Abstract
Background
Aging is accompanied by an alteration in myocardial contractility. However, its noninvasive detection is difficult. The effect of chronic exercise on this decrease is unknown. Murine models of senescence are increasingly used to test therapies in aging. We tested whether strain rate imaging detected left ventricular (LV) systolic dysfunction in aging mice and was able to assess a potential improvement after exercise.
Methods and Results
Young (3 weeks), adult (2 to 3 months), and old (6 to 18 months) C57BL6 male mice underwent echocardiograms with strain rate imaging, either in sedentary conditions or before, 2 weeks and 4 weeks after chronic swimming. Hemodynamic parameters of LV function including maximal and end-systolic elastance were obtained before euthanizing. LV fibrosis was measured using Sirius red staining. Conventional echocardiography was unable to detect LV systolic dysfunction in old mice, whereas both systolic strain rate and load-independent hemodynamic parameters such as preload recruitable stroke work and end-systolic elastance were significantly decreased. Both strain rate and load-independent hemodynamic parameters normalized after 4 weeks of exercise. Both endocardial and epicardial fibrosis were increased in the LV of aging mice. Endocardial fibrosis decreased in exercised aged mice.
Conclusions
Strain rate noninvasively detects LV systolic dysfunction associated with aging in mice, whereas conventional echocardiography does not. Chronic exercise normalizes LV systolic function and decreases fibrosis in old mice. Strain rate imaging in mice may be a useful tool to monitor the effect of new therapeutic strategies preventing the myocardial dysfunction associated with aging.
Keywords: aging, echocardiography, exercise
Alarge variety of cardiovascular alterations occurs as the mature mammal ages to senescence,1 including structural remodeling and functional alterations of the left ventricle (LV).2 In several cross-sectional clinical studies, echocardiographic measurements have shown an increase in the thickness of the LV myocardial walls and a subsequent increase in LV mass with advancing age.3 In addition to these morphological changes, myocardial function is progressively altered.1 The decline in cardiac performance with aging may result from the diminution of the intrinsic contractile properties of the cardiac muscle related to numerous factors, including the β-myosin heavy chain reexpression, the alteration in excitation-contraction coupling responsible for the prolongation of the duration of contraction and the lengthened relaxation time, the slower calcium transport by cardiac sarcoplasmic reticulum and the impairment in mitochondrial function.1,4 As a consequence, a loss of adaptive capacity is observed when mechanical stresses or acute ischemia are imposed on the older heart.5,6 Interestingly, in a model of senescent rat as well in the elderly patients, exercise training attenuates age-associated myocardial diastolic dysfunction7–10 and restores the cardiac protective effect of preconditioning otherwise lost during aging.11 However, no improvement in systolic function has yet been demonstrated in vivo with exercise training.
Although the molecular changes associated with age are expected to affect the systolic mechanics of the left ventricle, accurate noninvasive assessment of myocardial dysfunction related to senescence remains difficult. Whereas alterations of LV filling are well documented by transmitral velocity profile, echocardiographic parameters such as LV ejection fraction at rest are not sensitive enough to identify the subtle changes in systolic function induced by aging.1,12–14 Strain rate imaging provides new indexes of regional myocardial function and may accurately detect subtle changes in cardiac contractility otherwise undetectable by conventional echocardiography.15–18 Recently, strain rate imaging has been validated in small animals.19,20 With the widespread use of genetically modified mice in cardiovascular research, accurate characterization of cardiac anatomy and function in mice throughout the lifespan is crucial. Therefore, the purpose of this study was to test whether age-induced cardiac alterations could be noninvasively detected in mice by strain rate imaging and to evaluate the effect of exercise training on age-associated systolic myocardial dysfunction.
Methods
All experiments performed in this study conformed to the guiding principles in the care and use of animals approved by the American Physiological Society. The protocol was approved by the Massachusetts General Hospital Subcommittee on Research Animal Care.
Animals
Young (3 weeks old, n=19), adult (2 to 3 months old, n=37), and old (6 to 18 months old, n=41) C57BL6 male mice were studied. Twenty adult and 21 old mice underwent 4 weeks of physical training, consisting of 2 daily swimming sessions in a water tank maintained at 30°C to 32°C separated by a 6-hour break. Swimming sessions were supervised to avoid floating and/or clinging of individual animals and increased by 10 minutes each day until 90 minutes sessions were reached. All mice underwent a baseline echocardiogram. The exercised mice, 7 adult sedentary mice, and 8 sedentary old mice underwent echocardiograms 2 and 4 weeks after the baseline echocardiogram.
Echocardiography
Mice were lightly sedated with an intraperitoneal injection of 80 mg/kg of ketamine. Echocardiography was obtained using an echocardiogram equipped with a 13-MHz linear transducer (Vivid 7, GE Medical Systems, Milwaukee, Wis). Wall thickness and LV dimensions were obtained from an M-mode at the level of the papillary muscles, and LV mass, fractional shortening (FS), and relative wall thickness were calculated.21 Strain rate imaging was performed as previously described.20,22 Strain rate curves were obtained from a parasternal short-axis view at midventricular level, at a frame rate of 450 frames per second and a depth of 1 cm.
Peak systolic radial strain rate (SR, s−1) was computed from a region of interest positioned in the mid posterior wall and was measured over an axial distance of 0.6 mm. The temporal smoothing filters were turned off for all measurements. Three consecutive cardiac cycles were selected and the peak systolic velocities and peak SR were measured and averaged. Strain rate imaging analysis was performed offline (EchoPac Software, GE Medical Systems) by an observer (H.T.) blinded to the age of the animals.
Hemodynamic Measurements
Twenty-two control mice (10 adult and 12 old) and 23 exercise trained mice (10 adult and 13 old mice after completion of 4 physical training weeks) were anesthetized with intraperitoneal injections of urethane (750 to 1000 mg/kg), etomidate (5 to 10 mg/kg), and morphine (1 to 2 mg/kg). The jugular vein was cannulated and fluid supplementation (12.5% human albumin at a constant rate of 10 μL/min) was provided. A 1.4-French high-fidelity Millar conductance catheter (SPR-839, Millar Instruments, Houston, Tex) was inserted through the LV apex to obtain steady-state and caval occlusion pressure-volume loops.22 PVAN software (Conductance Technologies, San Antonio, Tex, and Millar, Houston, Tex) was used to analyze all pressure-volume loop data. The maximal rate of developed LV pressure (LVdP/dtmax), the maximal value of the time-varying elastance (Emax), the end-systolic volume elastance (Ees) and preload recruitable stroke work (PRSW) were measured as previously described.23 In addition, the maximal rate of pressure decay (LVdP/dtmin), the time constant of isovolumic relaxation (τ), and the ventricular-to-vascular coupling ratio (arterial elastance to Ees ratio) were also measured.
Fibrosis Measurement
After the invasive measurements were performed, mice were euthanized, and the LV was blotted, weighed, and frozen. Sections of 5-μm thick were obtained at midventricular level and stained using Sirius red.24 Myocardial fibrosis was assessed in sedentary mice (4 young, 4 adult, and 5 old) and in exercise trained mice (6 adult and 7 old) both in the endocardium and the epicardium as previously described.19 The area of collagen deposition indicated by red staining was outlined and quantitated by an automated image analysis program (IP Laboratory Spectrum, Signal Analytics, Vienna, Va), and the ratio of the area of collagen deposition to the total field area was calculated and expressed as percent fibrosis. Staining and digitizing were performed by a blinded observer (M.J.R.). Images were analyzed by an independent blinded observer (M.S.C.).
Statistical Analysis
All data are reported as mean ± SEM. The echocardiographic and hemodynamic parameters were correlated using simple regression. Comparison between the echocardiographic parameters of young, adult, and old sedentary mice was performed using 1-way ANOVA. To assess the effect of time, comparison between the echocardiographic parameters at baseline and at 2 and 4 weeks was performed using ANOVA for repeated measurements in each age group. The effect of exercise on echocardiographic variables according to age was tested using ANOVA for repeated measurements. If the interaction of age and exercise was significant, exercised animals were compared with sedentary animals for each of the age groups using the t tests for the least squares means. Comparison of the hemodynamic parameters and of the amount of fibrosis was undertaken by comparing all groups using 1-way ANOVA. If the ANOVA was significant, the groups were compared using Student t tests.25 A probability value of <0.05 was considered as indicative of a statistically significant difference.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
LV Size and Weight
Cardiac morphological changes that accompanied aging in sedentary mice included increased diastolic wall thickness, LV mass, and relative wall thickness (Table 1). However, LV mass to body weight ratio was decreased in adult and old mice compared with young mice (Table 1).
Table 1.
Characteristics of the Groups at Baseline
| Young n=19 | Adult n=37 | Old n=41 | ANOVA | |
|---|---|---|---|---|
| BW, g | 11.8±0.4 | 26.4±0.5* | 39.9±1.2†‡ | 0.008 |
| Heart rate, bpm | 648±19 | 652±16 | 624±17 | 0.63 |
| LVEDD, mm | 2.8±0.6 | 3.2±0.7 | 3.1±0.6 | 0.06 |
| LVESD, mm | 1.1±0.6 | 1.4±0.4 | 1.5±0.6‡ | 0.04 |
| PWT, mm | 0.7±0.02 | 0.9±0.02* | 1±0.02†‡ | <0.001 |
| LV mass, mg | 50±2 | 84±2* | 107±5†‡ | 0.0021 |
| LV mass/BW, mg/g | 4±0.2 | 3±0.1* | 2.6±0.1†‡ | 0.0008 |
| RWT, % | 47±2 | 55±1* | 67±3†‡ | 0.037 |
| LV FS, % | 62±1.3 | 55±1.5* | 53±1.2‡ | 0.012 |
| SR, s−1 | 39±3 | 25±0.9* | 18±0.5†‡ | <0.0001 |
BW indicates body weight; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; PWT, end-diastolic posterior wall thickness; RWT, relative wall thickness.
P<0.05, adult versus young.
P<0.05, old versus adult.
P<0.05, old versus young.
After 4 weeks of exercise, LV mass, and LV mass to body weight ratio increased in old mice compared with baseline values and sedentary animals (Table 2). Relative wall thickness decreased in old mice after exercise because of LV dilatation (Table 2).
Table 2.
Characteristics of the Exercise Groups Before and After 4 Weeks of Training
| Adult (n=20) |
Old (n=21) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 2 Weeks | 4 Weeks | ANOVA | Baseline | 2 Weeks | 4 Weeks | ANOVA | |
| BW, g | 25±0.3 | 25±0.5 | 26±0.5 | 0.12 | 40±1.5 | 37±1 | 38±0.9* | 0.034 |
| Heart rate, bpm | 598±28 | 632±32 | 628±25 | 0.63 | 617±22 | 621±28 | 603±21 | 0.06 |
| LVEDD, mm | 3.2±0.7 | 3.1±0.5 | 3.1±0.4 | 0.12 | 3±0.9 | 3.2±0.7 | 3.5±0.9* | 0.012 |
| LVESD, mm | 1.5±0.6 | 1.4±0.6 | 1.4±0.4 | 0.75 | 1.5±0.9 | 1.6±0.69 | 1.8±0.9* | <0.0001 |
| PWT, mm | 0.82±0.02 | 0.87±0.02 | 0.95±0.02 | 0.47 | 0.99±0.03 | 1.05±0.02 | 1.03±0.03 | 0.07 |
| LV mass, mg | 82±3 | 83±5 | 95±3 | 0.18 | 102±3 | 112±4 | 129±6* | 0.01 |
| LV Mass/BW, mg/g | 3.3±0.09 | 3.3±0.2 | 3.6±0.3 | 0.54 | 2.4±0.8 | 3±0.4 | 3.4±0.5* | 0.0003 |
| RWT, % | 51±2 | 55±6 | 61±9* | 0.006 | 69±4 | 65±7 | 58±6* | 0.006 |
| LV FS, % | 55±1 | 58±1 | 57±2 | 0.95 | 53±2 | 51±1 | 52±1 | 0.30 |
| SR, s−1 | 26±1 | 30±2 | 29±0.8 | 0.29 | 19±0.6 | 25±1.5 | 29±1.2* | <0.0001 |
BW indicates body weight; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; PWT, end-diastolic posterior wall thickness; RWT, relative wall thickness.
P<0.05, 4 weeks versus baseline.
No significant changes in LV size and weight were observed in the sedentary adult and old mice followed serially during 4 weeks (data not shown).
LV Systolic Function
Echocardiography
LV FS was similar between adult and old sedentary mice. However, LV FS was greater in young mice compared with old mice (Table 1). No significant changes in LV FS were observed in the sedentary adult and old mice followed serially (data not shown).
Representative strain rate curves in young, adult, and old sedentary mice are shown in Figure 1. Peak systolic strain rate progressively decreased with age, demonstrating an impairment of systolic function with aging (Table 1; Figure 1). Interestingly, in old mice, systolic SR peak was followed by a second positive peak, which may suggest a postsystolic contraction.
Figure 1.
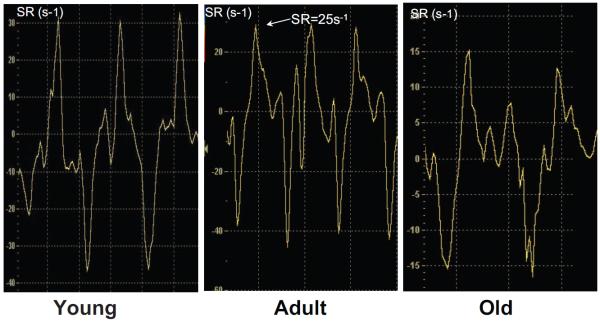
Representative strain rate time curve in young, adult, and old mice. SR (arrow) was significantly decreased in old mice as compared with young and adult mice.
With training, systolic function as evaluated by LV FS did not change in adult or old mice (Table 2). However, a significant increase in systolic SR was noted in old mice after training. Representative M-mode echocardiograms and SR curves in an old mouse, before and after 4 weeks of exercise training are shown in Figure 2. After 4 weeks of physical training, systolic SR in old mice was similar to SR in sedentary and exercised adult mice (Table 2; Figures 2 and 3). No significant change in SR was noted in the sedentary adult and old mice followed for 4 weeks (Figure 3).
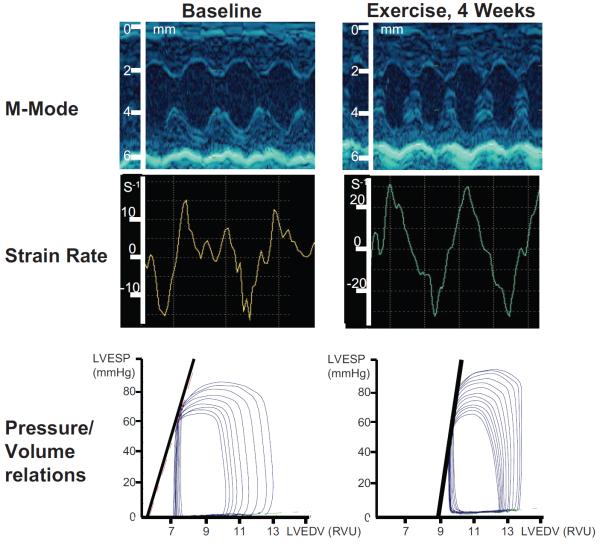
Figure 2.
M-mode echocardiogram, strain rate time curve, and pressure-volume relationship in old mice before and after 4 weeks of exercise. There was no change in LV shortening fraction on M-mode echocardiogram. Both peak systolic strain rate and end-systolic elastance were improved after training as compared with baseline.
Figure 3.
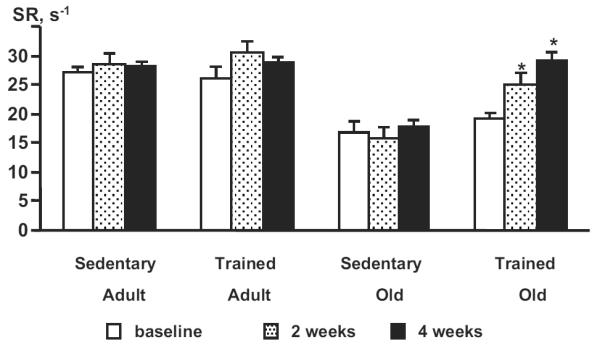
SR obtained in control and exercised animals at baseline and at 2 and 4 weeks. SR was not modified by exercise in adult mice but was significantly improved in old mice when compared with values in old sedentary animals at the same time points. N=7 adult sedentary mice, 8 old sedentary mice, 20 adult exercised mice, and 21 old exercised mice. *P<0.05.
Hemodynamic Measurements
Systolic function assessed by Emax, Ees, and preload recruitable stroke work was significantly less in old sedentary mice compared with adult sedentary mice (Table 3). Emax, Ees, and preload recruitable stroke work were greater in exercised old mice compared with sedentary old mice (Table 3; Figure 2). Conversely, in adult mice, Emax, Ees, and preload recruitable stroke work were similar in exercised and sedentary animals. Interestingly, systolic SR correlated strongly with Emax (r=0.72; P<0.0001) and Ees (r=0.74; P<0.0001; Figure 4).
Table 3.
Hemodynamic Parameters
| N | HR, bpm | LVESP, mm Hg | LVEDP, mm Hg | dP/dtmax, mm Hg/s | dP/dtmin, mm Hg/s | τ, ms | Emax, mm Hg/RVU | Ees, mm Hg/RVU | PRSW, mm Hg | Ea, mm Hg/RVU | Ea/Ees | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adult | ||||||||||||
| Control | 10 | 664±9 | 93±2 | 6±1 | 14269±822 | −7739±430 | 4.9±0.3 | 86±8 | 66±9 | 92±5 | 21±1 | 0.42±0.05 |
| Exercise | 10 | 651±7 | 106±9 | 6±1 | 13847±1166 | −9812±892 | 4.8±0.1 | 125±34 | 51±6 | 105±5 | 21±3 | 0.60±0.11 |
| Old | ||||||||||||
| Control | 12 | 683±14 | 87±3 | 5±1 | 12023±720 | −7280±389 | 4.5±0.3 | 48±6* | 21±3* | 59±9* | 20±3 | 1.61±0.5* |
| Exercise | 13 | 663±16 | 84±3* | 5±1 | 10486±892‡ | −7957±519 | 4.9±0.2 | 94±5† | 55±7† | 103±10† | 18±1 | 0.44±0.05† |
| ANOVA | 0.74 | 0.03 | 0.79 | 0.03 | 0.09 | 0.77 | 0.03 | 0.0005 | 0.002 | 0.65 | 0.02 |
HR indicates heart rate; LVESP, left ventricular end-systolic pressure; LVEDP, left ventricular end-diastolic pressure; Ea, arterial elastance.
P<0.05, control old versus control and exercised adult.
P<0.05, exercised old versus control old.
P<0.05, exercised old versus control and exercised adult.
Figure 4.
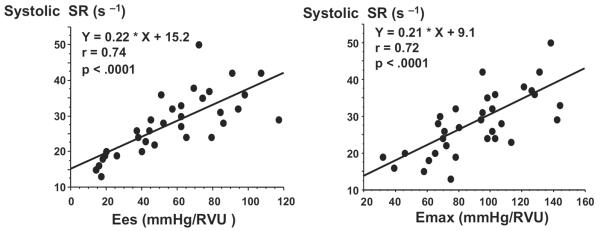
Correlation between SR and Ees (left) and Emax (right). Systolic SR correlates closely with Ees and Emax measured in both sedentary and exercised adult and old mice.
The ratio of arterial elastance to ventricular elastance (arterial elastance/Ees) was significantly increased in the old mice compared with adult mice in the sedentary groups (P<0.02). This ratio decreased in exercised old mice and was similar to that of exercised adult mice (Table 3). Arterial elastance values were similar in old and adult mice both in sedentary and exercised groups.
Diastolic function was similar between old and adult mice at high heart rates (approximately 650 beats per minute). To take into account the possible masking of a dysfunction in relaxation at high heart rates, additional measurements of dP/dtmin were obtained in 5 adult and 5 old mice at 2 heart rates, “low” (approximately 550 beats per minute) and “high” (approximately 650 bpm). Whereas no significant change in dP/dtmin values was noted between heart rates in adult mice, a significant decrease in dP/dtmin occurred with the lower heart rate in old mice.
Myocardial Fibrosis
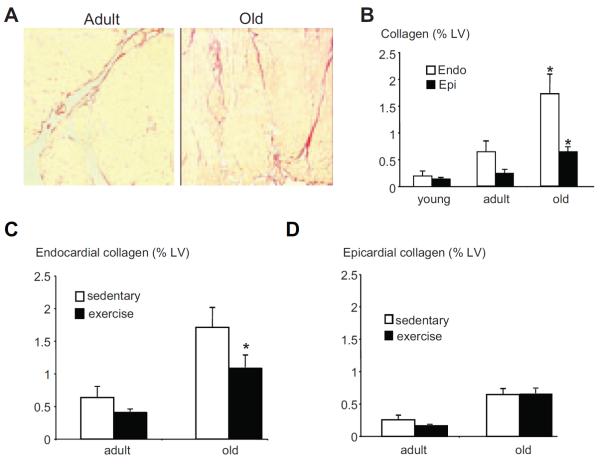
Representative views of the endocardial fibrosis of a sedentary adult and a sedentary old mouse are shown in Figure 5A. Both endocardial and epicardial collagen deposition was increased in sedentary old mice compared with adult and young mice (Figure 5A and 5B). Exercise decreased endocardial fibrosis in old mice without affecting epicardial fibrosis (Figure 5C and 5D).
Figure 5.
A, Representative endocardial fibrosis in sedentary adult and old mice (magnification ×100). B, In sedentary animals, collagen deposition was significantly higher in old mice as compared with young and adult mice in both endocardium and epicardium. *P<0.05 versus adult and young mice. C and D, In old mice, exercise training significantly decreased the collagen content in endocardium but not epicardium. *P<0.05 versus old sedentary mice.
Discussion
The present study demonstrates that systolic SR, an index of myocardial deformation obtained noninvasively using ultrasound imaging, accurately detects LV systolic dysfunction in old mice and its improvement after exercise, whereas conventional echocardiographic parameters are similar in sedentary old and adult mice and do not change after exercise.
Noninvasively identifying senescent heart dysfunction has become increasingly important because of the augmentation of the aging population and the increased incidence of age-related heart failure. Using papillary muscle preparations, aging has been associated to functional, biochemical, and electric performance alterations of the heart.1 Cardiac senescence is characterized by functional lowering of myocardial strength and contraction speed,26,27 prolongation of the relaxation phase and stiffening of both myocytes and mural connective tissue.28–30 Although these changes associated with age are expected to affect LV systolic function, LV ejection fraction and FS at rest are preserved in aging asymptomatic human subjects.14 Of note, exhaustive upright exercise unmasks systolic myocardial dysfunction in healthy senescent subjects.14
The first goal of our study was to compare conventional indices of LV systolic function such as LV FS and systolic strain rate in young, adult, and old mice. When we compared relative extremes in age (3 weeks and 6 to 18 months), we found lower values in LV FS in old versus young mice. Similar findings were reported in female rats by Boluyt et al,31 and the authors concluded that a mild decrement in systolic function occurred with advanced age and appeared to accelerate after 22 months of age. The same authors indicated that these changes in FS were modest but were supported by the decline in the velocity of circumferential shortening, an index less preload dependent than the FS.31
However, our data also demonstrated that LV FS values in old mice were within the normal range and could not discriminate between old and adult mice. Conversely, systolic SR was decreased in old mice, accurately identifying systolic myocardial dysfunction. Our findings suggest that aging is associated with a decrease in regional contractile function without obvious loss of the global LV pump function. This alteration is not detected by LV ejection fraction measurement.
Furthermore, the load-dependency of LV ejection fraction limits its use as an accurate tool for the evaluation of myocardial contractility. A reliable estimate of overall contractility is the slope of the end-systolic pressure/end-systolic volume relationship (end-systolic volume elastance, Ees) measured from pressure-volume loops.32,33 These in vivo assessments of systolic function, however, are invasive and involve a terminal procedure. In the present study, the alteration in systolic function detected noninvasively by SR in old mice was confirmed by the decrease in parameters of LV systolic function obtained by invasive hemodynamics, in particular Ees. Similarly, Yang et al23 were able to demonstrate a decreased contractile function at rest in senescent mice using a conductance catheter inserted in the LV to measure Ees.
Strain rate, the rate of deformation of the myocardium, reflects the local elastic deformation properties of the myocardium and is relatively independent of overall cardiac motion and the motion of adjacent myocardial segments. In large mammals, systolic strain rate correlates with peak elastance, a load-independent index of myocardial contractility.34 In the mouse, we found that systolic SR correlated closely with Emax and Ees, 2 fairly load-independent indices of contractility.32,33
Previous studies have already shown the ability of strain rate to accurately quantify regional contractile dysfunction in cardiomyopathy and ischemia before and independently of the development of conventional echocardiographic abnormalities.17,18,22,35 Recent studies have reported that tissue Doppler imaging is feasible, reproducible, and accurate in small animal models such as rats19 and mice,20,22,36 and that murine systolic strain rate correlates closely with dP/dtmax.20,36 Of note, changes in dP/dtmax in the present model of physiological aging were small and did not reflect the changes in SR. These findings are different than those observed in previous models of endotoxin shock, ischemia, or doxorubicin-induced cardiomyopathy20,22,36 where greater variations of dP/dtmax were observed and correlated with SR.
In the present study, the alteration in systolic function detected by SR and hemodynamic measurements was associated with an increase in myocardial collagen content in endocardial but also epicardial layers. Such an increase may participate in a loss of contractile function. During the aging process, the extensive reparative process of fibrosis, which is associated with myocyte loss and hypertrophy of remaining myocytes, has been implicated in contractile dysfunction.37,38 Other factors are known to contribute to the alteration of contractility detected in senescence such as prolonged calcium transients and diminished calcium reuptake mechanisms4,8 and mitochondrial dysfunction.39
Surprisingly, we did not observe any dysfunction in myocardial relaxation in old mice. One possible explanation for this finding is that we recorded hemodynamic parameters at heart rates >650 beats per minute, to be within the physiological range of heart rate in awake mice and avoid depression of myocardial contractility.40 Indeed, a decrease of contractility parameters at slow heart rates is expected because of the powerful role of the force-frequency relationship in mice.41,42 Moreover, high heart rates may also modify diastolic parameters42 and mask underlying abnormalities by shortening the relaxation time. To explore the possibility that high heart rate was masking differences in diastolic function between adult and old mice, we obtained hemodynamic measurements of dP/dtmin in 5 adult and 5 old mice at 2 heart rates, “low” (approximately 550 beats per minute) and “high” (approximately 650 bpm). Whereas no significant change in dP/dtmin values was noted between high and low heart rates in adult mice, a significant decrease in dP/dtmin occurred with low heart rate in old mice, unmasking an underlying relaxation abnormality in the old animals.
The second goal of the study was to determine whether exercise training was able to reverse these contractile abnormalities. In a broad variety of species, from rat to humans, chronic exercise by swimming has been shown to increase left ventricular mass, to decrease resting heart rate, to enhance rates of diastolic relaxation and to increase coronary capacitance. Although experimental and clinical investigations have demonstrated an improvement in diastolic function after training in senescent hearts, no study has addressed the effect of exercise on systolic function.9,10 We report that 4 weeks of exercise training reversed the age-related impairment in contractility as assessed by both systolic strain rate and invasive hemodynamic parameters. This improvement in contractility was associated with a decrease in endocardial fibrosis. The underlying mechanism for the improvement in contractility function is probably multifactorial including modulation of collagen gene expression,43 refined calcium handling,8 increased myocardial catecholamine stores,44 and improvement in myocardial metabolic enzyme activity45 and in mitochondrial function.46
This study has important clinical applications, suggesting that prolonged and sustained endurance training preserves systolic LV function and may help to prevent heart failure in the elderly.
In mice, strain rate imaging allows accurate quantification of contractile function. Strain rate imaging is able to detect age-related contractile abnormalities and to assess restoration of normal systolic function after chronic exercise. Strain rate may be a useful parameter in murine models to serially follow-up the effects of aging on LV function and monitor the effect of therapies on senescence related alterations of myocardial contractility.
CLINICAL PERSPECTIVE.
Aging may be associated with a decrease in cardiac function, including myocardial contraction and relaxation, and the risk of cardiovascular morbidity is increased n the aged heart. Our study aimed to demonstrate that chronic exercise training improves the aging-induced decrease in myocardial function. However, both the impaired systolic fucntion and the improvement in cardiac function by exercise training are difficult to demonstrate using conventional assessment of myocardial function by left ventricular ejection fraction.
In this study performed in mice, we demonstrated that systolic strain rate, an index of myocardial deformation obtained noninvasively using ultrasound imaging, accurately detects left ventricular systolic dysfunction in old mice and its improvement after exercise, whereas conventional ultrasound parameters are similar in sedentary old and adult mice, and do not change after exercise. In addition, we demonstrated that this aging-related alteration in systolic function was associated with an increase in myocardial collagen content in both endocardial and epicardial layers.
This exercise training-induced improvement of cardiac function is a beneficial adaptation that may lessen morbidity in the aged heart. Specifically, endurance exercise could help provide cardioprotection against cardiac insults in both young and old animals.
Acknowledgments
The authors are grateful to Dr Stuart Houser for his advice regarding the technique of fibrosis analysis and to Dr Elkan F. Halpern for his statistical advice.
Sources of Funding
This study was supported by a Scientist Development Grant (M.S.C.) as well as grants HL-42397, HL-70896, and HL-71987 from the National Heart, Lung, and Blood Institute Public Health Service (K.D.B.).
Footnotes
Disclosures
None.
References
- 1.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. Part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 2.Hees PS, Fleg JL, Lakatta EG, Shapiro EP. Left ventricular remodeling with age in normal men versus women: novel insights using three-dimensional magnetic resonance imaging. Am J Cardiol. 2002;90:1231–1236. doi: 10.1016/s0002-9149(02)02840-0. [DOI] [PubMed] [Google Scholar]
- 3.Shub C, Klein AL, Zachariah PK, Bailey KR, Tajik AJ. Determination of LV mass by echocardiography in a normal population: effect of age and sex in addition to body size. Mayo Clin Proc. 1994;69:205–211. doi: 10.1016/s0025-6196(12)61058-1. [DOI] [PubMed] [Google Scholar]
- 4.Navarro A, Boveris A. The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol. 2007;292:C670–C686. doi: 10.1152/ajpcell.00213.2006. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi T, Schunkert H, Isoyama S, Wei JY, Nadal-Ginard B, Grossman W, Izumo S. Age-related differences in the expression of proto-oncogene and contractile protein genes in response to pressure overload in the rat myocardium. J Clin Invest. 1992;89:939–945. doi: 10.1172/JCI115675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abete P, Ferrara N, Cioppa A, Ferrara P, Bianco S, Calabrese C, Cacciatore F, Longobardi G, Rengo F. Preconditioning does not prevent post-ischemic dysfunction in aging heart. J Am Coll Cardiol. 1996;27:1777–1786. doi: 10.1016/0735-1097(96)00070-8. [DOI] [PubMed] [Google Scholar]
- 7.Spurgeon HA, Steinbach MF, Lakatta EG. Chronic exercise prevents characteristic age-related changes in rat cardiac contraction. Am J Physiol. 1983;244:H513–H518. doi: 10.1152/ajpheart.1983.244.4.H513. [DOI] [PubMed] [Google Scholar]
- 8.Tate CA, Taffet GE, Hudson EK, Blaylock SL, McBride RP, Michael LH. Enhanced calcium uptake of cardiac sarcoplasmic reticulum in exercise-trained old rats. Am J Physiol. 1990;258:H431–H435. doi: 10.1152/ajpheart.1990.258.2.H431. [DOI] [PubMed] [Google Scholar]
- 9.Brenner DA, Apstein CS, Saupe KW. Exercise training attenuates age-associated diastolic dysfunction in rats. Circulation. 2001;104:221–226. doi: 10.1161/01.cir.104.2.221. [DOI] [PubMed] [Google Scholar]
- 10.Palka P, Lange A, Nihoyannopoulos P. The effect of long-term training on age-related left ventricular changes by Doppler myocardial velocity gradient. Am J Cardiol. 1999;84:1061–1067. doi: 10.1016/s0002-9149(99)00499-3. [DOI] [PubMed] [Google Scholar]
- 11.Abete P, Calabrese C, Ferrara N, Cioppa A, Pisanelli P, Cacciatore F, Longobardi G, Napoli C, Rengo F. Exercise training restores ischemic preconditioning in aging heart. J Am Coll Cardiol. 2000;36:643–650. doi: 10.1016/s0735-1097(00)00722-1. [DOI] [PubMed] [Google Scholar]
- 12.Schulman SP, Lakatta EG, Fleg JL, Lakatta L, Becker LC, Gerstenblith Age-related decline in left ventricular filling at rest and exercise. Am J Physiol. 1992;263:H1932–H1938. doi: 10.1152/ajpheart.1992.263.6.H1932. [DOI] [PubMed] [Google Scholar]
- 13.Swinne CJ, Shapiro EP, Lima SD, Fleg JL. Age-associated changes in left ventricular diastolic performance during isometric exercise in normal subjects. Am J Cardiol. 1992;69:823–826. doi: 10.1016/0002-9149(92)90518-4. [DOI] [PubMed] [Google Scholar]
- 14.Fleg JL, O'Connor F, Gerstenblith G, Becker LC, Clulow J, Schulman SP, Lakatta EG. Impact of age on the cardiovascular response to dynamic upright exercise in healthy men and women. J Appl Physiol. 1995;69:890–900. doi: 10.1152/jappl.1995.78.3.890. [DOI] [PubMed] [Google Scholar]
- 15.Gorcsan J, III, Strum DP, Mandarino WA, Gulati VK, Pinsky MR. Quantitative assessment of alterations in regional contractility with color-coded tissue Doppler echocardiography. Comparison with sonomicrometry and pressure-volume relations. Circulation. 1997;95:2423–2433. doi: 10.1161/01.cir.95.10.2423. [DOI] [PubMed] [Google Scholar]
- 16.Urheim S, Edvardsen T, Torp H, Angelsen B, Smiseth OA. Myocardial strain by Doppler echocardiography: validation of a new method to quantify regional myocardial function. Circulation. 2000;102:1158–1164. doi: 10.1161/01.cir.102.10.1158. [DOI] [PubMed] [Google Scholar]
- 17.Derumeaux G, Loufoua J, Pontier G, Cribier A, Ovize M. Tissue Doppler imaging differentiates transmural from nontransmural acute myocardial infarction after reperfusion therapy. Circulation. 2001;103:589–596. doi: 10.1161/01.cir.103.4.589. [DOI] [PubMed] [Google Scholar]
- 18.Nagueh SF, Bachinski LL, Meyer D, Hill R, Zoghbi WA, Tam JW, Quinones MA, Roberts R, Marian AJ. Tissue Doppler imaging consistently detects myocardial abnormalities in patients with hypertrophic cardiomyopathy and provides a novel means for an early diagnosis before and independently of hypertrophy. Circulation. 2001;104:128–130. doi: 10.1161/01.cir.104.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Derumeaux G, Mulder P, Richard V, Chagraoui A, Nafeh C, Bauer F, Henry JP, Thuillez C. Tissue Doppler imaging differentiates physiological from pathological pressure-overload left ventricular hypertrophy in rats. Circulation. 2002;105:1602–1608. doi: 10.1161/01.cir.0000012943.91101.d7. [DOI] [PubMed] [Google Scholar]
- 20.Sebag IA, Handschumacher MD, Ichinose F, Morgan JG, Hataishi R, Rodrigues AC, Guerrero JL, Steudel W, Raher MJ, Halpern EF, Derumeaux G, Bloch KD, Picard MH, Scherrer-Crosbie M. Quantitative assessment of regional myocardial function in mice by tissue Doppler imaging: comparison with hemodynamics and sonomicrometry. Circulation. 2005;111:2611–2616. doi: 10.1161/CIRCULATIONAHA.104.474411. [DOI] [PubMed] [Google Scholar]
- 21.Gardin JM, Siri FM, Kitsis RN, Edwards JG, Leinwand LA. Echocardio-graphic assessment of left ventricular mass and systolic function mice. Circ Res. 1995;76:907–914. doi: 10.1161/01.res.76.5.907. [DOI] [PubMed] [Google Scholar]
- 22.Thibault H, Gomez L, Donal E, Pontier G, Scherrer-Crosbie M, Ovize M, Derumeaux G. Acute myocardial infarction in mice: assessment of transmurality by strain rate imaging. Am J Physiol Heart Circ Physiol. 2007;293:H496–H502. doi: 10.1152/ajpheart.00087.2007. [DOI] [PubMed] [Google Scholar]
- 23.Yang B, Larson DF, Watson R. Age-related left ventricular function in the mouse: analysis based on in vivo pressure-volume relationships. Am J Physiol Heart Circ Physiol. 1999;46:H1906–H1913. doi: 10.1152/ajpheart.1999.277.5.H1906. [DOI] [PubMed] [Google Scholar]
- 24.Ichinose F, Bloch KD, Wu JC, Hataishi R, Aretz HT, Picard MH, Scherrer-Crosbie M. Pressure overload-induced LV hypertrophy and dysfunction in mice are exacerbated by congenital NOS3 deficiency. Am J Physiol Heart Circ Physiol. 2004;286:H1070–H1075. doi: 10.1152/ajpheart.00940.2003. [DOI] [PubMed] [Google Scholar]
- 25.Scheffé . The Analysis of Variance. Wiley; New York: 1959. p. 70. [Google Scholar]
- 26.Anversa P, Puntillo E, Nikitin P, Olivetti G, Capasso JM, Sonnenblick EH. Effects of age on mechanical and structural properties of myocardium of Fisher 344 rats. Am J Physiol Heart Circ Physiol. 1989;256:H1440–H1449. doi: 10.1152/ajpheart.1989.256.5.H1440. [DOI] [PubMed] [Google Scholar]
- 27.Rozenberg S, Tavernier B, Riou B, Swinghedaw B, Le Page C, Boucher F, de Leiris J, Besse S. Severe impairment of ventricular compliance accounts for advanced age-associated hemodynamic dysfunction in rats. Exp Geront. 2006;41:289–295. doi: 10.1016/j.exger.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. Part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–497. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 29.Wei JY, Spurgeon HA, Lakatta EG. Excitation-contraction in rat myocardium: alterations with adult aging. Am J Physiol. 1984;246:H784–H791. doi: 10.1152/ajpheart.1984.246.6.H784. [DOI] [PubMed] [Google Scholar]
- 30.Assayag P, Charlemagne D, de Leiris J, Boucher F, Valere PE, Lortet S, Swynghedauw B, Besse S. Senescent heart compared with pressure overload-induced hypertrophy. Hypertension. 1997;29:15–21. doi: 10.1161/01.hyp.29.1.15. [DOI] [PubMed] [Google Scholar]
- 31.Boluyt MO, Converso K, Hwang HS, Mikkor A, Russell Echocardio-graphic assessement of age-associated changes in systolic and diastolic function in the female F344 rat heart. J Appl Physiol. 2004;96:822–828. doi: 10.1152/japplphysiol.01026.2003. [DOI] [PubMed] [Google Scholar]
- 32.Suga H, Sagawa K. Instantaneous pressure-volume relationships and their ratio in the excised, supported canine left ventricle. Circ Res. 1974;35:117–126. doi: 10.1161/01.res.35.1.117. [DOI] [PubMed] [Google Scholar]
- 33.Georgakopoulos D, Mitzner WA, Chen CH, Byrne BJ, Millar HD, Hare JM, Kass DA. In vivo murine left ventricular pressure-volume relations by miniaturized conductance micromanometry. Am J Physiol. 1998;274:H1416–H1422. doi: 10.1152/ajpheart.1998.274.4.H1416. [DOI] [PubMed] [Google Scholar]
- 34.Greenberg NL, Firstenberg MS, Castro PL, Main M, Travaglini A, Odabashian JA, Drinko JK, Rodriguez LL, Thomas JD, Garcia MJ. Doppler-derived myocardial systolic strain rate is a strong index of left ventricular contractility. Circulation. 2002;105:99–105. doi: 10.1161/hc0102.101396. [DOI] [PubMed] [Google Scholar]
- 35.Pieroni M, Chimenti C, Ricci R, Sale P, Russo MA, Frustaci A. Early detection of Fabry cardiomyopathy by tissue Doppler imaging. Circulation. 2003;107:1978–1984. doi: 10.1161/01.CIR.0000061952.27445.A0. [DOI] [PubMed] [Google Scholar]
- 36.Neilan TG, Jassal DS, Perez-Sanz TM, Raher MJ, Pradhan AD, Buys ES, Ichinose F, Bayne DB, Halpern EF, Weyman AE, Derumeaux G, Bloch KD, Picard MH, Scherrer-Crosbie M. Tissue Doppler imaging predicts left ventricular dysfunction and mortality in a murine model of cardiac injury. Eur Heart J. 2006;27:1868–1875. doi: 10.1093/eurheartj/ehl013. [DOI] [PubMed] [Google Scholar]
- 37.Anversa P, Capasso JM. Cellular basis of aging in the mammalian heart. Scanning Microsc. 1991;5:1065–1074. [PubMed] [Google Scholar]
- 38.Besse S, Assayag P, Delcayre PC, Carre F, Cheav SL, Lecarpentier Y, Swynghedauw B. Normal and hypertrophied senescent rat heart: mechanical and molecular characteristics. Am J Physiol. 1993;265:H183–H190. doi: 10.1152/ajpheart.1993.265.1.H183. [DOI] [PubMed] [Google Scholar]
- 39.Judge S, Leeuwenburgh C. Cardiac mitochondrial bioenergetics, oxidative stress, and aging. Am J Physiol Cell Physiol. 2007;292:C1983–C1992. doi: 10.1152/ajpcell.00285.2006. [DOI] [PubMed] [Google Scholar]
- 40.Rottman JN, Ni G, Khoo M, Wang Z, Zhang W, Anderson ME, Madu EC. Temporal changes in ventricular function assessed echocardiographically in conscious and anesthetized mice. J Am Soc Echocardiogr. 2003;16:1150–1157. doi: 10.1067/S0894-7317(03)00471-1. [DOI] [PubMed] [Google Scholar]
- 41.Palakodeti V, Oh S, Oh BH, Mao L, Hongo M, Peterson LL, Ross J. Force-frequency effect is a powerful determinant of myocardial contractility in the mouse. Am J Physiol Heart Circ Physiol. 1997;273:H-1283–H-1290. doi: 10.1152/ajpheart.1997.273.3.H1283. [DOI] [PubMed] [Google Scholar]
- 42.Takimoto E, Soergel DG, Janssen PM, Stull LB, Kass DA, Murphy AM. Frequency- and afterload-dependent cardiac modulation in vivo by troponin I with constitutively active protein kinase A phosphorylation sites. Circ Res. 2004;94:496–504. doi: 10.1161/01.RES.0000117307.57798.F5. [DOI] [PubMed] [Google Scholar]
- 43.Thomas DP, Zimmerman SD, Hansen TR, Martin DT, McCormick RJ. Collagen gene expression in rat left ventricle: interactive effect of age and exercise training. J Appl Physiol. 2000;89:1462–1468. doi: 10.1152/jappl.2000.89.4.1462. [DOI] [PubMed] [Google Scholar]
- 44.Geenen D, Buttrick P, Scheuer J. Cardiovascular and hormonal responses to swimming and running in the rat. J Appl Physiol. 1988;65:116–123. doi: 10.1152/jappl.1988.65.1.116. [DOI] [PubMed] [Google Scholar]
- 45.Iemitsu M, Miyauchi T, Maeda S, Tanabe T, Takanashi M, Irukayama-Tomobe Y, Sakai S, Ohmori H, Matsuda M, Yamaguchi I. Aging-induced decrease in the PPAR-A level in hearts is improved by exercise training. Am J Physiol. 2002;283:H1750–H1760. doi: 10.1152/ajpheart.01051.2001. [DOI] [PubMed] [Google Scholar]
- 46.Judge S, Jang YM, Smith A, Selman C, Phillips T, Speakman JR, Hagen T, Leeuwenburgh C. Exercise by lifelong voluntary wheel running reduces subsarcolemmal and interfibrillar mitochondrial hydrogen peroxide production in the heart. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1564–R1572. doi: 10.1152/ajpregu.00396.2005. [DOI] [PubMed] [Google Scholar]