The unique optical characteristics of lanthanide ions (LnIII) have driven their use in a wide range of applications, however the efficiency of populating directly their electronic states is limited so that the creation of an antenna to capture energy and generate excited LnIII ions is broadly important. In this context, metal-organic frameworks, hybrid materials, and nanoparticles randomly doped with homo- or heterometallic mixtures of luminescent lanthanide cations, LnIII, are intensively being investigated for engineering luminescent devices for bright white lighting, for upconversion, and as sensing agents.[1] Although the exact location of the various metals in the final material is crucial for dual ligand-centered/metal-centered emission,[1l,m] for upconversion[1g] and for directional light-conversion[2a] processes, the preparation of organized polymetallic 4f-4f oligomers and polymers remains rare and challenging.[2] A statistical mechanics (Ising model) analysis suggests that standard repulsive nearest neighbor intermetallic interactions operating in linear polymers with regularly spaced binding sites should provide the targeted ordered …-Ln1-Ln2-Ln1-Ln2-… microstates.[3,4] Pioneering work in this field has relied on the bulk electropolymerization of didentate 1,10-phenanthroline with thienyl spacers[5] and the acyclic diene metathesis of tridentate 2,6-bis(benzimidazol-2-yl)pyridine,[6] followed by reaction with Eu(β-diketonate)3 or Eu(NO3)3 to yield red-emitting metallopolymers. A reliable exploitation of this concept for the development of luminescent materials, however, requires the efficient sensitization of the luminophore via the rational optimization of each photophysical step by using chemical tools.
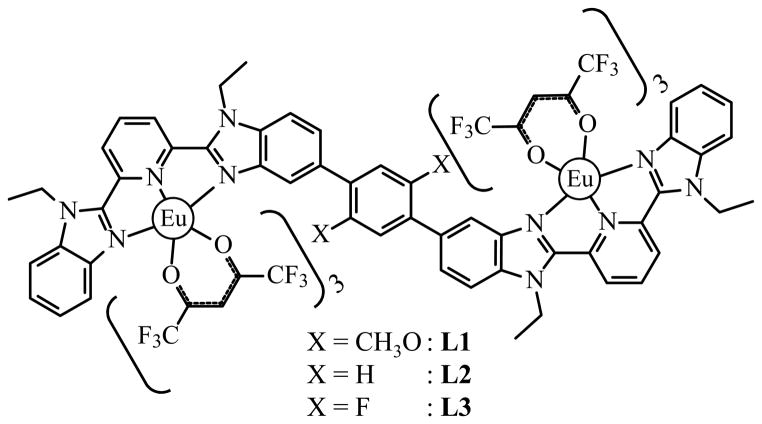
As a first step toward this goal, the rigid segmental ligand strands L1–L3, made of two tridentate binding units separated by a rigid and electronically-tunable aromatic spacer, have been reacted with trivalent europium to give the dinuclear complexes [Eu2(Lk)(hfac)6] (Scheme 1).[7] The use of a simple method for deciphering the various contributions to the sensitization mechanism clearly showed that [Eu2(L3)(hfac)6] displayed the largest global emission quantum yield ( , eq. 1) because of an efficient L3→Eu energy transfer step ( eq. 3, see the dark grey histograms in Fig. 1).[7]
Scheme 1.
Chemical structures of complexes [Eu2(Lk)(hfac)6]
Figure 1.
This simplified Jablonski diagram for [Eu2(Lk)(hfac)6] (k = 2–4) shows the ligand-centered triplet-mediated sensitization mechanism of the two Eu3+ ions.[10] The photophysical processes are described by the rate constants: for ligand fluorescence, for ligand internal non-radiative conversion, for ligand phosphorescence, for non-radiative relaxation from the ligand triplet state, for emission of Eu*, for nonradiative decay of Eu*, kISC for ligand intersystem crossing, and for the ligand-to-metal energy transfer. Efficiencies of intersystem crossing (ηISC), energy transfer ( ), intrinsic quantum yield ( ), and quantum yield ( ) for the global ligand-mediated sensitization of EuIII in [Eu2(Lk)(hfac)6] (solid-state, 293 K).
Theoretical considerations suggest that could benefit from a shift of the L(3π*) state to higher energy produced by the perfluorination of the central aromatic spacer in L4 (Scheme 2).[8] Correspondingly, the expected decrease of and , and of the so-called π-conjugation length Aπ (eq. 5)[9] in [Eu2(L4)(hfac)6],[7] should optimize both intersystem crossing efficiency (ηISC = 0.6(1), eq. 2) and intrinsic Eu-centered quantum yield ( , eq. 4) that were previously measured in [Eu2(L3)(hfac)6] (dark grey histograms in Fig. 1). Note that
Scheme 2.
Synthesis of the perfluorinated ligand L4.
| (1) |
| (2) |
| (3) |
| (4) |
and
| (5) |
The centrosymmetrical perfluorinated ligand L4 is obtained through two successive Pd-catalyzed Suzuki-Myaura cross coupling reactions (Scheme 2, Figs. S1–S2). Stoichiometric mixing of L4 with [Ln(hfac)3(diglyme)] (2.0 eq) in chloroform gives [Ln2(L4)(hfac)6] (Ln = Gd, Eu) with a 80% yield. Slow evaporation from concentrated acetonitrile/chloroform solutions provides X-ray quality prisms (Table S1) containing S-shaped centrosymmetrical neutral [Eu2(L4)(hfac)6] complexes, in which the Eu atoms are separated by 14.667(1) Å (Fig. 2). Each metal is nine-coordinate in a highly distorted monocapped square antiprismatic polyhedron, produced by the three nitrogen atoms of the bound tridentate aromatic unit and by the six oxygen atoms of the three didentate hexafluoroacetylacetonates, N1 occupying the capping position (see Appendix 1). All bond distances and bond angles are standard (Tables S2–S4) and the solid-state molecular structures of [Yb2(L3)(hfac)6] and [Eu2(L4)(hfac)6] are almost superimposable, except for the interannular phenyl-benzimidazole twist angle, which increases from 54.1(1)° to 66.2(1)° (Fig. S3 and Table S5).
Figure 2.

Perspective view of the molecular structure of [Eu2(L4)(hfac)6] as obtained from X-ray diffraction with numbering scheme. Thermal ellipsoids are represented at the 30% probability level and hydrogen atoms are omitted for clarity.
Irradiation into the allowed ligand-centered 1π*←1π transition of [Eu2(L4)(hfac)6] at ν̄exc = 28170 cm−1 produces an intense long-lived red emission signal, arising from L4→Eu(III) energy transfer followed by Eu(5D1) and Eu(5D0)-centered luminescence (Fig. S4). The emission spectrum is dominated by the hypersensitive forced electric dipolar Eu(5D0→7F2) transition centered at 16340 cm−1 leading to the largest global absolute quantum yields in this series ( , solid state, 293K, Table 1, entry 6).[11] Using Einstein’s result for the spontaneous radiative emission rate,[12] the radiative rate constant (Table 1, entry 2) is deduced from the Itot/IMD ratio, where Itot is the integrated emission for the Eu(5D0) level (5D0 → 7FJ, J = 0 – 4) and IMD is the integrated intensity of the magnetic dipolar Eu(5D0→7F1) transition (Table 1, entry 1). Combined with the characteristic lifetime τEu = 0.90(1) ms (Table 1, entry 3), we calculate for the intrinsic Eu-centered quantum yield (eq. 4, Table 1, entry 4), a value identical to that obtained for [Eu2(L3)(hfac)6], which implies that the gain in global quantum yields can be specifically assigned to the improved sensitization process operating in [Eu2(L4)(hfac)6] (eq. 1, Table 1, entry 7). Given the experimental ligand-centered fluorescence lifetimes of only 30–60 ps that are measured for the [Gd2(Lk)(hfac)6] complexes (Lk = L1–L4, Table S6), one can assume that the energy migration processes in [Eu2(Lk)(hfac)6] is well described by the exclusive contribution of the triplet state as shown in Fig. 1.[7,13] Considering that (i) the sum of the radiative and internal non-radiative conversion rate constants controlling the relaxation of the 1π* excited state in the free ligand are the same in the gadolinium complex [Gd2(L4)(hfac)6] and that (ii) because of the paramagnetic and heavy atom effects generated by Gd(III),[14] the introduction of the experimental characteristic 1π* lifetimes measured in L4 ( ) and in [Gd2(L4)(hfac)6] ( , Table S6) into eq. 6 gives (Table 1, entry 8) and (Table 1, entry 9), from which ηISC = 0.88(15) can be deduced with eq. 2 (Table 1, entry 10).[7]
Table 1.
Experimental global ( ) and intrinsic ( ) quantum yields, luminescence lifetimes (τEu) and calculated energy migration efficiencies (ηISC, ) and rate constants ( , kISC) for [Eu2(Lk)(hfac)6] in the solid state at 293 K (k = 2–4).[10]
| Compound | [Eu2(L2)(hfac)6] | [Eu2(L3)(hfac)6] | [Eu2(L4)(hfac)6] | |
|---|---|---|---|---|
| Eu-centered luminescence | ||||
| Itot/IMD | 17.5(3) | 18.2(3) | 17.2(2) | |
|
|
0.86(2) | 0.90(2) | 0.85(1) | |
| τEu/ms | 0.88(4) | 0.83(15) | 0.90(1) | |
|
|
0.76(4) | 0.75(1) | 0.77(1) | |
|
|
0.21(1) | 0.23(4) | 0.200(4) | |
| Global quantum yield and sensitization efficiency | ||||
|
|
0.092(3) | 0.206(7) | 0.26(1) | |
|
|
0.122(7) | 0.28(5) | 0.34(1) | |
| Energy migration and associated rate constants | ||||
|
|
10.0(6) | 9(2) | 30(4) | |
|
|
6.71(5) | 6.25(3) | 4.00(5) | |
| η ISC | 0.60(3) | 0.59(12) | 0.88(15) | |
|
|
0.20(2) | 0.47(14) | 0.39(6) | |
|
|
2.1(3) | 5.5(2.3) | 5.7(2.1) | |
|
|
8.1(5) | 6.3(8) | 9.1(2.5) | |
| Reference | [7] | [7] | This work | |
| (6) |
Finally, the energy transfer efficiency and the associated rate constant (eq. 3 with ) calculated for [Eu2(L4)(hfac)6] (Table 1, entries 11 and 12) indicates no noticeable improvement of these parameters on changing from the difluorinated (L3) to the perfluorinated (L4) spacer, despite the 500–1000 cm−1 energy blue shift of the ligand-centered 1π* (Fig. S5) and 3π* (Fig. S6) excited states. Our simple method for dissecting the sensitization mechanism[7] shows that the gain in the global quantum yield , for the complex [Eu2(L4)(hfac)6] as compared to [Eu2(L3)(hfac)6], indeed results from an optimization of the intersystem crossing process ηISC (Fig. 1).
Scrutiny of the various rate constants (Tables 1 and S7) reveals that the decrease of the radiative and internal conversion rate constant for the ligand-centered L(1π*) state along the series L2 > L3 > L4 acts to improve ηISC (eq. 2), but it is the remarkable increase of in [Ln2(L4)(hfac)6], which eventually controls the overall intersystem crossing efficiency.
The physical origin of this beneficial effect can be traced back to the golden-rule expression for radiationless transitions:[15]
| (7) |
where FCWDS is the Franck-Condon Weighted Density of States. It accounts for the density of vibrational states in the triplet and their vibrational overlap with the singlet vibrational state. A model that accounts for the thermal population of levels and uses a single quantum mode of frequency ω, is commonly associated with the Marcus-Levich-Jortner theory for electron transfer expressed in eq. 8:[16]
| (8) |
The spin-orbit coupling matrix element 〈1π* |HSO| 3π*〉 reaches a maximum for non-planar polyaromatic molecules containing heavy paramagnetic atoms in the molecular frame;[16,17] two conditions which are fulfilled by all [Eu2(Lk)(hfac)6] complexes described in this study. We however note that the deviation from the planarity, as measured by the interplanar phenyl-benzimidazole angles, increases along the series L2 (25.26(4)°) < L3 (54.1(1)°) and L4 (66.2(1)°) in line with kISC (eq. 7 and Table 1). The Franck-Condon weighted density of states (FCWDS) depends on the singlet-triplet energy splitting ΔE = E(1π*)−E(3π*) and on the reorganization energy λ, which corresponds to the energy difference between the triplet and the singlet state at its equilibrium geometry (eq. 8).[18] In the limit of parabolic surfaces, this energy parameter, along with ΔE, provides the energy gap for n = 0. The successive fluorination of the ligands along the series [Eu2(Lk)(hfac)6] (k = 2–4) is known to significantly affect the frontier orbitals, hence λ and ΔE and FCWDS in eq. 8.[7] While a quantitative understanding of the changes in λ requires sophisticated theoretical calculations of the vibrational coupling scheme controlling the Huang-Rhys factors (S),[15,16] eqs (7)–(8) predict that the increasing ligand-centered energy gap ΔE observed along the series L2 (3550 cm−1) ≈ L3 (3230 cm−1) < L4 (5200 cm−1) should lower kISC and η ISC. The apparent contradiction with our experimental results (Table 1, entries 8 and 10) can be resolved by including higher lying triplet states in the model; an approach used successfully for oligothiophenes[15] and helicenes.[15b] This counter-intuitive correlation was empirically noticed for other polyaromatic chromophores, and it was suggested as a ‘rule-of-thumb’ that a singlet-triplet gap of E(1π*)−E(3π*) ≥ 5000 cm−1 warrants quantitative intersystem crossing processes in Tb- and Eu-complexes.[19] Given that the singlet-triplet energy gap can be readily calculated by using DFT[7] or semi-empirical[8] methods, computations may be useful for identifying simple chemical and structural modifications that will enhance the quantum efficiency further.
In conclusion, the application of this simple method for analyzing the various contributions to EuIII luminescence sensitization shows that perfluorination of the remote phenyl spacer in the rigid single-stranded dumbbell-shaped [Eu2(L4)(hfac)6] oligomer optimizes both intersystem crossing efficiency and intrinsic EuIII quantum yield, thus maximizing the global quantum yields in these polyaromatic rigid complexes (red histograms in Fig. 1).
Supplementary Material
Footnotes
This work was supported through grants from the Swiss National Science Foundation, the National Institute of Health, via NIH grant R21-EB008257-01A1, the National Science Foundation (CHE-0718755), la Ligue contre le Cancer and the Institut National de la Santé et de la Recherche Médicale (INSERM). The work was carried out within the COST Actions D38 and CM1006.
Supporting Information for this article is available on the WWW under http://www.angewandte.org.
Contributor Information
Dr. Jean-François Lemonnier, Department of Inorganic, Analytical and Applied Chemistry, University of Geneva, 30 quai E. Ansermet, CH-1211 Geneva 4 (Switzerland)
Ms. Lucille Babel, Department of Inorganic, Analytical and Applied Chemistry, University of Geneva, 30 quai E. Ansermet, CH-1211 Geneva 4 (Switzerland)
Dr. Laure Guénée, Laboratory of Crystallography, University of Geneva, 24 quai E. Ansermet, CH-1211 Geneva 4 (Switzerland)
Dr. Prasun Mukherjee, Department of Chemistry, University of Pittsburgh, 219 Parkman Avenue, Pittsburgh, PA 15260, USA
Prof. Dr. David H. Waldeck, Email: dave@pitt.edu, Department of Chemistry, University of Pittsburgh, 219 Parkman Avenue, Pittsburgh, PA 15260, USA
Dr. Svetlana V. Eliseeva, Centre de Biophysique Moléculaire, CNRS UPR 4301, Rue Charles Sadron, F-45071 Orléans Cedex 2 (France)
Prof. Dr. Stéphane Petoud, Email: stephane.petoud@cnrs-orleans.fr, Centre de Biophysique Moléculaire, CNRS UPR 4301, Rue Charles Sadron, F-45071 Orléans Cedex 2 (France)
Prof. Dr. Claude Piguet, Email: claude.piguet@unige.ch, Department of Inorganic, Analytical and Applied Chemistry, University of Geneva, 30 quai E. Ansermet, CH-1211 Geneva 4 (Switzerland)
References and Notes
- 1.a) Kido J, Okamoto Y. Chem Rev. 2002;102:2357–2368. doi: 10.1021/cr010448y. [DOI] [PubMed] [Google Scholar]; b) Evans RC, Douglas P, Winscom CJ. Coord Chem Rev. 2006;250:2093–2126. [Google Scholar]; c) de Bettencourt-Dias A. Dalton Trans. 2007:2229–2241. doi: 10.1039/b702341c. [DOI] [PubMed] [Google Scholar]; d) Binnemans K. Chem Rev. 2009;109:4283–4374. doi: 10.1021/cr8003983. [DOI] [PubMed] [Google Scholar]; (e) Kerbellec N, Kustaryono D, Haquin V, Etienne M, Daiguebonne C, Guillou O. Inorg Chem. 2009;48:2837–2843. doi: 10.1021/ic801616y. [DOI] [PubMed] [Google Scholar]; f) Law GL, Wong KL, Tam HL, Cheah KW, Wong WT. Inorg Chem. 2009;48:10492–10494. doi: 10.1021/ic9018037. [DOI] [PubMed] [Google Scholar]; g) Zheng K, Zhang D, Zhao D, Liu N, Shi F, Qin W. Phys Chem Chem Phys. 2010;12:7620–7625. doi: 10.1039/b922230h. [DOI] [PubMed] [Google Scholar]; h) Katkova MA, Bochkarev MN. Dalton Trans. 2010;39:6599–6612. doi: 10.1039/c001152e. [DOI] [PubMed] [Google Scholar]; i) Eliseeva SV, Bünzli JCG. Chem Soc Rev. 2010;39:189–227. doi: 10.1039/b905604c. [DOI] [PubMed] [Google Scholar]; j) Farinola GM, Ragni R. Chem Soc Rev. 2011;40:3467–3482. doi: 10.1039/c0cs00204f. [DOI] [PubMed] [Google Scholar]; k) Cui Y, Yue Y, Qian G, Chen B. Chem Rev. 2012;112:1126–1162. doi: 10.1021/cr200101d. [DOI] [PubMed] [Google Scholar]; l) Liu Y, Pan M, Yang QY, Fu L, Li K, Wei SC, Su CY. Chem Mater. 2012;24:1954–1960. [Google Scholar]; m) Sava DF, Rohwer LES, Rodriguez MA, Nenoff TM. J Am Chem Soc. 2012;134:3983–3986. doi: 10.1021/ja211230p. [DOI] [PubMed] [Google Scholar]
- 2.a) Bünzli J-CG, Piguet CC. Chem Rev. 2002;102:1897–1928. doi: 10.1021/cr010299j. [DOI] [PubMed] [Google Scholar]; b) dos Santos CMG, Harte AJ, Quinn SJ, Gunnlaugsson T. Coord Chem Rev. 2008;252:2512–2527. [Google Scholar]; c) Swavey S, Swavey R. Coord Chem Rev. 2009;253:2627–2638. [Google Scholar]; d) Faulkner S, Natrajan LS, Perry WS, Sykes D. Dalton Trans. 2009:3890–3899. doi: 10.1039/b902006c. [DOI] [PubMed] [Google Scholar]; e) Vigato PA, Peruzzo V, Tamburini S. Coord Chem Rev. 2009;253:1099–1201. [Google Scholar]; f) Lincheneau C, Stomeo F, Comby S, Gunnlaugsson T. Aust J Chem. 2011;64:1315–1326. [Google Scholar]
- 3.a) Borkovec M, Hamacek J, Piguet C. Dalton Trans. 2004:4096–4105. doi: 10.1039/b413603a. [DOI] [PubMed] [Google Scholar]; b) Piguet C, Borkovec M, Hamacek J, Zeckert K. Coord Chem Rev. 2005;249:705–726. [Google Scholar]
- 4.Dalla Favera N, Hamacek J, Borkovec M, Jeannerat D, Ercolani G, Piguet C. Inorg Chem. 2007;46:9312–9322. doi: 10.1021/ic701308h. [DOI] [PubMed] [Google Scholar]
- 5.Chen XY, Yang X, Holliday BJ. J Am Chem Soc. 2008;130:1546–1547. doi: 10.1021/ja077626a. [DOI] [PubMed] [Google Scholar]
- 6.McKenzie BM, Wojtecki RJ, Burke KA, Zhang C, Jakli A, Mather PT, Rowan SJ. Chem Mater. 2011;23:3525–3533. [Google Scholar]
- 7.Lemonnier JF, Guénée L, Beuchat C, Wesolowski TA, Mukherjee P, Waldeck DH, Gogik KA, Petoud S, Piguet C. J Am Chem Soc. 2011;133:16219–16234. doi: 10.1021/ja206806t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freire RO, Albuquerque RQ, Junior SA, Rocha GB, Mesquita ME. Chem Phys Lett. 2005;405:123–126. [Google Scholar]
- 9.Yamaguchi Y, Matsubara Y, Ochi T, Wakamiya T, Yoshida ZI. J Am Chem Soc. 2008;130:13867–13869. doi: 10.1021/ja8040493. [DOI] [PubMed] [Google Scholar]
- 10.For the sake of clarity and conciseness in the discussions, the kinetic data collected for L1, which bears electron-donating methoxy groups, are not further considered in Fig. 1 and Table 1.
- 11.Global quantum yields up to 64% have been reported for polynuclear Eu(III) complexes, see Miyata K, Ohba T, Kobayashi A, Kato M, Nakanishi T, Fushimi K, Hasegawa Y. ChemPlusChem. 2012;77:277–280.
- 12. with AMD,0 = 14.65 s−1 for the magnetic dipolar Eu(5D0→7F1) transition and a refractive index n = 1.5. Aebischer A, Gumy F, Bünzli J-CG. Phys Chem Chem Phys. 2009;11:1346–1353. doi: 10.1039/b816131c. and references therein.
- 13.a) Sabbatini N, Guardigli M, Manet I. In: Handbook on the Physics and Chemistry of Rare Earths. Gschneidner KA Jr, Eyring L, editors. Vol. 23. Elsevier Science; Amsterdam: 1996. pp. 69–120. [Google Scholar]; b) Faulkner S, Pope SJ, Burton-Pye BP. Appl Spectrosc Rev. 2005;40:1–35. [Google Scholar]; c) Ward MD. Coord Chem Rev. 2010;254:2634–2642. [Google Scholar]
- 14.a) Tobita S, Arakawa M, Tanaka I. J Phys Chem. 1984;88:2697–2702. [Google Scholar]; b) Tobita S, Arakawa M, Tanaka I. J Phys Chem. 1985;89:5649–5654. [Google Scholar]
- 15.Beljonne D, Shuai Z, Pourtois G, Brédas JL. J Phys Chem A. 2001;105:3899–3907.and references therein. [16] Jortner J, Bixon M. Adv Chem Phys. 1999;106:35–202.Brédas JL, Beljonne D, Coropceanu V, Cornil J. Chem Rev. 2004;104:4971–5003. doi: 10.1021/cr040084k.Schmidt K, Brovelli S, Coropceanu V, Beljonne D, Cornil J, Bazzini C, Caronna T, Tubino R, Meinardi F, Shuai Z, Brédas JL. J Chem Phys A. 2007;111:10490–10499. doi: 10.1021/jp075248q.
- 17.Monguzzi A, Tubino R, Hoseinkhani S, Campione M, Meinardi F. Phys Chem Chem Phys. 2012;14:4322–4332. doi: 10.1039/c2cp23900k. [DOI] [PubMed] [Google Scholar]
- 18.For intersystem crossing, solvent effects are expected to be small and are not considered in λ. As a first approximation, λ= 1500–2000 cm−1 are realistic values for polyaromatic scaffolds.[15],[16]
- 19.Steemers FJ, Verboom W, Reinhoudt DN, van der Tol EB, Verhoeven JW. J Am Chem Soc. 1995;117:9408–9414. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.