Abstract
Purpose
Describe frequencies and risk factors of altered oral health and odontogenesis in childhood cancer survivors.
Patients and Methods
9308 survivors, diagnosed between 1970–1986, and 2951 siblings from Childhood Cancer Survivor Study completed a survey containing oral-dental health information. We analyzed treatment impact, socioeconomic data and patient demographics on dental outcomes using univariate and multivariate logistic regression models to estimate odds ratios (OR).
Results
In multivariate analysis, survivors more likely reported microdontia (OR 3.0, 95% confidence interval [CI] 2.4–3.8), hypodontia (OR 1.7, 95% CI 1.4–2.0), root abnormalities (OR 3.0, 95% CI 2.2–4.0), abnormal enamel (OR 2.4, 95% CI 2.0–2.9), teeth loss ≥6 (OR 2.6, 95% CI 1.9–3.6), severe gingivitis (OR 1.2, 95% CI 1.0–1.5), xerostomia (OR 9.7, 95% CI 4.8–19.7). Controlling for chemotherapy and socio-economic factors, radiation exposure of ≥20Gy to dentition was significantly associated with increased risk of ≥1 dental abnormality. Dose-dependent alkylating agent therapy significantly increased risk ≥1 anatomic/developmental dental abnormalities in survivors diagnosed <5 years of age (OR 1.7, 2.7, 3.3 for alkylating agent score of 1, 2, 3, respectively).
Conclusion
Radiation and chemotherapy are independent risk factors for adverse oral-dental sequelae among childhood cancer survivors. Patients receiving alkylating agents at < 5 years should be closely monitored.
Keywords: radiation, chemotherapy, pediatric oncology, dental abnormalities
Introduction
Current multimodality therapies have increased the survival of childhood cancer patients. Many are associated with delayed toxicities resulting in long-term complications, but little has been published about the effects of pediatric cancer therapy on developing dentition of children. Reported abnormalities include hypodontia (developmentally missing teeth), microdontia (small teeth), enamel hypoplasia, root stunting, taurodontia (enlarged pulp chambers), over-retention of primary teeth, an increased caries index (1–8), malocclusion (9), and decreased temporomandibular joint mobility (10). Local effects of radiation therapy on craniofacial and dental development has been described (1;2;11–15). Existing reports have found that children may be at greater risks for odontogenic developmental abnormalities if treated with chemotherapy when younger than 5 years because of proliferation of dental stem cells during this period (14–16). However, the odontogenic toxicities induced by individual chemotherapy agents remain obscure. Thus, we describe the types and frequencies of altered dental development in adult survivors of pediatric cancers and associate disodontogenesis with treatment agents, socioeconomic demographics and type of malignancy.
Methods
Subject selection and contact
The Childhood Cancer Survivor Study (CCSS) is a multi-institutional study (see Appendix 1) of individuals who survived 5 or more years following diagnosis of cancer during childhood or adolescence (17). Eligibility criteria for this cohort are: a) diagnosis of leukemia, CNS malignancy (all histologies), Hodgkin's disease, non-Hodgkin's lymphoma, kidney cancer, neuroblastoma, soft tissue sarcoma, or malignant bone tumor (list of eligible ICD-O codes can be found at www.stjude.org/ccss, see Supplemental Information for CCSS Publications); b) diagnosis and initial treatment at one of the 26 collaborating CCSS institutions; c) diagnosis date between January 1, 1970 and December 31, 1986; d) age less than 21 years at diagnosis; e) survival five or more years from diagnosis.
The CCSS protocol and contact documents were reviewed and approved by the Human Subjects Committee at each participating institution. Baseline data were collected for the study cohort using a 24-page self-reported questionnaire. The questionnaire was designed to capture a wide range of information including demographic characteristics, health habits (smoking, alcohol consumption, physical activity), frequency of diagnosed medical conditions, recurrent cancer, and subsequent primary neoplasms. The medical records of all members of the cohort were abstracted. Detailed data regarding the chemotherapeutic agents administered to the patient for treatment of the original cancer, and for any recurrences of the cancer, the cumulative dose of drug administered for several drugs of interest, and the doses, volumes and dates of administration of all radiation therapy were recorded. Of the 20,626 five-year survivors, 3,058 (14.8%) were lost to follow-up and never offered enrollment. Of the remaining 17,568 survivors, 14,363 (81.8%) completed the baseline survey. Complete medical record abstraction was successful for 12,492 participants. A random sample of participating survivors was selected and asked to contact their sibling closest in age to inform them about the study and ask them to participate. Of the 4790 siblings selected, 3899 (81.4%) completed a baseline survey.
A follow-up survey was distributed beginning in 2003, which updated information on medical conditions and included questions regarding dental health (questionnaires are available for review and download at www.stjude.org/ccss). Of the 11,723 survivors enrolled in CCSS who were not lost to follow-up, 9308 (79%) completed the 2003 follow-up questionnaire, of whom 8522 had complete treatment data available from the medical record abstraction process and are included in the current analysis (Questions utilized for this study are available in the Appendix). Of the 3899 siblings who were eligible for the follow-up survey; 2951 (85%) completed the second follow-up questionnaire, and of these 2831 did not report a cancer event. This report includes data available at the time of the analysis from these 8522 survivors and 2831 siblings.
Statistical analysis
Descriptive statistics of demographic and treatment characteristics were calculated for the 8522 survivors and 2831 siblings. Self-reported dental outcomes including hypodontia, microdontia, enamel hypoplasia, abnormal root development, 6 or more missing teeth, denture use, and dental prosthesis use, xerostomia, gingivitis, and 6 or more cavities were considered in this analysis. Three additional outcomes were created from a combination of these individual outcomes and included in the analyses. The presence of one or more of hypodontia, microdontia, enamel hypoplasia, abnormal root development, 6 or more missing teeth, denture use, and dental prosthesis use was defined as “at least one dental health issue”. Similarly, at least one of xerostomia, gingivitis, and 6 or more cavities were defined as “at least one soft tissue issue” and dental bridge use, denture use, or oral prosthesis use defined the outcome “at least one appliance use”.
Among patients exposed to an alkylating agent, the alkylating agent dose (AAD) score was calculated by adding the tertile score for each of the alkylating agents given to a particular patient (18). Radiation dose to the teeth or teeth buds was estimated for each patient by reviewing and abstracting details of the radiation therapy from radiation oncology records submitted to the Radiation Physics Center at M.D. Anderson Cancer Center. The radiation dose used in the analysis was the mean of dose to 12 points throughout the teeth or teeth bud region. Dosimetry methods are described fully elsewhere (19)
We cross-sectionally compared demographic characteristics and dental outcomes between survivors and siblings, using logistic regression models adjusted for potential intra-family correlation with robust sandwich variance estimates (20). Sensitivity analyses were carried out to assess the impact of missing or unknown responses to dental outcomes questions, assuming all unknown/missing outcomes to be either “no” or “yes” responses. Analyses within the survivor cohort were performed to assess the impact of treatment- and diagnosis-related factors using subjects with available treatment data and within age-at-diagnosis strata 0–5 years, 6–10 years, and >10 years. Analyses were conducted in SAS version 9.1.3 (SAS Institute Inc., Cary, NC, USA).
Results
Of the 8522 survivors in the current analysis, 49.9% were female, and 86.8% white non-Hispanic. The median age at cancer diagnosis was 6.0 years (range, 0–20 years) and the median time from diagnosis to interview was 22.0 years (range, 15–34 years) (Table 1). The distribution of survivors by diagnosis and details of treatment received are shown in Table 2. The majority of survivors received chemotherapy with or without radiation therapy. Compared to survivors, siblings were significantly more likely to be white, female, ≥ 30 years of age, high school graduates, to have annual household incomes exceeding $20, 000, and to have health or dental insurance.
Table 1.
Characteristics of Survivor and Sibling Cohorts.
| Characteristic | Survivors N (%) |
Siblings N (%) |
P-value* |
|---|---|---|---|
| Total Cohort | 8522 | 2831 | |
| History of at least one dental health issue | |||
| Yes | 2539 (32.6) | 530 (19.9) | |
| No | 5249 (67.4) | 2132 (80.1) | <0.01 |
| History of at least one soft tissue issue (includes caries) | |||
| Yes | 4662 (57.4) | 1511 (55.8) | |
| No | 3458 (42.6) | 1197 (44.2) | 0.12 |
| History of Dental Appliance use | |||
| Yes | 625 (7.4) | 168 (6.0) | |
| No | 7821 (92.6) | 2642 (94.0) | 0.01 |
| Socio-economic and Demographic Characteristics | |||
| Race | |||
| White not Hispanic | 7367(86.8) | 2441 (91.7) | |
| Other | 1124 (13.2) | 220 (8.3) | <0.01 |
| Gender | |||
| Male | 4273 (50.1) | 1325 (46.8) | |
| Female | 4249 (49.9) | 1506 (53.2) | <0.01 |
| Age at follow-up (yrs) | |||
| 17–29 years | 3746 (44.0) | 1082 (39.2) | |
| ≥ 30 years | 4776 (56.0) | 1675 (60.8) | <0.01 |
| Education level | |||
| No Post High School | 1691 (20.1) | 489 (17.3) | |
| Post High School | 6720 (79.9) | 2333 (82.7) | <0.01 |
| Household income | |||
| <$20, 000 per year | 2643 (31.9) | 635 (22.9) | |
| >$20, 000 per year | 5647 (68.1) | 2134(77.1) | <0.01 |
| Health insurance | |||
| Yes | 7477 (88.5) | 2550 (90.4) | |
| No | 971 (11.5) | 271 (9.6) | <0.01 |
| Dental insurance | |||
| Yes | 5582 (67.6) | 1963 (70.9) | |
| No | 2680 (32.4) | 806 (29.1) | <0.01 |
| Preventive Care | |||
| Annual cleaning | |||
| Yes | 5666 (67.4) | 1946 (69.4) | |
| No | 2739 (32.6) | 859 (30.6) | 0.05 |
| Clinic visit within 1 year | |||
| Yes | 6079 (71.7) | 2027 (72.1) | |
| No | 2395 (28.3) | 785 (27.9) | 0.72 |
| Difficulty finding a dentist because of previous cancer | |||
| Yes | 247 (3.0) | - | |
| No | 8091 (97.0) | - | NA |
P-values are from GEE models accounting for inter-family correlations.
Table 2.
Characteristics of Cancer Diagnosis Treatment.
| Characteristic | Survivors N (%) |
|---|---|
| Diagnosis | |
| Leukemia | 2910 (34.2) |
| CNS | 1076 (12.6) |
| Hodgkin’s disease | 1086 (12.7) |
| Non-Hodgkin’s lymphoma | 628 (7.4) |
| Kidney (Wilms) | 794 (9.3) |
| Neuroblastoma | 575 (6.8) |
| Soft tissue sarcoma | 750 (8.8) |
| Bone cancer | 702 (8.2) |
| Year of diagnosis | |
| 1970−1973 | 1078 (12.7) |
| 1974−1977 | 1704 (20.0) |
| 1978−1981 | 2213 (26.0) |
| 1982−1986 | 3526 (41.4) |
| Age at Diagnosis (years) | |
| 0 – 5 years | 3971 (46.6) |
| 6 – 10 years | 1661 (19.5) |
| 11+ years | 2889 (33.9) |
| Treatment with: | |
| Radiation therapy only | 1067(12.5) |
| Chemotherapy only | 2165 (25.4) |
| Chemotherapy + radiation therapy | 4412 (51.8) |
| Surgery only involving oral cavity | 13 (0.2) |
| Chemotherapy + radiation + Surgery involving oral cavity | 124 (1.5) |
| Radiation+surgery involving oral cavity | 17 (0.2) |
| Other or Unknown | 724 (8.5) |
| Alkylating agent score | |
| 0 | 4214 (52.7) |
| 1 | 1841 (23.0) |
| 2 | 1195 (15.0) |
| 3 | 740 (9.3) |
| Cyclophosphamide dose | |
| 0 | 4828 (59.1) |
| 1–3999 mg | 998 (12.2) |
| 4000–7999 mg | 969 (11.9) |
| ≥8000 mg | 1374 (16.8) |
| Antimetabolites | |
| Yes | 4063 (47.7) |
| No | 4452 (52.3) |
| Steroids | |
| Yes | 4121 (48.4) |
| No | 4394 (51.6) |
| Vincristine | |
| Yes | 6064 (71.2) |
| No | 2451 (28.8) |
| Treatment with Radiation: | |
| None | 2900 (35.2) |
| >0 to <20 Gy | 5198 (63.1) |
| 20+ Gy | 143 (1.7) |
Comparison to Sibling Cohort
When compared to the sibling cohort, survivors reported a higher frequency of all adverse dental outcomes with the exception of dental bridge use and need for oral prosthesis (Table 3). However, certain differences were more striking than others. In multivariate analysis adjusted for gender, race, education, household income, health insurance status, and age at study, survivors were statistically significantly more likely than siblings to report dental developmental issues including microdontia (9.2% vs 3.3%)), hypodontia (8.2 vs. 5.3%), abnormal root development (5.4% vs. 1.9%), enamel hypoplasia (11.7% vs. 5.3%), loss of >6 teeth due to decay or gum disease (4.8% vs. 1.8%), and to have had severe gingivitis (6.7% vs. 5.7%). Additionally, survivors reported a greater need for dentures (3.0% vs. 1.7%) and an increased frequency of xerostomia (2.8% vs. 0.3%). Additional multivariate models, adjusting for dental insurance and composite dental care utilization in multivariate models, provided virtually identical odds ratios (data not shown). Sensitivity analyses were conducted to evaluate the potential impact of missing or unknown outcome responses on associations. Replacement of missing/unknown responses with all “no” responses resulted in no qualitative, or marked quantitative differences in p-values or point estimates of ORs. The less plausible replacement of missing/unknown with “yes” responses did result in reduced ORs for abnormal roots, microdontia, oral prothesis, and zerostomia, but there were no changes to significance of p-values.
Table 3.
Comparison of dental outcomes between survivors and siblings*
| Survivor cohort (%) | Sibling cohort (%) | Adjusted OR >(95% CI) |
Adjusted P-value |
|||||
|---|---|---|---|---|---|---|---|---|
| Dental characteristic | Yes | No | Unknown | Yes | No | Unknown | ||
| > 1 dental abnormality | 2539 (29.8) | 5249 (61.6) | 734 (8.6) | 530 (18.7) | 2132 (75.3) | 169 (6.0) | 1.9 (1.7–2.1) | <0.01 |
| > 1 dental soft tissue abnormality | 4662 (54.7) | 3458 (40.6) | 402 (4.7) | 1511 (53.4) | 1197 (42.3) | 123 (4.3) | 1.2 (1.1–1.3) | <0.01 |
| Dental appliance use | 625 ( 7.3) | 7821 (91.8) | 76 (0.9) | 168 ( 5.9) | 2642 (93.3) | 21 (0.7) | 1.3 (1.1–1.6) | 0.01 |
| Microdontia | 785 ( 9.2) | 7498 (88.0) | 239 (2.8) | 92 ( 3.3) | 2698 (95.3) | 41 (1.1) | 3.0 (2.4–3.8) | <0.01 |
| Hypodontia | 698 ( 8.2) | 7613 (89.3) | 211 (2.5) | 149 ( 5.3) | 2640 (93.3) | 42 (1.5) | 1.7 (1.4–2.0) | <0.01 |
| >5 caries | 4429 (52.0) | 3696 (43.4) | 397 (4.7) | 1445 (51.0) | 1263 (44.6) | 123 (4.3) | 1.2 (1.1–1.3) | <0.01 |
| Abnormal roots | 464 ( 5.4) | 7585 (89.0) | 473 (5.5) | 53 ( 1.9) | 2708 (95.7) | 70 (2.5) | 3.0 (2.2–4.0) | <0.01 |
| Enamel hypoplasia | 998 (11.7) | 6706 (79.7) | 818 (9.6) | 151 ( 5.3) | 2535 (89.5) | 145 (5.1) | 2.4 (2.0–2.9) | <0.01 |
| ≥6 teeth lost | 410 ( 4.8) | 8030 (94.2) | 82 (1.0) | 51 ( 1.8) | 2768 (97.8) | 12 (0.4) | 2.6 (1.9–3.6) | <0.01 |
| Gingivitis | 572 ( 6.7) | 7808 ( 1.6) | 142 (1.7) | 161 ( 5.7) | 2647 (93.5) | 23 (0.8) | 1.2 (1.0–1.5) | 0.03 |
| Xerostomia | 237 ( 2.8) | 8203 (96.3) | 82 (1.0) | 8 ( 0.3) | 2813 (99.4) | 10 (0.4) | 9.7 (4.8–19.7) | <0.01 |
| Dental bridge | 458 ( 5.4) | 8003 (93.9) | 61 (0.7) | 140 ( 5.0) | 2675 (94.5) | 16 (0.6) | 1.2 (1.0–1.4) | 0.12 |
| Dentures | 252 ( 3.0) | 8224 (96.5) | 46 (0.5) | 47 ( 1.7) | 2774 (97.8) | 10 (0.4) | 1.7 (1.2–2.4) | <0.01 |
| Oral prosthesis | 30 ( 0.4) | 8430 (98.9) | 62 (0.7) | 2 ( 0.1) | 2815 (99.4) | 14 (0.5) | 3.9 (0.9–16.7) | 0.07 |
Models were adjusted for gender, race, education, household income, health insurance, and age at follow-up, which included GEE methods for intra-family correlations. Additional models, also including adjustment for dental insurance and dental care utilization (as a composite) provided virtually no change in observed ORs. Sensitivity analyses to impact the potential impact of unknown responses were also conducted (see Results Section).
Utilization of Dental Services
Acknowledging that reporting of many dental abnormalities may be partly dependent upon their diagnosis by a dental healthcare professional, we examined associations between utilization of dental services and socio-economic factors. We found no significant difference in utilization of dental services between survivors and siblings (Table 1) but significantly higher frequencies of both cleaning visits and seeing a dentist in the past year for subjects who are white, those with educational level beyond high school, income levels greater than $20,000 and female (Table 4).
Table 4.
Survivors’ Utilization of Dental Services.
| Yes N (%) |
No N (%) |
OR | (95% CI) | P-value | |
|---|---|---|---|---|---|
| Teeth Cleaned in Last Year | |||||
| Education | |||||
| Post High School | 4655 (83.3) | 1981 (73.2) | 1.8 | (1.6, 2.0) | <0.01 |
| No Post High School | 936 (16.7) | 727 (26.9) | 1.0 | ||
| Gender | |||||
| Female | 2986 (52.7) | 1213 (44.3) | 1.4 | (1.3, 1.5) | <0.01 |
| Male | 2680 (47.3) | 1526 (55.7) | 1.0 | ||
| Household Income | |||||
| ≥ $20,000 and higher | 4123 (74.7) | 1456 (54.7) | 2.5 | (2.2, 2.7) | <0.01 |
| <$20,000 | 1396 (25.3) | 1207 (45.3) | 1.0 | ||
| Race | |||||
| White non-Hispanic | 4942 (87.5) | 2323 (85.1) | 1.2 | (1.1,1.4) | <0.01 |
| Other | 704 (12.5) | 406 (14.9) | 1.0 | ||
| Visit to Dentist in Last Year | |||||
| Education | |||||
| Post High School | 4938 (82.4) | 1755 (74.0) | 1.6 | (1.5, 1.8) | <0.01 |
| No Post-High School | 1057(17.6) | 617 (26.0) | 1.0 | ||
| Gender | |||||
| Female | 3193 (52.5) | 1038 (43.3) | 1.5 | (1.3,1.6) | <0.01 |
| Male | 2886 (47.5) | 1357 (56.7) | 1.0 | ||
| Household Income | |||||
| ≥$20,000 | 4320 (73.1) | 1301 (55.7) | 2.2 | (2.0,2.4) | <0.01 |
| <$20,000 | 1591 (26.9) | 1037 (44.4) | 1.0 | ||
| Race | |||||
| White non-Hispanic | 5291 (87.3) | 2032 (85.2) | 1.2 | (1.1, 1.4) | <0.01 |
| Other | 766 (12.7) | 354 (14.8) | 1.0 |
Risk Factors for Dental Abnormalities
To examine the influence of demographic (gender, race/ethnicity), socioeconomic factors (education, income, insurance status) and the impact of treatment exposures (radiation and chemotherapy including alkylating agents, antimetabolites, steroids and vincristine), we performed a multivariate logistic regression analysis (Tables 5 and 6). A low frequency of events prohibited analysis of some outcomes including the need for oral appliance use. Increased risk of at least one reported dental health issue was associated with younger age at study, female gender, race/ethnicity White non-Hispanic, educational attainment of high school or less, and annual household income of less than $20,000. Excepting age at study and educational attainment level, similar risks were observed for at least one reported soft tissue issue. Dose of radiation to the teeth was statistically significantly associated with an increased risk of one or more dental health issues (OR 1.3 and 5.6, exposure of >0 to <20 Gy and ≥20 Gy, respectively) and one or more soft tissue issues (OR 1.2 and 2.2, exposure of>0 to <20 Gy and ≥20 Gy, respectively). Similarly, increasing cumulative exposure to alkylating agent therapy was associated with an increased risk for reporting at least one dental abnormality (Table 6).
Table 5.
Odds Ratios for socio-economic variables - multivariate logistic regression model adjusted for radiation dose, alkylating agents score, steroids, vincristine, and antimetabolites.
| At least one dental health issue |
At least one soft tissue issue |
Dental appliance | Microdontia | Hypodontia | 6+ cavities | Abnormal roots | Enamel hypoplasia |
Lost 6+ teeth | Xerostomia | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI |
| Age at Follow-up | ||||||||||||||||||||
| <30 years | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||||||||||
| ≥30 years | 0.9a | 0.8 – 1.0 | 2.6b | 2.4 – 2.9 | 2.6b | 2.1 – 3.2 | 0.4b | 0.3 – 0.5 | 0.6b | 0.5 – 0.7 | 2.4b | 2.2 – 2.7 | 0.5b | 0.4 – 0.6 | 0.9 | 0.8 – 1.1 | 2.7b | 2.1 – 3.5 | 2.7b | 1.8 – 3.9 |
| Gender | ||||||||||||||||||||
| Male | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||||||||||
| Female | 1.3b | 1.2 – 1.4 | 1.4b | 1.3 – 1.6 | 1.2 | 1.0 – .4 | 1.2a | 1.0 – 1.4 | 1.6b | 1.3 – 1.9 | 1.5b | 1.3 – 1.6 | 1.1 | 0.9 – 1.4 | 1.3b | 1.1 – 1.5 | 1.1 | 0.9 – 1.4 | 1.1 | 0.8 – 1.5 |
| Race/Ethnicity | ||||||||||||||||||||
| Other | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||||||||||
| White, non- Hispanic | 1.2b | 1.0 – 1.4 | 1.2b | 1.0 – 1.4 | 1.4a | 1.0 – 1.9 | 1.1 | 0.9 – 1.4 | 1.4b | 1.1 – 1.9 | 1.2b | 1.1 – 1.4 | 1.4 | 1.0 – 1.9 | 1.4b | 1.1 – 1.7 | 1.1 | 0.8 – 1.5 | 1.5 | 0.9 – 2.4 |
| Education | ||||||||||||||||||||
| No Post High School | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||||||||||
| Post High School | 0.8b | 0.7 – 0.9 | 1.1 | 0.9 – 1.2 | 0.8a | 0.6 – 1.0 | 0.7b | 0.5 – 0.8 | 0.9 | 0.8 – 1.2 | 1.1 | 1.0 – 1.2 | 0.7b | 0.5 – 0.9 | 0.8a | 0.7 – 1.0 | 0.4b | 0.3 – 0.5 | 1.6a | 1.0 – 2.5 |
| Household Income | ||||||||||||||||||||
| < $20,000 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||||||||||
| ≥ $20,000 | 0.8b | 0.7 – 0.9 | 0.9a | 0.8 – 1.0 | 0.8 | 0.7 – 1.0 | 0.8b | 0.7 – 0.9 | 1.1 | 0.9 – 1.3 | 0.9a | 0.8 – 1.0 | 0.7b | 0.6 – 0.9 | 0.7b | 0.6 – 0.8 | 0.5b | 0.4 – 0.6 | 0.6b | 0.5 – 0.8 |
| Health Insurance | ||||||||||||||||||||
| No Insurance | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||||||||||
| Yes or Canadian | 0.9 | 0.7 – 1.0 | 0.9 | 0.8 – 1.1 | 0.9 | 0.7 – 1.2 | 1.1 | 0.9 – 1.4 | 1.1 | 0.8–1.4 | 0.9 | 0.8 – 1.1 | 0.9 | 0.6 – 1.2 | 0.8a | 0.6 – 1.0 | 0.7b | 0.5 – 0.9 | 1.0 | 0.6 – 1.6 |
0.01 > p < 0.05
p ≤ 0.01
Table 6.
Odds Ratios for radiation and chemotherapy exposure in a multivariate logistic regression model adjusting for age at follow-up, gender, race/ethnicity, education, household income and health insurance.
| At least one dental health issue | At least one soft tissue issue | |||||
|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | p-value | OR | 95% CI | p-value |
| Radiation Dose to the Jaw | ||||||
| 0 | 1.0 | 1.0 | ||||
| >0 to <20 Gy | 1.3 | 1.2 – 1.5 | <0.01 | 1.2 | 1.1 – 1.3 | <0.01 |
| ≥ 20 Gy | 5.6 | 3.7 – 8.5 | <0.01 | 2.2 | 1.4 – 3.4 | <0.01 |
| Cumulative Alyklating Agent Dose Score | ||||||
| 0 | 1.0 | 1.0 | ||||
| 1 | 1.4 | 1.2 – 1.6 | <0.01 | 1.0 | 0.9 – 1.1 | 0.77 |
| 2 | 1.7 | 1.5 – 2.0 | <0.01 | 1.4 | 1.2 – 1.6 | <0.01 |
| 3 | 2.0 | 1.6 – 2.4 | <0.01 | 1.3 | 1.1 – 1.6 | <0.01 |
| Antimetabolite Therapy | ||||||
| No | 1.0 | 1.0 | ||||
| Yes | 1.0 | 0.9 – 1.2 | 0.89 | 0.9 | 0.8 – 1.1 | 0.43 |
| Steroid Therapy | ||||||
| No | 1.0 | 1.0 | ||||
| Yes | 0.9 | 0.8 – 1.1 | 0.18 | 1.1 | 1.0 – 1.3 | 0.13 |
| Vincristine Therapy | ||||||
| No | 1.0 | 1.0 | ||||
| Yes | 1.0 | 0.8 – 1.1 | 0.68 | 0.9 | 0.8 – 1.0 | 0.19 |
Treatment factors were examined in these same multivariate models for all ages combined, as well as stratified on age at cancer treatment. Radiation therapy to the teeth significantly increased the risk for development of one or more dental abnormalities in a dose dependent pattern (OR 1.32, 95% CI 1.17 – 1.48 for dose of >0 to <20 Gy and OR 5.61, 95% CI 3.71 – 8.47 for dose ≥20 Gy). This dose-dependent risk was present within all age strata, except for those survivors older than 10 years at diagnosis who received >0 to <20 Gy (age 0–5 years: OR 1.32, 95% CI 1.17 – 1.48 for dose of >0 to <20 Gy and OR 5.61, 95% CI 3.71 – 8.47 for dose ≥20 Gy; age 6–10 years: OR 1.45, 95% CI 1.09 – 1.91 for dose of >0 to <20 Gy and OR 9.64, 95% CI 4.14 – 22.44 for dose ≥20 Gy; and age >10 years: OR 1.15, 95% CI 0.93 – 1.42 for dose of >0 to <20 Gy and OR 4.30, 95% CI 2.22 – 8.33 for dose ≥20 Gy). Investigation of risk for specific dental issues according to cumulative exposure to alkylating agent therapy and for cumulative dose of cyclophosphamide, demonstrated a striking association with increased risk, independent of radiation exposure to the teeth, among survivors diagnosed and treated for their cancer when younger than 5 years (Figure 1).
FIGURE 1.
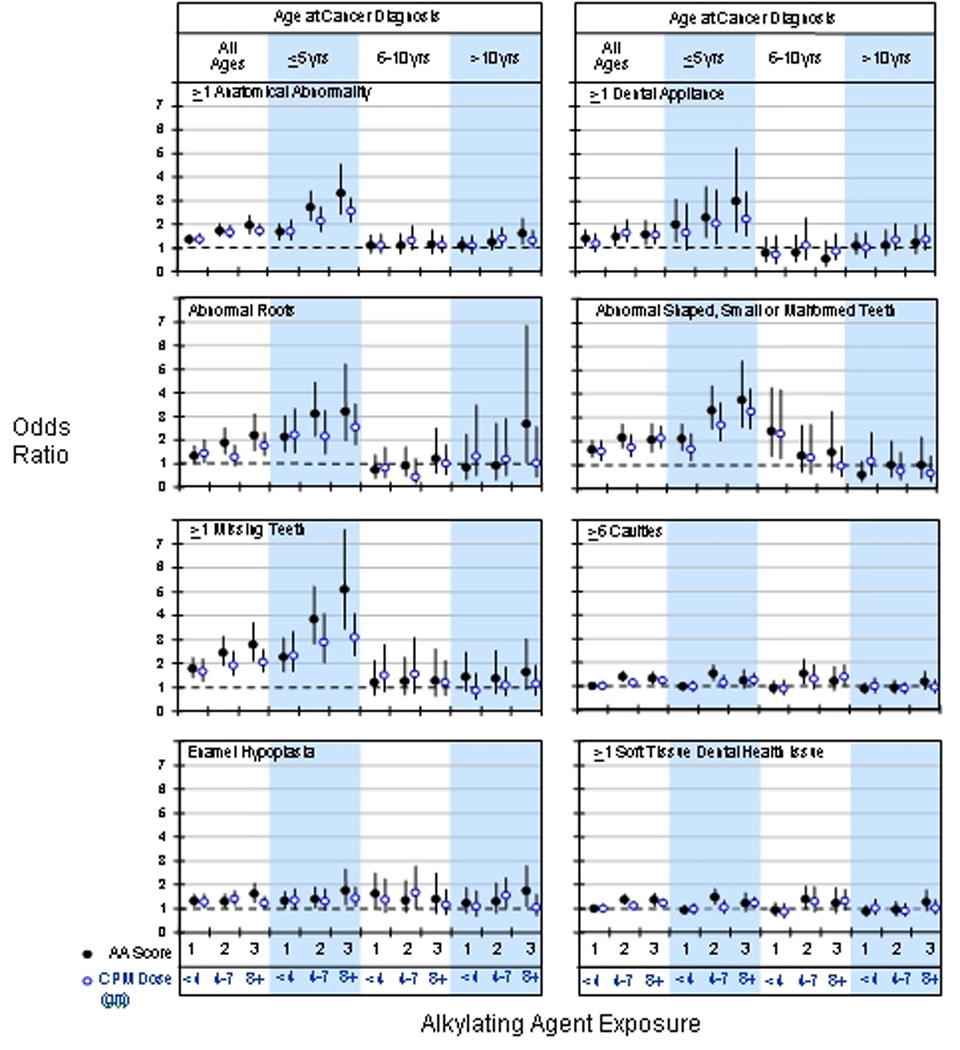
Risk of dental abnormalities by alkylating agent exposure is shown. Solid circles represent alkylating agent score as described in the methods with referent as a score of 0. Open circles represent cumulative cyclophosphamide exposure in grams, with referent as no cyclophosphamide. Odds ratios and 95% confidence limits are adjusted for age at follow-up, sex, race, education, household income, insurance, radiation exposure to the jaw, steroids, antimetabolites, and vincristine
While radiation exposure to the teeth and alkylating agent therapy were independent risk factors for dental abnormalities, analyses were conducted to determine if there was a potential interaction effect. These tests indicated that only the outcomes of microdontia (p = 0.01) and enamel hypoplasia (p=0.02) had a statistically significant interactive effect between radiation to the teeth and use of alkylating agents.
Discussion
Delayed toxicities resulting from modern cancer therapy are increasingly recognized and many have the potential to compromise long-term health. Through the CCSS, with the large number of participants and detailed treatment information, we identified dose- and age-associated exposure to alkylating agents, independent of radiation exposure, as one of the primary causes of adverse dental outcomes in childhood cancer survivors.
Alkylating agents, and specifically, cyclophosphamide, have been shown in animal studies to be toxic to normal dentinogenesis by its binding to DNA in the S-phase of mitosis, ultimately resulting in early apoptosis (21–23). The dental effect of cyclophosphamide-induced cell death predominates in primitive mesenchymal cells and preodontoblasts of the pulp (24). Resulting outcomes may reflect the stage of dental development occurring at the time of exposure to this agent such that the younger the animal, the greater the effect on dentition. Upon completion of dental development, mature teeth, particularly incisors and molars, may exhibit a variety of abnormalities including foreshortened root development, small soft crowns, abnormal partially calcified pulp chambers, miss-shapened teeth, and enamel defects (25–28). Although findings from animal studies have been supported through case reports and investigations among small cohorts of pediatric cancer patients (25;29), the current CCSS study definitively identifies young age and increased exposure to alkylating agents as risk factors for developmental dental abnormalities in long-term survivors of childhood cancer.
Altered odontogenesis and exfoliation may be accompanied by altered dental eruption. In rat studies, corticosteroids accelerated and cyclophosphamide slowed eruption of rat incisors during the normal phase of eruption; neither drug affected eruption during the initial slow phase (30). As the rapid growth of rat dentition has been likened to the rapid dental growth and development in children (23), similar effects might be expected to manifest in children also exposed to this agent. In fact, a patient is at greater risk for odontogenic developmental abnormalities if treated with chemotherapy when younger than 5 years of age because of prolific activity of dental stem cells during this period (14–16). Our data emphasizes the significant vulnerability of developing dentition in young children when treated with alkylator therapy. When either cyclophosphamide or the alkylator index was examined, a dose response was demonstrated with greater frequency of adverse dental outcomes with higher exposure to alkylator chemotherapy. Children beyond age 5 years failed to demonstrate these findings even at the highest levels of alkylator exposure.
Radiation therapy may alter dental integrity (1;2) and craniofacial development (12;31). Xerostomia is a common side effect during radiation treatment (32) and contributes to altered oral flora which in turn can be associated with an increased number of caries (33;34). Radiation therapy has also been shown to damage the tooth bud thereby causing microdontia, growth retardation of teeth, malocclusion and arrested root development (1;2;35;36). Atrophy of underlying soft tissue, enamel hypoplasia or incomplete calcification can also result. As supported by our current findings, the degree and severity of these effects depend on the child’s age at diagnosis and type and dose of radiation (37–39). Sonis et al. found that acute lymphoblastic leukemia survivors who received 2400 cGy were more severely affected than those who received1800 cGy (16). We, too, found that exposure to radiation therapy doses exceeding 20 Gy contributed to a four to 10-fold higher risk of developing dental abnormalities.
Further, we found that gender and socioeconomic factors correlated with odontogenic toxicity after adjustment for treatment differences and were also associated with significant differences in the utilization of health care (40). Female gender, white non-Hispanic race, lower education level, and lower household income level are associated with an increased risk of adverse dental health in childhood cancer survivors. In contrast, patients with higher incomes and more education actually reported fewer dental abnormalities. Increased risk of dental abnormalities may reflect decreased access to dental care (particularly, limited access to dentists trained in caring for these complex patients) and potentially, decreased use of preventive care. Individuals of white race, those with educational level beyond high school, income levels greater than $20,000 and female sex all reported a higher frequency of having both cleaning visits and seeing a dentist in the last year. Such an increase in dental care utilization may result in a greater likelihood of diagnosis and, hence, knowledge of specific dental conditions rather than reflecting a difference in biologic susceptibility. These patients who presumably have better access to dental care and possibly greater health awareness, may have also received preventative dental care that minimized some adverse outcomes.
In interpreting the results of this study, it is important to consider the limitations and strengths. The validity of self-reported dental abnormalities in this population is unknown. The ability to self-report many abnormalities described in this report may be dependent upon the level of dental care. Missing teeth, number of cavities and use of dental appliances are readily apparent to the individual. However, recognition of abnormalities such as lack of enamel (enamel hypoplasia), small teeth (microdontia) and root abnormalities (root stunting) require professional diagnosis. Strengths of this study include the large survivor and sibling cohorts, known cumulative radiation and chemotherapy dose exposures and long-term follow-up that allows detailed analysis of factors contributing to dental developmental abnormalities.
Our data indicate that children less than 5 years of age when exposed to alkylating agents, particularly those receiving high cumulative doses, are at high risk for developmental dental abnormalities. While this may not be avoidable for many, given the wide use of alkylating agents in pediatric oncology, these data can be utilized to inform parents, patients, and dental care providers that close monitoring and follow-up of these children will be necessary. The same caveats hold true for children exposed to radiation therapy to the teeth, particularly if the dose is over 20 Gy. The need for ongoing close dental follow-up should be conveyed to cancer survivors and their parents during therapy and re-emphasized upon completion of therapy.
Acknowledgements
We thank Sandra Gaither for manuscript preparation.
Supported by Grant No. U24-CA-55727 (L.L. Robison, Principal Investigator) from the Department of Health and Human Services, funding to the University of Minnesota from the Children’s Cancer Research Fund, and funding to St. Jude Children’s Research Hospital from the American Lebanese Syrian Associated Charities – ALSAC.
APPENDIX
The Childhood Cancer Survivor Study (CCSS) is a collaborative, multi-institutional project, funded as a resource by the National Cancer Institute, of individuals who survived five or more years after diagnosis of childhood cancer. For participating investigators see www.stjude.org/ccss
Questions asked of study participants regarding dental and oral abnormalities and corresponding abnormality
Have you ever …
| Had one or more missing teeth because they did not develop? | Hypodontia |
| Had a lack of or decreased amount of enamel on surface of teeth? | Enamel hypoplasia |
| Had abnormal shaped (small or malformed) teeth? | Microdontia |
| Had difficulty producing saliva (dry mouth) that required treatment such as artificial saliva? | Xerostomia |
| Had severe gingivitis or gum disease requiring surgery or deep cleaning? | Gingivitis |
| Had more than 5 cavities? | |
| Lost 6 or more teeth due to decay or gum disease? | |
| Worn a dental bridge (for missing or removed teeth)? | |
| Worn removable dentures (complete or partial upper or lower or both)? | |
| Worn a prosthesis to lift your palate to improve the quality of your voice? | |
Reference List
- 1.Kaste SC, Hopkins KP, Jenkins JJ., III Abnormal odontogenesis in children treated with radiation and chemotherapy: imaging findings. AJR Am. J Roentgenol. 1994;162:1407–1411. doi: 10.2214/ajr.162.6.8192008. [DOI] [PubMed] [Google Scholar]
- 2.Kaste SC, Hopkins KP, Jones D, Crom D, Greenwald CA, Santana VM. Dental abnormalities in children treated for acute lymphoblastic leukemia. Leukemia. 1997;11:792–796. doi: 10.1038/sj.leu.2400670. [DOI] [PubMed] [Google Scholar]
- 3.Kaste SC, Hopkins KP, Bowman LC, Santana VM. Dental abnormalities in children treated for neuroblastoma. Med Pediatr.Oncol. 1998;30:22–27. doi: 10.1002/(sici)1096-911x(199801)30:1<22::aid-mpo8>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Nasman M, Bjork O, Soderhall S, Ringden O, Dahllof G. Disturbances in the oral cavity in pediatric long-term survivors after different forms of antineoplastic therapy. Pediatr.Dent. 1994;16:217–223. [PubMed] [Google Scholar]
- 5.Marec-Berard P, Azzi D, Chaux-Bodard AG, Lagrange H, Gourmet R, Bergeron C. Long-term effects of chemotherapy on dental status in children treated for nephroblastoma. Pediatr.Hematol.Oncol. 2005;22:581–588. doi: 10.1080/08880010500198848. [DOI] [PubMed] [Google Scholar]
- 6.Vaughan MD, Rowland CC, Tong X, Srivastava DK, Hale GA, Rochester R, et al. Dental abnormalities after pediatric bone marrow transplantation. Bone Marrow Transplant. 2005;36:725–729. doi: 10.1038/sj.bmt.1705136. [DOI] [PubMed] [Google Scholar]
- 7.Vaughan MD, Rowland CC, Tong X, Srivastava DK, Hale GA, Rochester R, et al. Dental abnormalities in children preparing for pediatric bone marrow transplantation. Bone Marrow Transplant. 2005;36:863–866. doi: 10.1038/sj.bmt.1705111. [DOI] [PubMed] [Google Scholar]
- 8.Wogelius P, Dahllof G, Gorst-Rasmussen A, Sorensen HT, Rosthoj S, Poulsen S. A population-based observational study of dental caries among survivors of childhood cancer. Pediatr.Blood Cancer. 2008;50:1221–1226. doi: 10.1002/pbc.21464. [DOI] [PubMed] [Google Scholar]
- 9.Sheller B, Williams B. Orthodontic management of patients with hematologic malignancies. Am.J Orthod.Dentofacial Orthop. 1996;109:575–580. doi: 10.1016/s0889-5406(96)70068-9. [DOI] [PubMed] [Google Scholar]
- 10.Dahllof G, Krekmanova L, Kopp S, Borgstrom B, Forsberg CM, Ringden O. Craniomandibular dysfunction in children treated with total-body irradiation and bone marrow transplantation. Acta Odontol.Scand. 1994;52:99–105. doi: 10.3109/00016359409029062. [DOI] [PubMed] [Google Scholar]
- 11.Kaste SC, Hopkins KP, Bowman LC. Dental abnormalities in long-term survivors of head and neck rhabdomyosarcoma. Med Pediatr.Oncol. 1995;25:96–101. doi: 10.1002/mpo.2950250209. [DOI] [PubMed] [Google Scholar]
- 12.Denys D, Kaste SC, Kun LE, Chaudhary MA, Bowman LC, Robbins KT. The effects of radiation on craniofacial skeletal growth: a quantitative study. Int J Pediatr.Otorhinolaryngol. 1998;45:7–13. doi: 10.1016/s0165-5876(98)00028-7. [DOI] [PubMed] [Google Scholar]
- 13.Berkowitz RJ, Neuman P, Spalding P, Novak L, Strandjord S, Coccia PF. Developmental orofacial deficits associated with multimodal cancer therapy: case report. Pediatr.Dent. 1989;11:227–231. [PubMed] [Google Scholar]
- 14.Ubios AM, Piloni MJ, Cabrini RL. Mandibular growth and tooth eruption after localized xradiation. J Oral Maxillofac.Surg. 1992;50:153–156. doi: 10.1016/0278-2391(92)90361-3. [DOI] [PubMed] [Google Scholar]
- 15.McGinnis JP, Jr, Hopkins KP, Thompson EI, Hustu HO. Tooth root growth impairment after mantle radiation in long-term survivors of Hodgkin's disease. J. Am. Dent.Assoc. 1985;111:584–588. doi: 10.14219/jada.archive.1985.0164. [DOI] [PubMed] [Google Scholar]
- 16.Sonis AL, Tarbell N, Valachovic RW, Gelber R, Schwenn M, Sallan S. Dentofacial development in long-term survivors of acute lymphoblastic leukemia. A comparison of three treatment modalities. Cancer. 1990;66:2645–2652. doi: 10.1002/1097-0142(19901215)66:12<2645::aid-cncr2820661230>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 17.Robison LL, Mertens AC, Boice JD, Breslow NE, Donaldson SS, Green DM, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr.Oncol. 2002;38:229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 18.Tucker MA, Meadows AT, Boice JD, Jr, Stovall M, Oberlin O, Stone BJ. Leukemia after therapy with alkylating agents for childhood cancer. J Natl.Cancer Inst. 1987;78:459–464. [PubMed] [Google Scholar]
- 19.Stovall M, Weathers R, Kasper C, Smith SA, Travis L, Ron E, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat.Res. 2006;166:141–157. doi: 10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 20.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 21.Wei X, Senders C, Owiti GO, Liu X, Wei ZN, Dillard-Telm L, et al. The origin and development of the upper lateral incisor and premaxilla in normal and cleft lip/palate monkeys induced with cyclophosphamide. Cleft Palate Craniofac.J. 2000;37:571–583. doi: 10.1597/1545-1569_2000_037_0571_toadot_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 22.Orams HJ. Cyclophosphamide-induced changes in rodent odontogenesis. A light- and electron-microscopic study. Cell Tissue Res. 1983;234:679–689. doi: 10.1007/BF00218659. [DOI] [PubMed] [Google Scholar]
- 23.Vahlsing HL, Feringa ER, Britten AG, Kinning WK. Dental abnormalities in rats after a single large dose of cyclophosphamide. Cancer Res. 1975;35:2199–2202. [PubMed] [Google Scholar]
- 24.Anton E. Ultrastructural study of the effect of cyclophosphamide on the growth area of incisor teeth of DBA/2 and C57BL/6 mice. Int J Exp.Pathol. 1996;77:83–88. doi: 10.1046/j.1365-2613.1996.00967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alpaslan G, Alpaslan C, Gogen H, Oguz A, Cetiner S, Karadeniz C. Disturbances in oral and dental structures in patients with pediatric lymphoma after chemotherapy: a preliminary report. Oral Surg.Oral Med Oral Pathol.Oral Radiol Endod. 1999;87:317–321. doi: 10.1016/s1079-2104(99)70215-5. [DOI] [PubMed] [Google Scholar]
- 26.Koppang HS. Effect of cyclophosphamide on dentinogenesis in the rat incisor: fluorescence microscopic and microradiographic investigations. Scand.J Dent.Res. 1981;89:59–70. doi: 10.1111/j.1600-0722.1981.tb01278.x. [DOI] [PubMed] [Google Scholar]
- 27.Nasman M, Forsberg CM, Dahllof G. Long-term dental development in children after treatment for malignant disease. Eur.J Orthod. 1997;19:151–159. doi: 10.1093/ejo/19.2.151. [DOI] [PubMed] [Google Scholar]
- 28.Nasman M, Hammarstrom L. Influence of the antineoplastic agent cyclophosphamide on dental development in rat molars. Acta Odontol.Scand. 1996;54:287–294. doi: 10.3109/00016359609003540. [DOI] [PubMed] [Google Scholar]
- 29.Remmers D, Bokkerink JP, Katsaros C. Microdontia after chemotherapy in a child treated for neuroblastoma. Orthod.Craniofac.Res. 2006;9:206–210. doi: 10.1111/j.1601-6343.2006.00374.x. [DOI] [PubMed] [Google Scholar]
- 30.Burn-Murdoch RA. The effect of corticosteroids and cyclophosphamide on the eruption of resected incisor teeth in the rat. Arch.Oral Biol. 1988;33:661–667. doi: 10.1016/0003-9969(88)90121-5. [DOI] [PubMed] [Google Scholar]
- 31.Kaste SC, Hopkins KP. Micrognathia after radiation therapy for childhood facial tumors. Report of two cases with long-term follow-up. Oral Surg.Oral Med Oral Pathol. 1994;77:95–99. doi: 10.1016/s0030-4220(06)80115-5. [DOI] [PubMed] [Google Scholar]
- 32.Simon AR, Roberts MW. Management of oral complications associated with cancer therapy in pediatric patients. ASDC J Dent.Child. 1991;58:384–389. [PubMed] [Google Scholar]
- 33.Pajari U, Ollila P, Lanning M. Incidence of dental caries in children with acute lymphoblastic leukemia is related to the therapy used. ASDC J Dent.Child. 1995;62:349–352. [PubMed] [Google Scholar]
- 34.Miller EC, Vergo TJ, Jr, Feldman MI. Dental management of patients undergoing radiation therapy for cancer of the head and neck. Compend.Contin.Educ.Dent. 1981;2:350–356. [PubMed] [Google Scholar]
- 35.Kahl B. Odontogenesis and dentition development following irradiation of pediatric tumors of the maxillofacial area. Fortschr.Kieferorthop. 1989;50:127–135. doi: 10.1007/BF02203069. [DOI] [PubMed] [Google Scholar]
- 36.Dahllof G, Barr M, Bolme P, Modeer T, Lonnqvist B, Ringden O, et al. Disturbances in dental development after total body irradiation in bone marrow transplant recipients. Oral Surg.Oral Med Oral Pathol. 1988;65:41–44. doi: 10.1016/0030-4220(88)90189-2. [DOI] [PubMed] [Google Scholar]
- 37.Dahllof G, Jonsson A, Ulmner M, Huggare J. Orthodontic treatment in long-term survivors after pediatric bone marrow transplantation. Am.J Orthod.Dentofacial Orthop. 2001;120:459–465. doi: 10.1067/mod.2001.118102. [DOI] [PubMed] [Google Scholar]
- 38.Runge ME, Edwards DL. Orthodontic treatment for an adolescent with a history of acute lymphoblastic leukemia. Pediatr.Dent. 2000;22:494–498. [PubMed] [Google Scholar]
- 39.Burden D, Mullally B, Sandler J. Orthodontic treatment of patients with medical disorders. Eur.J Orthod. 2001;23:363–372. doi: 10.1093/ejo/23.4.363. [DOI] [PubMed] [Google Scholar]
- 40.Yeazel MW, Gurney JG, Oeffinger KC, Mitby PA, Mertens AC, Hudson MM, et al. An examination of the dental utilization practices of adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Public Health Dent. 2004;64:50–54. doi: 10.1111/j.1752-7325.2004.tb02726.x. [DOI] [PubMed] [Google Scholar]


