Interleukin-25 preferentially elicits multipotent progenitor type 2 cells, which are distinct from other populations of type 2 innate lymphoid cells.
Abstract
The predominantly epithelial cell–derived cytokines IL-25, IL-33, and thymic stromal lymphopoietin (TSLP) can promote CD4+ Th2 cell–dependent immunity, inflammation, and tissue repair at barrier surfaces through the induction of multiple innate immune cell populations. IL-25 and IL-33 were previously shown to elicit four innate cell populations, named natural helper cells, nuocytes, innate type 2 helper cells, and multipotent progenitor type 2 (MPPtype2) cells, now collectively termed group 2 innate lymphoid cells (ILC2). In contrast to other types of ILC2, MPPtype2 cells exhibit multipotent potential and do not express T1/ST2 or IL-7Rα, suggesting that MPPtype2 cells may be a distinct population. Here, we show that IL-33 elicits robust ILC2 responses, whereas IL-25 predominantly promotes MPPtype2 cell responses at multiple tissue sites with limited effects on ILC2 responses. MPPtype2 cells were distinguished from ILC2 by their differential developmental requirements for specific transcription factors, distinct genome-wide transcriptional profile, and functional potential. Furthermore, IL-25–induced MPPtype2 cells promoted Th2 cytokine–associated inflammation after depletion of ILC2. These findings indicate that IL-25 simultaneously elicits phenotypically and functionally distinct innate lymphoid– and nonlymphoid-associated cell populations and implicate IL-25–elicited MPPtype2 cells and extramedullary hematopoiesis in the promotion of Th2 cytokine responses at mucosal surfaces.
CD4pos Th2 cells are characterized by the production of IL-4, IL-5, IL-9, and IL-13 and promote immunity to helminth infections and allergen-induced inflammation (Anthony et al., 2007; Kim et al., 2010; Palm et al., 2012; Pulendran and Artis, 2012). Emerging studies indicate that the primarily epithelial cell–derived cytokines thymic stromal lymphopoietin (TSLP), IL-25 (IL-17E), and IL-33 are critical in orchestrating distinct modules of the innate immune response that promote Th2 cell–dependent immunity, inflammation and tissue repair (Saenz et al., 2010a; Ziegler and Artis, 2010; Koyasu and Moro, 2011; Oliphant et al., 2011; Spits and Di Santo, 2011; Monticelli et al., 2012; Palm et al., 2012; Pulendran and Artis, 2012). For example, previous studies have shown that TSLP can induce Th2 cytokine–mediated inflammation by activating and promoting the accumulation of multiple cell types including DCs, lymphocytes, mast cells, and basophils (Park et al., 2000; Reche et al., 2001; Al-Shami et al., 2004; Allakhverdi et al., 2007; Liu et al., 2007; Rochman et al., 2007; Iliev et al., 2009; Perrigoue et al., 2009; Ziegler and Artis, 2010; Siracusa et al., 2011).
Recently, four independent laboratories identified previously unrecognized innate immune cell populations that were capable of contributing to Th2 cytokine responses in vivo (Moro et al., 2010; Neill et al., 2010; Price et al., 2010; Saenz et al., 2010b). These cell populations, termed natural helper cells (NHCs), nuocytes, innate type 2 helper (Ih2) cells, or multipotent progenitor type 2 (MPPtype2) cells, were shown to exhibit many similar phenotypic characteristics. For example, all four cell populations were characterized as being lineage negative (Linneg; lacking expression of hematopoietic cell lineage–associated markers for T cells, B cells, macrophages, DCs, NK cells, lymphoid tissue inducer (LTi) cells, neutrophils, mast cells, basophils, and eosinophils) but expressed Sca1 and c-kit (Moro et al., 2010; Neill et al., 2010; Price et al., 2010; Saenz et al., 2010b). Furthermore, these cell populations were elicited after helminth infection, were dependent on IL-25 and/or IL-33 signaling pathways, and could promote Th2 cytokine–mediated inflammation and immunity after exposure to Trichuris muris or Nippostrongylus brasiliensis (Moro et al., 2010; Neill et al., 2010; Price et al., 2010; Saenz et al., 2010b). Based on developmental, phenotypic, and functional similarities, NHCs, nuocytes, and Ih2 cells have been collectively categorized as group 2 innate lymphoid cells (ILC2; Spits and Di Santo, 2011; Monticelli et al., 2012; Sonnenberg and Artis, 2012; Spits and Cupedo, 2012; Tait Wojno and Artis, 2012; Walker et al., 2013). Work from this laboratory and many others went on to show that ILC2 are present in multiple tissues in both mice and humans and play critical roles in promoting immunity to helminth parasites, allergic inflammation, and the resolution of pulmonary inflammation (Moro et al., 2010; Neill et al., 2010; Price et al., 2010; Saenz et al., 2010b; Mjösberg et al., 2011; Monticelli et al., 2011; Chang et al., 2011; Hoorweg et al., 2012; Kim et al., 2012; Yasuda et al., 2012).
Although MPPtype2 cells share some phenotypic and functional characteristics with other ILC2 populations, their discordant expression of T1/ST2 (IL-33R), IL-7Rα, and CD90 (Thy1) and distinct multipotent potential suggest that MPPtype2 cells may differ from ILC2 family members. In this study, we use genetic approaches, genome-wide transcriptional profiling, and in vitro and in vivo functional assays to demonstrate that IL-25 simultaneously elicits phenotypically, functionally, and developmentally distinct populations of lymphoid-derived ILC2 and nonlymphoid MPPtype2 cells. Critically, IL-25–induced MPPtype2 cells could promote Th2 cytokine–associated inflammation even when ILC2 populations were depleted. These findings indicate that IL-25–elicited MPPtype2 cells are distinct from ILC2 and suggest that IL-25 simultaneously elicits both lymphoid- and nonlymphoid-associated innate immune cell populations that can promote type 2 inflammation at barrier surfaces.
RESULTS
IL-33 and IL-25 differentially regulate ILC2 and MPPtype2 cell responses
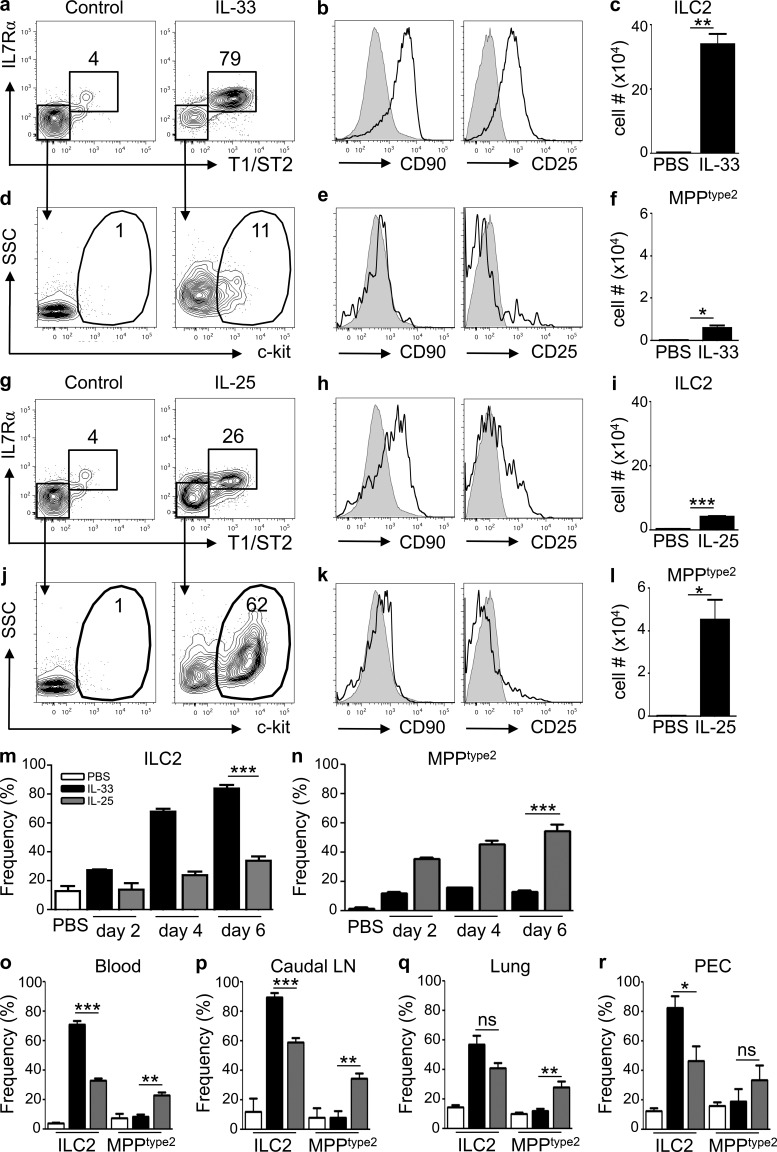
IL-33 and IL-25 were recently shown to elicit previously unrecognized innate immune cell populations that contribute to Th2 cytokine–associated inflammation. These cells express c-kit and Sca1 and were termed NHCs, nuocytes, or Ih2 cells (Moro et al., 2010; Neill et al., 2010; Price et al., 2010). In addition, a fourth previously unrecognized IL-25–responsive innate cell population has been described, termed MPPtype2 cells, given their capacity to exhibit multipotent potential to yield macrophages, mast cells, and basophils that can promote Th2 cytokine–mediated immunity (Saenz et al., 2010b). The identification of multiple IL-25– or IL-33–responsive innate immune cell populations provokes the question of whether these are four different cell populations or the same cell population identified by four different groups. To directly test whether IL-25 or IL-33 elicits similar or distinct innate cell populations, WT mice were treated with exogenous IL-33 or IL-25 and Linneg cells in the mesenteric LNs (MLNs) were analyzed for expression of surface markers that define ILC2 versus MPPtype2 cells (T1/ST2, IL-7Rα, Sca1, CD90, CD25, and c-kit). As previously reported (Neill et al., 2010), administration of recombinant IL-33 elicited increased frequencies of Linneg T1/ST2pos IL-7Rαpos cells in the MLN (Fig. 1 a), which express CD90, CD25, and Sca1 (Fig. 1 b and not depicted), a phenotype consistent with ILC2. In addition, IL-33 treatment resulted in increased total numbers of ILC2 in the MLN (Fig. 1 c).
Figure 1.
IL-25 simultaneously elicits phenotypically distinct populations of MPPtype2 cells and ILC2. (a–r) C57BL/6 WT mice (The Jackson Laboratory) were treated i.p. with PBS (control), 0.3 µg of recombinant IL-33, or 0.3 µg of recombinant IL-25 daily for 2, 4, or 7 d. Indicated tissues were harvested at day 7 (or as indicated), and the frequency and total cell numbers of ILC2 and MPPtype2 cells were assessed by flow cytometry. (a) Frequency of T1/ST2pos IL-7Rαpos ILC2 in the Linneg cell compartment from the MLNs of control or IL-33–treated mice. (b) CD90.2 (Thy1.2) and CD25 expression on ILC2 (black histograms) from IL-33–treated mice. (c) Total cell numbers of ILC2 from control or IL-33–treated mice. (d) Expression of c-kit on gated T1/ST2neg IL-7Rαneg cells from control or IL-33–treated mice. (e) CD90 and CD25 expression on c-kitpos MPPtype2 cells (black histograms) from IL-33–treated mice. (f) Total cell numbers of MPPtype2 cells from control or IL-33–treated mice. (g) Frequency of ILC2 in the Linneg cell compartment from the MLN of control or IL-25–treated mice. (h) CD90 and CD25 expression on ILC2 (black histograms) from IL-25–treated mice. (i) Total cell numbers of ILC2 from control or IL-25–treated mice. (j) Expression of c-kit on gated T1/ST2neg IL-7Rαneg cells from control or IL-25–treated mice. (k) CD90 and CD25 expression on c-kitpos MPPtype2 cells (black histograms) from IL-25–treated mice. (l) Total cell numbers of MPPtype2 cells from control or IL-25–treated mice. Plots are gated on live, Linneg (CD4, CD8, CD11b, CD11c, and CD19) cells or as indicated. Gray shaded histograms represent CD90 or CD25 expression on Linneg cells from control mice. (m and n) Frequencies of ILC2 (m) or MPPtype2 cells (n) as gated in a, g, d, and i from the MLNs of control (open bars), IL-33–treated, or IL-25–treated mice at days 2, 4, and 6. (o–r) Frequencies of ILC2 and MPPtype2 cells as gated in a and g and d and i from the blood (o), caudal LN (p), lung (q), and PEC (r) of control (open bars), IL-33–treated (black bars), or IL-25–treated (gray bars) mice at day 6. Data in a–n are representative of two or more independent experiments (control, n = 4; IL-33 treated, n = 8; IL-25 treated, n = 8). Data in o–r are representative of two independent experiments (control, n = 4; IL-25 treated, n = 8; IL-33 treated, n = 8). *, P < 0.05; **, P < 0.01; ***, P < 0.0001. Lung IL-33–elicited ILC2 versus IL-25–elicited ILC2, P = 0.07; PEC IL-33–elicited MPPtype2 cells versus IL-25–elicited MPPtype2 cells, P = 0.31. Error bars indicate SEM.
Although IL-33–mediated induction of ILC2 populations is well characterized (Barlow et al., 2012; Neill et al., 2010), whether IL-33 also elicits MPPtype2 cells remains unclear. To test this, the frequency and number of Linneg T1/ST2neg IL-7Rαneg cells that express c-kit were analyzed. Compared with controls, IL-33–treated mice exhibited modest increases in the frequencies (Fig. 1 d) and total numbers (Fig. 1 f) of Linneg T1/ST2neg IL-7Rαneg c-kitpos cells, which was independent of IL-25 (not depicted). In addition, these cells expressed Sca1 (not depicted) but lacked expression of CD90 and CD25 (Fig. 1 e), a phenotype consistent with that of MPPtype2 cells but distinct from Linneg T1/ST2pos IL-7Rαpos ILC2 cells.
Previous studies demonstrated redundancy between IL-33 and IL-25 to induce ILC2 populations but have also shown that treatment with exogenous IL-33 elicits a more robust ILC2 response in the lung than IL-25 (Neill et al., 2010; Barlow et al., 2012). Therefore, to address whether IL-25 elicits similar innate immune cell populations and with a similar magnitude to IL-33, WT mice were treated with exogenous IL-25 at the same time as mice that were treated with IL-33 as depicted above, and the frequency of ILC2 and MPPtype2 cells were assessed. IL-25 treatment was associated with modest increases in the frequencies (Fig. 1 g) and total numbers of ILC2 populations in the MLN (Fig. 1 i), which displayed heterogeneous expression of CD90 and CD25 (Fig. 1 h). In contrast to eliciting modest ILC2 responses, administration of IL-25 elicited increased frequencies (Fig. 1, j and k) and significantly elevated total numbers of Linneg c-kitpos MPPtype2 cells in the MLN (Fig. 1 l).
Increased frequencies of ILC2 were observed as early as 2 d after IL-33 treatment and continued to increase at days 4 and 6 (Fig. 1 m, black bars). In contrast, the IL-25–mediated induction of ILC2 was not apparent until days 4–6 (Fig. 1 m, gray bars) and remained modest in comparison with IL-33–elicited ILC2 responses. In addition, although administration of IL-33 resulted in an early (day 2) modest increase in the frequency of T1/ST2neg IL-7Rαneg c-kitpos MPPtype2 cells in the MLN, the frequency did not increase at days 4 or 6 (Fig. 1 n, black bars). However, induction of MPPtype2 cells after IL-25 treatment was observed as early as day 2 after treatment and continued to increase through day 6 (Fig. 1 n, gray bars). Moreover, the selectivity of IL-33–elicited ILC2 and IL-25–elicited MPPtype2 cells was observed in multiple anatomical sites including the blood (Fig. 1 o), caudal LN (Fig. 1 p), lung (Fig. 1 q), and peritoneal cavity (PEC; Fig. 1 r). Collectively, these data indicate that IL-33 predominantly elicits ILC2 responses with limited effects on MPPtype2 cells, whereas IL-25 robustly promotes MPPtype2 cell responses in multiple anatomical sites.
IL-25 elicits MPPtype2 cells independently of IL-33–IL-33R interactions
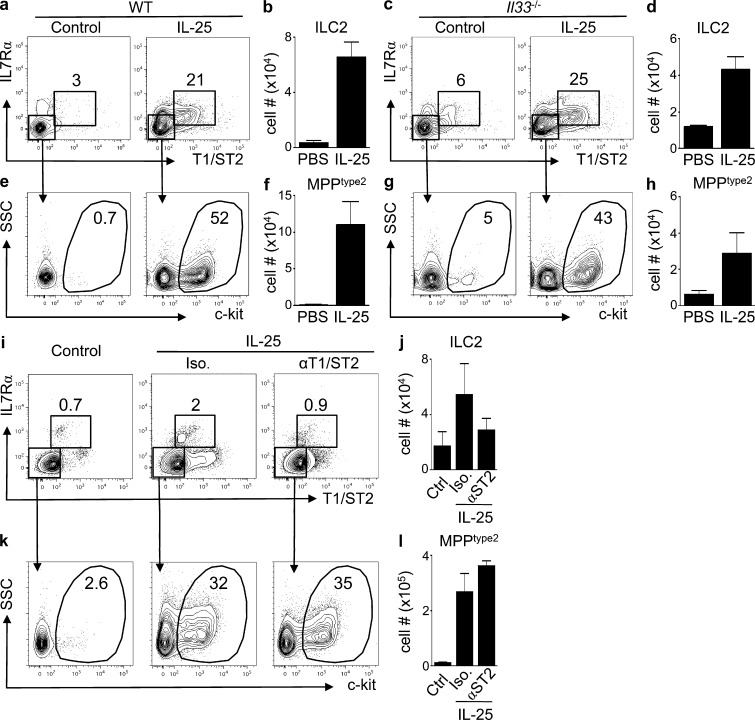
IL-33 and IL-25 were shown to redundantly elicit ILC2 (Neill et al., 2010). To test whether IL-25 can promote the induction of ILC2 or MPPtype2 cells independently of IL-33–IL-33R interactions, WT and Il33−/− mice were treated with IL-25 and ILC2, and MPPtype2 cell responses were assessed. IL-25–treated WT mice displayed a modest increase in the frequency and total cell number of ILC2 cells in the MLN compared with control mice (Fig. 2, a and b). Furthermore, IL-33–deficient mice treated with IL-25 exhibited a similar increase in the frequencies and total cell numbers of ILC2 cells to those observed in WT mice (Fig. 2, c and d). Critically, IL-25 treatment also resulted in increased frequencies and total cell numbers of MPPtype2 cells in both WT (Fig. 2, e and f) and IL-33–deficient mice (Fig. 2, g and h). Similarly, mice treated with IL-25 in the presence of a neutralizing mAb against the IL-33 receptor (T1/ST2) exhibited increased frequencies and total cell numbers of MPPtype2 cells at levels comparable with those observed in mice treated with IL-25 and an isotype control mAb (Fig. 2, i–l). Collectively, these data indicate that IL-25 simultaneously elicits distinct ILC2 and MPPtype2 cell populations in IL-33–sufficient and –deficient environments.
Figure 2.
IL-25 elicits ILC2 and MPPtype2 cells independent of IL-33 signaling. (a–d) C57BL/6 WT mice and C57BL/6 Il33−/− mice (Taconic) were treated i.p. with PBS (control) or 0.3 µg of recombinant IL-25 daily for 7 d. MLNs were harvested at day 7, and the frequency and total cell numbers of ILC2 and MPPtype2 cells were assessed by flow cytometry. (a and c) Frequency of ILC2 in control or IL-25–treated WT (a) or Il33−/− mice (c). (b and d) Total cell numbers of ILC2 in control or IL-25–treated WT (b) or Il33−/− mice (d). (e and g) Frequency of MPPtype2 cells in control or IL-25–treated WT (e) or Il33−/− mice (g). (f and h) Total cell numbers of MPPtype2 cells in control or IL-25–treated WT (f) or Il33−/− mice (h). Data in a–h are representative of two independent experiments (control, n = 4; IL-25–treated WT, n = 6; IL-25–treated Il33−/−, n = 6). (i–l) BALB/c WT mice (The Jackson Laboratory) were treated with PBS (control) or 0.3 µg of recombinant IL-25 plus isotype (IgG) or anti-T1/ST2 mAbs. MLNs were harvested at day 7, and the frequency and total cell numbers of ILC2 and MPPtype2 cells were assessed by flow cytometry. Frequency and total numbers of T1/ST2pos IL-7Rαpos ILC2 (i and j) and T1/ST2neg IL-7Rαneg c-kitpos MPPtype2 cells (k and l). Plots are gated on live, Linneg (CD4, CD8α, CD11b, CD11c, and CD19) cells. Data in i–l are representative of two independent experiments (control, n = 6; IL-25 treated, n = 9; anti-T1/ST2 IL-25 treated, n = 9). Error bars indicate SEM.
MPPtype2 cells exhibit distinct transcriptional profiles from ILCs
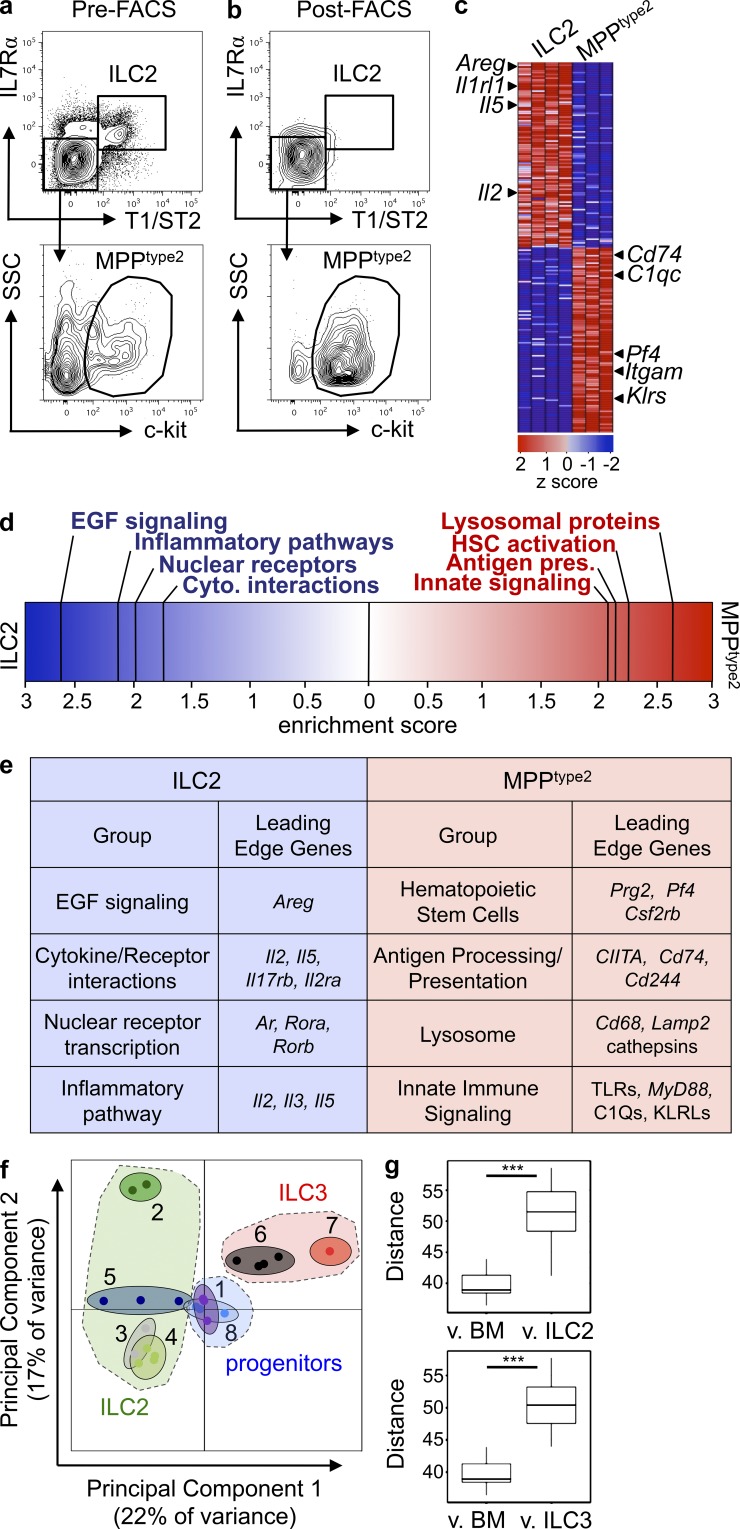
The capacity of IL-25 to elicit MPPtype2 cells independently of IL-33–IL-33R signaling and the finding that MPPtype2 cells possess a distinct surface phenotype from ILC2 provoked the hypothesis that MPPtype2 cells may be distinct from ILC2. To investigate potential differences between MPPtype2 cells and ILC2, genome-wide transcriptional profiling was performed. IL-25–elicited MPPtype2 cells (defined as Linneg T1/ST2neg IL-7Rαneg CD90neg CD25neg c-kitpos) were sort-purified to a purity of ≥95% (Fig. 3, a and b), mRNA was hybridized onto microarray chips, and the transcriptional profile of MPPtype2 cells was compared against the transcriptional profile of Linneg T1/ST2pos IL-7Rαpos CD90pos CD25pos lung-resident ILC2 (Monticelli et al., 2011). Examination of the top 100 most differentially expressed genes between MPPtype2 cells and lung-resident ILC2 revealed substantial differences between these two populations (Fig. 3 c). Consistent with previous studies (Neill et al., 2010; Monticelli et al., 2011), ILC2 cells were characterized by expression of genes encoding T1/ST2 (Il1rl1), the cytokines IL-2 (Il2) and IL-5 (Il5), and the wound-healing response protein amphiregulin (Areg; Fig. 3 c). In contrast, MPPtype2 cells were enriched for genes encoding innate immune signaling pathways (Klr and C1q proteins), hematopoietic stem cell (HSC)–associated genes (Pf4), and myeloid-associated genes (Itgam and Cd74; Fig. 3 c).
Figure 3.
IL-25–elicited MPPtype2 cells possess a unique transcriptional profile from ILC2 cells. (a and b) C57BL/6 WT mice (The Jackson Laboratory) were treated with 0.3 µg of recombinant IL-25 daily for 7 d. MLNs and PECs were harvested from IL-25–treated mice and MPPtype2 cells (Linneg T1/ST2neg IL-7Rαneg CD4neg CD90neg CD25neg c-kitpos; a) were FACS purified to ≥95% purity (b). Three biological replicates of MPPtype2 cells were collected, and mRNA was isolated, amplified, and hybridized to Affymetrix gene chips for microarray analysis. The previously published microarray gene expression profile of lung-resident ILC2 was used for comparison (GEO series no. GSE46468; Monticelli et al., 2011). (c) Heat map representing gene expression profiles of the top 100 differentially expressed genes between MPPtype2 cells and ILC2. Red indicates high expression, and blue indicates low expression. (d) GSEA comparing the gene expression signatures of MPPtype2 cells and ILC2. (e) List of leading edge genes from GSEA analysis from d. (f) PCA plot comparing transcriptional profiles for MPPtype2 cells (1), ex vivo nuocytes (2; GSE25890), NHCs (3; GSE18752), lung-resident ILC2 (4; GSE46468), unstimulated lung NHCs (5; GSE36057), splenic LTi cells (6, GSE46468; and 7, GSE18752), and BM-GMPs (8). Categories of ILC2 (green shaded area), ILC3 (red shaded area), and progenitors (blue shaded area) were grouped (as indicated in f, dashed lines), and Euclidean distance measurements between MPPtype2 cells and BM-GMP versus ILC2 or ILC3 were calculated (g). ***, P < 0.0001. MPPtype2 cells versus BM-GMP versus ILC2, P = 8.5 × 10−8; MPPtype2 cells versus BM-GMP versus ILC3s, P = 3.1 × 10−6.
To further assess the differences in transcriptional profiles between MPPtype2 and ILC2 cells, gene set enrichment analysis (GSEA) was performed (Subramanian et al., 2005). GSEA demonstrated that lung-resident ILC2 cells were enriched for gene expression associated with epidermal growth factor (EGF) signaling, cytokine–receptor interactions, nuclear receptor transcription, and inflammatory pathways (Fig. 3, d and e). In contrast, IL-25–elicited MPPtype2 cells exhibited a gene expression profile enriched for HSCs, antigen processing/presentation, lysosomal proteins, and innate immune signaling pathways (Fig. 3, d and e), a finding consistent with the potential of MPPtype2 cells to differentiate into macrophage and granulocyte populations.
In addition, to assess the differences in transcriptional profiles between MPPtype2 cells and ILC2 populations, we undertook a more comprehensive characterization of the genome-wide transcriptional profiles for MPPtype2 cells (Fig. 3 f, 1) and compared them against microarray datasets for ILC2 generated by multiple laboratories (including nuocytes [2], NHCs [3], lung-resident ILC2 [4], and lung NHCs [5]; Moro et al., 2010; Neill et al., 2010; Monticelli et al., 2011; Halim et al., 2012). In addition, ILC3 microarray datasets were included from multiple laboratories (Fig. 3 f, 6 and 7, LTi-like cells; Monticelli et al. [2011] and Moro et al. [2010], respectively) as a comparison to a known population of distinct innate immune cells (Fig. 3 f). Finally, given the phenotypic and genome-wide transcriptional profile of the IL-25–elicited MPPtype2 cells (Fig. 3, c–e), a BM-resident granulocyte/monocyte precursor (BM-GMP) cell population was also included (Fig. 3 f, 8) in these unbiased analyses of transcriptional profiles.
Principal component analysis (PCA) was performed using batch-corrected microarray datasets from ILC2, ILC3, MPPtype2, and BM-GMP cells to generate a plot of the first two components that accounted for ∼40% of the total variance between all groups (Fig. 3 f). As expected, ILC3 populations clustered together (Fig. 3 f). Notably, although nuocytes did not cluster as closely with other ILC2 populations, as expected based on surface marker phenotype and function, ILC2 reported by multiple groups (NHCs, lung-resident ILC2, and lung NHCs) clustered tightly together (Fig. 3 f, green shaded area) and did not cluster with ILC3 (Fig. 3 f, red shaded area). Critically, however, IL-25–elicited MPPtype2 cells (Fig. 3 f, 1) did not cluster with either ILC2 or ILC3, but instead clustered with BM-GMP (Fig. 3 f), suggesting that MPPtype2 cells represent a distinct innate cell population from ILC2 and ILC3. Analysis of the Euclidean distances between MPPtype2 cells and BM-GMP (Fig. 3, f and g, blue shaded area) showed that they were significantly shorter than the distances between MPPtype2 cells and ILC2 (Fig. 3, f and g, green shaded area) or ILC3 (Fig. 3, f and g, red shaded area), indicating that MPPtype2 cells are more similar to BM-GMP than to ILC2 or ILC3. Together, these data suggest that MPPtype2 cells exhibit a transcriptional profile similar to that of BM-derived hematopoietic progenitor populations and are thus distinct from known ILC populations.
MPPtype2 cells, but not ILC2, exhibit progenitor potential
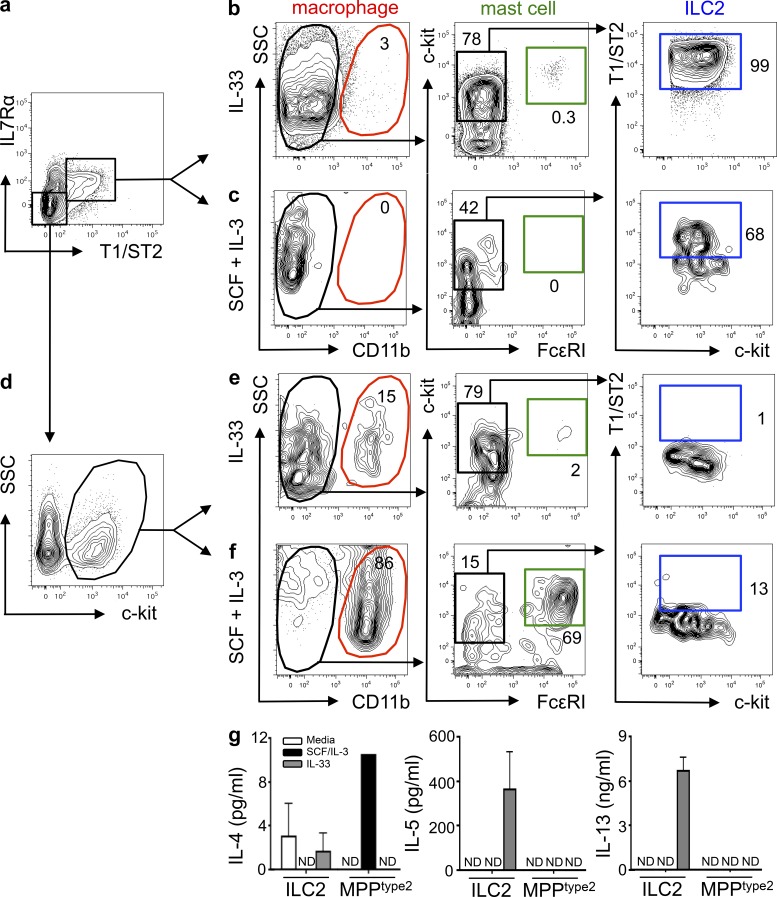
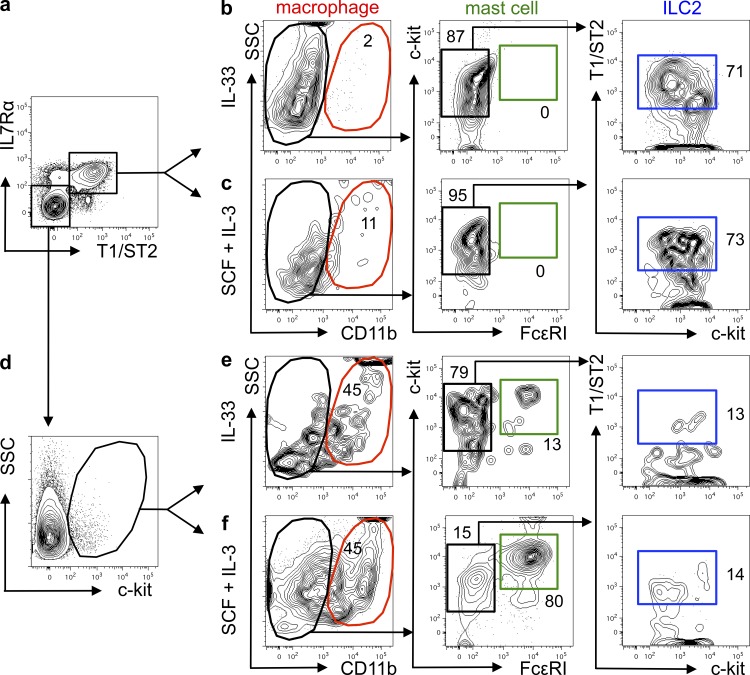
The transcriptional profiles coupled with clustering analyses of MPPtype2 cells suggested that MPPtype2 cells might represent a progenitor cell population capable of differentiating into multiple cell lineages, in agreement with previously reported findings (Saenz et al., 2010b). To test whether ILC2 and MPPtype2 cells exhibit similar progenitor cell capacities, cells were sort-purified from IL-25–treated mice and cultured in the presence of IL-33 alone or SCF and IL-3. The resultant progeny were analyzed for differentiation into multiple cell lineages by flow cytometry based on surface marker expression. Consistent with previous studies (Moro et al., 2010; Neill et al., 2010), analysis of FACS-purified ILC2 (Fig. 4 a) cultured in the presence of IL-33 revealed that ILC2 proliferated but did not differentiate into macrophages or mast cells (Fig. 4 b and not depicted). However, IL-33–stimulated ILC2 retained their T1/ST2 expression (Fig. 4 b) and produced elevated levels of IL-5 and IL-13 but little IL-4 (Fig. 4 g). In addition, ILC2 cultured in the presence of SCF and IL-3 did not differentiate into macrophages or mast cells, but a small proportion of the cells retained their ILC2 surface phenotype (Fig. 4 c), indicating that ILC2 cells are a terminally differentiated cell population unable to give rise to other cell lineages.
Figure 4.
IL-25–elicited MPPtype2 cells, but not ILC2, possess multipotent potential. BALB/c WT mice (The Jackson Laboratory) were treated with 0.3 µg of recombinant IL-25 daily for 7 d. MLNs and PECs were harvested from IL-25–treated mice, and ILC2 and MPPtype2 cells were sort-purified and cultured in the presence of IL-33 alone or SCF plus IL-3. (a and d) FACS purification gating of ILC2 (Linneg T1/ST2pos IL-7Rαpos; a) or MPPtype2 cells (Linneg T1/ST2neg IL-7Rαneg c-kitpos; d) from IL-25–treated mice. Plots shown are gated on live, Linneg cells (CD3ε, CD8α, CD19, CD11b, CD11c, Gr-1). (b and c) Flow cytometric analysis of myeloid, granulocyte, and ILC2 cell differentiation of day 8–12 cultures seeded with FACS-purified ILC2 in the presence of IL-33 (b) or SCF and IL-3 (c). (e and f) Flow cytometric analysis of macrophage, granulocyte, and ILC2 cell differentiation of day 8–12 cultures seeded with FACS-purified MPPtype2 cells in the presence of IL-33 (e) or SCF and IL-3 (f). (g) Culture supernatants from b, c, e, and f were collected, and IL-4, IL-5, and IL-13 protein levels were measured by ELISA. Data in a–g are representative of at least three independent experiments. Error bars indicate SEM.
In contrast to ILC2, when cultured in the presence of IL-33 alone, FACS-purified MPPtype2 cells (Fig. 4 d) yielded a small but identifiable CD11bpos macrophage-like cell population but did not give rise to a CD11bneg c-kitpos FcεRIpos mast cell population or a CD11bneg T1/ST2pos ILC2 population (Fig. 4 e). Furthermore, after culture with IL-33, MPPtype2 cells and their progeny did not produce IL-4, IL-5, or IL-13 (Fig. 4 g), indicating that MPPtype2 cells do not differentiate and produce cytokines in response to IL-33. However, in vitro cultures seeded with MPPtype2 cells in the presence of SCF and IL-3 contained substantial frequencies of CD11bpos macrophages as well as CD11bneg c-kitpos FcεRIpos mast cells but did not contain T1/ST2pos IL-7Rαpos ILC2 (Fig. 4 f). Critically, in contrast to IL-25–elicited ILC2, progeny derived from IL-25–elicited MPPtype2 cells produced elevated levels of IL-4 but not IL-5 or IL-13 (Fig. 4 g). Combined with the differences observed in surface phenotype, transcriptional profile and the finding that MPPtype2 cells but not ILC2 possess multipotent potential, these data support the hypothesis that MPPtype2 cells are distinct from ILC2.
Consistent with a degree of redundancy between IL-33 and IL-25, IL-33 also elicited modest increases in the frequencies of MPPtype2-like cells (Fig. 1, d–f). Therefore, to investigate whether IL-33–elicited ILC2 and MPPtype2 cells display similar functional potential compared with the IL-25–elicited cell populations, we sort-purified ILC2 or MPPtype2 cell populations from the MLNs of IL-33–treated mice (Fig. 5, a and d) and cultured them in the presence of IL-33 alone or SCF and IL-3. After culture, resultant progeny were assessed for differentiation into myeloid, granulocyte, or ILC2 lineages (Fig. 5, b, c, e, and f). Consistent with IL-25–elicited ILC2, IL-33–elicited ILC2 proliferated and retained their expression of T1/ST2 in response to IL-33 (Fig. 5 b). However, when cultured in the presence of SCF and IL-3, ILC2 from IL-33–treated mice did not differentiate into macrophages, mast cells, or basophils but did retain an ILC2 surface phenotype (Fig. 5 c), suggesting that consistent with the phenotype of IL-25–induced ILC2 and previously published data, IL-33–elicited ILC2 are a terminally differentiated cell population and cannot develop into myeloid lineages (Moro et al., 2010).
Figure 5.
IL-33–elicited MPPtype2 cells, but not ILC2, possess multipotent potential. BALB/c WT mice (The Jackson Laboratory) were treated with 0.3 µg of recombinant IL-33 daily for 7 d. MLNs and PECs were harvested from IL-33–treated mice, and ILC2 and MPPtype2 cells were sort-purified and cultured in the presence of IL-33 alone or SCF plus IL-3. (a and d) FACS purification gating of ILC2 (Linneg T1/ST2pos IL-7Rαpos; a) or MPPtype2 cells (Linneg T1/ST2neg IL-7Rαneg c-kitpos; d) from IL-33–treated mice. Plots shown are gated on live, Linneg cells (CD3ε, CD8α, CD19, CD11b, CD11c, and Gr-1). (b and c) Flow cytometric analysis of myeloid, granulocyte, and ILC2 cell differentiation of day 8–12 cultures seeded with FACS-purified ILC2 in the presence of IL-33 (b) or SCF and IL-3 (c). (e and f) Flow cytometric analysis of myeloid, granulocyte, and ILC2 cell differentiation of day 8 cultures seeded with FACS-purified MPPtype2 cells in the presence of IL-33 (e) or SCF and IL-3 (f). Data in a–f are representative of at least two independent experiments.
Similar to IL-25–elicited MPPtype2 cells, when cultured in the presence of IL-33 alone, MPPtype2 cells from IL-33–treated mice did not give rise to substantial populations of macrophages, mast cells, or ILC2 (Fig. 5 e). However, when cultured in the presence of SCF and IL-3, IL-33–elicited MPPtype2 cells yielded CD11bpos macrophages and CD11bneg c-kitpos FcεRIpos mast cells but not ILC2 (Fig. 5 f). Combined, these data suggest that IL-33–elicited ILC2 and MPPtype2 cells respond to IL-33 or SCF + IL-3 in a similar fashion to their IL-25–elicited counterparts and further demonstrate that, in contrast to ILC2, MPPtype2 cells uniquely exhibit multipotent potential.
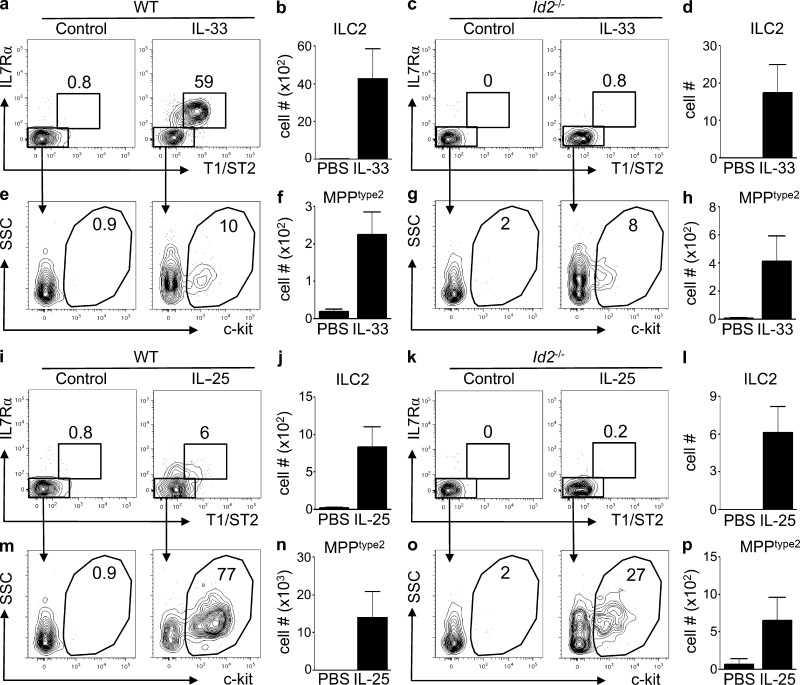
MPPtype2 cell responses are partially independent of Id2
ILC2 are developmentally dependent on the lymphoid lineage specifying transcription factor inhibitor of DNA binding 2 (Id2; Moro et al., 2010; Monticelli et al., 2011). Therefore, to test whether MPPtype2 cell responses could be induced in the absence of Id2, BM chimeras were generated in which either WT or Id2-deficient BM cells were transplanted into lethally irradiated congenic WT recipients. 8 wk after transplantation, chimeric mice were treated daily with either IL-33 or IL-25 for 7 d, and the induction of ILC2 populations and MPPtype2 cells was evaluated. Congenic mice that received WT BM exhibited increased frequencies and total cell numbers of donor-derived ILC2 cells in the MLN after treatment with IL-33 (Fig. 6, a and b). Consistent with the developmental dependence of ILC2 cells on Id2, administration of IL-33 failed to induce donor-derived ILC2 cells in mice that received Id2−/− BM (Fig. 6, c and d). In contrast, IL-33 elicited donor-derived MPPtype2 cells in both WT and Id2-deficient chimeras (Fig. 6, e–h), suggesting that MPPtype2 cells do not require Id2 for their development.
Figure 6.
IL-25–mediated induction of MPPtype2 cells occurs independently of Id2. (a–p) CD5/B220-depleted donor BM (WT, CD45.1; Id2−/−, CD45.1.2) was transferred into lethally irradiated C57BL/6 WT (CD45.2) mice. After reconstitution for 8 wk after transplant, mice were treated i.p. with PBS (control), 0.3 µg of recombinant IL-33, or 0.3 µg of recombinant IL-25 daily for 7 d. MLNs were harvested, and the frequency and total cell numbers of ILC2 and MPPtype2 cells were assessed by flow cytometry. (a and c) Frequency of T1/ST2pos IL-7Rαpos ILC2 from control or IL-33–treated WT BM chimera mice (a) or Id2-deficient BM chimera mice (c). (b and d) Total cell numbers of ILC2 from control or IL-33–treated WT BM chimera mice (b) or Id2-deficient BM chimera mice (d). (e and g) Frequency of c-kitpos MPPtype2 cells from control or IL-33–treated WT BM chimera mice (e) or Id2-deficient BM chimera mice (g). (f and h) Total cell numbers of MPPtype2 cells from control or IL-33–treated WT BM chimera mice (f) or Id2-deficient BM chimera mice (h). (i and k) Frequency of T1/ST2pos IL-7Rαpos ILC2 cells from control or IL-25–treated WT BM chimera mice (i) or Id2-deficient BM chimera mice (k). (j and l) Total cell numbers of ILC2 from control or IL-25–treated WT BM chimera mice (j) or Id2-deficient BM chimera mice (l). (m and o) Frequency of c-kitpos MPPtype2 cells from control or IL-25–treated WT BM chimera mice (m) or Id2-deficient BM chimera mice (o). (n and p) Total cell numbers of MPPtype2 cells from control or IL-25–treated WT BM chimera mice (n) or Id2-deficient BM chimera mice (p). Plots are gated on live, Linneg (CD4, CD8, CD11b, CD11c, and CD19) donor-derived cells. Data in a–p are representative of two or more independent experiments (control, n = 4; IL-33 treated, n = 6; IL-25 treated, n = 6). Error bars indicate SEM.
IL-25 induced the modest increase in the frequency and total cell numbers of donor-derived ILC2 in WT chimeras (Fig. 6, i and j) but did not elicit an ILC2 population from Id2-deficient donor cells (Fig. 6, k and l). Administration of IL-25 also resulted in increased frequencies and total cell numbers of donor-derived MPPtype2 cells in mice that received WT BM (Fig. 6, m and n). Furthermore, although diminished compared with the induction observed in WT mice (27% compared with 77%), treatment of Id2−/− BM chimeras with IL-25 resulted in the population expansion and increased total cell numbers of MPPtype2 cells from donor-derived cells (Fig. 6, o and p). Thus, in contrast to ILC2, which are critically dependent on Id2 for their development, administration of IL-25 could partially promote MPPtype2 cell responses in both the presence and absence of Id2, supporting the hypothesis that IL-25 elicits two developmentally distinct innate immune cell populations, ILC2 and MPPtype2 cells.
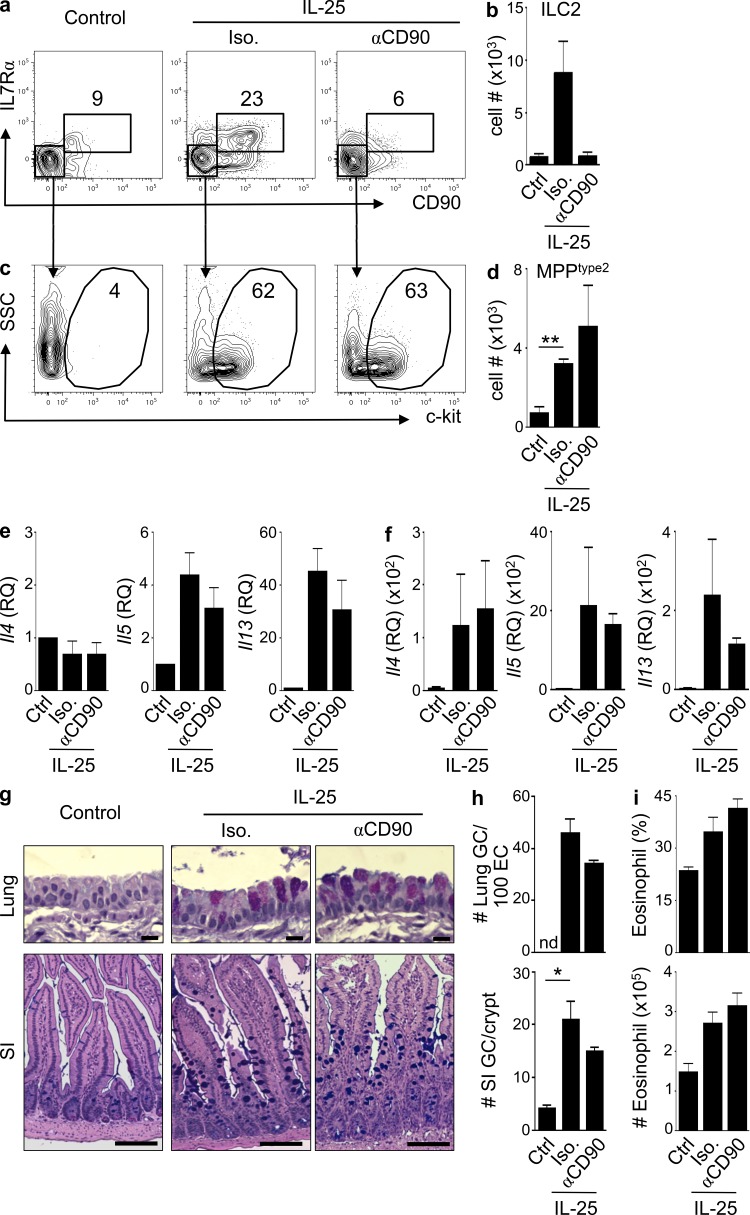
MPPtype2 cells can promote type 2 inflammation in the absence of ILC2
To investigate the innate immune mechanisms through which IL-25 and IL-33 promote type 2 inflammation, the function of MPPtype2 cells was assessed in vivo in mice depleted of ILC2. Using the anti-CD90 mAb–mediated depletion protocol in Rag1−/− mice, ILCs can be depleted in vivo without influencing the frequencies of MPPtype2 cells (Fig. 7), which do not express CD90 (Fig. 1 k). Rag1−/− mice were treated with IL-25 and simultaneously administered either an isotype control antibody or anti-CD90 mAb. After IL-25 treatment, the frequencies of ILC2 and MPPtype2 cells and Th2 cytokine–associated inflammation were assessed. As expected, compared with control-treated mice, IL-25 treatment induced increased frequencies and total cell numbers of CD90pos T1/ST2pos IL-7Rαpos ILC2 cells (Fig. 7, a and b) and a c-kitpos MPPtype2 cell population in the MLN (Fig. 7, c and d). These responses were associated with increased expression of Il4, Il5, and Il13 in the lung and small intestine (Fig. 7, e and f), goblet cell hyperplasia in the lung and small intestine (Fig. 7, g and h), and increased frequencies and total cell numbers of eosinophils in the lung (Fig. 7 i). Treatment with anti-CD90 mAb resulted in the depletion of CD90pos T1/ST2pos IL-7Rαpos ILC2 cells (Fig. 7, a and b). Critically, despite depletion of ILC2, IL-25–mediated induction of c-kitpos MPPtype2 cells (Fig. 7, c and d), induction of Il4, Il5, and Il13 expression in the lung and intestine (Fig. 7, e and f), promotion of goblet cell hyperplasia in the lung and small intestine (Fig. 7, g and h), and eosinophil responses (Fig. 7 i) were not affected. Thus, these data indicate that after depletion of ILC2, administration of IL-25 can still promote the induction of MPPtype2 cells and type 2 inflammation and suggest that IL-25–elicited MPPtype2 cells can promote Th2 cell–dependent immune responses independent of ILC2.
Figure 7.
IL-25–mediated induction of MPPtype2 cells and type 2 inflammation occurs independently of ILC2. (a–i) C57BL/6 Rag1−/− mice (The Jackson Laboratory) were treated i.p. with PBS (control) or 0.3 µg of IL-25 plus isotype mAb (Iso.) or anti-CD90 mAb. (a and b) Frequencies (a) and total cell numbers (b) of CD90pos T1/ST2pos IL-7Rαpos ILC2 in the MLNs of control or IL-25–treated mice given either isotype or anti-CD90 mAb. (c and d) Frequencies (c) and total cell numbers (d) of CD90neg T1/ST2neg IL-7Rαneg MPPtype2 cells in the MLNs of control or IL-25–treated mice given either isotype or anti-CD90 mAb. Plots are gated on live, Linneg (CD4, CD8, CD11b, CD11c, and CD19) cells or as indicated. (e and f) Quantitative real-time PCR of Il4, Il5, and Il13 gene expression levels from lung (e) or small intestinal tissue (f) of control or IL-25–treated mice receiving either isotype or anti-CD90 mAb. RQ, relative quantification. (g) Periodic acid–Schiff/Alcian blue staining of lung and small intestine (SI) tissue from control or IL-25–treated mice receiving either isotype or anti-CD90 mAb. Bars: (top) 50 µm; (bottom) 100 µm. (h) Goblet cell (GC) counts from g. nd, not detected. (i) Frequency and total cell numbers of eosinophils in the lung from control or IL-25–treated mice given either isotype or anti-CD90 mAb. Frequency is percentage of SSChi SiglecFpos cells gated on live, Linneg CD11bpos cells. Data in a–i are representative of three independent experiments (control + Iso., n = 6; control + anti-CD90, n = 6; IL-25 treated + Iso., n = 6; IL-25 treated + anti-CD90, n = 6). *, P < 0.05; **, P < 0.01. Control ILC2 versus IL-25 + Iso. ILC2, P = 0.057; IL-25 + Iso. ILC2 versus IL-25 + αCD90 ILC2, P = 0.059; IL-25 + Iso. MPPtype2 versus IL-25 + αCD90 MPPtype2, P = 0.41; lung GC#: IL-25 + Iso. versus IL-25 + αCD90, P = 0.10; SI GC#: IL-25 + Iso. versus IL-25 + αCD90, P = 0.16. Error bars indicate SEM.
MPPtype2 cells promote Th2 cytokine responses and protective immunity
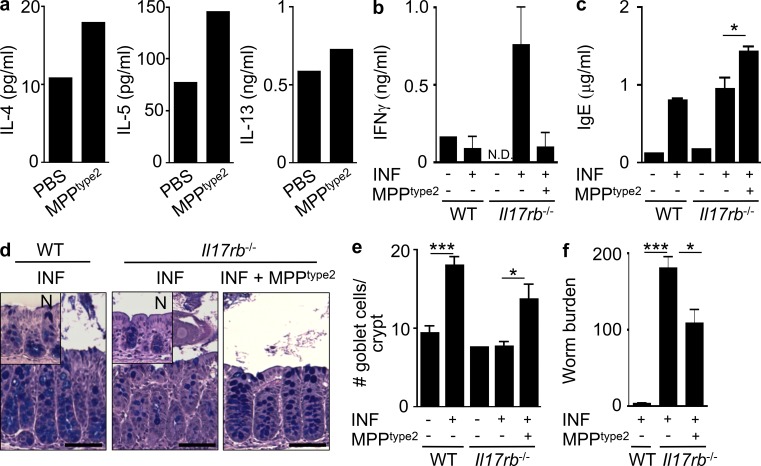
To test whether MPPtype2 cells were sufficient to promote Th2 cytokine–associated immune responses in a lymphocyte-sufficient environment, MPPtype2 cells were FACS purified from IL-25–treated mice and injected intradermally into naive WT C57BL/6 mice. At day 5 after injection, skin-draining LNs were collected and stimulated with αCD3/αCD28, and cytokine production was assessed. After adoptive transfer, cells isolated from the skin-draining LNs of mice that had received MPPtype2 cells exhibited elevated IL-4 and IL-5 production with only a modest increase in IL-13 protein levels compared with mice that received intradermal injection of PBS alone (Fig. 8 a), suggesting that MPPtype2 cells can promote conditions permissive for the development of Th2 cell responses.
Figure 8.
IL-25–elicited MPPtype2 cells promote Th2 cytokine–dependent responses in vivo. C57BL/6 WT mice (The Jackson Laboratory) were treated i.p. with 0.3 µg IL-25 daily for 7 d. MLNs were harvested, and MPPtype2 cells were sort-purified and injected intradermally into naive C57BL/6 WT mice. (a) IL-4, IL-5, and IL-13 cytokine production from skin-draining LN cells from mice receiving intradermal injection of control or IL-25–elicited MPPtype2 cells after 48-h αCD3/αCD28 stimulation measured by ELISA. Data in a are representative of two independent experiments. (b–f) C57BL/6 WT mice were treated i.p. with 0.3 µg IL-25 daily for 7 d. MLNs were harvested, and MPPtype2 cells were sort-purified and injected into T. muris–infected (INF) Il17rb−/− mice (Charles River). (b) IFN-γ cytokine production by T. muris antigen–stimulated MLN cells. (c) Total serum IgE antibody titers measured by ELISA. (d) Periodic acid–Schiff/Alcian blue–stained colon sections of intestine tissue from naive or infected WT or Il17rb−/− mice ± MPPtype2 cells. N, naive (inset). Bars, 100 µm. (e) Goblet cell counts from d. (f) Worm burdens from T. muris–infected mice were assessed at day 21 after infection. Data in b–f are representative of two independent experiments (WT naive, n = 2–3; WT INF, n = 6–8; Il17rb−/− naive, n = 2–3; Il17rb−/− INF, n = 6–7; Il17rb−/− INF + MPPtype2 cells, n = 6). *, P < 0.05; ***, P < 0.001. IFN-γ production between Il17rb−/− INF and Il17rb−/− INF + MPPtype2 cells, P = 0.068. Error bars indicate SEM.
To test whether MPPtype2 cells could promote protective Th2 cell–dependent immunity in the context of helminth infection, IL-25–elicited MPPtype2 cells were sort-purified and adoptively transferred into Il17rb−/− mice that are normally susceptible to the intestinal helminth parasite T. muris. Protective anti-helminth immune responses were assessed on day 21 after infection. As expected, based on our previous study (Owyang et al., 2006), T. muris–infected Il17rb−/− mice exhibited heightened antigen-specific IFN-γ responses (Fig. 8 b), did not exhibit goblet cell hyperplasia (Fig. 8, d and e), and were unable to successfully clear infection (Fig. 8 f). In contrast, T. muris–infected Il17rb−/− mice that received adoptively transferred MPPtype2 cells displayed decreased antigen-specific IFN-γ responses (Fig. 8 b), increased levels of total serum IgE (Fig. 8 c), and increased goblet cell hyperplasia (Fig. 8, d and e). Moreover, adoptive transfer of IL-25–elicited MPPtype2 cells partially restored protective immunity in normally susceptible Il17rb−/− mice (Fig. 8 f). Collectively, these data demonstrate that MPPtype2 cells are sufficient to partially restore Th2 cytokine responses and protective immunity in vivo.
DISCUSSION
Although ILC2 and MPPtype2 cell responses can be elicited by IL-33 and IL-25 and promote type 2 inflammation, the functional potential and relationship between these cell populations has remained unclear (Moro et al., 2010; Neill et al., 2010; Price et al., 2010; Saenz et al., 2010b). In this study, we demonstrate that although IL-33 predominantly elicits ILC2 responses, IL-25 simultaneously elicits phenotypically and functionally distinct ILC2 and MPPtype2 cell populations at multiple tissue sites. MPPtype2 cells were distinguished from ILC2 by lack of surface expression of T1/ST2, IL-7Rα, CD90, and CD25, their genome-wide transcriptional profile, their multipotent potential, and their developmental dependence on Id2. Furthermore, IL-25–induced MPPtype2 cells could promote Th2 cytokine–associated inflammation and Th2 cell–dependent immunity to helminth infection in mice in which the endogenous ILC2 response had been depleted.
Recent studies have reported differential induction of ILC2 responses by IL-33 and IL-25 (Barlow et al., 2012), suggesting that these cytokines promote type 2 cytokine–dependent inflammation through distinct innate immune mechanisms. Consistent with this, we found that IL-33 predominantly elicited ILC2 responses of greater magnitude to those observed after administration of IL-25. Notably, IL-33 also elicited a small population of MPPtype2 cells despite their lack of IL-33 receptor expression. The IL-33–elicited MPPtype2 cells were independent of IL-25 signaling, suggesting that this response may be regulated via alternate pathways such as IL-33–dependent induction of other hematopoietic growth factors including SCF, GM-CSF, or IL-3. However, future studies will be required to address this hypothesis. In contrast to IL-33, although IL-25 promotes modest ILC2 responses, IL-25 simultaneously elicits MPPtype2 cells at multiple tissue sites. MPPtype2 cells exhibited remarkable differences compared with ILC2 in their genome-wide transcriptional profile, multipotent potential, and Id2 dependence. Furthermore, the finding that IL-25–induced MPPtype2 cells could promote type 2 cytokine–dependent inflammation in mice depleted of endogenous ILC2s identifies MPPtype2 cells, independent of ILC2, as a critical innate cellular component in the development of Th2 cytokine–mediated inflammation at mucosal surfaces.
MPPtype2 cells possess the potential to differentiate into multiple innate cell populations such as macrophages and mast cells and basophils capable of producing IL-4 and IL-13, thus implicating extramedullary hematopoiesis (EMH) as a mechanism through which IL-25 can promote type 2 immune responses. It is likely that MPPtype2 cells and their progeny act cooperatively with other immune cell populations (including ILC2, granulocytes, macrophages, and/or T cells) to promote Th2 cytokine–associated immune responses and that immune cell populations might regulate the development and lineage potential of MPPtype2 cells. However, additional studies will be required to further define the cellular interactions, mediators, and signaling pathways that regulate these processes.
Although we found that MPPtype2 cells differed from ILC2 in their dependence on Id2, whether MPPtype2 cells require other transcription factors necessary for the development of ILC2 such as RORα, GATA3, or TCF1 remains to be tested. Of note, our current data demonstrate that IL-25 promotes MPPtype2 cell responses and type 2 inflammation in the absence of ILC2. However, Halim et al. (2012) and Wong et al. (2012) demonstrated that RORα is necessary for the IL-25–mediated induction of type 2 inflammation. Although these studies identified a role for RORα in the development of ILC2 responses, they did not investigate MPPtype2 cell responses in RORα-deficient mice. Given both of these findings, it is possible that MPPtype2 cells are themselves dependent on RORα. However, additional studies will be required to further characterize the molecular pathways involved in the development of IL-25–elicited MPPtype2 cell responses.
Consistent with our functional analyses, PCA demonstrated that IL-25–elicited MPPtype2 cells were more similar to BM-GMPs than to ILC2 or ILC3 populations. The significance of this finding is highlighted by reports of HSC mobilization out of the BM in response to microbial signals (Nagai et al., 2006; Massberg et al., 2007), where it is hypothesized that HSCs act as sentinels and contribute to immunosurveillance (Massberg et al., 2007). A recent study has also demonstrated that EMH is a key pathway in the IFN-dependent pathogenesis of IL-23–mediated colitis (Griseri et al., 2012). Collectively, these studies implicate EMH as an evolutionarily conserved mechanism of innate immunity that can direct the scope and intensity of adaptive immune responses in the context of infection and chronic inflammation.
The recent identification of CD34pos progenitor-like cells in human asthmatic patients that share some similarities to IL-25–elicited MPPtype2 cells including localization in peripheral tissues and responsiveness to Th2-associated cytokines (Allakhverdi et al., 2009; Saenz et al., 2010b) provokes the hypothesis that the IL-25–EMH–Th2 axis might also function in human disease. In support of this, increased expression of IL-25 and IL-25R has been reported in lung tissue of asthmatic patients (Liu et al., 2007). Although human homologues of MPPtype2 cells have yet to be identified, these data implicate IL-25–dependent EMH as a mechanism that influences the development of type 2 cytokine–dependent inflammation both in mice and humans.
TSLP, IL-33, and IL-25 are commonly coexpressed in epithelial cells and have multiple distinct cellular targets in vivo, including effects on mast cells, basophils, eosinophils, DC, macrophages, and T cells (Löhning et al., 1998; Moritz et al., 1998; Reche et al., 2001; Allakhverdi et al., 2007; Iikura et al., 2007; Liu et al., 2007; Rochman et al., 2007; Iliev et al., 2009; Perrigoue et al., 2009; Ziegler and Artis, 2010; Siracusa et al., 2011). However, the apparent differences between the innate immune pathways elicited by TSLP, IL-33, and IL-25 suggest that epithelial cell–derived cytokines from different cytokine families regulate distinct modules of innate immune responses. For instance, whereas the IL-7–like cytokine TSLP has been shown to regulate DC and basophil responses (Perrigoue et al., 2009; Taylor et al., 2009; Siracusa et al., 2011) and the IL-1 family member IL-33 promotes type 2 inflammation through IL-5 and IL-13 production from ILC2 (Moro et al., 2010; Monticelli et al., 2011; Yang et al., 2011; Wong et al., 2012), the IL-17 family member IL-25 is capable of promoting type 2 inflammation through the induction of a progenitor cell population that promotes EMH and subsequent IL-4 production. Consistent with the effects of IL-25 on progenitor cells that can promote monocyte and granulocyte responses, IL-17A, another member of the IL-17 cytokine family, promotes the expansion of other myeloid lineages (Schwarzenberger et al., 1998), indicating that promotion of granulopoiesis may be a characteristic of multiple IL-17 family members.
Despite the distinct cellular targets of IL-25, IL-33, and TSLP, this triad of epithelial cell–derived cytokines also exhibits a high degree of cross-regulation. Recent studies have shown that although not necessary for ILC2 development (Hoyler et al., 2012), TSLP can synergize with IL-33, resulting in greater cytokine production from ILC2 (Halim et al., 2012; Mjösberg et al., 2012). In addition, ILC2 populations in the skin have recently been identified and found to be dependent on TSLP–TSLPR interactions (Kim et al., 2013), indicating that TSLP can directly influence ILC2 responses. However, although TSLP–TSLPR interactions were not required for the IL-25–mediated induction of MPPtype2 cells responses in vivo, MPPtype2 cells did express the TSLPRα chain (unpublished data). This suggests that similar to ILC2, TSLP might regulate MPPtype2 cell responses; however, the role TSLP plays in MPPtype2 cell biology remains unknown and warrants further investigation.
The coordinated expression of TSLP, IL-25, and IL-33 by epithelial cells in response to diverse allergens or helminth infections and interactions between these epithelial cell–derived cytokines may represent a mechanism by which epithelial cells can simultaneously induce multiple modules of the innate immune response that promote Th2 cell–dependent immunity, inflammation, and tissue repair by inducing distinct modules of the innate immune response (Saenz et al., 2010a; Ziegler and Artis, 2010; Koyasu and Moro, 2011; Oliphant et al., 2011; Spits and Di Santo, 2011; Monticelli et al., 2012; Pulendran and Artis, 2012). Although the identification of these previously unrecognized innate immune cell populations provides new insights into the cellular mechanisms through which CD4pos Th2 cell–dependent cytokine responses are initiated and regulated, the finding that MPPtype2 cells represent a distinct population from ILC2 highlights the need for further investigation into the identity, function, and cell lineage relationships between MPPtype2 cells, ILC2, and other progenitor-like cell populations. Understanding these relationships in the steady-state and in the context of infectious or inflammatory diseases may help establish novel therapeutic approaches for the treatment of helminth infections and allergic diseases in humans.
MATERIALS AND METHODS
Mice.
WT BALB/c and WT C57BL/6 and Rag1−/− mice were obtained from Jackson Laboratory, and C57BL/6 Ly5.2/Cr (CD45.1) mice were obtained from the National Cancer Institute. C57BL/6 WT and C57BL/6 Il33−/− mice were from Taconic and were provided by D.E. Smith (Amgen). C57BL/6 WT and C57BL/6 Il17rb−/− mice (Charles River) were provided by A.L. Budelsky (Amgen). Animals were bred and housed in specific pathogen–free conditions at the University of Pennsylvania. All experiments were performed under Institutional Animal Care and Use Committee (IACUC)–approved protocols and in accordance with the guidelines of the IACUC of the University of Pennsylvania. All experiments were performed with age-, gender-, and genetically matched mice between the ages of 4 and 12 wk. Mice were treated i.p. with PBS, 0.3 µg of recombinant IL-25 (endotoxin level: 0.029 EU/ml; R&D Systems), or 0.3 µg of recombinant IL-33 (endotoxin level: 0.0285 EU/ml; eBioscience) daily for 2, 4, or 7 d. For anti-T1/ST2 antibody–mediated blockade, mice were treated i.p. with 0.25 mg/day of control IgG or anti-T1/ST2 (from D.E. Smith) on days −3, −1, 0, 2, and 4. For anti-CD90 antibody–mediated blockade, mice were treated i.p. with 0.3 mg/day of control IgG isotype (Iso.) or anti-CD90 (30H12; Bio X Cell) every 2 d beginning on day −3.
BM chimera generation.
For all BM chimeras, BM was harvested from the tibia and femur and depleted of TCRβpos, Thy1pos cells or CD5pos and B220pos by magnetic beads (Miltenyi Biotec). For WT BM chimeras, purified donor cells (CD45.2) were injected i.v. into lethally irradiated (900 rad) recipient mice (CD45.1) through retroorbital injections. For Id2-deficient BM chimeras, purified donor cells (CD45.1.2pos) were injected i.v. into lethally irradiated (900 rad) recipient mice (CD45.2pos) through retroorbital injections. All Id2-deficient BM chimeras used were either second or third generation from fetal liver chimeras (Cannarile et al., 2006). Generation of Id2-deficient mice and fetal liver chimeras has been described previously (Cannarile et al., 2006). All other chimeras used were first generation. Reconstitution was allowed to proceed for 8 wk after transplant in all chimeras, and mice were maintained on antibiotics (1%) in the drinking water for 2 wk after transplant (Hi-Tech Pharmacal).
Flow cytometry and cell sorting.
MLNs were separated from the mesentery, homogenized by passing through a 70-µm nylon mesh filter, and stained with anti–mouse fluorochrome-conjugated mAbs against CD3ε, CD4, CD8α, TCRβ, CD19, CD11b, CD11c, NK1.1, FcεRIα, c-kit, Sca-1, CD127 (IL-7Rα), CD90, CD25, and CD45.2 (eBioscience and BD). T1/ST2 staining was performed using T1/ST2 biotinylated mAb (MD Biosciences) and eFluor 450–conjugated streptavidin (eBioscience). Cells were run on an LSR II using DiVa software (BD) and analyzed with FlowJo software (version 9.4.11; Tree Star). For cell sorting, MLNs from IL-25–treated mice were isolated and stained with 1 µg/ml DAPI (Molecular Probes) as described above, and live (DAPIneg) MPPtype2 cells (Linneg T1/ST2neg IL-7Rαneg CD90neg CD25neg c-kitpos), ILC2 cell populations (Linneg T1/ST2pos IL-7Rαpos), and BM-GMP (Linneg Sca1neg c-kitpos CD34pos CD16/32pos) were sorted using a FACSAria II (BD).
Adoptive transfers and helminth infections.
For intradermal injections, 2 × 104 FACS-purified IL-25–elicited MPPtype2 cells were suspended in 50 µl PBS and injected intradermally into naive C57BL/6 WT mice. At day 5 after injection, skin-draining LNs were collected and polyclonally stimulated with 1 µg/ml each of αCD3 and αCD28 (eBioscience). After 48 h, cell-free supernatants were assessed for cytokine production by sandwich ELISA (eBioscience). For helminth infections, WT and Il17rb−/− mice (Charles River) were infected with 250 embryonated T. muris eggs via oral gavage. T. muris–infected Il17rb−/− mice were left untreated or given 7 × 104 to 2 × 105 FACS-purified IL-25–elicited MPPtype2 cells at days 10, 12, 14, 16, and 18 after infection. Worm counts were performed at day 21 after infection. MLN cells were collected at necropsy and plated with 50 µg/ml 4h T. muris antigen as previously described (Owyang et al., 2006). After 48 h, cell-free supernatants were assessed for cytokine production by sandwich ELISA (eBioscience). Total serum IgE was measured using the OptEIA IgE ELISA kit (BD) according to the manufacturer’s instructions.
Microarray gene expression profiling and GSEA.
IL-25–elicited MPPtype2 cells (Linneg T1/ST2neg IL-7Rαneg CD90neg CD25neg c-kitpos) were FACS purified from the MLNs and PECs of C57BL/6 mice, and BM-GMP (Linneg Sca1neg c-kitpos CD34pos CD16/32pos) were sorted from the BM of naive C57BL/6 mice. Lineage markers included CD3ε, CD4, CD8α, CD19, CD11b, CD11c, and NK1.1. Three biological replicates were collected, each consisting of 50,000–70,000 cells. mRNA was isolated, amplified, and hybridized to the Mouse Gene 1.0ST GeneChip (Affymetrix). Using the ClassNeighbors function in GenePattern, differentially expressed genes (fold change greater than two, P < 0.05) were identified. GSEA of gene expression profiles from each biological group was performed (Broad Institute). All microarray datasets were batch-corrected using the ComBat (Johnson et al., 2007) R script to normalize differences between samples caused by Affymetrix Chip. PCA (Culhane et al., 2005) and Euclidean distance measurements were performed using R (R Core Team), and hierarchical clustering analysis was performed using the unweighted pair group method with arithmetic mean (UPGMA) algorithm.
In vitro differentiation assays.
Cell populations (MPPtype2 cells or ILC2) were FACS purified as described above, seeded into 96-well flat bottom TC plates (Falcon; BD), and incubated in the presence of 50 ng/ml SCF (R&D Systems) and 10 ng/ml IL-3 (R&D Systems) or 50 ng/ml IL-33 (R&D Systems) for 8 d. Cytokines and culture media were replenished at days 3 and 6 after culture. After in vitro culture, progeny were assessed for expression of lineage-associated markers CD11b, FcεRIα, c-kit, T1/ST2, IL-7Rα (CD127), and CD90 by flow cytometry as described above. Cell-free supernatants were assessed for IL-4, IL-5, and IL-13 cytokine production by standard sandwich ELISA (eBioscience). Limits of detection are as follows: IL-4, 4.39 pg/ml; IL-5, 10 pg/ml; and IL-13, 8 pg/ml.
Histology.
Lung and small intestinal tissues were fixed in 4% (vol/vol) paraformaldehyde and embedded in paraffin wax. 4-µm sections were stained with periodic acid–Schiff/Alcian blue.
Real-time RT-PCR.
RNA from intestinal tissues of mice was isolated by TRIzol extraction (Invitrogen). Whole tissues were homogenized with a tissue homogenizer (TissueLyzer; QIAGEN), and cDNA was prepared with SuperScript reverse transcription (Invitrogen). Quantitative real-time PCR analysis used commercial QuantiTect primer sets for Il4, Il5, Il13 (QIAGEN), and SYBR Green chemistry (Applied Biosystems). All reactions were run on an ABI 7500 Fast Real-Time PCR System (Applied Biosystems). Target genes were normalized for endogenous β-actin levels, and relative quantification of samples was compared with controls.
Statistical analysis.
Representative plots for each sample group are shown. Statistical significance for total cell numbers was determined by two-tailed Student’s t test using means ± SEM for individual groups. Results were considered significant at P < 0.05.
Acknowledgments
We thank members of the Artis laboratory for discussions and critical reading of the manuscript. We thank Kyle Bittinger and Frederic D. Bushman for assistance with hierarchical clustering analysis and Dirk E. Smith and Alison L. Budelsky for providing reagents. We also thank the Penn Microarray Facility and the Abramson Cancer Center (ACC) Flow Cytometry and Cell Sorting Resource Laboratory for technical advice and support.
Research in the Artis laboratory is supported by the National Institutes of Health (NIH; grants AI061570, AI087990, AI074878, AI083480, AI095466, AI095608, AI102942, and AI097333 to D. Artis; F32-AI085828 to M.C. Siracusa; T32-AI007532 to L.A. Monticelli; KL2-RR024132 to B.S. Kim; and T32-AI060516 to J.R. Brestoff) and the Burroughs Wellcome Fund Investigator in Pathogenesis of Infectious Disease Award (to D. Artis). The ACC Flow Cytometry and Cell Sorting Shared Resource is partially supported by a National Cancer Institute Comprehensive Cancer Center Support Grant (#2-P30 CA016520). This work was supported by the National Institute of Allergy and Infectious Diseases Mucosal Immunology Studies Team (MIST) consortium (www.mucosal.org; grant U01 AI095608), the NIH/National Institute of Diabetes and Digestive and Kidney Diseases P30 Center for Molecular Studies in Digestive and Liver Diseases (grant P30-DK050306), its pilot grant program and scientific core facilities (Molecular Pathology and Imaging, Molecular Biology, Cell Culture, and Mouse), as well as the Joint CHOP-Penn Center in Digestive, Liver, and Pancreatic Medicine and its pilot grant program.
The authors declare no competing financial interests.
Author contributions: S.A. Saenz, M.C. Siracusa, L.A. Monticelli, B.S. Kim, J.R. Brestoff, L.W. Peterson, A. Bhandoola, and D. Artis designed and performed the research. A.W. Goldrath provided new reagents. S.A. Saenz, C.G.K. Ziegler, E.J. Wherry, and D. Artis analyzed the data. S.A. Saenz and D. Artis wrote the paper.
Footnotes
Abbreviations used:
- BM-GMP
- BM-resident granulocyte/monocyte precursor
- EMH
- extramedullary hematopoiesis
- GSEA
- gene set enrichment analysis
- HSC
- hematopoietic stem cell
- Ih2 cell
- innate type 2 helper cell
- LTi
- lymphoid tissue inducer
- MLN
- mesenteric LN
- MPPtype2
- multipotent progenitor type 2
- NHC
- natural helper cell
- PCA
- principal component analysis
- PEC
- peritoneal cavity
- TSLP
- thymic stromal lymphopoietin
References
- Al-Shami A., Spolski R., Kelly J., Fry T., Schwartzberg P.L., Pandey A., Mackall C.L., Leonard W.J. 2004. A role for thymic stromal lymphopoietin in CD4+ T cell development. J. Exp. Med. 200:159–168 10.1084/jem.20031975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allakhverdi Z., Comeau M.R., Jessup H.K., Yoon B.R., Brewer A., Chartier S., Paquette N., Ziegler S.F., Sarfati M., Delespesse G. 2007. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J. Exp. Med. 204:253–258 10.1084/jem.20062211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allakhverdi Z., Comeau M.R., Smith D.E., Toy D., Endam L.M., Desrosiers M., Liu Y.J., Howie K.J., Denburg J.A., Gauvreau G.M., Delespesse G. 2009. CD34+ hemopoietic progenitor cells are potent effectors of allergic inflammation. J. Allergy Clin. Immunol. 123:472–478 10.1016/j.jaci.2008.10.022 [DOI] [PubMed] [Google Scholar]
- Anthony R.M., Rutitzky L.I., Urban J.F., Jr, Stadecker M.J., Gause W.C. 2007. Protective immune mechanisms in helminth infection. Nat. Rev. Immunol. 7:975–987 10.1038/nri2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow J.L., Bellosi A., Hardman C.S., Drynan L.F., Wong S.H., Cruickshank J.P., McKenzie A.N. 2012. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J. Allergy Clin. Immunol. 129:191–198 10.1016/j.jaci.2011.09.041 [DOI] [PubMed] [Google Scholar]
- Cannarile M.A., Lind N.A., Rivera R., Sheridan A.D., Camfield K.A., Wu B.B., Cheung K.P., Ding Z., Goldrath A.W. 2006. Transcriptional regulator Id2 mediates CD8+ T cell immunity. Nat. Immunol. 7:1317–1325 10.1038/ni1403 [DOI] [PubMed] [Google Scholar]
- Chang Y.J., Kim H.Y., Albacker L.A., Baumgarth N., McKenzie A.N., Smith D.E., Dekruyff R.H., Umetsu D.T. 2011. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat. Immunol. 12:631–638 10.1038/ni.2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culhane A.C., Thioulouse J., Perrière G., Higgins D.G. 2005. MADE4: an R package for multivariate analysis of gene expression data. Bioinformatics. 21:2789–2790 10.1093/bioinformatics/bti394 [DOI] [PubMed] [Google Scholar]
- Griseri T., McKenzie B.S., Schiering C., Powrie F. 2012. Dysregulated hematopoietic stem and progenitor cell activity promotes interleukin-23-driven chronic intestinal inflammation. Immunity. 37:1116–1129 10.1016/j.immuni.2012.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halim T.Y., Krauss R.H., Sun A.C., Takei F. 2012. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 36:451–463 10.1016/j.immuni.2011.12.020 [DOI] [PubMed] [Google Scholar]
- Hoorweg K., Peters C.P., Cornelissen F., Aparicio-Domingo P., Papazian N., Kazemier G., Mjösberg J.M., Spits H., Cupedo T. 2012. Functional differences between human NKp44(-) and NKp44(+) RORC(+) innate lymphoid cells. Front Immunol. 3:72 10.3389/fimmu.2012.00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyler T., Klose C.S., Souabni A., Turqueti-Neves A., Pfeifer D., Rawlins E.L., Voehringer D., Busslinger M., Diefenbach A. 2012. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 37:634–648 10.1016/j.immuni.2012.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iikura M., Suto H., Kajiwara N., Oboki K., Ohno T., Okayama Y., Saito H., Galli S.J., Nakae S. 2007. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab. Invest. 87:971–978 10.1038/labinvest.3700663 [DOI] [PubMed] [Google Scholar]
- Iliev I.D., Mileti E., Matteoli G., Chieppa M., Rescigno M. 2009. Intestinal epithelial cells promote colitis-protective regulatory T-cell differentiation through dendritic cell conditioning. Mucosal Immunol. 2:340–350 10.1038/mi.2009.13 [DOI] [PubMed] [Google Scholar]
- Johnson W.E., Li C., Rabinovic A. 2007. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 8:118–127 10.1093/biostatistics/kxj037 [DOI] [PubMed] [Google Scholar]
- Kim B.S., Siracusa M.C., Saenz S.A., Noti M., Monticelli L.A., Sonnenberg G.F., Hepworth M.R., Van Voorhees A.S., Comeau M.R., Artis D. 2013. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci. Transl. Med. 5:70ra16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.Y., DeKruyff R.H., Umetsu D.T. 2010. The many paths to asthma: phenotype shaped by innate and adaptive immunity. Nat. Immunol. 11:577–584 10.1038/ni.1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.Y., Chang Y.J., Subramanian S., Lee H.H., Albacker L.A., Matangkasombut P., Savage P.B., McKenzie A.N., Smith D.E., Rottman J.B., et al. 2012. Innate lymphoid cells responding to IL-33 mediate airway hyperreactivity independently of adaptive immunity. J. Allergy Clin. Immunol. 129:216–227 10.1016/j.jaci.2011.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyasu S., Moro K. 2011. Type 2 innate immune responses and the natural helper cell. Immunology. 132:475–481 10.1111/j.1365-2567.2011.03413.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.J., Soumelis V., Watanabe N., Ito T., Wang Y.H., Malefyt Rde.W., Omori M., Zhou B., Ziegler S.F. 2007. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu. Rev. Immunol. 25:193–219 10.1146/annurev.immunol.25.022106.141718 [DOI] [PubMed] [Google Scholar]
- Löhning M., Stroehmann A., Coyle A.J., Grogan J.L., Lin S., Gutierrez-Ramos J.C., Levinson D., Radbruch A., Kamradt T. 1998. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc. Natl. Acad. Sci. USA. 95:6930–6935 10.1073/pnas.95.12.6930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massberg S., Schaerli P., Knezevic-Maramica I., Köllnberger M., Tubo N., Moseman E.A., Huff I.V., Junt T., Wagers A.J., Mazo I.B., von Andrian U.H. 2007. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 131:994–1008 10.1016/j.cell.2007.09.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mjösberg J.M., Trifari S., Crellin N.K., Peters C.P., van Drunen C.M., Piet B., Fokkens W.J., Cupedo T., Spits H. 2011. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat. Immunol. 12:1055–1062 10.1038/ni.2104 [DOI] [PubMed] [Google Scholar]
- Mjösberg J., Bernink J., Golebski K., Karrich J.J., Peters C.P., Blom B., te Velde A.A., Fokkens W.J., van Drunen C.M., Spits H. 2012. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 37:649–659 10.1016/j.immuni.2012.08.015 [DOI] [PubMed] [Google Scholar]
- Monticelli L.A., Sonnenberg G.F., Abt M.C., Alenghat T., Ziegler C.G., Doering T.A., Angelosanto J.M., Laidlaw B.J., Yang C.Y., Sathaliyawala T., et al. 2011. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat. Immunol. 12:1045–1054 10.1038/ni.2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monticelli L.A., Sonnenberg G.F., Artis D. 2012. Innate lymphoid cells: critical regulators of allergic inflammation and tissue repair in the lung. Curr. Opin. Immunol. 24:284–289 10.1016/j.coi.2012.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz D.R., Rodewald H.R., Gheyselinck J., Klemenz R. 1998. The IL-1 receptor-related T1 antigen is expressed on immature and mature mast cells and on fetal blood mast cell progenitors. J. Immunol. 161:4866–4874 [PubMed] [Google Scholar]
- Moro K., Yamada T., Tanabe M., Takeuchi T., Ikawa T., Kawamoto H., Furusawa J.-i., Ohtani M., Fujii H., Koyasu S. 2010. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 463:540–544 10.1038/nature08636 [DOI] [PubMed] [Google Scholar]
- Nagai Y., Garrett K.P., Ohta S., Bahrun U., Kouro T., Akira S., Takatsu K., Kincade P.W. 2006. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 24:801–812 10.1016/j.immuni.2006.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill D.R., Wong S.H., Bellosi A., Flynn R.J., Daly M., Langford T.K., Bucks C., Kane C.M., Fallon P.G., Pannell R., et al. 2010. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 464:1367–1370 10.1038/nature08900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliphant C.J., Barlow J.L., McKenzie A.N. 2011. Insights into the initiation of type 2 immune responses. Immunology. 134:378–385 10.1111/j.1365-2567.2011.03499.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owyang A.M., Zaph C., Wilson E.H., Guild K.J., McClanahan T., Miller H.R., Cua D.J., Goldschmidt M., Hunter C.A., Kastelein R.A., Artis D. 2006. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J. Exp. Med. 203:843–849 10.1084/jem.20051496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm N.W., Rosenstein R.K., Medzhitov R. 2012. Allergic host defences. Nature. 484:465–472 10.1038/nature11047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L.S., Martin U., Garka K., Gliniak B., Di Santo J.P., Muller W., Largaespada D.A., Copeland N.G., Jenkins N.A., Farr A.G., et al. 2000. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor. J. Exp. Med. 192:659–670 10.1084/jem.192.5.659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrigoue J.G., Saenz S.A., Siracusa M.C., Allenspach E.J., Taylor B.C., Giacomin P.R., Nair M.G., Du Y., Zaph C., van Rooijen N., et al. 2009. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat. Immunol. 10:697–705 10.1038/ni.1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price A.E., Liang H.E., Sullivan B.M., Reinhardt R.L., Eisley C.J., Erle D.J., Locksley R.M. 2010. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl. Acad. Sci. USA. 107:11489–11494 10.1073/pnas.1003988107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B., Artis D. 2012. New paradigms in type 2 immunity. Science. 337:431–435 10.1126/science.1221064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reche P.A., Soumelis V., Gorman D.M., Clifford T., Liu Mr M., Travis M., Zurawski S.M., Johnston J., Liu Y.J., Spits H., et al. 2001. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J. Immunol. 167:336–343 [DOI] [PubMed] [Google Scholar]
- Rochman I., Watanabe N., Arima K., Liu Y.J., Leonard W.J. 2007. Cutting edge: direct action of thymic stromal lymphopoietin on activated human CD4+ T cells. J. Immunol. 178:6720–6724 [DOI] [PubMed] [Google Scholar]
- Saenz S.A., Noti M., Artis D. 2010a. Innate immune cell populations function as initiators and effectors in Th2 cytokine responses. Trends Immunol. 31:407–413 10.1016/j.it.2010.09.001 [DOI] [PubMed] [Google Scholar]
- Saenz S.A., Siracusa M.C., Perrigoue J.G., Spencer S.P., Urban J.F., Jr, Tocker J.E., Budelsky A.L., Kleinschek M.A., Kastelein R.A., Kambayashi T., et al. 2010b. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 464:1362–1366 10.1038/nature08901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzenberger P., La Russa V., Miller A., Ye P., Huang W., Zieske A., Nelson S., Bagby G.J., Stoltz D., Mynatt R.L., et al. 1998. IL-17 stimulates granulopoiesis in mice: use of an alternate, novel gene therapy-derived method for in vivo evaluation of cytokines. J. Immunol. 161:6383–6389 [PubMed] [Google Scholar]
- Siracusa M.C., Saenz S.A., Hill D.A., Kim B.S., Headley M.B., Doering T.A., Wherry E.J., Jessup H.K., Siegel L.A., Kambayashi T., et al. 2011. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. 477:229–233 10.1038/nature10329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg G.F., Artis D. 2012. Innate lymphoid cell interactions with microbiota: implications for intestinal health and disease. Immunity. 37:601–610 10.1016/j.immuni.2012.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H., Cupedo T. 2012. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu. Rev. Immunol. 30:647–675 10.1146/annurev-immunol-020711-075053 [DOI] [PubMed] [Google Scholar]
- Spits H., Di Santo J.P. 2011. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat. Immunol. 12:21–27 10.1038/ni.1962 [DOI] [PubMed] [Google Scholar]
- Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., Paulovich A., Pomeroy S.L., Golub T.R., Lander E.S., Mesirov J.P. 2005. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA. 102:15545–15550 10.1073/pnas.0506580102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait Wojno E.D., Artis D. 2012. Innate lymphoid cells: balancing immunity, inflammation, and tissue repair in the intestine. Cell Host Microbe. 12:445–457 10.1016/j.chom.2012.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B.C., Zaph C., Troy A.E., Du Y., Guild K.J., Comeau M.R., Artis D. 2009. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J. Exp. Med. 206:655–667 10.1084/jem.20081499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J.A., Barlow J.L., McKenzie A.N. 2013. Innate lymphoid cells—how did we miss them? Nat. Rev. Immunol. 13:75–87 10.1038/nri3349 [DOI] [PubMed] [Google Scholar]
- Wong S.H., Walker J.A., Jolin H.E., Drynan L.F., Hams E., Camelo A., Barlow J.L., Neill D.R., Panova V., Koch U., et al. 2012. Transcription factor RORα is critical for nuocyte development. Nat. Immunol. 13:229–236 10.1038/ni.2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Saenz S.A., Zlotoff D.A., Artis D., Bhandoola A. 2011. Cutting edge: Natural helper cells derive from lymphoid progenitors. J. Immunol. 187:5505–5509 10.4049/jimmunol.1102039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda K., Muto T., Kawagoe T., Matsumoto M., Sasaki Y., Matsushita K., Taki Y., Futatsugi-Yumikura S., Tsutsui H., Ishii K.J., et al. 2012. Contribution of IL-33-activated type II innate lymphoid cells to pulmonary eosinophilia in intestinal nematode-infected mice. Proc. Natl. Acad. Sci. USA. 109:3451–3456 10.1073/pnas.1201042109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler S.F., Artis D. 2010. Sensing the outside world: TSLP regulates barrier immunity. Nat. Immunol. 11:289–293 10.1038/ni.1852 [DOI] [PMC free article] [PubMed] [Google Scholar]