Abstract
Herein we report a metal-free method for the direct anti-Markovnikov hydroamination of unsaturated amines. Irradiation of the amine substrates with visible light in the presence of catalytic quantities of easily synthesized 9-mesityl-10-methylacridinium tetrafluoroborate and thiophenol as a hydrogen atom donor furnished the nitrogen containing heterocycles with complete regio-control. Two examples of in termolecular anti-Markovnikov alkene hydroamination are also disclosed.
The direct addition of N-H across an alkene1 provides an efficient, atom-economical route to highly valuable, biologically active nitrogen containing compounds.2 Considerable effort has been devoted to the development of catalyst systems for alkene hydroamination, with the majority of these strategies exhibiting preferential Markovnikov selectivity.3, 4 Thus, accessing anti-Markovnikov reactivity has proven quite challenging and considerably fewer reports exist in this arena. Catalytic intermolecular anti-Markovnikov olefin hydroamination reactions have been demonstrated using transition metals,5 alkaline earth metals,1, 5 and to a limited extent, photosensitizers.6, 7To our knowledge, there exists a single report of an intramolecular anti-Markovnikov hydroamination of styrenes reported by the Hartwig group in 2006 employing a Rh catalyst at elevated temperatures (eq. 1).8, 9 Recently, our group reported an anti-Markovnikov hydroalkoxylation reaction using a photocatalyst and hydrogen atom donor system.10 Given the paucity of intramolecular anti-Markovnikov hydroamination reports, we saw an opportunity to further demonstrate the utility of our catalytic strategy towards this end. Here, we report a metal-free, anti-Markovnikov hydroamination of unsaturated amines using 9-mesityl-10-methylacridinium and thiophenol as a hydrogen atom donor (Figure 1, eq. 2).
Figure 1.
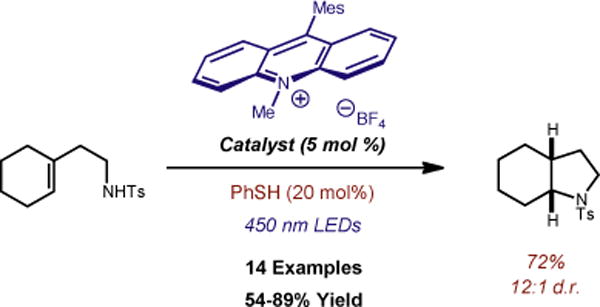
Catalytic Anti-Markovnikov Intramolecular Hydroamination
In our previously reported hydroalkoxylation reaction, we took advantage of the well-documented single electron oxidation of alkenes to provide unique radical cations that give rise to anti-Markovnikov reactivity.11–13 We proposed to apply this strategy to the hydroamination reaction, although we anticipated some challenges associated with amine oxidation.14 Sufficiently electron-rich amines are susceptible to oxidation at nitrogen and numerous groups have taken advantage of this reactivity.15While this pathway could lead to productive hydroamination, it may also result in undesirable side reactions stemming from amine cation radical intermediates. Judicious selection of the amine protecting group could circumvent these potential issues and for this reason, we elected to first examine the use of a sulfonyl group as it should be adequately withdrawing to suppress amine oxidation, yet still render the amine nucleophilic.
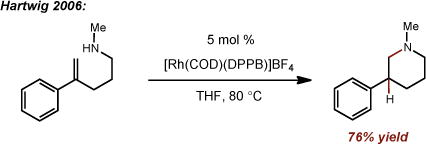 |
(1) |
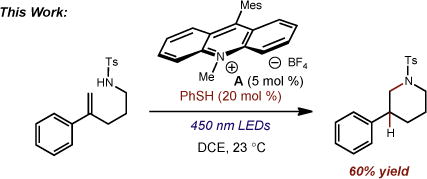 |
(2) |
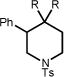
We began our studies by submitting the p-toluenesulfonyl-protected isoprenyl amine 9a to our previously reported conditions for the anti-Markovnikov hydroalkoxylation reaction. Despite the low yield obtained, complete anti-Markovnikov regioselection (>20:1) was observed in the formation of the desired pyrrolidine product after 3 days (16%, Table 1, entry 1). After additional efforts at reaction optimization (solvent, addition of organic and inorganic bases, concentration, etc.) failed to increase the reaction efficiency, we turned our attention to the identity of the hydrogen atom donor. While 9-cyanofluorene gave essentially the same result (entry 2) as did phenylmalononitrile, heteroatom hydrogen donor thiophenol provided a 2-fold increase in yield (entry 3).16 Upon decreasing the thiophenol loading to 20 mol %, we were pleased to find that pyrrolidine 9b could be obtained in a 70% yield as a single regioisomer (entry 4). To our knowledge, this result represents a rare example of an intramolecular anti-Markovnikov hydroamination of a non-activated olefin.3b, 4a
Table 1.
Optimization Studiesa
|
| ||||
|---|---|---|---|---|
|
| ||||
| Entry | R | H-Atom Donor | Time | Yieldb |
| 1 | Ts | 1.0 equiv PhCH(CN)2 | 72 | 16% |
| 2 | Ts | 1.0 equiv 9-Cyanofluorene | 72 | 12% |
| 3 | Ts | 1.0 equiv PhSH | 72 | 41% |
| 4 | Ts | 0.2 equiv PhSH | 96 | 70%c |
| 5 | H | 0.2 equiv PhSH | 96 | <5% |
| 6 | Bn | 0.2 equiv PhSH | 96 | 15% |
| 7 | Boc | 0.2 equiv PhSH | 96 | 65% |
| 8 | Ts | None | 96 | <5% |
| 9 | Ts | 0.2 equiv PhSH without photocatalyst | 24 | <5% |
| 10 | Ts | 0.2 equiv PhSH without light | 24 | <5% |
All reactions irradiated with a 15W 450 nm LED flood lamp.
Determined by 1H NMR analysis.
Isolated yield
Control experiments revealed that the thiophenol, light and photocatalyst were all necessary for productive reactivity (entries 8–10). The use of thiophenol as the hydrogen atom donor allowed us to explore the use of alternative common protecting groups for amines. While a benzyl protecting group afforded only small amounts of the pyrrolidine adduct (15% yield, entry 6), presumably due to the formation of numerous unidentified side products, we found that the Boc protecting group was suitable in this context (65% yield, entry 7). This supports our hypothesis that electron rich amines would be poor substrates as they are susceptible to oxidation. While the use of a Boc protecting group was quite appealing owing to the ease of its removal, we elected to evaluate tosylamine substrates for their straightforward characterization.
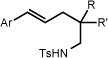
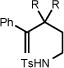
We next shifted our focus to the investigation of the alkene hydroamination reaction scope. Amine substrates bearing pendant styrenes underwent smooth 5-exo cyclization to furnish the corresponding regioisomerically pure pyrrolidines (entries 1–6). It should be noted that similar reactivity can be obtained with catalytic quantities of strong bases.17 The presence of electron releasing (-OMe) and withdrawing (-F) groups had little effect on the reaction efficiencies (entries 2–5). Substitution at the ortho position of the styrene was tolerated, giving desired pyrrolidine 4b in 69% yield (entry 4). Importantly, 6-endo cyclizations of 1,1 disubstituted styrenes to give tosylpiperidine products 7b and 8b also proceeded in good yields and with complete regiocontrol (entry 7, 8). We observed that geminal substitution in the backbone was not required for reactivity, and saw only slight decreases in yield as compared to their dimethyl substituted analogs, albeit longer reaction times were generally required (cf. entries 3&5; 7&8). For styrenyl substrates, the major byproduct was the cyclized deprotected product. Re-protection or deprotection of the reaction mixture can easily convert the remainder of the mass balance as desired.
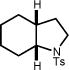
Inclusion of a stereocenter neighboring the amino group (10a) gave stereocontrol during the ring-forming event, albeit in modest levels (3:1 d.r., entry 10). We were pleased to find that a more geometrically-challenging 5-endo cyclization could be achieved employing unsaturated amine 11a to afford the fully-saturated indole derivative 11b in 72% yield and 12:1 dr (entry 11). Furthermore, the method is not limited to tosylamine as the sulfamate proved to be a competent nucleophile, givingaccess to a unique 6-exo cyclization (entry 12).
From the beginning of our studies, we presumed that the role of the thiophenol was to act as a hydrogen atom donor and the subsequent thiyl radical could serve to reoxidize the reduced form of A. To exclude alternative mechanistic pathways, we conducted several control experiments. We considered that thiophenol could participate in a thiol-ene reaction that could be catalyzed by the acridinium catalyst.18 Following the thiol-ene reaction, subsequent nucleophilic displacement of the resultant phenylthioether could furnish the observed products. However, this prospect seemed unlikely given that the limited examples of this reactivity require either strong exogenous base or elevated temperatures. To probe the potential involvement of this reaction pathway, we prepared Boc-protected unsaturated amine 13 and submitted it to the reaction conditions shown in eq 3. As we only observed unchanged starting material and no incorporation of thiophenol into the molecule, we believe that the thiol-ene pathway is likely not operative in this transformation.
We also considered that thiophenol was acting solely as a hydrogen atom shuttle.19 In this context, we submitted isoprenyl amine 9a to thiophenol with either di-tert-butyl peroxide or AIBN as thermal radical initiators (Conditions A and B, eq 4). No reactivity was observed in either case, suggesting that formation of nitrogen-centered radical intermediates was also unlikely.
 |
(3) |
 |
(4) |
Finally, after the observation of varying quantities of PhSSPh in the crude reaction mixtures, we questioned whether this byproduct was active in the catalytic cycle. Subjection of 9a to the reaction conditions employing PhSSPh instead of PhSH afforded the anti-Markovnikov hydroamination product in 55% yield (eq 5). While this observation is not fully understood at this time, it is conceivable that diphenyl disulfide could serve as a reservoir of phenyl thiyl radical via oxidation of the disulfide (Epox = +1.51 V vs. SCE)20 and subsequent fragmentation. It is possible that the phenyl thiyl radical then can act as an oxidant for the reduced form of catalyst A in a manner similar to the mechanism invoked in our prior communication.10
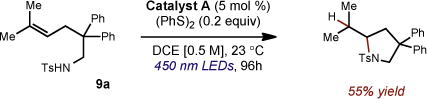 |
(5) |
Based on these experiments and the reactivity observed in this study, we have developed the working mechanistic hypothesis depicted in Scheme 1. After oxidation of the unsaturated amine (9a) by the excited state of the catalyst (A*), anti-Markovnikov addition of the amine would furnish intermediate cation radical 14. Hydrogen atom transfer from thiophenol to 14 would furnish the desired amine heterocycle (9a) after proton loss. The subsequent thiyl radical (15) could serve as an oxidant for 14 to reset catalyst A and generate thiophenoxide anion. Given the known reduction potential of 16 (Ep = +0.45 V vs. SCE)21 and the oxidation potential of 15 (E1/2 = −0.57 V vs. SCE),22 we estimate this electron transfer should be exergonic. Thiophenoxide then should serve as a mild base to neutralize the acid generated during the course of the reaction.
Scheme 1.
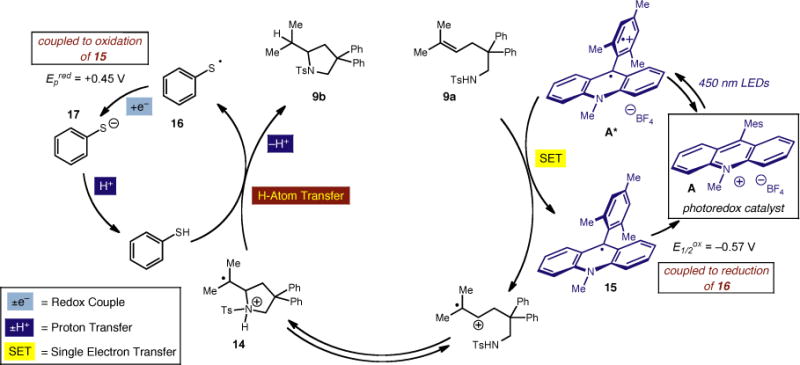
Working Mechanism for Direct Anti-Markovnikov Hydroamination of Alkenes
We were pleased to find that this protocol could be extended to include examples of in termolecular alkene hydroamination (eqns 6 & 7). Treatment of a styrenyl and alkenyl substrate with 3.0 equiv trifylamide under our standard reaction conditions with the addition of 0.25 equiv of 2,6-lutidine gave the desired products in modest yields as single regioisomers. To our knowledge, this represents the first example of an organocatalytic intermolecular anti-Markovnikov alkene hydroamination.
 |
(6) |
 |
(7) |
In conclusion, we have reported an intramolecular anti-Markovnikov hydroamination method using catalytic amounts of thiophenol and an organic photocatalyst promoted by visible light. The reaction conditions are mild and effect a range of cyclization modes to give important nitrogen-containing heterocycles. We have also demonstrated that this protocol can be extended to inter-mo lecular reactions. Efforts to further understand the mechanism of this transformation are currently underway.
Supplementary Material
Table 2.
Scope of the Intramolecular Anti-Markovnikov Hydroamination Reactions of Unsaturated Aminesa
|
| ||||
|---|---|---|---|---|
|
| ||||
| Entry | Alkenol | Product | Time | Yield |
|
|
|||
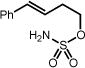
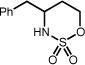
| 1 | 1a Ar = C6H5, R, R′ = Me | 1b | 24h | 82% |
| 2 | 2a Ar = 4-(F)C6H4, R, R′ = Me | 2b | 24h | 89% |
| 3 | 3a Ar = 4-(MeO)C6H4, R, R′ = Me | 3b | 30h | 88% |
| 4 | 4a Ar = 2-(MeO)C6H4, R, R′ = Me | 4b | 40h | 69% |
| 5 | 5a Ar = 4-(MeO)C6H4, R, R′ = H | 5b | 48h | 79% |
| 6 | 6a Ar = 4-(MeO)C6H4, R = H, R′ = i-Pr | 6b | 39h | 88% |
|
|
1.6:1 d.r.b | ||
| 7 | 7a R = Me | 7b | 48h | 79% |
| 8 | 8a R = H | 8b | 96h | 60% |
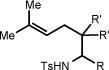
|

|
|||
| 9 | 9a R = H; R′ = Ph | 9b | 96h | 70% |
| 10 | 10a R = Me; R′ = H | 10b | 96h | 56% |
|
|
|
3:1 d.r.b | ||
| 11 | 11a | 11b | 96h | 72% |
|
|
12:1 d.r.b | ||
| 12 | 12a | 12b | 96h | 54% |
All reactions irradiated with a 15W 450 nm LED flood lamp. Reported as isolated yields, average of two trials.
Determined by 1H NMR analysis of the crude reaction mixtures.
Acknowledgments
The authors are grateful to M. Geier for aid in obtaining the cyclic voltammograms and N. Manohar for the preparation of some of the unsaturated amine substrates. The project described was supported by Award No. R01 GM098340 from the National Institute of General Medical Sciences, UNC-CH and an Eli Lilly New Faculty Award.
Footnotes
Supporting Information Placeholder
ASSOCIATED CONTENT
Experimental procedures and spectral data. This material is available free of charge via the Internet at http://pubs.acs.org.
Funding Sources
The authors declare no competing financial interests.
References
- 1.Müller TE, Hultzsch KC, Yus M, Foubelo F, Tada M. Chem Rev. 2008;108:3795. doi: 10.1021/cr0306788. [DOI] [PubMed] [Google Scholar]
- 2.O’Hagan D. Nat Prod Rep. 2000;17:435. doi: 10.1039/a707613d. [DOI] [PubMed] [Google Scholar]
- 3.For selected examples of intramolecular Markovnikov hydroaminations catalyzed by complexes of group IV metals and lanthanides, see:; (a) Manna K, Xu S, Sadow AD. Angew Chem, Int Ed. 2011;50:1865. doi: 10.1002/anie.201006163. [DOI] [PubMed] [Google Scholar]; (b) Leitch DC, Payne PR, Dunbar CR, Schafer LL. J Am Chem Soc. 2009;131:18246. doi: 10.1021/ja906955b. [DOI] [PubMed] [Google Scholar]; (c) Wood MC, Leitch DC, Yeung CS, Kozak JA, Schafer LL. Angew Chem, Int Ed. 2007;46:354. doi: 10.1002/anie.200603017. [DOI] [PubMed] [Google Scholar]; (d) Watson DA, Chiu M, Bergman RG. Organometallics. 2006;25:4731. doi: 10.1021/om0606791. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Gribkov DV, Hultzsch KC, Hampel F. J Am Chem Soc. 2006;128:3748. doi: 10.1021/ja058287t. [DOI] [PubMed] [Google Scholar]; (f) Kim JY, Livinghouse T. Org Lett. 2005;7:1737. doi: 10.1021/ol050294z. [DOI] [PubMed] [Google Scholar]; (g) Hong S, Marks T. J Acc Chem Res. 2004;37:673. doi: 10.1021/ar040051r. [DOI] [PubMed] [Google Scholar]; (h) Hong S, Tian S, Metz MV, Marks TJ. J Am Chem Soc. 2003;125:14768. doi: 10.1021/ja0364672. [DOI] [PubMed] [Google Scholar]
- 4.For selected examples of intramolecular Markovnikov hydroaminations catalyzed by late transition metals and lanthanides, see:; (a) Chapurina Y, Ibrahim H, Guillot R, Kolodziej E, Collin J, Trifonov A, Schultz E, Hannedouche J. J Org Chem. 2011;76:10163. doi: 10.1021/jo202009q. [DOI] [PubMed] [Google Scholar]; (b) Shen X, Buchwald SL. Angew Chem, Int Ed. 2010;49:564. doi: 10.1002/anie.200905402. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Julian LD, Hartwig JF. J Am Chem Soc. 2010;132:13813. doi: 10.1021/ja1052126. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Ohmiya H, Moriya T, Sawamura M. Org Lett. 2009;11:2145. doi: 10.1021/ol9007712. [DOI] [PubMed] [Google Scholar]; (e) Hesp KD, Tobisch S, Stradiotto M. J Am Chem Soc. 2009;132:413. doi: 10.1021/ja908316n. [DOI] [PubMed] [Google Scholar]; (f) Liu Z, Hartwig JF. J Am Chem Soc. 2008;130:1570. doi: 10.1021/ja710126x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Cochran BM, Michael FE. J Am Chem Soc. 2008;130:2786. doi: 10.1021/ja0734997. [DOI] [PubMed] [Google Scholar]; (h) Michael FE, Cochran BM. J Am Chem Soc. 2006;128:4246. doi: 10.1021/ja060126h. [DOI] [PubMed] [Google Scholar]; (i) Han X, Widenhoefer RA. Angew Chem, Int Ed. 2006;45:1747. doi: 10.1002/anie.200600052. [DOI] [PubMed] [Google Scholar]; (j) Bender CF, Widenhoefer RA. J Am Chem Soc. 2005;127:1070. doi: 10.1021/ja043278q. [DOI] [PubMed] [Google Scholar]; For selected examples of intermolecular Markovnikov hydroaminations, see:; (k) Zhou J, Hartwig JF. J Am Chem Soc. 2008;130:12220. doi: 10.1021/ja803523z. [DOI] [PubMed] [Google Scholar]; (l) McBee JL, Bell AT, Tilley TD. J Am Chem Soc. 2008;130:16562. doi: 10.1021/ja8030104. [DOI] [PubMed] [Google Scholar]; (m) Brunet J-J, Chu N-C, Rodriguez-Zubiri M. Eur J Inorg Chem. 2007:4711. [Google Scholar]; (n) Zhang J, Yang C-G, He C. J Am Chem Soc. 2006;128:1798. doi: 10.1021/ja053864z. [DOI] [PubMed] [Google Scholar]; (o) Rosenfeld DC, Shekhar S, Takemiya A, Utsunomiya M, Hartwig JF. Org Lett. 2006;8:4179. doi: 10.1021/ol061174+. [DOI] [PubMed] [Google Scholar]; (p) Dorta R, Egli P, Zurcher F, Togni A. J Am Chem Soc. 1997;119:10857. [Google Scholar]; (q) Casalnuovo AL, Calabrese JC, Milstein D. J Am Chem Soc. 1988;110:6738. [Google Scholar]
- 5.For selected examples of intermolecular anti-Markovnikov hydroaminations, see:; (a) Utsunomiya M, Kuwano R, Kawatsura M, Hartwig JF. J Am Chem Soc. 2003;125:5608. doi: 10.1021/ja0293608. [DOI] [PubMed] [Google Scholar]; (b) Munro-Leighton C, Blue ED, Gunnoe TB. J Am Chem Soc. 2006;128:1446. doi: 10.1021/ja057622a. [DOI] [PubMed] [Google Scholar]; (c) Fadini L, Togni A. Chem Commun. 2003:30. doi: 10.1039/b210680a. [DOI] [PubMed] [Google Scholar]; (d) Seligson AL, Trogler WC. Organometallics. 1993;12:744. [Google Scholar]; (e) Castonguay A, Spasyuk DM, Madern N, Beauchamp AL, Zargarian D. Organometallics. 2009;28:2134. [Google Scholar]; (f) Kawatsura M, Hartwig JF. Organometallics. 2001;20:1960. [Google Scholar]; (g) Beller M, Trauthwein H, Eichberger M, Breindl C, Herwig J, Müller TE, Thiel OR. Chem Eur J. 1999;5:1306. [Google Scholar]; (h) Ryu J-S, Li GY, Marks TJ. J Am Chem Soc. 2003;125:12584. doi: 10.1021/ja035867m. [DOI] [PubMed] [Google Scholar]; (i) Utsunomiya M, Hartwig JF. J Am Chem Soc. 2004;126:2702. doi: 10.1021/ja031542u. [DOI] [PubMed] [Google Scholar]; (j) Takaya J, Hartwig JF. J Am Chem Soc. 2005;127:5756. doi: 10.1021/ja0506410. [DOI] [PubMed] [Google Scholar]; (k) Munro-Leighton C, Delp SA, Alsop NM, Blue ED, Gunnoe TB. Chem Commun. 2008;1:111. doi: 10.1039/b715507g. [DOI] [PubMed] [Google Scholar]; (l) Brinkmann C, Barrett AGM, Hill MS, Procopiou PA. J Am Chem Soc. 2012;134:2193. doi: 10.1021/ja209135t. [DOI] [PubMed] [Google Scholar]
- 6.Yasuda M, Yamashita T, Shima K, Pac C. J Org Chem. 1987;52:753. [Google Scholar]
- 7.For a recent example of a formal intermolecular anti-Markovnikov alkene hydroamination, see:; Rucker R, Whittaker AM, Dang H, Lalic G. J Am Chem Soc. 2012;134:6571. doi: 10.1021/ja3023829. [DOI] [PubMed] [Google Scholar]
- 8.Takemiya A, Hartwig JF. J Am Chem Soc. 2006;128:6042. doi: 10.1021/ja058299e. [DOI] [PubMed] [Google Scholar]
- 9.For examples of formal intramolecular anti-Markovnikov alkene hydroaminations, see:; (a) Pronin SV, Tabor MG, Jansen DJ, Shenvi RA. J Am Chem Soc. 2012;134:2012. doi: 10.1021/ja211090n. [DOI] [PubMed] [Google Scholar]; (b) Shirai M, Brebion F, Rumthao S, Crich D. Tetrahedron. 2006;62:6501. [Google Scholar]
- 10.Hamilton DS, Nicewicz DA. J Am Chem Soc. 2012;134:18577. doi: 10.1021/ja309635w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnold DR, Chan MSW, McManus KA. Can J Chem. 1996;74:2143. [Google Scholar]
- 12.Gassman PG, Bottorff KJ. J Am Chem Soc. 1987;109:7547. [Google Scholar]
- 13.Mangion D, Arnold DR. Acc Chem Res. 2002;35:297. doi: 10.1021/ar010108z. [DOI] [PubMed] [Google Scholar]
- 14.For reactions of amines with electrochemically-generated alkene cation radicals, see:; (a) Ashikari Y, Nokami T, Yoshida J. J Am Chem Soc. 2011;133:11840. doi: 10.1021/ja202880n. [DOI] [PubMed] [Google Scholar]; (b) Campbell JM, Xu H, Moeller KD. J Am Chem Soc. 2012;134:18338. doi: 10.1021/ja307046j. [DOI] [PubMed] [Google Scholar]; (c) Xu H, Moeller KD. Org Lett. 2010;12:5174. doi: 10.1021/ol102193x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Xu H, Moeller KD. J Am Chem Soc. 2010;132:2839. doi: 10.1021/ja910586v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Xu H, Moeller KD. J Am Chem Soc. 2008;130:13542. doi: 10.1021/ja806259z. [DOI] [PubMed] [Google Scholar]
- 15.(a) Furst L, Matsuura BS, Narayanam JMR, Tucker JW, Stephenson CRJ. Org Lett. 2010;12:3104. doi: 10.1021/ol101146f. [DOI] [PubMed] [Google Scholar]; (b) Condie AG, González-Gómez JC, Stephenson CRJ. J Am Chem Soc. 2010;132:1464. doi: 10.1021/ja909145y. [DOI] [PubMed] [Google Scholar]; (c) Dai C, Meschini F, Narayanam JMR, Stephenson CRJ. J Org Chem. 2012;77:4425. doi: 10.1021/jo300162c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Zhu S, Das A, Bui L, Zhou H, Curran DP, Rueping M. J Am Chem Soc. 2013;135:1823. doi: 10.1021/ja309580a. [DOI] [PubMed] [Google Scholar]; (e) Hari DP, König B. Org Lett. 2011;13:3852. doi: 10.1021/ol201376v. [DOI] [PubMed] [Google Scholar]; (f) Fu W, Guo W, Zou G, Xu C. J Fluorine Chem. 2012;140:88. [Google Scholar]
- 16.(a) Deeb TM, Newcomb M. J Am Chem Soc. 1987;109:3163. [Google Scholar]; (b) Newcomb M, Weber KA. J Org Chem. 1991;56:1309. [Google Scholar]
- 17.For lead references, see:; (a) Tokuda M, Miyamoto T, Fujita H, Suginome H. Tetrahedron. 1991;47:747. [Google Scholar]; (b) Tokuda M, Fujita H, Miyamoto T, Suginome H. Tetrahedron. 1993;49:2413. [Google Scholar]; For an excellent recent enantioselective example, see:; (c) Zhang X, Emge TJ, Hultzsch KC. Angew Chem Int Ed. 2012;51:394. doi: 10.1002/anie.201105079. [DOI] [PubMed] [Google Scholar]
- 18.Tyson EL, Ament MS, Yoon TP. J Org Chem. 2013;78:2046. doi: 10.1021/jo3020825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts BP. Chem Soc Rev. 1999;28:25. [Google Scholar]
- 20.Value corrected from SHE:; Töteberg-Kaulen S, Steckhan E. Tetrahedron. 1988;44:4389. [Google Scholar]
- 21.Armstrong DA, Sun Q, Schuler RH. J Phys Chem. 1996;100:9892. [Google Scholar]
- 22.(a) Fukuzumi S, Kotani H, Ohkubo K, Ogo S, Tkachenko NV, Lemmetyinen H. J Am Chem Soc. 2004;126:1600. doi: 10.1021/ja038656q. [DOI] [PubMed] [Google Scholar]; (b) Ohkubo K, Mizushime K, Iwata R, Souma K, Suzuki S, Fukuzumi S. Chem Commun. 2010;46:601. doi: 10.1039/b920606j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


