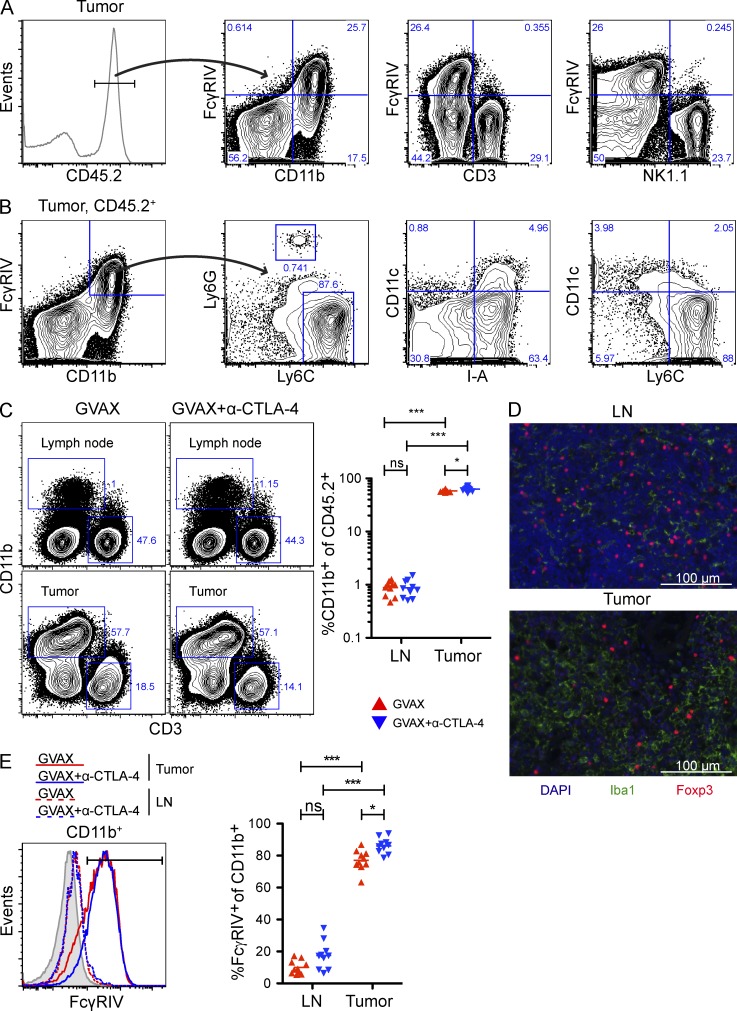
Anti–CTLA-4 antibody induces selective depletion of T reg cells within tumor lesions in a manner that is dependent on the presence of Fc gamma receptor-expressing macrophages within the tumor microenvironment.
Abstract
Treatment with monoclonal antibody specific for cytotoxic T lymphocyte–associated antigen 4 (CTLA-4), an inhibitory receptor expressed by T lymphocytes, has emerged as an effective therapy for the treatment of metastatic melanoma. Although subject to debate, current models favor a mechanism of activity involving blockade of the inhibitory activity of CTLA-4 on both effector (T eff) and regulatory (T reg) T cells, resulting in enhanced antitumor effector T cell activity capable of inducing tumor regression. We demonstrate, however, that the activity of anti–CTLA-4 antibody on the T reg cell compartment is mediated via selective depletion of T reg cells within tumor lesions. Importantly, T reg cell depletion is dependent on the presence of Fcγ receptor–expressing macrophages within the tumor microenvironment, indicating that T reg cells are depleted in trans in a context-dependent manner. Our results reveal further mechanistic insight into the activity of anti-CTLA-4–based cancer immunotherapy, and illustrate the importance of specific features of the local tumor environment on the final outcome of antibody-based immunomodulatory therapies.
The fully human anti–cytotoxic T lymphocyte–associated antigen 4 (CTLA-4) monoclonal antibody Ipilimumab represents the first of a new class of cancer therapies that function by enhancing immunological antitumor activity. Two pivotal phase III clinical trials demonstrated significant increases in survival in patients with melanoma treated with Ipilimumab (Hodi et al., 2010; Robert et al., 2011), which led to its recent approval by the FDA. Despite intensive investigation, however, the mechanism of action remains unclear. Although the initial premise was that anti–CTLA-4 antibodies (α–CTLA-4) function by blocking inhibitory signals into effector T cells (T eff cell; Krummel and Allison, 1996; Sutmuller et al., 2001), the demonstration that CD4+Foxp3+ regulatory T cells (T reg cell) express high levels of CTLA-4 led to the suggestion that α–CTLA-4 directly impacts the T reg cell compartment, either by mediating depletion, or by affecting their suppressive activity (Read et al., 2000, 2006; Takahashi et al., 2000; Wing et al., 2008). In this regard, we recently demonstrated that α–CTLA-4 needs to bind both T eff and T reg cells to elicit full tumor protection (Peggs et al., 2009). Several publications, however, have failed to support T reg cell depletion as a mechanism of action and have, to the contrary, demonstrated that α–CTLA-4 expands T reg cells in the secondary lymphoid organs (Quezada et al., 2006; Schmidt et al., 2009) and blood (Kavanagh et al., 2008) of both mice and humans, further supporting the notion that CTLA-4 restricts T cell proliferation. The mechanisms by which α–CTLA-4 directly affects the activity of the T reg cell compartment therefore remain obscure.
A common feature associated with α–CTLA-4–mediated tumor rejection is an increase in the ratio of T eff to T reg cells within the tumor (T eff/T reg cell ratio; Shrikant et al., 1999; Quezada et al., 2006; Kavanagh et al., 2008; Liakou et al., 2008; Chen et al., 2009; Curran and Allison, 2009; Waitz et al., 2012). This increase is thought to arise from the preferential expansion of T eff over T reg cells, although it remains unclear why this effect is restricted to the tumor microenvironment and why an antibody that simultaneously targets two cellular populations with opposing activities favors effector T cell function and promotes tumor rejection.
Here, we further define the mechanism underlying the antitumor activity of α–CTLA-4 by focusing on the factors controlling the selective increase in the T eff/T reg cell ratio within the tumor. By tracking tumor-specific CD4+ T cells, we show that α–CTLA-4 increases the absolute number of T eff and T reg cells in the lymph nodes and of T eff cells in the tumor, while selectively reducing the absolute number of T reg cells in the tumor. The reduction in T reg cells was consistent with a mechanism involving FcγRIV-dependent depletion associated with the presence of FcγR-expressing macrophages within the tumor, and elevated surface CTLA-4 expression by tumor-infiltrating T reg cells. Thus, α–CTLA-4 blocks inhibitory signals, resulting in the expansion and accumulation of T eff and T reg cells in the lymph node and of T eff cells in the tumor, but in parallel depletes tumor-infiltrating T reg cells, leading to an increase in the T eff/T reg cell ratio within the tumor. Collectively, these data explain the paradoxical effects of α–CTLA-4 on T eff and T reg cell accumulation in the lymph nodes and tumor. More importantly, they highlight the significant role played by the tumor microenvironment in determining the outcome of antibody-based immunotherapies, and how the impact on cellular compartments can differ in the periphery and in the tumor. Lastly, they suggest that approaches leveraging the capacity of the tumor microenvironment to deplete antibody-associated T reg cells could be used to enhance the antitumor activity of immunotherapies.
RESULTS
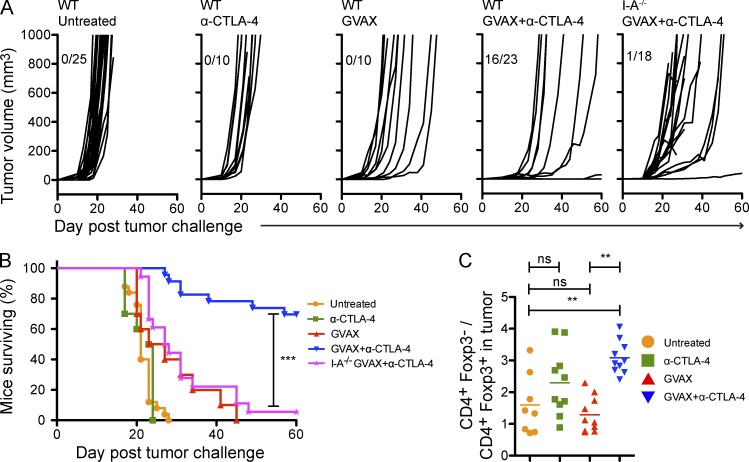
GVAX+α–CTLA-4 combination therapy protects against B16-BL6 melanoma through a CD4-dependent mechanism
To establish the involvement of the CD4+ T cell compartment in tumor protection, C57BL/6 wild-type and I-A−/− mice (lacking a CD4+ T cell compartment) were challenged with the transplantable B16-BL6 melanoma line. 3 d after implantation, mice were treated or not with an irradiated B16-BL6 tumor cell–based vaccine that secretes GM-CSF (GVAX) in the presence or absence of a monoclonal antibody blocking CTLA-4 (α–CTLA-4). In keeping with previous work (van Elsas et al., 1999), the combination of GVAX and α–CTLA-4 protected mice against tumor outgrowth, whereas each agent alone did not (Fig. 1 A). Protection was lost in I-A−/− mice (Fig. 1, A and B), indicating that CD4+ T cells were critical for tumor rejection. In agreement with prior work (Quezada et al., 2006, 2010; Liakou et al., 2008; Waitz et al., 2012), α–CTLA-4 significantly enhanced the intratumoral CD4+Foxp3−/CD4+Foxp3+ (T eff/T reg cell) ratio (Fig. 1 C).
Figure 1.
GVAX+α–CTLA-4 combination therapy protects against tumor outgrowth through a CD4+ T cell–dependent mechanism. (A) Mice were challenged with 104 B16-BL6 on day 0, and then left untreated or treated with GVAX, α–CTLA-4, or GVAX+α–CTLA-4 on day 3, 6, and 8. Results depict tumor growth curves of individual mice (A) and cumulative survival (B). n = 10–25 mice per group, death events correspond to a tumor volume >350 mm3 or death of the mouse. (C) Mice were challenged with 5 × 104 B16-BL6 on day 0 and were treated as described above. The CD4+Foxp3−/CD4+Foxp3+ ratio in the lymph node and tumor was calculated. n = 8–10 mice per group. **, P < 0.01; ***, P < 0.001. Experiments were repeated three times and pooled.
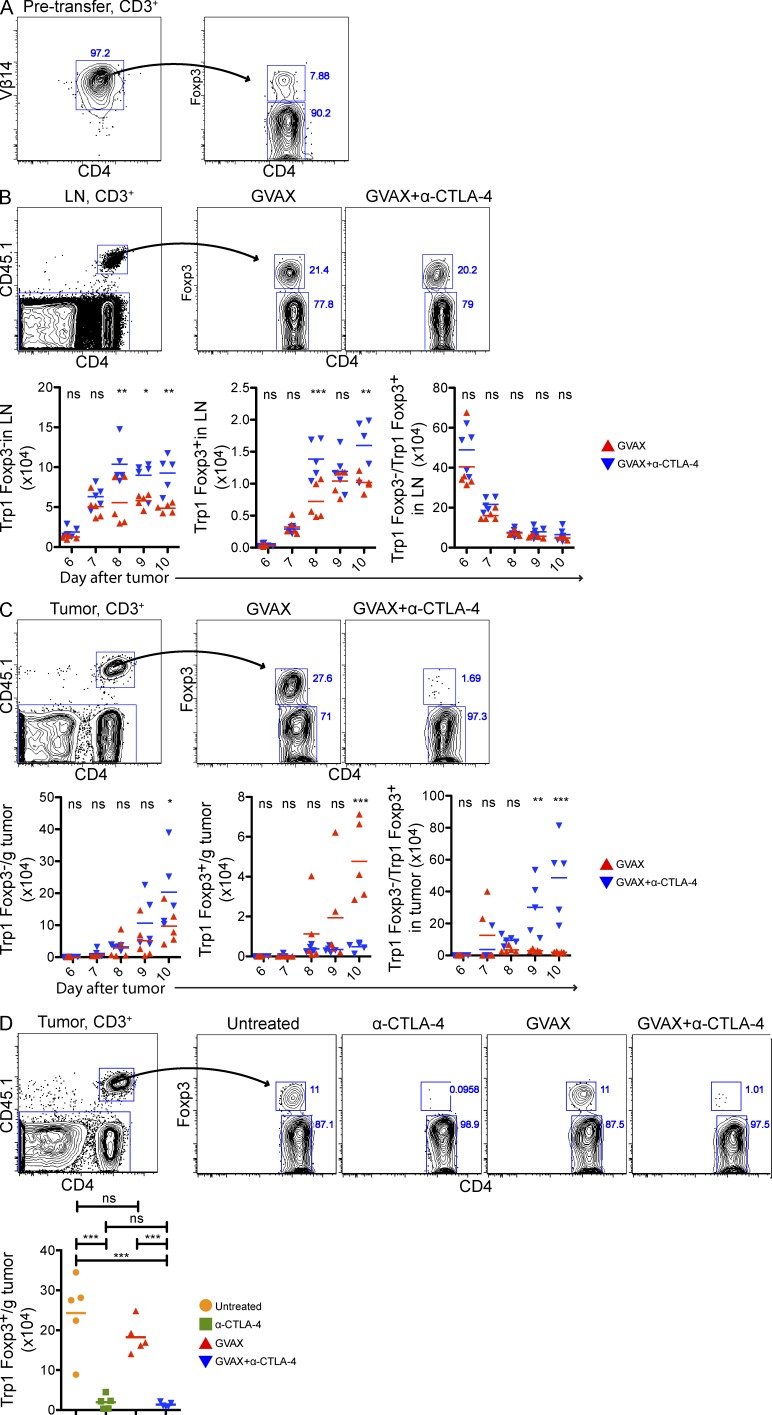
α–CTLA-4 increases the number of tumor-specific CD4+ T eff and T reg cells in lymph nodes, but prevents intratumoral T reg cell accumulation
Given that the T eff/T reg cell ratio is thought to influence the outcome of immunotherapy (Quezada et al., 2011), we focused on the mechanisms regulating T eff and T reg cell numbers in response to α–CTLA-4. To track tumor-/self-antigen–specific CD4+ T cells, we made use of a recently developed TCR transgenic mouse, which generates CD4+ T cells specific for the melanocyte differentiation antigen Trp1/gp75 presented on I-Ab (herein referred to as Trp1 T cells; Muranski et al., 2008). Approximately 10% of Trp1 T cells express the regulatory transcription factor Foxp3 (Fig. 2 A), and previous studies have demonstrated that Trp1Foxp3− cells (Trp1T eff cell) promote the development of autoimmune vitiligo whereas Trp1Foxp3+ cells (Trp1T reg cell) suppress it (Xie et al., 2010).
Figure 2.
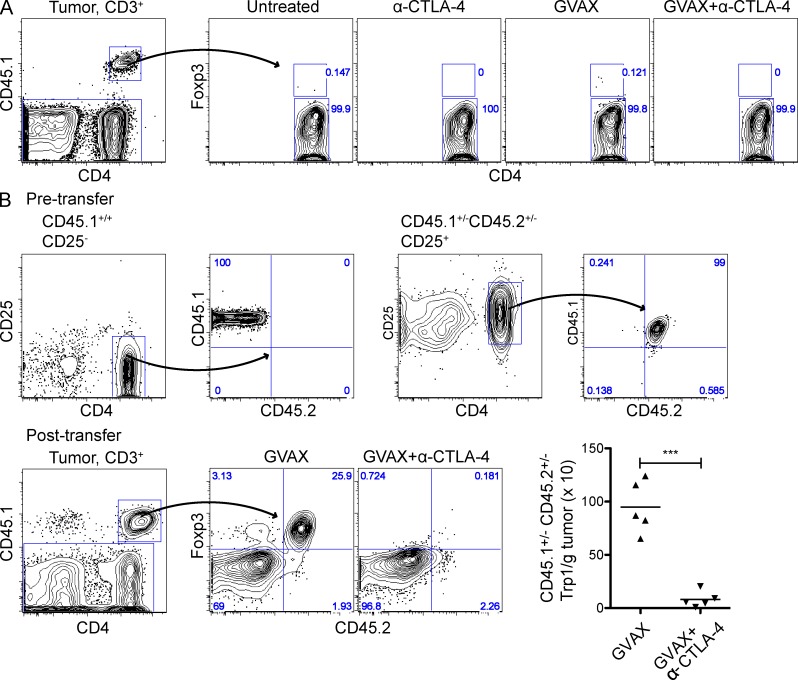
α–CTLA-4 increases the number of tumor-specific CD4+ T eff and T reg cells in lymph nodes while preventing intratumoral T reg cell accumulation. Mice were challenged with 1.5 × 105 B16-BL6 on day 0, adoptively transferred with 5 × 104 CD4+CD45.1+ Trp1 T on day 3, and treated with GVAX or GVAX+α–CTLA-4 on day 3, 6, and 8. (A) Foxp3 expression by CD4+Vβ14+ cell isolated from Trp1 SJL RAG tan mice before transfer. (B and C) Absolute cell numbers and Foxp3 expression by CD4+CD45.1+ Trp1 T cells from the lymph node (B) and tumor (C). (D) Foxp3 expression by intratumoral CD4+CD45.1+ Trp1 T cells. Mice were treated as described above, but were also left untreated or treated with α–CTLA-4 monotherapy. n = 5 mice per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Experiments were repeated two times.
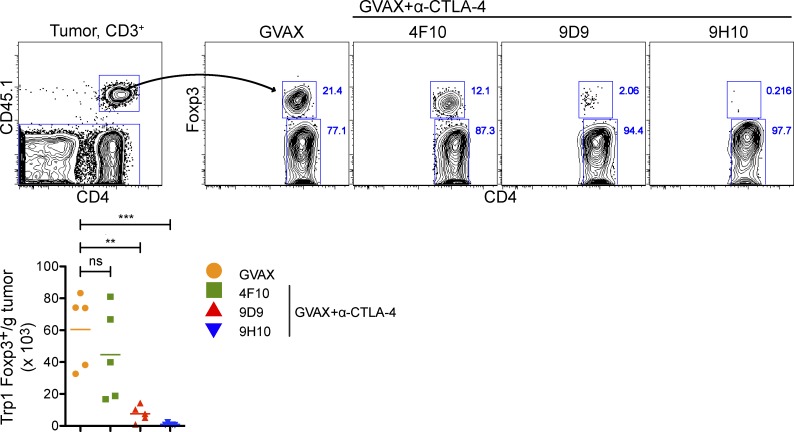
3 d after B16-BL6 implantation, mice received 50,000 CD45.1+Trp1 T cells intravenously in combination with GVAX, or GVAX and α–CTLA-4. Trp1 accumulation was quantified using the CD45.1 marker. Both Trp1T eff and Trp1T reg cell accumulated in the lymph nodes of GVAX-treated mice during the course of the experiment (Fig. 2 B). However, Trp1T reg cell accumulated preferentially (in association with expression of high levels of the proliferation-associated antigen Ki67; unpublished data), resulting in a depressed T eff/T reg cell ratio. α–CTLA-4 increased the absolute numbers of both populations, without changing the Trp1T eff cell/Trp1T reg cell ratio (Fig. 2 B). In contrast, whereas Trp1T eff cell and Trp1T reg cells accumulated in the tumors of GVAX-treated mice, α–CTLA-4 markedly reduced the number of Trp1T reg cells, significantly increasing the intratumoral Trp1T eff cell/Trp1T reg cell ratio (Fig. 2 C). Importantly, α–CTLA-4 as a monotherapy also reduced intratumoral Trp1T reg cell accumulation (Fig. 2 D), indicating that the reduction in T reg cell numbers within the tumor was not dependent on the administration of GVAX. Three different α–CTLA-4 clones reduced the number of tumor-infiltrating Trp1T reg cell to varying levels (Fig. 3), suggesting that these results may not be solely dependent on the capacity of α–CTLA-4 monoclonal antibodies to block CTLA-4.
Figure 3.
Multiple α–CTLA-4 clones reduce the intratumoral Trp1 T reg cell population. Mice were treated as described in Fig. 2 with α–CTLA-4 clone 4F10, 9D9, or 9H10. Results depict absolute cell numbers and Foxp3 expression by intratumoral CD4+CD45.1+ Trp1 T cells. n = 5 mice per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Experiment was repeated two times.
α–CTLA-4 induces rapid elimination of tumor-infiltrating CTLA-4+ T reg cell
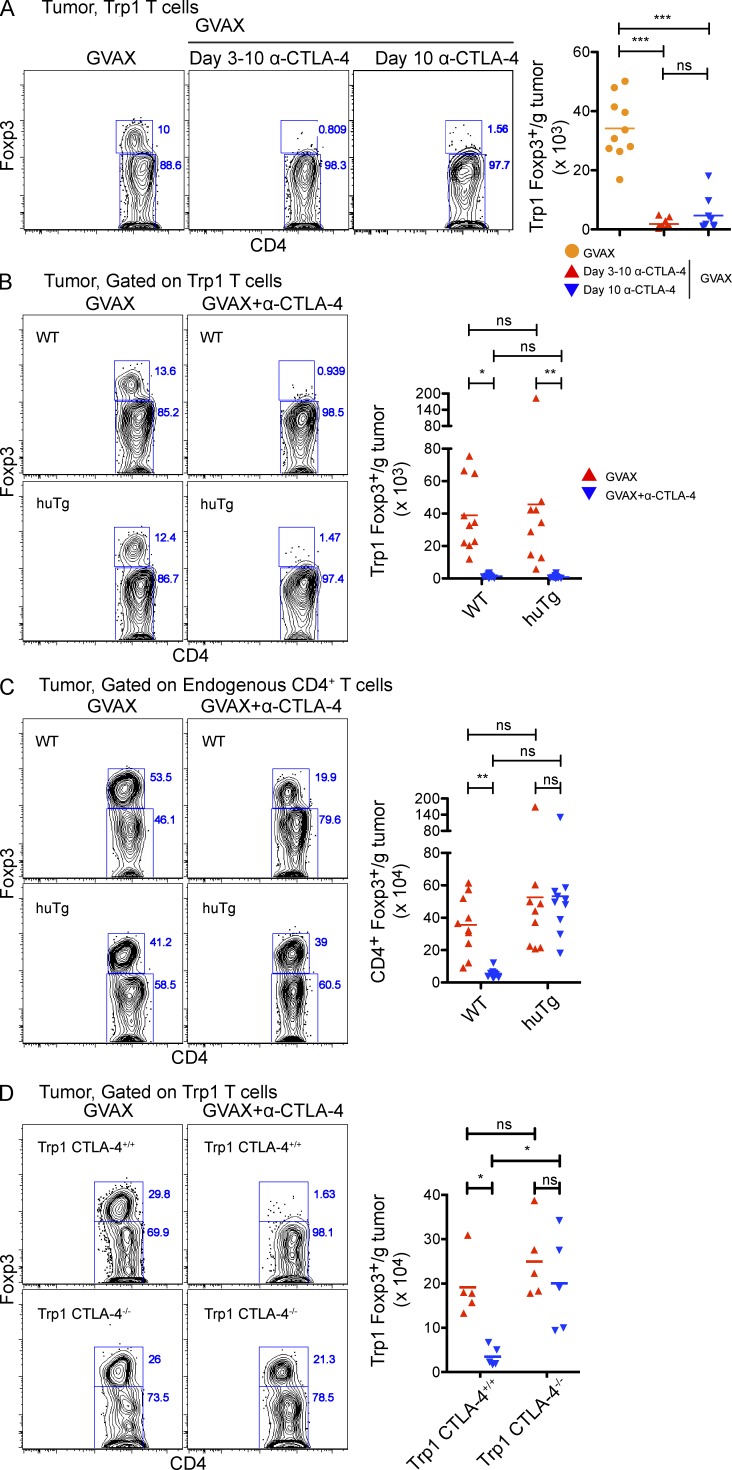
To understand the mechanisms regulating Trp1T reg cell numbers within the tumor after α–CTLA-4 therapy, we evaluated the kinetics of Trp1T reg cell elimination. Mice were treated with GVAX monotherapy until day 10 to allow robust Trp1T reg cell infiltration (Fig. 4 A). Mice were treated with either α–CTLA-4 throughout the experiment or with a single dose on day 10, and then sacrificed 1 d later. Surprisingly, both treatments significantly reduced the numbers of tumor-infiltrating Trp1T reg cells (Fig. 4 A), suggesting depletion as a potential mechanism.
Figure 4.
Tumor-specific T reg cells are rapidly and cell autonomously eliminated from the tumor by α–CTLA-4. (A) Mice were challenged with 1.5 × 105 B16-BL6 on day 0, adoptively transferred with 5 × 104 CD4+CD45.1+ Trp1 T on day 3, and treated with GVAX or GVAX+α–CTLA-4 on day 3, 6, and 8. Some mice received no further treatment or were treated with α–CTLA-4 on day 3–10, or on day 10 alone, and then sacrificed on day 11. Results depict absolute cell numbers and Foxp3 expression by intratumoral CD4+CD45.1+ Trp1 T cells. n = 9–10 mice per group. (B and C) Wild-type and CTLA-4-huTg mice were treated as described in Fig. 2. Results depict absolute cell numbers and Foxp3 expression by intratumoral CD4+CD45.1+ Trp1 (B) and CD4+CD45.1− endogenous polyclonal T cells (C). (D) CTLA-4+/+ and CTLA-4−/− Trp1 T cells were transferred into wild-type mice, and then treated as described in Fig. 2. Results depict absolute cell numbers and Foxp3 expression by intratumoral CD4+CD45.1+ Trp1 T cells. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Experiments were repeated two times and pooled.
To address whether direct binding of α–CTLA-4 mAb to Trp1T reg cell cells mediated depletion, we transferred Trp1 T cells into mice expressing human rather than mouse CTLA-4 (CTLA-4-huTg; Peggs et al., 2009). After treatment with GVAX and anti–mouse CTLA-4 (the 9H10 clone does not bind to human CTLA-4), Trp1T reg cell–expressing mouse CTLA-4 (Fig. 4 B), but not endogenous polyclonal T reg cell–expressing human CTLA-4 (Fig. 4 C), were depleted from the tumors, indicating that direct interactions between the α–CTLA-4 antibody and CTLA-4+Trp1T reg cell were required to mediate depletion from the tumor.
To corroborate whether α–CTLA-4 treatment eliminated tumor-infiltrating Trp1T reg cell by directly binding and depleting CTLA-4–expressing T reg cells instead of blocking the function of CTLA-4, we adoptively transferred Trp1 T cells from CTLA-4+/+ or CTLA-4−/− Trp1 TCR transgenic animals, and then measured intratumoral Trp1T reg cell accumulation in response to GVAX or GVAX and α–CTLA-4. Whereas the addition of α–CTLA-4 resulted in a marked reduction in the number of tumor-infiltrating CTLA-4+/+Trp1 T reg cells, no changes in the number of tumor-infiltrating CTLA-4−/− Trp1 cells were observed after α–CTLA-4 therapy (Fig. 4 D). These data demonstrate that the reduction in the number of tumor-reactive T reg cells in the tumor does not depend on “blocking” CTLA-4 mediated regulation, as genetic ablation of CTLA-4 on tumor-reactive Trp1 cells did not result in elimination of Trp1T reg cell from the tumor.
The rapid elimination of CTLA-4+Trp1T reg cell supported depletion as the underlying mechanism, although alternative mechanisms, such as loss of Foxp3 expression by Foxp3+ cells (Zhou et al., 2009), or reduced differentiation of Foxp3− to Foxp3+ cells (conversion; Benigni et al., 2006; Moo-Young et al., 2009) could also account for the reduction in Trp1T reg cell. To test these possibilities, we used Trp1Foxp3GFP reporter mice expressing GFP under the Foxp3 promoter to measure conversion, and congenically marked (CD45.1 and/or CD45.2) Trp1T eff cell (Foxp3−) and Trp1T reg cell (Foxp3+) subsets to track the fate of each compartment in response to therapy. Trp1GFP− (Foxp3−) cells transferred into tumor-bearing mice left untreated or treated with GVAX, α–CTLA-4, or GVAX+α–CTLA-4 failed to up-regulate GFP or Foxp3 (Fig. 5 A), indicating that Foxp3− to Foxp3+ conversion did not contribute to intratumoral Trp1T reg cell accumulation. Accordingly, these data also suggest that α–CTLA-4 does not eliminate Trp1T reg cells from the tumor by preventing conversion. To test potential loss of Foxp3 expression within the T reg cell compartment, mice were given a mixture of CD45.1+/+ CD25− Trp1T eff cells and CD45.1+/− CD45.2+/− CD25+ Trp1T reg cells, followed by treatment with GVAX or GVAX+α–CTLA-4 (Fig. 5 B). After GVAX+α–CTLA-4 treatment, the CD45.1+/− CD45.2+/− CD25+ Trp1T reg cell population was completely eliminated from the tumor to prevent loss of Foxp3 expression (Fig. 5 B). Furthermore, no significant differences in the expression of the proliferation marker Ki-67 were observed in T reg cell (not shown), excluding this as a potential mechanism for the loss of tumor-infiltrating T reg cell. These data support a model where α–CTLA-4 eliminates Trp1T reg cell from the tumor through depletion and not through changes in T reg cell proliferation or loss of Foxp3 expression.
Figure 5.
Tumor-specific T reg cells are depleted from the tumor by α–CTLA-4. (A) CD4+GFP− cells were FACS purified from CD45.1+/+ BW RAG−/− Trp1 Foxp3-GFP mice, adoptively transferred into mice bearing 3-d established tumors and treated as described in Fig. 2. Results depict Foxp3 expression by CD4+CD45.1+ Trp1 cells in the tumor. (B) Mice were challenged with 1.5 × 105 B16-BL6 on day 0, a mixture of CD25− CD45.1+/+ and CD25+ CD45.1+/− CD45.2+/− Trp1 T cells was adoptively transferred on day 3, and mice were then treated as described in Fig. 2. (top) Phenotype of sorted CD25− and CD25+ Trp1 T cells before mixing and transfer. (bottom) Absolute cell numbers and CD45.2 expression by intratumoral CD4+CD45.1+ Trp1 T cells. n = 5 mice per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Experiments were repeated two times.
T reg cell depletion is mediated largely by FcγRIV
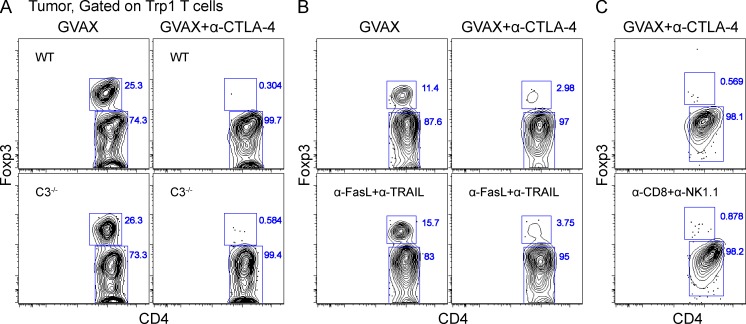
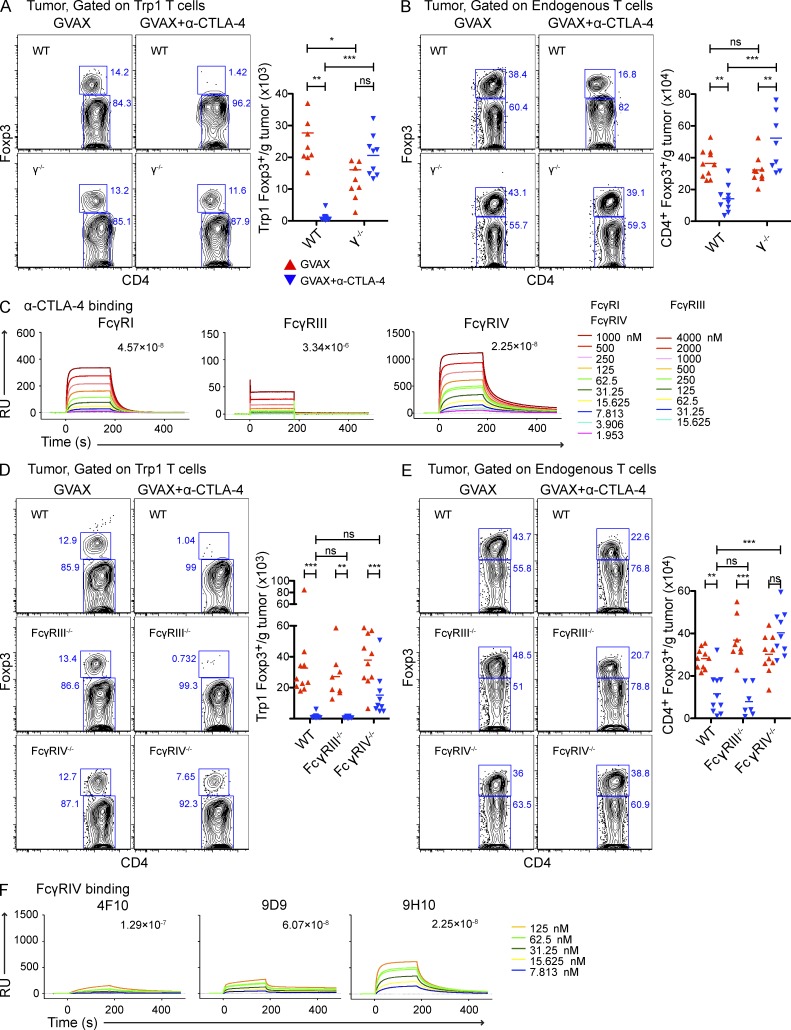
Antibody-mediated depletion can occur via complement-mediated lysis (Reff et al., 1994; Giorgini et al., 2008; Wang and Weiner, 2008) or antibody-dependent cell-mediated cytotoxicity (ADCC; Swanson and Hoppe, 2004; Stuart and Ezekowitz, 2005; Nimmerjahn and Ravetch, 2008). We first used complement deficient C3−/− mice (Giorgini et al., 2008) as recipients of tumor-reactive Trp1 T cells and still observed depletion of tumor-infiltrating Trp1T reg cell in response to α–CTLA-4 (Fig. 6 A). We therefore investigated the potential involvement of ADCC in our system. In mice, ADCC is regulated predominantly by the low-affinity receptors FcγRIII and FcγRIV (Nimmerjahn et al., 2005; Nimmerjahn et al., 2010; Setiady et al., 2010), and to a lesser extent by the high-affinity FcγRI (Fossati-Jimack et al., 2000; Tedder et al., 2006; Hamaguchi et al., 2006). To determine whether FcγRs mediated T reg cell depletion, we used γ−/− chain mice lacking all three activating FcγRs as recipients (Takai et al., 1994; Clynes et al., 1998). Although both the Trp1T reg cell (Fig. 7 A) and the endogenous polyclonal T reg cell compartments (Fig. 7 B) were significantly reduced in the tumors of wild-type mice treated with α–CTLA-4, no depletion was observed in γ−/− chain recipient mice treated with α–CTLA-4, suggesting depletion through an FcγR-dependent mechanism. Depletion also occurred independently of the FasL and TRAIL cytotoxicity pathways (Fig. 6 B).
Figure 6.
Tumor-specific T reg cells are not depleted by complement, the FasL–TRAIL pathway, or NK/CD8+ T cells. (A–C) Mice were treated as described in Fig. 2. Results depict Foxp3 expression by CD4+CD45.1+ Trp1 cells in complement C3−/− mice (A), mice treated with blocking α-FasL, and α-TRAIL antibodies (B), or mice treated with depleting α-CD8 and α-NK1.1 antibodies (C). Experiments were repeated two times.
Figure 7.
T reg cell depletion is mediated largely by FcγRIV. (A and B) Wild-type and γ−/− mice were challenged with B16-BL6 and treated as described in Fig. 2. Absolute cell numbers and Foxp3 expression by intratumoral CD4+CD45.1+ Trp1 (A) and CD4+CD45.1− endogenous polyclonal T cells (B). n = 9–10 mice per group. (C) SPR analysis of α–CTLA-4 clone 9H10 binding to FcγRI, FcγRIII, and FcγRIV. (D and E) Wild-type, FcγRIII−/−, and FcγRIV−/− mice were challenged with B16-BL6 and treated as described in Fig. 2. Absolute cell numbers and Foxp3 expression by intratumoral CD4+CD45.1+ Trp1 (D) and CD4+CD45.1− endogenous polyclonal T cells (E). (F) SPR analysis of three different α–CTLA-4 clones (4F10, 9D9, 9H10) binding to FcγRIV. n = 7–10 mice per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Experiments were repeated three times and pooled.
Using surface plasmon resonance (SPR) we determined the binding of α–CTLA-4 to different FcγRs. The α–CTLA-4 clone 9H10 bound to FcγRI with the highest affinity (Fig. 7 C). Of the two low-affinity FcγRs implicated in ADCC, 9H10 bound with higher affinity to FcγRIV, suggesting a greater involvement in depletion. When we compared in vivo depletion of tumor-infiltrating T reg cells by α–CTLA-4 in wild-type versus FcγRIII−/− and FcγRIV−/− mice, we observed that Trp1T reg cell numbers were partially restored and endogenous polyclonal T reg cell numbers were fully restored in FcγRIV−/−, but not in FcγRIII−/− mice (Fig. 7, D and E), supporting a major role for FcγRIV in the depletion of tumor-infiltrating T reg cells after α–CTLA-4 therapy. Interestingly, binding affinities of our three different α–CTLA-4 clones to FcγRIV (Fig. 7 F) correlated with their ability to deplete tumor-infiltrating T reg cells in vivo (Fig. 4 A). Depletion of NK cells, which express FcγRIII, but no other activating FcγRs (Takai et al., 1994; Nimmerjahn and Ravetch, 2008), did not prevent Trp1T reg cell depletion in response to α–CTLA-4 (Fig. 6 C), further supporting the dispensability of FcγRIII in T reg cell depletion in this model. However, the partial restoration of Trp1T reg cell in FcγRIV−/− mice compared with the full restoration of the endogenous polyclonal T reg cell compartment suggests potential redundancy in the FcγR system. This could be explained by differences in the expression level of the target molecule as Trp1 T reg cell express higher levels of surface CTLA-4 than endogenous T reg cell (see Fig. 9, A and B), potentially increasing their susceptibility to depletion by other FcγRs compensating for the loss of FcγRIV. In keeping with this, FcγRI, which bound with high affinity to the 9H10 clone (Fig. 7 C), is also capable of inducing ADCC (Otten et al., 2008), suggesting a potential role of this FcγR in Trp1T reg cell depletion. Nonetheless, our data point to FcγRIV as a key mediator in the depletion of endogenous tumor-infiltrating T reg cells.
Figure 9.
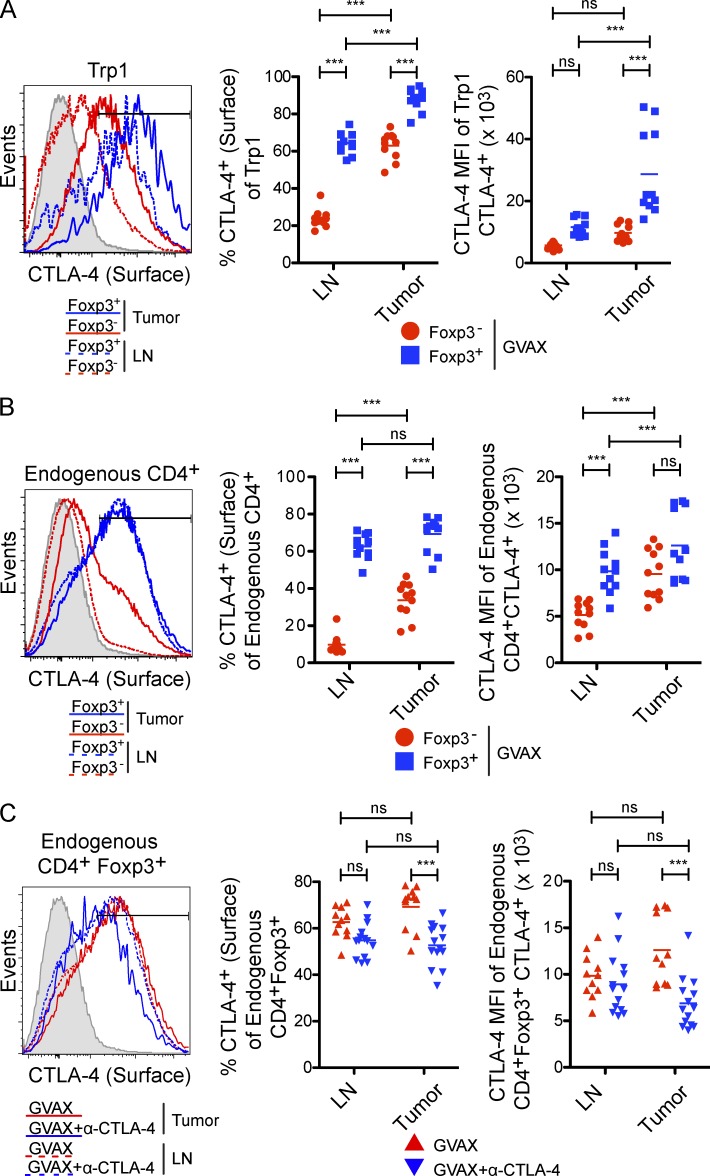
Tumor-infiltrating T reg cells express high levels of surface CTLA-4. (A and B) Surface CTLA-4 expression by CD4+CD45.1+ Trp1 (A) and CD4+CD45.1− endogenous polyclonal T cells (B) in the tumor and lymph node of mice treated with GVAX (as described in Fig. 2). The shaded histogram represents CTLA-4-huTg mice. n = 11 mice per group. (C) Quantification of surface CTLA-4 expression on CD4+Foxp3+ polyclonal T reg cell in the lymph node and tumor after treatment with GVAX or GVAX+α–CTLA-4. Mice were treated with GVAX or GVAX+α–CTLA-4 (clone 9H10) as described in Fig. 2 and sacrificed on day 10. Surface CTLA-4 expression on endogenous CD4+Foxp3+ T reg cells was detected by staining with α–CTLA-4 clone 4F10, which is not cross-blocked by clone 9H10. n = 11–14 mice per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Experiments were repeated two times and pooled.
CD11b+FcγRIV+ macrophages are enriched in the tumor microenvironment
Considering that ADCC in mice is predominantly mediated by macrophages (Uchida et al., 2004; Tedder et al., 2006; Setiady et al., 2010), we next evaluated the expression of FcγRIV on macrophages and other immune subsets within the tumor. As previously shown (Nimmerjahn et al., 2005), FcγRIV expression was restricted to CD11b+ cells (Fig. 8 A). Ly6ChiLy6G− macrophages (Shi et al., 2011) constituted the majority of the CD11b+FcγRIV+ population, whereas Ly6loLy6G+ neutrophils and CD11c+I-A+ dendritic cells were only a minor component (Fig. 8 B). Of particular note, CD11b+ cells were enriched 70-fold in the tumor relative to the lymph node (Fig. 8 C), where depletion did not occur (Fig. 2 B). The presence of macrophages in the tumor was confirmed by staining tissue sections for the macrophage specific marker Iba1 (Graeber et al., 1998), which showed a much higher density of macrophages in the tumor compared with lymph nodes (Fig. 8 D). Lastly, FcγRIV expression was significantly elevated on intratumoral CD11b+ cells, but almost undetectable on CD11b+ cells from lymph nodes (Fig. 8 E). Importantly, α–CTLA-4 did not alter the percentage of CD11b+ cells in the tumor (Fig. 8 C), or FcγRIV expression by tumor-infiltrating CD11b+ cells (Fig. 8 E), suggesting that T reg cell depletion did not result from a general increase in the number or function of macrophages in the tumor in response to α–CTLA-4.
Figure 8.
Phagocytic cells expressing high levels of FcγRIV are enriched in the tumor microenvironment. (A–E) Mice were treated as described in Fig. 2. (A) Representative FcγRIV expression by intratumoral CD11b+, NK1.1+, and CD3+ cells. (B) Representative CD11c, I-A, Ly6C, and Ly6G expression by intratumoral CD45.2+CD11b+FcγRIV+ cells. (C) CD3 and CD11b expression on CD45.2+ cells in the lymph node and tumor. n = 10 mice per group. (D) Iba1 and Foxp3 expression in the lymph and tumor of GVAX-treated mice. Bar, 100 µm. (E) FcγRIV expression by CD11b+ cells in the lymph node and tumor. The shaded histogram represents FcγRIV expression by CD11b+ cells from FcγRIV−/− mice. n = 10 mice per group. *, P < 0.05; ***, P < 0.001. Experiments were repeated two times and pooled.
Surface CTLA-4 expression is elevated on tumor-infiltrating T reg cells
The selective depletion of intratumoral Trp1T reg cells and endogenous T reg cells by α–CTLA-4 (Figs. 2 C and 7 B) suggests that α–CTLA-4 preferentially associates with the T reg cell compartment. In keeping with this, surface expression of CTLA-4 was significantly higher on Trp1T reg cell (Fig. 9 A) and endogenous T reg cell (Fig. 9 B) compared with their effector counterparts. Furthermore, Trp1T reg cell expressed higher levels of surface CTLA-4 compared with endogenous T reg cells (Fig. 9, A and B) and were more susceptible to depletion by α–CTLA-4 (Fig. 7, A and B). Moreover, surface CTLA-4 expression was increased in the tumor compared with the lymph node, where depletion did not occur (Fig. 2, B and C). Finally, levels of surface CTLA-4 on the T reg cell compartment were significantly reduced only at the tumor site after α–CTLA-4 treatment (Fig. 9 C). These data demonstrate that T reg cell depletion in the tumor is associated with the elevated surface expression of CTLA-4 by the T reg cell subset and enrichment of CD11b+FcγRIV+ macrophages in the tumor microenvironment.
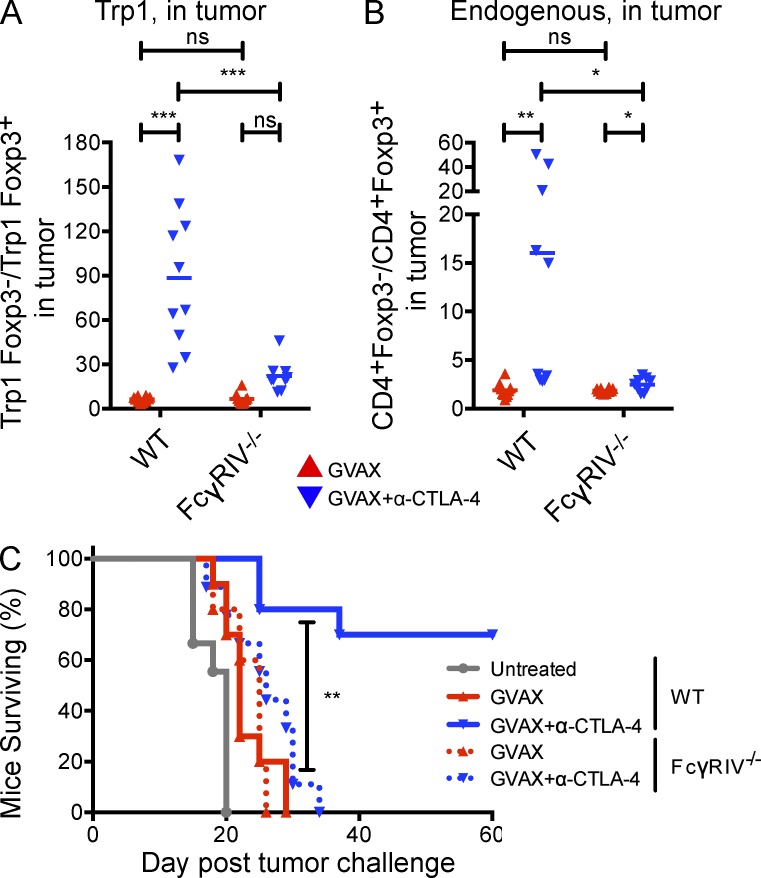
α–CTLA-4 treatment does not increase the intratumoral T eff/T reg cell ratio in FcγRIV−/− mice, and fails to elicit tumor protection
To determine whether the loss of FcγRIV expression and intratumoral T reg cell depletion affected tumor protection we evaluated the antitumor activity of GVAX and α–CTLA-4 in WT and FcγRIV−/− mice. Whereas α–CTLA-4 significantly increased the intratumoral ratio of T eff/T reg cells (Trp1 and endogenous) in wild-type mice, it failed to do so in FcγRIV−/− mice (Fig. 10, A and B), suggesting that T reg cell depletion by FcγRIV significantly contributes to the relative increase in T eff over T reg cells within the tumor. Importantly, FcγRIV−/− mice also failed to reject B16-BL6 melanoma after treatment with GVAX and α–CTLA-4. (Fig. 10 C), supporting the relevance of the depleting activity of α–CTLA-4 to its antitumor activity. Collectively, our data illustrate the critical importance of specific features of the tumor microenvironment in mediating T reg cell depletion and determining outcomes after α–CTLA-4 therapy.
Figure 10.
α–CTLA-4 therapy does not increase the intratumoral T eff/T reg cell ratio in FcγRIV−/− mice, and fails to elicit tumor protection. (A and B) The T eff/T reg cell ratio from CD4+CD45.1+ Trp1 (A) and CD4+CD45.1− endogenous polyclonal T cells (B) in the tumor. n = 9–10 mice per group. (C) Survival of wild-type and FcγRIV−/− mice treated with GVAX or GVAX+α–CTLA-4, as described in Fig. 1 A. n = 10–11 mice per group. *, P < 0.05; ***, P < 0.001. Experiments were repeated two times and pooled.
DISCUSSION
α–CTLA-4 therapy has demonstrated significant antitumor activity in preclinical tumor models (Leach et al., 1996; van Elsas et al., 1999; Quezada et al., 2011) and in clinical trials as an immunotherapy for metastatic melanoma (Hodi et al., 2010; Robert et al., 2011). In animal models and in the clinic, we and others have shown that α–CTLA-4 increases the intratumoral CD8+/T reg cell and T eff/T reg cell ratios (Fig. 2 C; Quezada et al., 2006; Hodi et al., 2008; Liakou et al., 2008), which is thought to drive tumor rejection given the anti- and pro-tumor growth activity of T eff and T reg cells, respectively (Griffiths et al., 2007; Quezada et al., 2008).
Here, we further interrogated the mechanisms by which α–CTLA-4 therapy increases the intratumoral T eff/T reg cell ratio, focusing on the impact on the T reg cell compartment. We demonstrate that α–CTLA-4 therapy increases this ratio by depleting T reg cells through an FcγR-dependent mechanism. The striking compartmentalization of α–CTLA-4–mediated T reg cell depletion reveals a previously unexpected characteristic of α–CTLA-4 mAb functionality, which is important for several reasons. First, it may help to define specific therapeutically relevant attributes of clinically effective mAb, allowing such characteristics to be engineered into second-generation antibodies being taken into the clinic. Second, the critical importance of the microenvironment in facilitating this α–CTLA-4 activity may help to inform the development of rational combinatorial therapies that aim to optimize the microenvironmental cues associated with maximal α–CTLA-4–mediated depletion, i.e., specific patterns of FcγR expression on tumor-infiltrating antigen-presenting cells/macrophages. Third, evaluation of the tumor microenvironment for these features may provide a valuable biomarker for predicting responsiveness to α–CTLA-4 therapy. Based on the importance of the T reg cell subset in restraining antitumor immune responses in a variety of malignancies (Antony et al., 2005; Chen et al., 2007; Li and Yee, 2008; Quezada et al., 2008; Klages et al., 2010), we predict that tumors containing few macrophages, or macrophages expressing low levels of FcγRs would respond less favorably to α–CTLA-4 therapy, and that therapies that enhance tumor infiltration by such cells would likely synergize, making them ideal candidates for further clinical evaluation. It is important to note that in a clinical setting, intratumoral NK cell abundance is also likely to impact the activity of α–CTLA-4, given that human NK cells express FcγRIIIA, a homologue of FcγRIV in mice, and a major mediator of ADCC (Nimmerjahn and Ravetch, 2008).
Genetic ablation of FcγRIV partially restored tumor-specific Trp1 T reg cells and fully restored endogenous polyclonal T reg cell infiltrating the tumor (Fig. 7, D and E), indicating a predominant role for this FcγR in depletion. Moreover, recent work demonstrating that genetic deletion of FcγRIV results in complete loss of ADCC in vivo (Nimmerjahn et al., 2010) further supports the role of FcγRIV in the depletion of tumor infiltrating T reg cells in response to α–CTLA-4. FcγRIV expression was restricted to CD11b+ cells within the tumor (Fig. 8 A), which were mostly Ly6ChiLy6G− (Fig. 8 B). Depletion of T reg cell by macrophages is consistent with previous work demonstrating that in mice, ADCC is predominantly mediated by this immune subset (Stockmeyer et al., 2001; Uchida et al., 2004; Tedder et al., 2006; Oflazoglu et al., 2007), with NK cells playing a more restricted role caused by the expression of a single FcγR, FcγRIII (Nimmerjahn and Ravetch, 2008). In agreement with this model, T reg cells were depleted from the tumor (Fig. 2 C), Peyer’s patches, and vaccine site (not depicted), which contained a large fraction of CD11b+ cells (Fig. 8 C and not depicted), but not from the lymph nodes (Fig. 2 B) and spleen (not depicted), which contained few CD11b+ cells with almost undetectable levels of FcγRIV (Fig. 8 C). Although the association between T reg cell depletion and the presence of CD11b+FcγRIV+ cells in the tumor appears clear, the additional contribution of GVAX to the high frequency of CD11b+FcγRIV+ cells found in the tumor (Fig. 8, C–E) requires further evaluation as secretion of GM-CSF by GVAX is known to contribute to an inflammatory macrophage phenotype (Stockmeyer et al., 2001). Of importance, depletion of tumor-reactive T reg cells depended on α–CTLA-4 administration and not on GVAX (Fig. 2 D). Although our data support the notion that α–CTLA-4 mediates depletion of tumor infiltrating T reg cells, it also suggest that for poorly immunogenic tumors (such as the B16 model), GVAX or other combinatorial approaches may be necessary to increase the frequency of tumor-reactive effector T cells in tumors and lymph nodes (Fig. 2, B and C). The addition of α–CTLA-4 would further increase the number of tumor-reactive effector T cells in LN and tumor (Fig. 2, B and C), while at the same time depleting tumor-infiltrating T reg cells (Fig. 2 C, 2 D).
Finally, we recently evaluated the impact of α–CTLA-4 on tumor infiltrating T reg cells in a mouse model of colon carcinoma (CT26) known to respond to α–CTLA-4 monotherapy. In this model, we also observed a significant reduction in the number of tumor-infiltrating T reg cells in response to α–CTLA-4 as a single agent. Furthermore, the reduction in tumor-infiltrating T reg cells in the CT26 model was also restricted to the tumor site and correlated with an increase in the intratumoral T eff/T reg cell ratio, tumor rejection, and tumor infiltration by CD11b+FcγRIV+ cells (unpublished data), in keeping with the results of another recent publication demonstrating the importance of antibody isotype in the antitumor activity of α–CTLA-4 in the CT26 model (Selby et al., 2013).
Our data also suggest that tumors that either express, or are induced to express, a subset of cells capable of driving ADCC will be those most likely to mediate T reg cell depletion in response to α–CTLA-4. The lack of additive or synergistic therapeutic benefit of the combination of α–CTLA-4 with peptide vaccination in the recent phase III clinical trial (Hodi et al., 2010) could at least be partially explained by a lack of vaccine-mediated impact on appropriate accessory cell populations. Ipilimumab (anti–human CTLA-4) monotherapy has been documented to cause a profound reduction in the intratumoral T reg cell population in patients with bladder cancer, which could relate to the particular microenvironment intrinsic to this tumor type (Liakou et al., 2008). It is also important to note that the binding affinity of the fully human α–CTLA-4 mAb Ipilimumab to FcγRIIIA (unpublished data) is comparable to the binding affinity of rituximab (an anti-CD20–depleting mAb), to FcγRIIIA (Kanda et al., 2007), further supporting the premise that a clinically active α–CTLA-4 mAb is capable of depleting T reg cell. Clearly, further investigation is warranted in the setting of the clinical use of α–CTLA-4 therapies, both in terms of documenting the potential depletion of tumor-infiltrating T reg cells, and in terms of defining the patterns of FcγR expression in human malignancies that might correlate with ADCC-mediated depletion.
The compartmentalization of α–CTLA-4–mediated T reg cell depletion with preferential elimination of T reg cell from sites containing higher levels of CD11b+ cells may be therapeutically advantageous. Such targeted activity might relieve immune suppression within the tumor while leaving systemic immunity largely intact. Widespread depletion of T reg cells can induce multiple autoimmune pathologies (Fontenot et al., 2003; Kim et al., 2007). Nevertheless, immune-related adverse events have been reported in a subset of patients treated with Ipilimumab (Hodi et al., 2010; Robert et al., 2011). Interestingly, colitis remains one of the more frequent adverse events, whereas Peyer’s patches were one of the few sites in which T reg cell were depleted in our murine model. Further evaluation of the impact of Ipilimumab on T reg cell populations in humans might help to explain potential toxicities, and also help in optimizing therapeutically relevant characteristics of α–CTLA-4 mAb.
We previously demonstrated that unicompartmental binding of CTLA-4 to the T eff cell compartment is capable of inducing tumor protection in some mice (Peggs et al., 2009), although protection was significantly reduced when compared to targeting both the T eff and T reg cell compartments. In contrast, unicompartmental binding to the T reg cell compartment failed to elicit tumor protection, highlighting the importance of the impact on the T eff cell compartment. These results can now be explained by the depletion of T reg cells within the tumor microenvironment by α–CTLA-4. In the current study, protection was ablated in FcγRIV−/− mice (Fig. 10 C) that are incapable of depleting T reg cells (Fig. 7 A and B), but should otherwise have a T eff cell compartment capable of responding to α–CTLA-4 treatment. This might indicate that cells expressing FcγRIV have other important roles in mediating tumor protection beyond depletion of T reg cell. Alternatively, the results may reflect an impact of α–CTLA-4 on T reg cell in other sites, as blockade induces a proliferation and accumulation in nodal sites that would not occur in the unicompartmental T eff cell blockade setting. Overall, the data presented here are consistent with a model where α–CTLA-4 immunotherapy releases cell-intrinsic inhibition of the T eff cell compartment while simultaneously targeting T reg cells for depletion within the tumor microenvironment.
Our data also raise the question of whether antibodies targeting other co-inhibitory or co-stimulatory receptors also potentiate antitumor immune activity through intratumoral T reg cell depletion. Both α-OX40 and α-GITR mAb have been shown to induce a loss of T reg cells from the tumor microenvironment (Coe et al., 2010; Hirschhorn-Cymerman et al., 2012), although the mechanisms remained obscure. Furthermore, the apparent duality of function of such antibodies, e.g., α-OX40 targeting both the T eff and T reg cell compartments (Piconese et al., 2008), has been previously noted.
In conclusion, our data illustrate that α–CTLA-4 mAb significantly enhances the intratumoral T eff/T reg cell ratio, in part by selectively depleting T reg cells in a manner that is dependent on the tumor microenvironment and the specific mAb clone. They support the further evaluation of clinical strategies leveraging the capacity of the tumor microenvironment to deplete antibody-associated cells and the design of depleting antibodies targeting co-stimulatory and co-inhibitory molecules preferentially expressed at high levels by T reg cells, optimized to enhance binding to the FcγR most relevant to the specific tumor type, and the particular microenvironment being targeted.
MATERIALS AND METHODS
Mice.
4-6-wk-old C57BL/6 and I-A−/−mice were purchased from The Jackson Laboratory and Charles River UK. 4–6-wk-old C57BL/6 and γ−/− mice were also purchased from Taconic. FcγRIII−/− and FcγRIV−/− mice were obtained from J.V. Ravetch at The Rockefeller University (New York, NY). Previously described BW RAG−/− Trp1 transgenic mice (Muranski et al., 2008) were provided by N. Restifo (National Cancer Institute, Bethesda, MD) and crossed to B6.SJL mice to create CD45.1+/+ BW RAG−/− Trp1 transgenic mice. The Trp1 mice were also crossed to Foxp3-GFP mice obtained from A. Rudensky (Memorial Sloan-Kettering Cancer Center, New York, NY) and to CTLA-4−/− mice to create CD45.1+/+ BW RAG−/− Trp1Foxp3GFP and CD45.1+/+ BW RAG−/− Trp1CTLA-4−/−, respectively. Mice were bred and maintained at Memorial Sloan-Kettering Cancer Center and Charles River UK. The Memorial Sloan-Kettering Cancer Center Institutional Animal Care and Use Committee and the University College London Animal Ethics Committee approved all animal experiments in this manuscript.
Cell lines.
The highly tumorigenic, poorly immunogenic B16-BL6 cell was originally obtained from I.J. Fidler (MD Anderson Cancer Center, Houston, TX) and cultured in complete RPMI (cRPMI).
Antibodies.
Most blocking and neutralizing antibodies were purchased from BioXCell and administered i.p. α-FasL (MFL4) and α-TRAIL (N2B2) antibodies were prepared by H. Yagita (Juntendo University School of Medicine, Tokyo, Japan). 9H10 and 4F10 are hamster anti-mouse CTLA-4 mAbs and 9D9 is a mouse anti–mouse CTLA-4 mAb generated in our laboratory. Antibodies for flow cytometry and immunofluorescence were purchased from eBioscience, BD, and BioLegend.
Trp1 T cell transfer.
Trp1 mice were sacrificed using CO2 euthanasia. CD4+ Trp1 cells were isolated from spleens and lymph nodes (inguinal, cervical, mesenteric, axillary, and brachial) using CD4+ selection (Miltenyi Biotec) according to the manufacturer’s instructions and adoptively transferred in PBS by tail vein injection.
Tumor challenge and treatment.
For functional experiments involving Trp1 T cells, mice were challenged intradermally (i.d.) with 1.5 × 105 B16-BL6 on day 0 on their right flank. 3 d after tumor implantation, 5 × 104 CD4+ Trp1 T cells were adoptively transferred i.v. and mice were treated with 106 irradiated GVAX cells (i.d. on their left flank) plus 200 µg α–CTLA-4 (i.p.). Mice received additional treatments of GVAX and 100 µg of α–CTLA-4 on day 6 and 8. Mice were sacrificed on day 10 by CO2 euthanasia. For protection experiments, mice were challenged (i.d.) with 104 B16-BL6 and treated with GVAX and α–CTLA-4 as described above. Tumors were measured every 2–3 d.
Tissue processing and flow cytometry.
Mice were sacrificed on day 10 with CO2. Lymph nodes (inguinal, axillary, and brachial) and tumors were dissected into RPMI. Lymph nodes were dispersed through a 70-µm filter whereas tumors were mechanically disrupted using scissors, digested with a mixture of 0.33 mg/ml DNase (Sigma-Aldrich) and 0.27 mg/ml Liberase TL (Roche) in serum-free RPMI for 25 min, and dispersed through a 70-µm filter. To identify T cell populations, samples were stained with α-CD4 APC-eFluor780, α-CD45.1 eFluor410, α-CD45.2 APC, α–CTLA-4 PE, and α-CD8 PerCP-eFluor710. In some experiments, α-CD8 Brilliant Violet 570 and fixable live/dead 660 dye were used. Cells were fixed and permeabilized with the Foxp3 buffer kit (eBioscience) and stained with α-CD3 PE-Cy7, α-Foxp3 Alexa Fluor 700, α-CD3 Pe-Cy7, and α-Ki67 Alexa Fluor 488. In some experiments, CTLA-4 surface signal was enhanced using the FASER kit (Miltenyi Biotec). To identify macrophages, samples were stained with α-CD3 PerCP-eFluor710, α-Ly6C APC, α-Ly6G PE-Cy7, α-NK1.1 APC, α-CD11c PE, α-I-A Alexa Fluor 700, α-CD45.2 eFluor410, α-FcγRIV Alexa488. Samples were mixed with Pacific Blue microbeads (Life Technologies) to normalize for sample volumes. Data were acquired on LSR II and Fortessa flow cytometers (BD). The absolute number of cells per tumor sample was quantified using fluorescent microbeads to normalize to a known sample volume, which was then normalized to tumor wet weight. The following formula was used for this calculation: absolute cell number = number of cells acquired/(tumor wet weight/[number of beads acquired/number of beads added to sample]).
Immunofluorescence.
Tumors and lymph nodes were dissected into 4% paraformaldehyde and fixed overnight. Samples were dehydrated in 70% ethanol, embedded in paraffin, and sectioned into 4-µm sections. Sections were stained with polyclonal rabbit α-Iba1 and polyclonal rat α-Foxp3, followed by secondary staining with α-rabbit Alexa Fluor 488 and α-rat Alexa Fluor 532.
SPR.
All SPR analyses were performed with a Biacore T100 SPR system (GE Healthcare) at 25°C in HBS EP+ buffer (10 mM Hepes, pH 7.4, 150 mM NaCl, 3.4 mM EDTA, 0.005% surfactant P20). His-tagged soluble mouse FcγR extracellular domains (Sino Biological Inc.) were immobilized on CM5 chips by amine coupling at pH 4.5, resulting in a density of ∼2,000 RU. Two-fold serially diluted 9H10 samples were injected through flow cells for 3 min at a flow rate of 30 ml/minute for association followed by a 5-min dissociation phase. The concentrations of 9H10 samples ranged from 4,000 nM to 15.63 nM for FcγRIIB and FcγRIII-binding analyses and from 1,000 nM to 1.95 nM for FcγRI and FcγRIV binding analyses. After each assay cycle, the sensor surface was regenerated with a 30-s injection of NaOH of optimized concentration at a flow rate of 50 ml/minute. Background binding to blank immobilized flow cells was subtracted and affinity constant Kd values were calculated using the 1:1 binding kinetics model built in BIAcore T100 Evaluation Software (version 1.1).
Statistical analyses.
Data were analyzed using Prism 5.0 (GraphPad Software). Experiments were repeated two to three times. Statistical significance was determined by Student’s t test (between two groups or conditions) or ANOVA with a post-hoc test (three or more groups or conditions). Tumor survival data were analyzed with the Kaplan-Meier method. The log-rank test was used to compare survival curves for different subgroups on univariate analyses. P values <0.05 were considered statistically significant.
Acknowledgments
We wish to thank Tsvetelina Pencheva-Hoang and Andrew Furness for critical review of this manuscript.
T.R. Simpson was supported by a Canadian Institutes of Health Research Doctoral Research Award. S.A. Quezada is funded by a Cancer Research UK Career Development Fellowship and is a recipient of a Cancer Research Institute Investigator Award. K.S. Peggs and F. Arce receive funding from Leukaemia Lymphoma Research UK.
J.P. Allison is an inventor of intellectual property licensed by the University of California to Bristol-Meyers Squib, and is a consultant for Bristol-Meyers Squibb.
The authors have no further conflicting financial interests.
Footnotes
Abbreviations used:
- ADCC
- antibody-dependent cell-mediated cytotoxicity
- CTLA-4
- cytotoxic T lymphocyte–associated antigen 4
- GVAX
- granulocyte-macrophage colony-stimulating factor secreting tumor cell-based vaccine
- SPR
- surface plasmon resonance
- T eff cell
- CD4+Foxp3− effector T cells
- T reg cell
- CD4+Foxp3+ regulatory T cells
References
- Antony P.A., Piccirillo C.A., Akpinarli A., Finkelstein S.E., Speiss P.J., Surman D.R., Palmer D.C., Chan C.-C., Klebanoff C.A., Overwijk W.W., et al. 2005. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J. Immunol. 174:2591–2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benigni A., Tomasoni S., Turka L.A., Longaretti L., Zentilin L., Mister M., Pezzotta A., Azzollini N., Noris M., Conti S., et al. 2006. Adeno-associated virus-mediated CTLA4Ig gene transfer protects MHC-mismatched renal allografts from chronic rejection. J. Am. Soc. Nephrol. 17:1665–1672 10.1681/ASN.2006010090 [DOI] [PubMed] [Google Scholar]
- Chen A., Liu S., Park D., Kang Y., Zheng G. 2007. Depleting intratumoral CD4+CD25+ regulatory T cells via FasL protein transfer enhances the therapeutic efficacy of adoptive T cell transfer. Cancer Res. 67:1291–1298 10.1158/0008-5472.CAN-06-2622 [DOI] [PubMed] [Google Scholar]
- Chen H., Liakou C.I., Kamat A., Pettaway C., Ward J.F., Tang D.N., Sun J., Jungbluth A.A., Troncoso P., Logothetis C., Sharma P. 2009. Anti-CTLA-4 therapy results in higher CD4+ICOShi T cell frequency and IFN-gamma levels in both nonmalignant and malignant prostate tissues. Proc. Natl. Acad. Sci. USA. 106:2729–2734 10.1073/pnas.0813175106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clynes R., Takechi Y., Moroi Y., Houghton A., Ravetch J.V. 1998. Fc receptors are required in passive and active immunity to melanoma. Proc. Natl. Acad. Sci. USA. 95:652–656 10.1073/pnas.95.2.652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe D., Begom S., Addey C., White M., Dyson J., Chai J.G. 2010. Depletion of regulatory T cells by anti-GITR mAb as a novel mechanism for cancer immunotherapy. Cancer Immunol. Immunother. 59:1367–1377 10.1007/s00262-010-0866-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran M.A., Allison J.P. 2009. Tumor vaccines expressing flt3 ligand synergize with ctla-4 blockade to reject preimplanted tumors. Cancer Res. 69:7747–7755 10.1158/0008-5472.CAN-08-3289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot J.D., Gavin M.A., Rudensky A.Y. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4:330–336 10.1038/ni904 [DOI] [PubMed] [Google Scholar]
- Fossati-Jimack L., Ioan-Facsinay A., Reininger L., Chicheportiche Y., Watanabe N., Saito T., Hofhuis F.M., Gessner J.E., Schiller C., Schmidt R.E., et al. 2000. Markedly different pathogenicity of four immunoglobulin G isotype-switch variants of an antierythrocyte autoantibody is based on their capacity to interact in vivo with the low-affinity Fcγ receptor III. J. Exp. Med. 191:1293–1302 10.1084/jem.191.8.1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgini A., Brown H.J., Lock H.R., Nimmerjahn F., Ravetch J.V., Verbeek J.S., Sacks S.H., Robson M.G. 2008. Fc gamma RIII and Fc gamma RIV are indispensable for acute glomerular inflammation induced by switch variant monoclonal antibodies. J. Immunol. 181:8745–8752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeber M.B., López-Redondo F., Ikoma E., Ishikawa M., Imai Y., Nakajima K., Kreutzberg G.W., Kohsaka S. 1998. The microglia/macrophage response in the neonatal rat facial nucleus following axotomy. Brain Res. 813:241–253 10.1016/S0006-8993(98)00859-2 [DOI] [PubMed] [Google Scholar]
- Griffiths R.W., Elkord E., Gilham D.E., Ramani V., Clarke N., Stern P.L., Hawkins R.E. 2007. Frequency of regulatory T cells in renal cell carcinoma patients and investigation of correlation with survival. Cancer Immunol. Immunother. 56:1743–1753 10.1007/s00262-007-0318-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi Y., Xiu Y., Komura K., Nimmerjahn F., Tedder T.F. 2006. Antibody isotype-specific engagement of Fcγ receptors regulates B lymphocyte depletion during CD20 immunotherapy. J. Exp. Med. 203:743–753 10.1084/jem.20052283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn-Cymerman D., Budhu S., Kitano S., Liu C., Zhao F., Zhong H., Lesokhin A.M., Avogadri-Connors F., Yuan J., Li Y., et al. 2012. Induction of tumoricidal function in CD4+ T cells is associated with concomitant memory and terminally differentiated phenotype. J. Exp. Med. 209:2113–2126 10.1084/jem.20120532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi F.S., Butler M., Oble D.A., Seiden M.V., Haluska F.G., Kruse A., Macrae S., Nelson M., Canning C., Lowy I., et al. 2008. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc. Natl. Acad. Sci. USA. 105:3005–3010 10.1073/pnas.0712237105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi F.S., O’Day S.J., McDermott D.F., Weber R.W., Sosman J.A., Haanen J.B., Gonzalez R., Robert C., Schadendorf D., Hassel J.C., et al. 2010. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363:711–723 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda Y., Yamada T., Mori K., Okazaki A., Inoue M., Kitajima-Miyama K., Kuni-Kamochi R., Nakano R., Yano K., Kakita S., et al. 2007. Comparison of biological activity among nonfucosylated therapeutic IgG1 antibodies with three different N-linked Fc oligosaccharides: the high-mannose, hybrid, and complex types. Glycobiology. 17:104–118 10.1093/glycob/cwl057 [DOI] [PubMed] [Google Scholar]
- Kavanagh B., O’Brien S., Lee D., Hou Y., Weinberg V., Rini B., Allison J.P., Small E.J., Fong L. 2008. CTLA4 blockade expands FoxP3+ regulatory and activated effector CD4+ T cells in a dose-dependent fashion. Blood. 112:1175–1183 10.1182/blood-2007-11-125435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.M., Rasmussen J.P., Rudensky A.Y. 2007. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 8:191–197 10.1038/ni1428 [DOI] [PubMed] [Google Scholar]
- Klages K., Mayer C.T., Lahl K., Loddenkemper C., Teng M.W.L., Ngiow S.F., Smyth M.J., Hamann A., Huehn J., Sparwasser T. 2010. Selective depletion of Foxp3+ regulatory T cells improves effective therapeutic vaccination against established melanoma. Cancer Res. 70:7788–7799 10.1158/0008-5472.CAN-10-1736 [DOI] [PubMed] [Google Scholar]
- Krummel M.F., Allison J.P. 1996. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J. Exp. Med. 183:2533–2540 10.1084/jem.183.6.2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach D.R., Krummel M.F., Allison J.P. 1996. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 271:1734–1736 10.1126/science.271.5256.1734 [DOI] [PubMed] [Google Scholar]
- Li Y., Yee C. 2008. IL-21 mediated Foxp3 suppression leads to enhanced generation of antigen-specific CD8+ cytotoxic T lymphocytes. Blood. 111:229–235 10.1182/blood-2007-05-089375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liakou C.I., Kamat A., Tang D.N., Chen H., Sun J., Troncoso P., Logothetis C., Sharma P. 2008. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc. Natl. Acad. Sci. USA. 105:14987–14992 10.1073/pnas.0806075105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moo-Young T.A., Larson J.W., Belt B.A., Tan M.C., Hawkins W.G., Eberlein T.J., Goedegebuure P.S., Linehan D.C. 2009. Tumor-derived TGF-beta mediates conversion of CD4+Foxp3+ regulatory T cells in a murine model of pancreas cancer. J. Immunother. 32:12–21 10.1097/CJI.0b013e318189f13c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muranski P., Boni A., Antony P.A., Cassard L., Irvine K.R., Kaiser A., Paulos C.M., Palmer D.C., Touloukian C.E., Ptak K., et al. 2008. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 112:362–373 10.1182/blood-2007-11-120998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn F., Ravetch J.V. 2008. Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 8:34–47 10.1038/nri2206 [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F., Bruhns P., Horiuchi K., Ravetch J.V. 2005. FcgammaRIV: a novel FcR with distinct IgG subclass specificity. Immunity. 23:41–51 10.1016/j.immuni.2005.05.010 [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F., Lux A., Albert H., Woigk M., Lehmann C., Dudziak D., Smith P., Ravetch J.V. 2010. FcγRIV deletion reveals its central role for IgG2a and IgG2b activity in vivo. Proc. Natl. Acad. Sci. USA. 107:19396–19401 10.1073/pnas.1014515107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oflazoglu E., Stone I.J., Gordon K.A., Grewal I.S., van Rooijen N., Law C.-L., Gerber H.-P. 2007. Macrophages contribute to the antitumor activity of the anti-CD30 antibody SGN-30. Blood. 110:4370–4372 10.1182/blood-2007-06-097014 [DOI] [PubMed] [Google Scholar]
- Otten M.A., van der Bij G.J., Verbeek S.J., Nimmerjahn F., Ravetch J.V., Beelen R.H.J., van de Winkel J.G.J., van Egmond M. 2008. Experimental antibody therapy of liver metastases reveals functional redundancy between Fc gammaRI and Fc gammaRIV. J. Immunol. 181:6829–6836 [DOI] [PubMed] [Google Scholar]
- Peggs K.S., Quezada S.A., Chambers C.A., Korman A.J., Allison J.P. 2009. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti–CTLA-4 antibodies. J. Exp. Med. 206:1717–1725 10.1084/jem.20082492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piconese S., Valzasina B., Colombo M.P. 2008. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J. Exp. Med. 205:825–839 10.1084/jem.20071341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezada S.A., Peggs K.S., Curran M.A., Allison J.P. 2006. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J. Clin. Invest. 116:1935–1945 10.1172/JCI27745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezada S.A., Peggs K.S., Simpson T.R., Shen Y., Littman D.R., Allison J.P. 2008. Limited tumor infiltration by activated T effector cells restricts the therapeutic activity of regulatory T cell depletion against established melanoma. J. Exp. Med. 205:2125–2138 10.1084/jem.20080099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezada S.A., Simpson T.R., Peggs K.S., Merghoub T., Vider J., Fan X., Blasberg R., Yagita H., Muranski P., Antony P.A., et al. 2010. Tumor-reactive CD4+ T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J. Exp. Med. 207:637–650 10.1084/jem.20091918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezada S.A., Peggs K.S., Simpson T.R., Allison J.P. 2011. Shifting the equilibrium in cancer immunoediting: from tumor tolerance to eradication. Immunol. Rev. 241:104–118 10.1111/j.1600-065X.2011.01007.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S., Malmström V., Powrie F. 2000. Cytotoxic T lymphocyte–associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 192:295–302 10.1084/jem.192.2.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S., Greenwald R., Izcue A., Robinson N., Mandelbrot D., Francisco L., Sharpe A.H., Powrie F. 2006. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J. Immunol. 177:4376–4383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reff M.E., Carner K., Chambers K.S., Chinn P.C., Leonard J.E., Raab R., Newman R.A., Hanna N., Anderson D.R. 1994. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 83:435–445 [PubMed] [Google Scholar]
- Robert C., Thomas L., Bondarenko I., O’Day S., M D J.W., Garbe C., Lebbe C., Baurain J.F., Testori A., Grob J.J., et al. 2011. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 364:2517–2526 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- Schmidt E.M., Wang C.J., Ryan G.A., Clough L.E., Qureshi O.S., Goodall M., Abbas A.K., Sharpe A.H., Sansom D.M., Walker L.S.K. 2009. Ctla-4 controls regulatory T cell peripheral homeostasis and is required for suppression of pancreatic islet autoimmunity. J. Immunol. 182:274–282 [DOI] [PubMed] [Google Scholar]
- Selby M.J., Engelhardt J.J., Quigley M., Henning K.A., Chen T., Srinivasan M., Korman A.J. 2013. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunology Research. 10.1158/2326-6066.CIR-13-0013 [DOI] [PubMed] [Google Scholar]
- Setiady Y.Y., Coccia J.A., Park P.U. 2010. In vivo depletion of CD4+FOXP3+ Treg cells by the PC61 anti-CD25 monoclonal antibody is mediated by FcgammaRIII+ phagocytes. Eur. J. Immunol. 40:780–786 10.1002/eji.200939613 [DOI] [PubMed] [Google Scholar]
- Shi C., Hohl T.M., Leiner I., Equinda M.J., Fan X., Pamer E.G. 2011. Ly6G+ neutrophils are dispensable for defense against systemic Listeria monocytogenes infection. J. Immunol. 187:5293–5298 10.4049/jimmunol.1101721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrikant P., Khoruts A., Mescher M.F. 1999. CTLA-4 blockade reverses CD8+ T cell tolerance to tumor by a CD4+ T cell- and IL-2-dependent mechanism. Immunity. 11:483–493 10.1016/S1074-7613(00)80123-5 [DOI] [PubMed] [Google Scholar]
- Stockmeyer B., Elsässer D., Dechant M., Repp R., Gramatzki M., Glennie M.J., van de Winkel J.G., Valerius T. 2001. Mechanisms of G-CSF- or GM-CSF-stimulated tumor cell killing by Fc receptor-directed bispecific antibodies. J. Immunol. Methods. 248:103–111 10.1016/S0022-1759(00)00346-X [DOI] [PubMed] [Google Scholar]
- Stuart L.M., Ezekowitz R.A.B. 2005. Phagocytosis: elegant complexity. Immunity. 22:539–550 10.1016/j.immuni.2005.05.002 [DOI] [PubMed] [Google Scholar]
- Sutmuller R.P., van Duivenvoorde L.M., van Elsas A., Schumacher T.N., Wildenberg M.E., Allison J.P., Toes R.E., Offringa R., Melief C.J. 2001. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J. Exp. Med. 194:823–832 10.1084/jem.194.6.823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J.A., Hoppe A.D. 2004. The coordination of signaling during Fc receptor-mediated phagocytosis. J. Leukoc. Biol. 76:1093–1103 10.1189/jlb.0804439 [DOI] [PubMed] [Google Scholar]
- Takahashi T., Tagami T., Yamazaki S., Uede T., Shimizu J., Sakaguchi N., Mak T.W., Sakaguchi S. 2000. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med. 192:303–310 10.1084/jem.192.2.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai T., Li M., Sylvestre D., Clynes R., Ravetch J.V. 1994. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell. 76:519–529 10.1016/0092-8674(94)90115-5 [DOI] [PubMed] [Google Scholar]
- Tedder T.F., Baras A., Xiu Y. 2006. Fcgamma receptor-dependent effector mechanisms regulate CD19 and CD20 antibody immunotherapies for B lymphocyte malignancies and autoimmunity. Springer Semin. Immunopathol. 28:351–364 10.1007/s00281-006-0057-9 [DOI] [PubMed] [Google Scholar]
- Uchida J., Hamaguchi Y., Oliver J.A., Ravetch J.V., Poe J.C., Haas K.M., Tedder T.F. 2004. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. J. Exp. Med. 199:1659–1669 10.1084/jem.20040119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Elsas A., Hurwitz A.A., Allison J.P. 1999. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J. Exp. Med. 190:355–366 10.1084/jem.190.3.355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waitz R., Solomon S.B., Petre E.N., Trumble A.E., Fassò M., Norton L., Allison J.P. 2012. Potent induction of tumor immunity by combining tumor cryoablation with anti-CTLA-4 therapy. Cancer Res. 72:430–439 10.1158/0008-5472.CAN-11-1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.-Y., Weiner G. 2008. Complement and cellular cytotoxicity in antibody therapy of cancer. Expert Opin. Biol. Ther. 8:759–768 10.1517/14712598.8.6.759 [DOI] [PubMed] [Google Scholar]
- Wing K., Onishi Y., Prieto-Martin P., Yamaguchi T., Miyara M., Fehervari Z., Nomura T., Sakaguchi S. 2008. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 322:271–275 10.1126/science.1160062 [DOI] [PubMed] [Google Scholar]
- Xie Y., Akpinarli A., Maris C., Hipkiss E.L., Lane M., Kwon E.-K.M., Muranski P., Restifo N.P., Antony P.A. 2010. Naive tumor-specific CD4(+) T cells differentiated in vivo eradicate established melanoma. J. Exp. Med. 207:651–667 10.1084/jem.20091921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Bailey-Bucktrout S.L., Jeker L.T., Penaranda C., Martínez-Llordella M., Ashby M., Nakayama M., Rosenthal W., Bluestone J.A. 2009. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 10:1000–1007 10.1038/ni.1774 [DOI] [PMC free article] [PubMed] [Google Scholar]