Diverse FcγR-dependent mechanisms mediate anticancer activity of proapoptotic and immunomodulatory antibodies.
Abstract
Antibodies have important roles in controlling cellular immunity through interaction with activating or inhibitory Fcγ receptors (FcγRs). FcγR engagement can facilitate receptor cross-linking on target cells, or induce retrograde FcγR signals to stimulate or suppress antibody-dependent, cell-mediated depletion of antigen-bearing target cells. Recent studies uncover unexpectedly important roles for FcγRs in the anticancer action of antibodies designed to trigger tumor cell apoptosis or enhance antitumor immunity. Here, we outline a conceptual framework for understanding these findings and discuss their mechanistic and translational implications.
The adaptive immune system is capable of recognizing an essentially limitless number of antigens via the combinatorial assembly of gene segments that encode antibody variable domains. This diversity has been exploited successfully in a growing number of therapeutic antibodies that bind to a wide range of clinically validated targets. Antibodies that recognize soluble antigens, such as the cytokines tumor necrosis factor (TNF), vascular endothelial growth factor, or interleukin-6, act as antagonists by blocking the interaction of a target ligand with its cognate receptor. In some cases, such antibodies may also augment clearance of the target antigen.
In contrast, antibodies that bind to cell surface antigens, often transmembrane receptors such as HER2, EGFR, or DR5, may act as antagonists or agonists, respectively, to block or stimulate the action of the cognate target. Alternatively, antibodies may bind a cell surface target that lacks signaling function, such as the CD20 antigen, and act as an anchor for FcγR-based recruitment of immune-effector cells to kill the antigen-expressing target by antibody-dependent, cell-mediated cytotoxicity (ADCC). Therefore, antibodies that recognize cell surface receptors can be categorized by their function of either mediating target cell killing or modulating target receptor signal transduction. However, two new studies in this issue demonstrate that these activities are not mutually exclusive and that antibodies harboring both properties may be advantageous for cancer immunotherapy. Due to shared expression of cell surface antigens, such as CTLA-4 or glucocorticoid-induced TNFR-related protein (GITR) on protumorigenic regulatory T (T reg) cells and antitumorigenic effector T (T eff) cells, antibodies that target such receptors are capable of inducing antitumor immunity both by depleting T reg cells and by stimulating T eff cells. However, antibodies that conform to this dual mechanism of action have the risk of depleting T eff cells, which are the final mediators of tumor cell killing. Therefore, understanding the principles that govern antibody–FcγR interactions is crucial for designing effective antibody-based immunotherapies.
Antibody–FcγR interactions
FcγRs fall into two functional classes: activating and inhibitory (Nimmerjahn and Ravetch, 2006). The FcγR family comprises three activating (mouse FcγRI, FcγRIII, and FcγRIV; human FcγRI, FcγRIIA, and FcγRIIIA) and one inhibitory (FcγRIIB) receptor. Activating FcγRs associate with a common signaling chain (FcRγ), containing an immunoreceptor tyrosine-based activation motif (ITAM) that recruits Syk family kinases to stimulate effector function. In contrast, FcγRIIB contains an immunoreceptor tyrosine-based inhibitory motif (ITIM) that recruits specific phosphatases to oppose signaling by activating FcγRs. Innate-immune cells, such as macrophages, monocytes, dendritic cells, mast cells, and granulocytes, express both activating and inhibitory FcγRIIB (Amigorena et al., 1992; Nimmerjahn and Ravetch, 2008). IgG subtypes differ in FcγR affinity: human IgG1 and IgG3 have higher affinity for activating than inhibitory FcγR, as do mouse IgG2a and IgG2b (Dijstelbloem et al., 2001; Nimmerjahn and Ravetch, 2005, 2006).
Antagonist antibodies may bind to a soluble ligand or a cell surface receptor to prevent signaling. Target inhibition per se typically does not require accessory FcγR-bearing cells; therefore, antagonist antibodies often act independently of FcγRs, and accordingly, IgG subtype. However, if the target is engaged at the cell surface and is sufficiently abundant, effector cells may be recruited via Fc–FcγR interactions to deplete the antigen-displaying cell, an outcome that can be desirable or undesirable depending on the context. Target cell depletion can be manipulated by selecting IgG subtypes that favor binding to activating or inhibitory FcγRs. Unwanted target cell depletion can be minimized by incorporating Fc mutations that decrease FcγR affinity (Presta et al., 2002; Carter, 2006; Lazar et al., 2006; Satoh et al., 2006; Jefferis, 2009). For example, asparagine 297, the site for N-linked glycosylation required for FcγR binding in the constant region, can be replaced by alanine. Further mutations to enhance or decrease specific FcγR interactions have also been reported. Alternatively, some antibody variants can be produced in Escherichia coli rather than mammalian cells to prevent Fc glycosylation. Fc effectorless antibodies have been demonstrated to be equally as potent at blocking ligand–receptor interactions as their wild-type counterparts.
Recent work has revealed unexpectedly that agonist antibodies designed to stimulate the tumor necrosis factor receptor superfamily (TNFRSF) members DR4, DR5, or CD40 depend on FcγR interaction for robust agonist activity (Li and Ravetch, 2011; Wilson et al., 2011; Smith et al., 2012). As TNFRSF members usually require ligand-induced super-clustering for signal transmission, bivalent IgG molecules are unable to induce their efficient stimulation. In vitro activity can be enhanced by artificial cross-linking of the primary antibody, with secondary anti-Fc antibodies, or—perhaps more importantly—by providing contact with FcγR-bearing cells. Pretreating FcγR-expressing cells with actin polymerization inhibitors blocks this enhancement, suggesting that FcγR clustering is important for antibody-mediated stimulation of the target receptor (Wilson et al., 2011). Studies with mice deficient in specific FcγR subsets demonstrate that expression of the inhibitory FcγRIIB is sufficient—if not superior—for enabling in vivo efficacy of agonist antibodies targeting CD40, or the death receptors DR4 and DR5 (Li and Ravetch, 2011; Wilson et al., 2011). Reliance on FcγRIIB circumvents the potential for FcγR-mediated target cell depletion and is therefore advantageous for inducing signal transduction on target cells, such as CD40 signaling in dendritic cells. CD40 engagement on dendritic cells enhances their antigen presentation capabilities, thereby increasing T cell responses. Therefore, cross-linking CD40 via FcγRIIB on DCs enhances their T cell priming function, while minimizing depletion. Importantly, anti-CD40–induced adjuvant activity was unabated in mice deficient in activating FcγRs, yet was abrogated in FcγRIIB-deficient mice (Li and Ravetch, 2011). However, FcγR-dependent depletion of DR4- or DR5-expressing tumor cells could be useful to induce antitumor activity. These findings suggest that agonist antibodies targeting either proapoptotic or co-stimulatory TNFRSF members can rely on FcγRs as a dynamic cross-linking scaffold—a function that, at least in mice, may be supported more effectively by FcγRIIB.
Agonist GITR antibodies require activating FcγRs for antitumor efficacy
In this issue, Bulliard et al. report an apparent exception to the latter paradigm: agonist antibodies targeting the TNFRSF family member GITR require activating FcγR effector function to promote tumor regression in mice. GITR expression is induced on T eff cells upon T cell receptor (TCR) stimulation, and GITR cross-linking by GITR ligand or agonist antibodies co-stimulates TCR signaling (McHugh et al., 2002; Shimizu et al., 2002; Tone et al., 2003; Ronchetti et al., 2004; Stephens et al., 2004). In transplantable tumor models, anti-GITR treatment is hypothesized to induce tumor regression through T eff cell co-stimulation (Turk et al., 2004; Ko et al., 2005). Distinct from antibodies that target other TNFR superfamily members, anti-GITR activity was unaltered in Fcgr2b−/− mice. However, anti-GITR treatment was ineffective in knockout mice lacking the ITAM-containing FcγR chain required for signaling by all activating FcγRs. Hence, an alternative (or additional) mechanism of action—distinct from FcγRIIB-mediated GITR cross-linking to promote co-stimulation—may be critical for antitumor efficacy. Given that GITR-expressing CD8+ T cells and CD4+ T eff cells are needed for tumor cell killing, this mechanism may involve FcγR-dependent depletion of GITR-expressing T reg cells.
T reg cells frequently express T cell activation markers induced by TCR signaling (Gavin et al., 2002; McHugh et al., 2002). In addition to their well-characterized role in maintaining peripheral tolerance to self-antigens, T reg cells have been demonstrated to suppress tumor immunity (Nishikawa and Sakaguchi, 2010; Josefowicz et al., 2012). T reg cells are highly enriched in tumors, both in mouse models and in various human cancers. Furthermore, in cancer patients, abundance of T reg cells within tumors is associated with poor prognosis, suggesting that these cells play an important role in suppressing antitumor immunity. Therefore, strategies to deplete intratumoral T reg cells may enhance the generation of tumor-directed T eff cell responses.
GITR is weakly expressed on naive T eff cells, but is present on resting T reg cells and up-regulated on activated T eff cells. Bulliard et al. (2013) observed a significant reduction in tumor-associated, but not peripheral, T reg cells upon anti-GITR treatment of tumor-bearing mice. Hence, anti-GITR antibodies may enhance antitumor immunity by depleting GITR-positive T reg cells, in addition to co-stimulating antigen-experienced T eff cells (Coe et al., 2010). However, the shared expression of GITR on T reg and T eff cells makes both populations potentially susceptible to antibody-dependent, FcγR-mediated depletion. Indeed, diminished effector CD4+ and CD8+ T cell numbers were reported to accompany T reg cell depletion in tumor tissues. Therefore, elimination of intratumoral T reg cells, coupled with T eff cell co-stimulation and minimal T eff cell depletion, might provide an integrated mechanism of action for anti-GITR antibodies. The relative contribution of each of these mechanistic components remains to be clarified.
CTLA-4 blocking antibodies mediate T reg cell depletion
CTLA-4 is an inhibitory receptor that is induced on antigen-experienced T eff cells as a negative feedback regulator (Walunas et al., 1994; Krummel and Allison, 1995). Whereas CTLA-4 expression is induced on activated T eff cells, CTLA-4 is constitutively expressed by T reg cells, which require CTLA-4 function to aggregate preferentially around dendritic cells and inhibit their antigen-presenting activity (Onishi et al., 2008). As such, the multiorgan autoimmunity detected in Ctla4 germline knockout mice is phenocopied in T reg cell–specific Ctla4 conditional knockout mice (Tivol et al., 1995; Waterhouse et al., 1995; Chambers et al., 1997; Wing et al., 2008). Although anti–CTLA-4 therapy is one of the first clinically validated examples of effective cancer immunotherapy, its mechanism of action is poorly understood (Hodi et al., 2010). It is hypothesized that antagonist anti–CTLA-4 antibodies enhance tumor immunity by relieving inhibitory CTLA-4 signals on antigen-experienced T eff cells, as well as curtailing the suppressive function of T reg cells. Following the paradigm of antagonist antibodies, CTLA-4–blocking antibodies would be expected to exert their efficacy in the absence of Fc effector function.
However, studies from Bulliard et al. (2013) and Simpson et al. in this issue provide compelling evidence that the activity of anti–CTLA-4 antibodies requires activating FcγR engagement to deplete T reg cells. Similar to anti-GITR therapy, anti–CTLA-4–mediated tumor regression was abrogated in FcγR-deficient mice, suggesting that retrograde signaling by activating FcγRs and consequent T reg cell depletion is important for antitumor activity. Accordingly, intratumoral T reg cell numbers were dramatically reduced in response to anti–CTLA-4 treatment. Importantly, this reduction was not associated with dedifferentiation or impaired generation of T reg cells, thereby supporting cellular depletion as the most plausible mechanism of efficacy.
Because activated T eff cells express CTLA-4, they are also susceptible to antibody-mediated depletion. In this regard, Bulliard et al. (2013) demonstrated that both T reg and T eff cell numbers were reduced in response to anti–CTLA-4 treatment. In contrast, Simpson et al. (2013) observed that in an adoptive T cell transfer model, intratumoral T eff cells were not depleted by anti–CTLA-4 treatment, but rather increased in number. Differences in tumor model, antibody isotype or clone, or vaccine co-administration could potentially account for these discrepant results. Nevertheless, T reg cells may be more sensitive to antibody-mediated depletion because of their increased expression of CTLA-4 within tumor tissues. Consistent with this notion, tumor-infiltrating T reg cells expressed nearly fourfold higher CTLA-4 levels than did tumor-associated T eff cells in the Colon26 carcinoma model. Additionally, in the B16-BL6 melanoma model, CTLA-4 surface expression was slightly elevated in tumor-associated versus peripheral T reg cells or intratumoral T eff cells. It remains to be investigated whether this variation in receptor expression is detected in human intratumoral T cells and leads to differential depletion in cancer patients.
Conclusions
Important roles have emerged for FcγRs in mediating the antitumor efficacy of a new generation of therapeutic antibodies aiming to attack cancer directly, by triggering tumor cell apoptosis, or indirectly, by enhancing antitumor immunity (Fig. 1). First, FcγRs on tumor-infiltrating innate immune cells provide a cross-linking scaffold to enhance antibody-mediated activation of proapoptotic TNFRSF members such as DR4 and DR5 on cancer cells. Similarly, FcγR-mediated cross-linking supports activation of T eff cells via co-stimulatory TNFRSF members such as GITR. In some instances, this cross-linking function can be performed redundantly by activating or inhibitory FcγRs; however, mouse studies suggest that the inhibitory FcγRIIB may provide a more effective molecular scaffold, for reasons that have yet to be defined. Fc mutations that enhance affinity for FcγRs may serve to improve the efficacy of agonist antibodies targeting various TNFRSF members. In addition to cross-linking cell surface receptors to support forward signaling in target cells, FcγRs can mediate antibody-driven reverse signaling to activate FcγR-bearing cells and promote target cell depletion. For cancer immunotherapy, antibodies that fulfill both functions may be beneficial to deplete immunosuppressive T reg cells and stimulate T eff cells. The high prevalence of T reg cells in tumor tissues poses a significant barrier to generating a productive antitumor T cell response. Expression of antigens such as GITR or CTLA-4 on T reg cells affords the opportunity for enhanced antibody-based depletion of such immune-suppressive cells from the tumor microenvironment, thereby augmenting antitumor immunity. A potential caveat is that desirable T eff cells also might express the same antigens and hence be subject to similar depletion. Thus, maximal therapeutic efficacy likely necessitates a delicate balance between depleting T reg cells while sparing T eff cells. Defining the FcγR interactions responsible for these different outcomes should help optimize the effectiveness of antibodies to biologically relevant T cell antigens, including GITR, CTLA-4, and beyond.
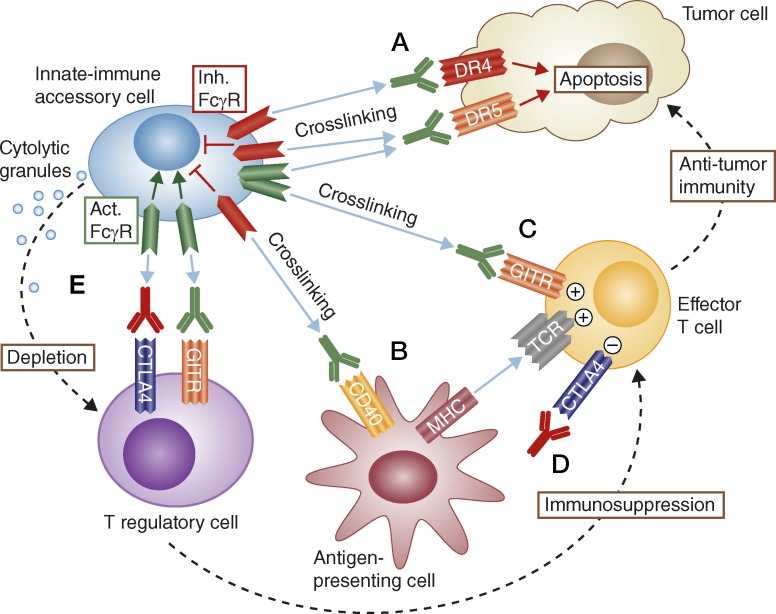
Figure 1.
How Fcγ receptors enable anticancer efficacy of proapoptotic and immune-modulatory antibodies. Inhibitory (Inh.) or activating (Act.) FcγRs can provide a dynamic scaffold for cross-linking agonist antibodies targeting proapoptotic receptors such as DR4 or DR5 on the surface of cancer cells, thereby promoting direct tumor cell apoptosis (A). Similarly, FcγRs can support agonist antibody-mediated cross-linking of immune cell co-stimulatory receptors, e.g., CD40 on antigen-presenting cells (B), or GITR on T eff cells (C), to augment antitumor immunity. T eff cell responses can be enhanced further, through direct antibody-based antagonism of the inhibitory molecule CTLA-4 (D). Finally, activating FcγRs can promote ADCC-based depletion of T reg cells upon antibody binding to GITR or CTLA-4, thus alleviating T reg suppression and strengthening antitumor immunity (E).
References
- Amigorena S., Bonnerot C., Drake J.R., Choquet D., Hunziker W., Guillet J.G., Webster P., Sautes C., Mellman I., Fridman W.H. 1992. Cytoplasmic domain heterogeneity and functions of IgG Fc receptors in B lymphocytes. Science. 256:1808–1812 10.1126/science.1535455 [DOI] [PubMed] [Google Scholar]
- Bulliard Y., Jolicouer R., Windman M., Rue S.M., Ettenberg S., Knee D.A., Wilson N.S., Dranoff G., Brogdon J.L. 2013. Activating Fc γ receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. J. Exp. Med. 210:1685–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P.J. 2006. Potent antibody therapeutics by design. Nat. Rev. Immunol. 6:343–357 10.1038/nri1837 [DOI] [PubMed] [Google Scholar]
- Chambers C.A., Sullivan T.J., Allison J.P. 1997. Lymphoproliferation in CTLA-4-deficient mice is mediated by costimulation-dependent activation of CD4+ T cells. Immunity. 7:885–895 10.1016/S1074-7613(00)80406-9 [DOI] [PubMed] [Google Scholar]
- Coe D., Begom S., Addey C., White M., Dyson J., Chai J.G. 2010. Depletion of regulatory T cells by anti-GITR mAb as a novel mechanism for cancer immunotherapy. Cancer Immunol. Immunother. 59:1367–1377 10.1007/s00262-010-0866-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijstelbloem H.M., van de Winkel J.G., Kallenberg C.G. 2001. Inflammation in autoimmunity: receptors for IgG revisited. Trends Immunol. 22:510–516 10.1016/S1471-4906(01)02014-2 [DOI] [PubMed] [Google Scholar]
- Gavin M.A., Clarke S.R., Negrou E., Gallegos A., Rudensky A. 2002. Homeostasis and anergy of CD4(+)CD25(+) suppressor T cells in vivo. Nat. Immunol. 3:33–41 10.1038/ni743 [DOI] [PubMed] [Google Scholar]
- Hodi F.S., O’Day S.J., McDermott D.F., Weber R.W., Sosman J.A., Haanen J.B., Gonzalez R., Robert C., Schadendorf D., Hassel J.C., et al. 2010. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363:711–723 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferis R. 2009. Glycosylation as a strategy to improve antibody-based therapeutics. Nat. Rev. Drug Discov. 8:226–234 10.1038/nrd2804 [DOI] [PubMed] [Google Scholar]
- Josefowicz S.Z., Lu L.F., Rudensky A.Y. 2012. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 30:531–564 10.1146/annurev.immunol.25.022106.141623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko K., Yamazaki S., Nakamura K., Nishioka T., Hirota K., Yamaguchi T., Shimizu J., Nomura T., Chiba T., Sakaguchi S. 2005. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells. J. Exp. Med. 202:885–891 10.1084/jem.20050940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummel M.F., Allison J.P. 1995. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 182:459–465 10.1084/jem.182.2.459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar G.A., Dang W., Karki S., Vafa O., Peng J.S., Hyun L., Chan C., Chung H.S., Eivazi A., Yoder S.C., et al. 2006. Engineered antibody Fc variants with enhanced effector function. Proc. Natl. Acad. Sci. USA. 103:4005–4010 10.1073/pnas.0508123103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Ravetch J.V. 2011. Inhibitory Fcγ receptor engagement drives adjuvant and anti-tumor activities of agonistic CD40 antibodies. Science. 333:1030–1034 10.1126/science.1206954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh R.S., Whitters M.J., Piccirillo C.A., Young D.A., Shevach E.M., Collins M., Byrne M.C. 2002. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 16:311–323 10.1016/S1074-7613(02)00280-7 [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F., Ravetch J.V. 2005. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 310:1510–1512 10.1126/science.1118948 [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F., Ravetch J.V. 2006. Fcgamma receptors: old friends and new family members. Immunity. 24:19–28 10.1016/j.immuni.2005.11.010 [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F., Ravetch J.V. 2008. Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 8:34–47 10.1038/nri2206 [DOI] [PubMed] [Google Scholar]
- Nishikawa H., Sakaguchi S. 2010. Regulatory T cells in tumor immunity. Int. J. Cancer. 127:759–767 [DOI] [PubMed] [Google Scholar]
- Onishi Y., Fehervari Z., Yamaguchi T., Sakaguchi S. 2008. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc. Natl. Acad. Sci. USA. 105:10113–10118 10.1073/pnas.0711106105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presta L.G., Shields R.L., Namenuk A.K., Hong K., Meng Y.G. 2002. Engineering therapeutic antibodies for improved function. Biochem. Soc. Trans. 30:487–490 10.1042/BST0300487 [DOI] [PubMed] [Google Scholar]
- Ronchetti S., Zollo O., Bruscoli S., Agostini M., Bianchini R., Nocentini G., Ayroldi E., Riccardi C. 2004. GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. Eur. J. Immunol. 34:613–622 10.1002/eji.200324804 [DOI] [PubMed] [Google Scholar]
- Satoh M., Iida S., Shitara K. 2006. Non-fucosylated therapeutic antibodies as next-generation therapeutic antibodies. Expert Opin. Biol. Ther. 6:1161–1173 10.1517/14712598.6.11.1161 [DOI] [PubMed] [Google Scholar]
- Shimizu J., Yamazaki S., Takahashi T., Ishida Y., Sakaguchi S. 2002. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat. Immunol. 3:135–142 10.1038/ni759 [DOI] [PubMed] [Google Scholar]
- Simpson T.R., Li F., Montalvo-Ortiz W., Sepulveda M.A., Bergerhoff K., Arce F., Roddie C., Henry J.Y., Yagita H., Wolchok J.D., et al. 2013. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti–CTLA-4 therapy against melanoma. J. Exp. Med. 210:1695–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P., DiLillo D.J., Bournazos S., Li F., Ravetch J.V. 2012. Mouse model recapitulating human Fcγ receptor structural and functional diversity. Proc. Natl. Acad. Sci. USA. 109:6181–6186 10.1073/pnas.1203954109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens G.L., McHugh R.S., Whitters M.J., Young D.A., Luxenberg D., Carreno B.M., Collins M., Shevach E.M. 2004. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J. Immunol. 173:5008–5020 [DOI] [PubMed] [Google Scholar]
- Tivol E.A., Borriello F., Schweitzer A.N., Lynch W.P., Bluestone J.A., Sharpe A.H. 1995. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 3:541–547 10.1016/1074-7613(95)90125-6 [DOI] [PubMed] [Google Scholar]
- Tone M., Tone Y., Adams E., Yates S.F., Frewin M.R., Cobbold S.P., Waldmann H. 2003. Mouse glucocorticoid-induced tumor necrosis factor receptor ligand is costimulatory for T cells. Proc. Natl. Acad. Sci. USA. 100:15059–15064 10.1073/pnas.2334901100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk M.J., Guevara-Patiño J.A., Rizzuto G.A., Engelhorn M.E., Sakaguchi S., Houghton A.N. 2004. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J. Exp. Med. 200:771–782 10.1084/jem.20041130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walunas T.L., Lenschow D.J., Bakker C.Y., Linsley P.S., Freeman G.J., Green J.M., Thompson C.B., Bluestone J.A. 1994. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1:405–413 10.1016/1074-7613(94)90071-X [DOI] [PubMed] [Google Scholar]
- Waterhouse P., Penninger J.M., Timms E., Wakeham A., Shahinian A., Lee K.P., Thompson C.B., Griesser H., Mak T.W. 1995. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 270:985–988 10.1126/science.270.5238.985 [DOI] [PubMed] [Google Scholar]
- Wilson N.S., Yang B., Yang A., Loeser S., Marsters S., Lawrence D., Li Y., Pitti R., Totpal K., Yee S., et al. 2011. An Fcγ receptor-dependent mechanism drives antibody-mediated target-receptor signaling in cancer cells. Cancer Cell. 19:101–113 10.1016/j.ccr.2010.11.012 [DOI] [PubMed] [Google Scholar]
- Wing K., Onishi Y., Prieto-Martin P., Yamaguchi T., Miyara M., Fehervari Z., Nomura T., Sakaguchi S. 2008. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 322:271–275 10.1126/science.1160062 [DOI] [PubMed] [Google Scholar]