H, Glacobbe P, Hamani C, Craddock R, Kennedy S. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64:461–467. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Phillips MD, Lurito JT, Mattson D, Dzemidzic M, Mathews VP. Multiple sclerosis: low-frequency temporal blood oxygen level-dependent fluctuations indicate reduced functional connectivity initial results. Radiology. 2002;224:184–192. doi: 10.1148/radiol.2241011005. [DOI] [PubMed] [Google Scholar]
- Lund I, Lundeberg T. Are minimal, superficial or sham acupuncture procedures acceptable as inert placebo controls? Acupunct Med. 2006;24:13–15. doi: 10.1136/aim.24.1.13. [DOI] [PubMed] [Google Scholar]
- Lundeberg T, Bondesson L, Lundström V. Relief of primary dysmenorrhea by transcutaneous electrical nerve stimulation. Acta Obstet Gynecol Scand. 1985;64:491–497. doi: 10.3109/00016348509156727. [DOI] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O'Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci U S A. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin A, Avants SK, Chang P, Kosten TR. Acupuncture for the treatment of cocaine dependence in methadone-maintained patients. Am J Addictions. 1993;2:194–201. [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Napadow V, Liu J, Kaptchuk T. A systematic study of acupuncture practice: acupoint usage in an outpatient setting in Beijing, China. Complement Ther Med. 2004;12:209–216. doi: 10.1016/j.ctim.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Napadow V, Dhond RP, Purdon PL, Kettner N, Makris N, Kwong KK, Hui KK. Correlating acupuncture FMRI in the human brainstem with heart rate variability. Conf Proc IEEE Eng Med Biol Soc. 2005a;5:4496–4499. doi: 10.1109/IEMBS.2005.1615466. [DOI] [PubMed] [Google Scholar]
- Napadow V, Makris N, Liu J, Kettner NW, Kwong KK, Hui KK. Effects of electro-acupuncture versus manual acupuncture on the human brain as measured by fMRI. Hum Brain Mapp. 2005b;24:193–205. doi: 10.1002/hbm.20081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V, Kettner N, Liu J, Li M, Kwong KK, Vangel M, Makris N, Audette J, Hui KK. Hypothalamus and amygdala response to acupuncture stimuli in Carpal Tunnel Syndrome. Pain. 2007;130:254–266. doi: 10.1016/j.pain.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olausson H, Wessberg J, Morrison I, McGlone F, Vallbo A. The neurophysiology of unmyelinated tactile afferents. Neurosci Biobehav Rev. 2010;34:185–191. doi: 10.1016/j.neubiorev.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The Enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Parvizi J, Van Hoesen GW, Buckwalter J, Damasio A. Neural connections of the posteromedial cortex in the macaque. Proc Natl Acad Sci U S A. 2006;103:1563–1568. doi: 10.1073/pnas.0507729103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts SA, Barkhof F, Goekoop R, Stam CJ, Sheltens P. Altered resting state networks in mild cognitive impairment and mild Alzheimer's disease: an fMRI study. Hum Brain Mapp. 2005;26:231–239. doi: 10.1002/hbm.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roschke J, Wolf CH, Muller MJ, Wagner P, Mann K, Grozinger M, Bech S. The benefit from whole body acupuncture in major depression. J Affect Disord. 2009;57:73–81. doi: 10.1016/s0165-0327(99)00061-0. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Fiber Pathways of the Brain. Oxford University Press; Oxford: 2006. [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosc. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi MT, Li L, He GR, Lu GW. Effect of somatosensory stimulation at specific points on levels of monoamines in cerebrospinal fluid. Acta Beijing Second Med Coll. 1986;7:15–18. [Google Scholar]
- Shulman GL, Corbetta M, Buckner RL, Raichle ME, Fiez JA, Miezin FM, Petersen SE. Top-down modulation of early sensory cortex. Cereb Cortex. 1997;7:193–206. doi: 10.1093/cercor/7.3.193. [DOI] [PubMed] [Google Scholar]
- Snell RS, editor. Clinical Neuroanatomy. 6th. Lipincott Williams & Wilkins; Philadelphia: 2006. pp. 142–144. [Google Scholar]
- Stein EA, Pankiewicz J, Harsch HH, Cho JK, Fuller SA, Hoffman RG, Hawkins M, Rao SM, Bandettini PA, Bloom AS. Nicotine induced limbic-cortical activation in the human brain: a functional MRI study. Am J Psychiatry. 1998;155:1009–1015. doi: 10.1176/ajp.155.8.1009. [DOI] [PubMed] [Google Scholar]
- Stoffers D, Bosboom JL, Deijen JB, Wolters EC, Berendse HW, Stam CJ. Slowing of oscillatory brain activity is a stable characteristic of Parkinson's disease without dementia. Brain. 2007;130:1847–1860. doi: 10.1093/brain/awm034. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-Dimensional proportional system: an approach to medical cerebral imaging. Thieme Medical; New York: 1988. [Google Scholar]
- Talbot JD, Marrett S, Evans AC, Meyer E, Bushnell MC, Duncan GH. Multiple representations of pain in human cerebral cortex. Science. 1991;251:1355–1358. doi: 10.1126/science.2003220. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Berger GR, Derbyshire SW. Structural and functional dichotomy of human mid- cingulate cortex. Eur J Neurosci. 2003;18:3134–3144. doi: 10.1111/j.1460-9568.2003.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Vogt L, Laureys S. Cytology and functionally correlated circuits of human posterior cingulate areas. Neuroimage. 2006;29:452–466. doi: 10.1016/j.neuroimage.2005.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zang Y, He Y, Liang M, Zhang X, Tian L, Wu T, Jiang T, Li K. Changes in hippocampal connectivity in the early stages of Alzheimer's disease: evidence from resting state fMRI. Neuroimage. 2006;31:496–504. doi: 10.1016/j.neuroimage.2005.12.033. [DOI] [PubMed] [Google Scholar]
- Wang W, Liu L, Zhi X, Huang JB, Liu DX, Wang H, Kong XQ, Xu HB. Study on the regulatory effect of electro-acupuncture on hegu point (LI4) in cerebral response with functional magnetic resonance imaging. Chin J Integ Med. 2007;13:10–16. doi: 10.1007/s11655-007-0010-3. [DOI] [PubMed] [Google Scholar]
- Wang H, Qi H, Wang BS, Cui YY, Zhu L, Rong ZX, Chen HZ. Is acupuncture beneficial in depression: A meta-analysis of 8 randomized controlled trials. J Affect Disord. 2008;111:125–134. doi: 10.1016/j.jad.2008.04.020. [DOI] [PubMed] [Google Scholar]
- Wedeen VJ, Wang RP, Schmahmann JD, Benner T, Tseng WY, Dai G, Pandya DN, Hagmann P, D'Arceuil H, de Crespigny AJ. Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage. 2008;41:1267–1277. doi: 10.1016/j.neuroimage.2008.03.036. [DOI] [PubMed] [Google Scholar]
- Willis WD. The origin and destination of pathways involved in pain transmission. In: Melzack R, Wall PD, editors. Textbook of Pain. 2nd. Churchill Livingstone; Edinburgh: 1989. pp. 112–127. [Google Scholar]
- Willis WD, Westlund KN. Neuroanatomy of the pain system and of the pathways that modulate pain. J Clin Neurophysiol. 1997;14:2–31. doi: 10.1097/00004691-199701000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MT, Hsieh JC, Xiong J, Yang CF, Pan HB, Chen YC, Tsai G, Rosen BR, Kwong KK. Central nervous pathway for acupuncture stimulation: localization of processing with functional MR imaging of the brain--preliminary experience. Radiology. 1999;212:133–141. doi: 10.1148/radiology.212.1.r99jl04133. [DOI] [PubMed] [Google Scholar]
- Yang CH, Shim I, Kim SY. Acupuncture attenuates the neurochemical and behavioral sensitization to repeated DA in cocaine treatment. Abstr Soc Neurosci. 2001;27:228. [Google Scholar]
- Yoon SS, Kwon YK, Kim MR, Shim I, Kim KJ, Lee MH, Lee YS, Golden GT, Yang CH. Acupuncture–mediated inhibition of ethanol-induced dopamine release in the rat nucleus accumbens through the GABAB receptor. Neurosci Lett. 2004;369:234–238. doi: 10.1016/j.neulet.2004.07.095. [DOI] [PubMed] [Google Scholar]
- Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, Tian LX, Jiang TZ, Wang YF. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Jiang T, Tian L, Liu Y, Liu Z, Liu H, Kuang F. Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neurosci Lett. 2007;417:297–302. doi: 10.1016/j.neulet.2007.02.081. [DOI] [PubMed] [Google Scholar]