Abstract
Intravasation, the active entry of primary tumor cells into the vasculature, remains the least studied step in the metastatic cascade. Protease-mediated escape and stromal invasion of tumor cells represent widely-accepted processes leading up to the intravasation step. However, molecular factors that contribute directly to tumor cell vascular penetration have not been identified. In this study, the in vivo role of the collagenolytic protease, MMP-1, in cancer cell intravasation and metastasis was analyzed by employing a highly-disseminating variant of human HEp3 epidermoid carcinoma, HEp3-hi/diss. Whereas naturally-acquired or experimentally-induced MMP-1 deficiency substantially suppressed HEp3-hi/diss intravasation, supplementation of recombinant MMP-1 to MMP-1-silenced primary tumors, restored their impaired vascular dissemination. Surprisingly, abrogation of MMP-1 production and activity did not affect significantly HEp3-hi/diss migration or matrix invasion, suggesting non-collagenolytic mechanisms underlying MMP-1-dependent cell intravasation. In support of such non-collagenolytic mechanisms, MMP-1 silencing in HEp3-hi/diss cells modulated the microarchitecture and integrity of the angiogenic vasculature in a novel microtumor model. Concomitantly, MMP-1 deficiency led to decreased levels of intratumoral vascular permeability, tumor cell intravasation and metastatic dissemination. Taking advantage of PAR1 deficiency of HEp3-hi/diss cells, we further demonstrate that endothelial PAR1 is a putative non-tumor-cell/non-matrix target, activation of which by carcinoma-produced MMP-1 regulates endothelial permeability and transendothelial migration. The inhibitory effects of specific PAR1 antagonists in live animals have also indicated that the mechanisms of MMP-1-dependent vascular permeability in tumors involve endothelial PAR1 activation. Together, our findings mechanistically underscore the contribution of a tumor MMP-1/endothelial PAR1 axis to actual intravasation events manifested by aggressive carcinoma cells.
Keywords: Matrix metalloproteinase-1, PAR1, metastasis, squamous cell carcinoma
Introduction
Deaths from cancer occur mainly because tumor cells metastasize from the primary tumor site to secondary vital tissues and organs likely by using the new angiogenic vasculature of the tumor as conduits. Metastasis, which is a complex multistep process, is still one of the major unsolved problems in cancer biology. Intravasation, the step in metastasis whereby escaping primary tumor cells enter the vasculature, is the least studied process in the entire metastatic cascade. Intravasation is experimentally understudied because there are very few if any in vivo models that accurately recapitulate the entry of tumor cells into the vasculature and also allow for quantification of the intravasation events. Furthermore, real-time imaging of escaping primary tumor cells and in vivo microscopic analysis of the structure and functionality of tumor-associated vasculature remain problematic for most laboratories. Because of these modeling and methodological issues, no clear signature molecules which directly contribute to the intravasation event have been identified. However, several mechanisms have been linked to the processes and events leading up to the intravasation step, such as primary tumor cell escape and migration and protease-mediated tumor cell invasion. In regard to the latter, proteolytic degradation of the basement membrane and stromal matrix by specific members of the matrix metalloproteinase (MMP) family of enzymes could provide functional molecular links to tumor cell escape, transendothelial migration and possibly to tumor cell-mediated active entry into the vasculature. The MMPs comprise a family of zinc-dependent endopeptidases that proteolytically modify the extracellular matrix in the primary tumors and metastatic sites as well as cleave distinct molecules on the surface of tumor and stromal cells (1-3).
A number of MMP genes have been linked to development and progression of squamous cell carcinomas (SCCs), which constitute 90% of head and neck cancers, the fifth leading cause of cancer-related deaths (4). The MMP genes that have been linked to SCC progression, include Mmp1, 2, 3, 7, 9, 10, 11, 12, and 13, most of which are overexpressed in the SCC tissue compared to normal mucosa (4, 5). However, a remarkable, more than 70-fold differential between normal oral mucosa and oral SCCs was demonstrated for the Mmp1 gene, which was found to be third best predictor among 25 signature genes (5), suggesting a critical role of MMP-1 protein in SCC progression Furthermore, while the expression of many MMPs in primary SCCs is associated with stromal or inflammatory cells rather than carcinoma cells, MMP-1 protein expression has been attributed to cancer cells at least in oral SCCs (5). In addition, MMP-1 has shown up as one of the signature genes for the metastatic phenotype for human breast cancers (6-8) and has also been validated as part of a set of 63 genes associated with the progression and metastasis of advanced cervical carcinomas (9). All these considerations clearly warrant mechanistic study of the functional contribution of tumor-produced MMP-1 to metastasis of SCCs.
To functionally analyze the role of MMP-1 in overall metastatic dissemination and specifically the intravasation step of SCCs, we employed the human epidermoid carcinoma cell line, HEp3, which is highly metastatic in both mice and chick embryos (10, 11). A distinctive feature of the chick embryo model, which is based on the grafting of human tumor cells on the chorioallantoic membrane (CAM), is that it uniquely allows for quantitative monitoring of intravasation into the CAM vasculature during spontaneous metastasis. With regard of intravasation, the HEp3 cells, when grafted onto the CAM at early passages, give rise to primary tumors and also disseminate to internal organs through the process of intravasation. These early passage-selected HEp3 cells have been referred to as the highly disseminating variant, HEp3-hi/diss. After 25 to 70 days in culture, the HEp3-hi/diss cells still maintain full tumorigenic capacity, but substantially reduce their metastatic potential and become low disseminating cells, denoted herein as HEp3-lo/diss. The lost metastatic potential of the HEp3-lo/diss cells can be recovered by in vivo passaging on the CAM, allowing for a continuous source of aggressive HEp3-hi/diss cells. These two HEp3 variants, presenting a distinct differential in their metastatic behavior, provide a suitable in vivo model system for identifying molecular factors that functionally contribute to the intravasation step of carcinoma cells.
By employing the low and high metastatic variants of HEp3 carcinoma, we have demonstrated in this study that tumor-produced MMP-1 functionally contributes to SCC vascular dissemination. However, in contrast to the established collagenous matrix remodeling and cell invasion-promoting functions of MMP-1, we have illuminated the mechanism whereby tumor-produced MMP-1 regulates development of an intravasation-sustaining intratumoral vasculature and assists carcinoma cell intravasation by inducing vascular permeability through the activation of endothelial PAR1. By placing MMP-1-producing carcinoma cells into an in vivo microenvironment where tumor cells confront intratumoral PAR1-positive angiogenic vasculature, our study for the first time presents evidence of how aggressive carcinoma cells not only induce tumor angiogenesis, but via secreted MMP-1 partially disrupt the endothelial barrier making it more amenable for the active entry of escaping cancer cells into the circulation.
Material and Methods
Cell lines and Maintenance
Human endothelial EA.hy926 cells (CRL-2922) were obtained from the American Type Culture Collection (ATCC, Manassas, MA). Prior to this study, the cells were verified for expression of human CD44, CD31, CD105 and Factor VIII-related antigen by immunocytochemistry. The cells were also tested and proved negative for mycoplasma contamination. Human head and neck carcinoma cell line Detroit 562 was obtained from ATCC and used within 6 month after purchasing. Unless specified, the cells were maintained as monolayer cultures in a humidified incubator at 5% CO2 and 37°C. Cell passaging was performed by brief exposure of cell monolayer to minimal volume of trypsin-EDTA solution followed by plating of detached cells in fresh DMEM-10% FCS.
Generation of HEp3 dissemination variants
Human epidermoid carcinoma HEp3-hi/diss cells were originally isolated from a neck lymph node metastasis of a patient diagnosed with head and neck carcinoma (12). The HEp3 cells were not deposited to any tissue or cell bank. The original HEp3 cell line was thoroughly characterized in animal models by Dr. L. Ossowski (13, 14) and became widely used in cancer research. In our laboratory, the parental HEp3 cells are maintained since 1986 (15) and have been repeatedly used in various cancer research studies (11, 16-19). The ability of HEp3 cells to form metastatic tumors after orthotopic implantations into the tongue and buccal mucosa of nude mice was verified in our laboratory in 2012.
The Hep3-hi/diss cells were generated from primary tumors developing from parental HEp3 cells grafted on the CAM of chick embryos as described (20). Briefly, CAM tumors were dissected from the CAM, minced and incubated with dispase (1.25 unit/mL) to generate single cell suspension. After washing, the cells were placed into Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% FBS (HyClone, Logan, UT), sodium pyruvate (Invitrogen) and 10 μg/ml gentamicin (Gibco) (D-10). Aliquots of primary cells were frozen at day 5 to provide a supply of HEp3 cells at low passage numbers. The cells, which were maintained in culture for 25 days or less, exhibited high levels of spontaneous dissemination and were designated as HEp3-hi/diss. The cells, which were cultured for more than 75 days, lost dissemination capacity and were designated as HEp3-lo/diss. The parental HEp3, HEp3-hi/diss and HEp3-lo/diss cells were tested for mycoplasma contamination and confirmed to be negative.
Intravasation and spontaneous metastasis model
Chick embryo spontaneous metastasis assays were performed as described (21). Briefly, 4×105 HEp3 cells were grafted on the top of the CAM. Where indicated, the developing tumors were treated topically on day 1 and 3 with 30-100 ng of recombinant MMP-1 (provided as a mixture of zymogen and activated species by Abcam, Cambridge, UK) or with MMP inhibitor GM6001 (Calbiochem-EMD Chemicals, Gibbstown, NJ). After 5 days, the primary tumors were excised, weighed and fixed in 10% Zn-formalin for histological examination. Portions of the distal CAM and the liver were examined by quantitative Alu-PCR to determine numbers of human tumor cells within chicken tissue background.
Experimental metastasis model
Embryos were inoculated with 5×104 tumor cells. At 2 hr (cell arrest) or 5 days (tissue colonization), the portions of the CAM and liver were analyzed by Alu-qPCR to determine numbers of human cells. CAM colonization was also examined by fluorescence microscopy as described (22). For this analysis, the cells were pre-labeled with 5 μM CellTracker Green, while the CAM vasculature was highlighted with Rhodamine-conjugated Lens culinaris agglutinin (LCA; Vector Labs, Burlingame, CA).
Intramesodermal microtumor model for tumor cell escape and stromal invasion
HEp3-hi/diss cells treated with control and MMP-1 siRNA constructs were labeled with 5 μM CellTracker Green and resuspended at 2×106/ml. Five to seven small boluses of tumor cells (3-5 μl) were injected directly into the CAM mesoderm of day 10 chick embryos incubated ex ovo as described (23). Five days after cell injections, embryos were inoculated with rhodamin-conjugated LCA to highlight the vasculature. Portions of the CAM with microtumors were imaged using a Carl Zeiss AxioImager microscope. Quantification of tumor cell escape and invasion was performed using ImageJ software (NIH, Bethesda, MD). The mean of invasion distances from the microtumor-CAM border was determined for individual microtumors. A total of 11 to 13 individual microtumors from 6 to 8 embryos were analyzed for each variable in 2-3 independent experiments.
Microtumor model and measurement of intratumoral vascular permeability
HEp3-hi/diss cells were treated with control or MMP-1 siRNA constructs, pre-labeled with CellTracker Green and mixed with neutralized type I collagen (Becton Dickinson, Bedford, MA) at 1×107 cells per ml and six 10-μl aliquots were placed separately on the top of the intact CAM of embryos developing for 9 or 10 days ex ovo to generate microtumors After 6-day incubation, Rhodamine-conjugated LCA was inoculated i.v. to highlight the CAM vasculature. The portions of the CAM containing microtumors were visualized in a fluorescent Olympus microscope. The images were analyzed for the overall structure of intratumoral vessels and their diameter.
To measure intratumoral vasculature permeability, CAM microtumors were generated from unlabeled HEp3-hi/diss cells. The developing microtumors were treated topically with 20 μL of vehicle (1%DMSO in PBS) or solutions of PAR1 antagonists, SCH79797 or RWJ56110 (Tocris Bioscience, UK) applied at 5 μM to 50 μM concentrations. The treatments were performed on days 1, 3 and 5 or days 2 and 4. On day 6, embryos were inoculated first with the permeable, TRITC-conjugated dextran with mol. wt. of 155 kDa. Non-permeable FITC-conjugated dextran with mol. wt. of 2,000 kDa was inoculated 45-60 min later, displacing the 155-kDa dextran from the vasculature. Images of microtumors and intratumoral vasculature were acquired in red and green fluorescence channels at the fixed exposure time, and individual microtumors were excised and lysed in 0.3 ml modified RIPA buffer. Levels of red fluorescence, reflecting amounts of tissue-retained low mol. wt. TRITC-conjugated dextran, were measured in lysates of individual tumors at 576 nm (excitation at 557 nm). Levels of green fluorescence, reflecting the volume of perfusable vasculature was measured at 516 nm (excitation at 492 nm).
Statistical analysis
Data processing and statistical analyses were performed using GraphPad Prism Software (GraphPad Software Inc., San Diego, CA). For the chick embryo assays, percent changes were calculated from the pooled differences determined as the ratios of numerical values for individual embryos over a mean of the control in the corresponding experiment. Statistical significance was evaluated using two-tailed unpaired Student’s t-test for P<0.05.
Quantitative real-time PCR (RT-qPCR), Alu-qPCR, MMP-1 silencing, cell maintenance, cell adhesion, chemotactic invasion in Transwells, endothelial permeability and transendothelial migration of HEp3-hi/diss cells, immunohistochemistry, western blotting, and gelatin zymography, are described in the Supplementary Information.
Results
Metastatic potential of HEp3 carcinoma variants correlates with MMP-1 expression
Comparative analysis of high and low disseminating HEp3 variants was performed in spontaneous versus experimental metastasis models to identify those temporal steps of the dissemination cascade where non-metastatic and metastatic carcinoma cells manifest most of their functional differential. In a spontaneous metastasis model, HEp3-hi/diss and HEp3-lo/diss cells gave rise to primary tumors with almost equal weight (approximately 166±6 mg), indicating similar tumorigenic potentials of HEp3 variants. However, only HEp3-hi/diss cells exhibited high levels of dissemination compared to their late-passage counterparts, reflected in a 25-fold differential in CAM intravasation and 10-fold differential in liver metastasis (P<0.0001) (Fig. 1A).
Figure 1. Characterization of HEp3-hi/diss and HEp3-lo/diss cell variants.
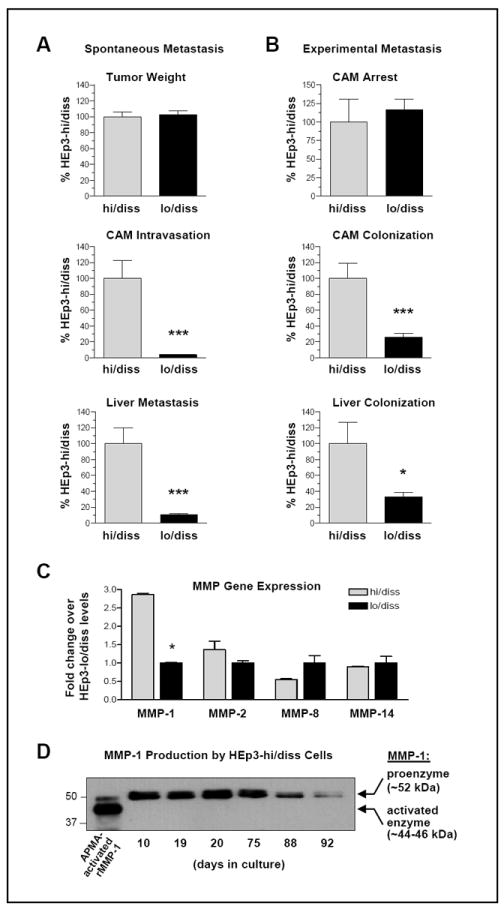
(A) Spontaneous dissemination model. HEp3-hi/diss and HEp3-lo/diss cells were grafted on the CAM of day 10 embryos (4×105 per embryo). Five days after cell grafting, primary tumors were excised and weighed (top panel). Portions of the distal CAM and liver were analyzed by quantitative Alu-qPCR for the levels of intravasation and metastasis (middle and bottom panels, respectively). Bars are means ± SEM of pooled data from two independent experiments employing 14 and 19 embryos per hi/diss and lo/diss variant, respectively. ***, P <0.001; two-tailed Student’s t test.
(B) Experimental metastasis assay. HEp3-hi/diss and HEp3-lo/diss cells were injected i.v. into the allantoic vein of day 12 embryos (5×104 cell per embryo). The CAM tissue was harvested 2 hours later (9 embryos for each cell variant) and analyzed by Alu-qPCR for the levels of vascular arrest (top panel). Levels of colonization in the CAM and liver were analyzed 5 days after cell injections (middle and bottom panels, respectively). Bars are means ± SEM of pooled data from two independent experiments employing 20 and 21 embryos per hi/diss and lo/diss variant, respectively. * and ***, P< 0.05 and < 0.001, respectively; two-tailed Student’s t test.
(C) MMP gene profiling of HEp3-hi/diss and HEp3-lo/diss cell variants. RT-qPCR was performed using 0.5 μg of total RNA in triplicate. The RNA levels for each MMP gene were normalized to GAPDH, producing ΔCt values (CtMMP –CtGAPDH). Difference in expression levels between two HEp3 dissemination variants was determined using the formula: 2-ΔΔCt, where ΔΔCt=(ΔCtlo/diss- ΔCthi/diss). Data are means ± SEM from two independent experiments where four relatively high expressed genes, namely, MMP-1, -2, -8 and -14, were analyzed in HEp3 dissemination variants. Data are expressed as the fold change expression of HEp3-hi/diss versus HEp3-lo/diss variant.
(D) Kinetic analysis of MMP-1 production by HEp3-hi/diss cells. HEp3-hi/diss cells were isolated from primary CAM tumors and cultured in vitro for the indicated time periods. Confluent cell layers were incubated in serum-free DMEM for 48 hr before collecting the conditioned medium. After conditioned medium was collected, the cells were trypsinized and counted. Aliquots of conditioned medium produced by equal number of cells were analyzed for MMP-1 by western blotting.
Whether overall metastatic potential of HEp3 variants depended on their ability to complete the late steps of cell dissemination was tested in an experimental metastasis model involving tumor cell inoculations directly into the blood circulation and thus bypassing the early events of metastasis, including primary tumor formation and tumor cell intravasation. The HEp3-lo/diss and HEp3-hi/diss cells did not differ in their 2-hr arrest in the CAM vasculature, indicating their similar ability to interact with the luminal surface of vascular endothelium. Furthermore, HEp3 variants differed only by 3 to 4 fold in their ability to colonize the CAM and liver tissue as measured at day 5 after cell inoculations (Fig. 1B). When compared with the 10-25 fold differentials in spontaneous metastasis, it appears that the dissemination disparity between the HEp3 variants lies primarily in their ability to complete early steps of the metastatic cascade, preceding and including the process of intravasation into the vasculature.
Since MMPs have been strongly implicated in progression of different cancer types, we thought to identify those distinct MMP(s), the expression of which would correlate with the dissemination potential of the HEp3 carcinoma variants. We first evaluated HEp3-hi/diss cells for overall expression of several candidate MMP genes, including Mmp1, 2, 3, 7, 8, 9, 10, 13, and 14. Whereas overall mRNA levels of Mmp3, 7, 9, 10, and 13 were relatively low (Ct values between 24 and 30; ΔCt values between 11 and 17), the expression levels of Mmp1, 2, 8 and 14 were relatively high (Ct values between 14 and 23; ΔCt values between 3 and 10). Comparative analysis demonstrated that levels of the highly expressed Mmp2 and Mmp14 were similar between the HEp3-lo/diss and HEp3-hi/diss variants, whereas the Mmp8 expression was higher in HEp3-lo/diss cells (Fig. 1C), the latter consistent with reports documenting reciprocal correlation between MMP-8 levels and tumor aggressiveness (24-26). In contrast, the levels of Mmp1 expression were consistently and significantly higher in HEp3-hi/diss cells (P<0.01), exceeding by 3-fold the levels observed in the HEp3-lo/diss variant (Fig. 1C) and suggesting that this collagenase may contribute to metastatic spread of aggressive HEp3 cells.
The relative MMP-1 production by HEp3-hi/diss and HEp3-lo/diss cells was analyzed by western blotting of conditioned medium (Fig. 1D). Comparison with the position of the residual zymogen and activated forms of recombinant proMMP-1 allows for a conclusion that MMP-1 is secreted by HEp3 cells mainly as a ~52-kDa proenzyme. Characteristically, the production of proMMP-1 gradually decreased with the time HEp3-hi/diss cells spent in culture, concomitantly with the loss of intravasation and metastatic capacity manifested in the resulting HEp3-lo/diss cells (>75 days in culture). Thus, the overexpressed MMP-1 gene and its protein product may determine in part the dissemination phenotype and metastatic fate of epidermoid carcinoma cells.
Functional significance of MMP-1 in HEp3-hi/diss intravasation and metastasis
To examine the specific contribution of MMP-1 to intravasation of carcinomas, HEp3-hi/diss cells were transfected with MMP-1 shRNA construct, generating a cell line that stably expressed 90% less MMP-1 protein as compared with control shRNA-treated cells (Fig. 2A, left). In addition, MMP-1 silencing was achieved by transient transfection of HEp3-hi/diss cells with siRNA constructs, resulting in a profound (95-99%) and relatively long-lasting downregulation of MMP-1 protein production (Fig. 2A, right). The lack of major off-target effects of MMP-1 silencing on other MMPs is indicated by similar levels of secreted MMP-2 in HEp3-hi/diss cells treated either with control or MMP-1 shRNA (Fig. 2A, zymograms). Furthermore, the unchanged levels of MMP-2 forms (namely, 68-kDa proenzyme, 64-kDa activation intermediate and 62-kDa fully activated enzyme) (Fig. 2A), are a valid indicator that the activity levels of MMP-14 also have not been affected by down-regulation of MMP-1 since MMP-14 is the only naturally expressed MMP responsible for MMP-2 processing and activation (27, 28).
Figure 2. Dependence of spontaneous dissemination of HEp-hi/diss cells on MMP-1 expression.
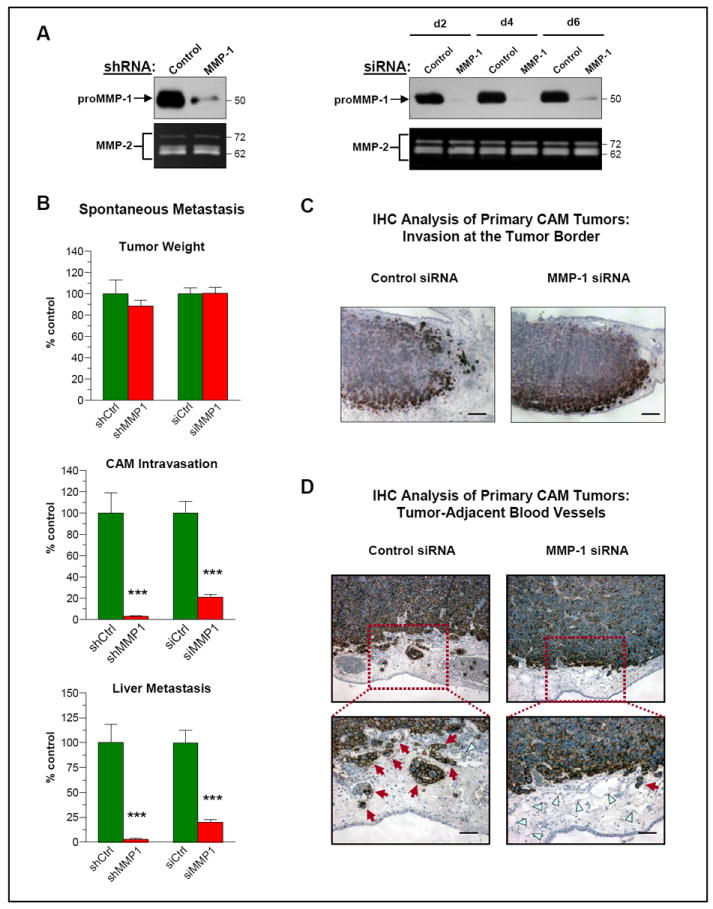
(A) Downregulation of MMP-1 by RNA interference. Left, MMP-1 was stably downregulated in HEp3-hi/diss cells by shRNA construct. Levels of proMMP-1 in 2-day conditioned medium from control and MMP-1 shRNA-transfected cells were analyzed by western blotting (upper panel). Right, HEp3-hi/diss cells were transiently transfected with control and MMP-1-specific siRNA constructs. Western blot analysis of conditioned medium for MMP-1 production was performed at indicated time points after cell transfections. Bottom panels, MMP-2 levels were analyzed by gelatin zymography to control for off-target effects of MMP-1 downregulation.
(B) Downregulation of MMP-1 inhibits spontaneous dissemination of HEp3-hi/diss cells. HEp3-hi/diss cells, treated with control (Ctrl) or MMP-1 specific shRNA and siRNA constructs, were inoculated onto the CAM of day 10 chick embryos and analyzed as described in Fig. 1A. Bars are means ± SEM of pooled data from three independent experiments employing 21 and 22 embryos respectively for control and MMP-1 shRNAs and 72 and 54 embryos respectively for control and MMP-1 siRNAs. ***, P <0.0001; two-tailed Student’s t test.
(C-D) IHC analysis of HEp3-hi/diss primary tumors. On day 5 after cell grafting, CAM tumors were excised and processed for staining with mAb 29-7 recognizing human CD44 (brown). C, While primary tumors have overall similar morphology, control HEp3-hi/diss tumors exhibit a more distorted tumor/CAM border (left panel) compared to tumors originating from MMP-1 siRNA-treated cells (right panel). Bar, 250 μm. D, IHC analysis of primary tumors for tumor-associated blood vessels (top panels; original magnification, 10x) indicated that control tumors harbor more intravascular tumor cells compared to MMP-1-deficient tumors (red arrows in bottom panels). In contrast, MMP-1-deficient tumors exhibit more blood vessels containing no detectable tumor cells (blue triangles). Bar, 50 μm.
Functional significance of MMP-1 expression for epidermoid carcinoma dissemination was evaluated by using the two types of MMP-1-silenced HEp3-hi/diss cells in both spontaneous and experimental metastasis models. MMP-1 silencing with either shRNA or siRNA constructs did not affect the tumorigenic capacity of HEp3-hi/diss cells (Fig. 2B, top). In agreement, control and MMP-1-silenced HEp3-hi/diss transfectants showed no significant difference in proliferation rates in 2D cultures and, more importantly, in 3D collagen gels (Suppl. Fig. S1). However, the deficiency in MMP-1 protein in HEp3-hi/diss cells caused either by shRNA or siRNA treatments was associated with a substantial, from 80% to 97% decrease in both CAM intravasation and liver metastasis (Fig. 2B, middle and bottom).
Immunohistological analysis demonstrated the HEp3-hi/diss tumors originating from control and MMP-1-silenced cells exhibited overall similar morphology. Although MMP-1-deficient tumors manifested a more intense staining for tumor cells at the tumor-stroma border, no significant differences were observed between invasive fronts of control and MMP-1 siRNA tumors (Fig. 2C and 2D). Interestingly, control CAM tumors, developing from the MMP-1-competent cells, displayed more blood vessels containing intravascular tumor cells as compared with primary tumors developed from HEp3-hi/diss cells treated with MMP-1 siRNA (Fig. 2D), further indicating a difference in tumor cell intravasation capacity. In agreement with Alu-qPCR data on intravasation, quantitative image analysis indeed indicated higher proportion of blood vessels harboring intravascular tumor cells in control, MMP-1-competent tumors (60.4±5.3%) as compared to tumors originating from MMP-1-deficient cells (31.8±2%; P=0.0008).
We next verified whether specific downregulation of MMP-1 would interfere with extravasation and colonization abilities of HEp3-hi/diss cells in the experimental metastasis model. MMP-1 deficiency due to shRNA or siRNA silencing did not affect either 2 hr vascular arrest or levels of CAM and liver colonization measured at 5 days after cell inoculations (Suppl. Fig. S2A). When analyzed by epifluorescence microscopy, siRNA-treated HEp3-hi/diss cells also showed no difference in the morphology of HEp3-hi/diss colonies in the CAM tissue (Suppl. Fig. S2B). These experimental metastasis findings strongly suggest that MMP-1 is functionally involved in early events in the metastatic cascade.
The contributory role of MMP-1 enzyme in HEp3-hi/diss intravasation and metastasis
To demonstrate functional contribution of MMP-1 to early steps of metastasis, we examined whether CAM intravasation and liver dissemination of HEp3-hi/diss cells, impaired by siRNA-induced MMP-1 protein deficiency, could be rescued by exogenously-supplied human recombinant MMP-1. Sustained MMP-1 deficiency in the HEp3-hi/diss cells was confirmed by western blot analysis (Fig. 3A), but while it did not affect significantly their ability to form primary tumors, it caused a considerable, from 60% to 70% decrease in the levels of CAM intravasation and liver metastasis as compared to the cells transfected with control siRNA (P<0.0001) (Fig. 3B). This significant impairment of MMP-1 siRNA-transfected cells in intravasation and liver metastasis was fully rescued by the supplementation of developing tumors with recombinant MMP-1, strengthening the notion that MMP-1 may be a functional contributor to dissemination of SCCs. These rescuing effects of exogenous MMP-1, which was supplied as a mixture of proenzyme and enzyme species (Figure 3A, first lane on the left), were completely abolished by a potent MMP inhibitor, GM6001 (P<0.05), indicating that the functional contribution of MMP-1 requires the proteolytic activity of the enzyme (Fig. 3B). Moreover, supplementation of MMP-1 protein to primary tumors also significantly increased intravasation levels of tumor cells escaping from HEp3-lo/diss primary tumors (Fig. 3C), supporting that MMP-1 deficiency was one of the contributing factors in low dissemination capacity of HEp3-lo/diss cells.
Figure 3. MMP-1 functionally contributes to HEp3-hi/diss intravasation and metastasis.
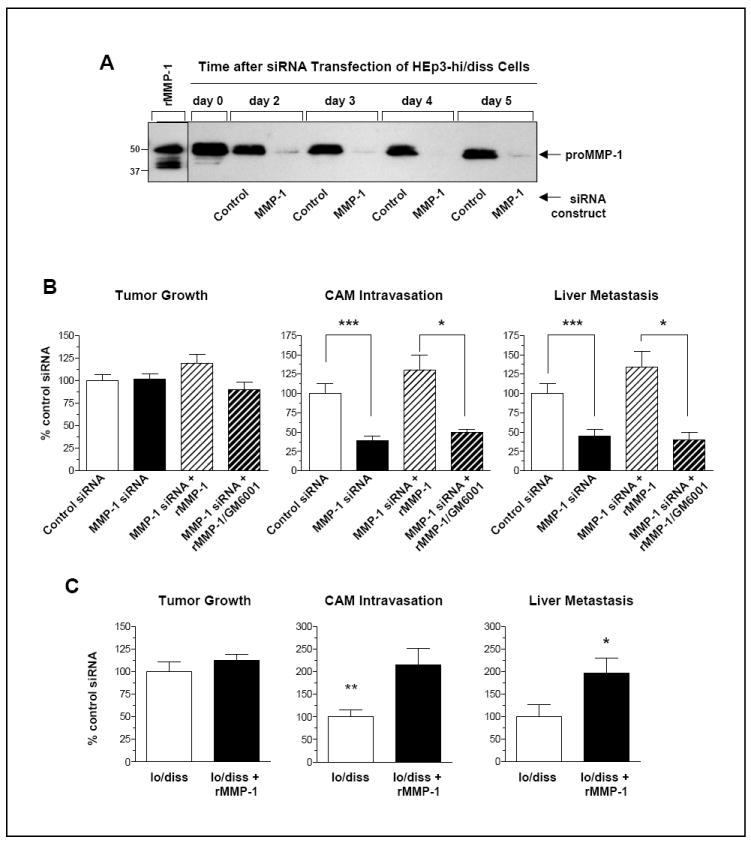
(A) Long-term downregulation of MMP-1 expression in HEp3-hi/diss cells by siRNA treatment. Western blot analysis for the levels of MMP-1 produced by HEp3-hi/diss cells treated with control siRNA or MMP-1-specific siRNA constructs. Serum-free conditioned medium was harvested at the indicated time points after siRNA treatments. Sample of recombinant MMP-1 (rMMP-1), containing both proenzyme and activated enzyme species, is shown on the left along with positions of mol. wt. markers.
(B) Rescue of HEp3-hi/diss intravasation and metastasis by exogenous MMP-1. HEp3-hi/diss cells treated with control and MMP-1-specific siRNAs, were grafted on the CAM of day 10 embryos. On day 1 and day 3 after cell grafting, a fraction of primary tumors developing from MMP-1-deficient cells were topically supplemented with 100 ng of human recombinant MMP-1, alone or pre-mixed with 0.5 mM GM6001. Tumor weights and levels of CAM intravasation and liver metastasis were determined on day 5 after cell grafting. Data from individual embryos were analyzed against the mean of control group (100%) in 3 independent experiments, each employing from 6 to 11 embryos per treatment. Bars are means ± SEM. * and ***, P <0.05 and <0.001, respectively; two-tailed Student’s t-test.
(C) Supplementation of exogenous MMP-1 induces intravasation and metastasis of HEp3-lo/diss variant. On day 1 and day 3 after grafting of HEp3-lo/diss cells on the CAM, the developing tumors were treated topically with 100 ng of human recombinant MMP-1. On day 5, primary tumors were excised and weighed (left graph) and intravasation to the distal CAM (middle graph) and metastasis to the liver (right graph) were quantified by Alu-qPCR. Data from individual embryos were analyzed against the mean of control group (100%) in 3 independent experiments, each employing from 7 to 8 embryos per treatment. Bars are means ± SEM. *, P<0.05; two-tailed Student’s t-test.
Functional contribution of MMP-1 to HEp3-hi/diss stromal invasion
We next analyzed whether MMP-1 produced by HEp3-hi/diss cells functionally contributes to the escape from primary tumors and stromal invasion since these processes are likely prerequisites of tumor cell intravasation. Putative involvement of MMP-1 in stromal invasion was examined in an in vivo stromal invasion model, in which human tumor cells are injected directly into the mesoderm of chick embryos developing ex ovo and allowed to form intramesodermal microtumors (22). Cell escape from these microtumors and invasion of the escaped cells within mesodermal stroma is then visualized by epifluorescence microscopy. In this assay, HEp3-hi/diss cells treated with MMP-1 siRNA constructs showed a diminished escape from microtumors and also demonstrated approximately 45% decrease in the migration distance compared to siRNA control (P<0.0001) (Fig. 4A). The most plausible mechanism for the observed effects would involve diminished collagen degradation mediated by MMP-1, a potent collagenase, which could modify the collagen-rich stroma of the CAM. However, this conclusion is not supported by a complete lack of any inhibitory effects of MMP-1 silencing on the overall adhesive, migratory and invasive capacities of HEp3-hi/diss cells tested in vitro (Suppl. Fig. S3). Therefore, although the inhibited invasion in vivo might indicate less escape and/or lower motility of MMP-1-depleted HEp3-hi/diss cells escaping from primary tumors, the relatively modest diminishment in stroma invasion is unlikely to account for the substantial, 80-97% reduction of tumor cell intravasation caused by stable (shRNA) or transient (siRNA) downregulation of MMP-1 (Figure 2B).
Figure 4. Effects of MMP-1 downregulation on stromal invasion of HEp3-hi/diss cells and microarchitecture of intratumoral vasculature.
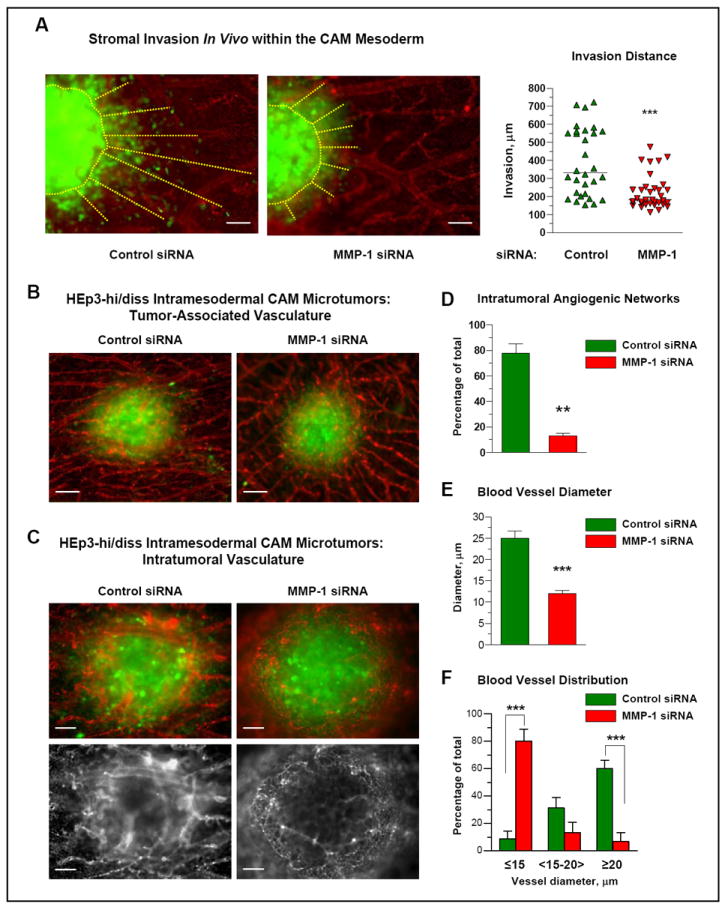
(A) MMP-1 deficiency decreases stromal invasion of HEp3-hi/diss cells in vivo. Small boluses of HEp3-hi/diss cells transfected with control or MMP-1-specific siRNA constructs were pre-labeled with CellTracker Green and injected directly into the CAM mesoderm of day 10 chick embryos. Five days after cell inoculations, the embryos were injected with Rhodamine-conjugated LCA to highlight the CAM vasculature (red) and portions of the CAM with microtumors (green) were excised and immediately imaged. Microtumor borders and invasion distances of green fluorescent tumor cells are indicated by yellow dotted lines. Bar, 100 μm. Quantification of microtumor invasion was performed in digitally captured images. Data from individual CAM microtumors are presented as scattergram. The mean of invasion distances (solid line) from the microtumor-CAM border was determined for 11-13 microtumors in 6-8 embryos in 3 independent experiments. ***, P<0.001; two-tailed Student’s t-test.
(B) MMP-1 silencing does not affect peripheral blood vessels converging onto primary tumors. Intramesodermal CAM microtumors were initiated from pre-labeled HEp3-hi/diss cells treated with control and MMP-1-specific siRNAs as described in (A). The images depict two representative microtumors and indicate no major differences in the appearance and the density of blood vessels coming towards microtumors. Bar, 100 μm.
(C) MMP-1 silencing affects the development and microarchitecture of intratumoral vasculature. Angiogenic vasculature (red) was imaged within primary tumors (green). Top, merged files recorded independently for green and red fluorescence. Bottom, signal for red fluorescence only is presented for more clear visualization of the vasculature. Bar, 50 μm.
(D-F) Image analysis of the intratumoral vasculature. Control and MMP-1-deficient microtumors were analyzed for the presence of well-developed intratumoral angiogenic networks and lumen diameter. Quantitative data from one representative experiment depict the percentage of highly-vascularized microtumors (D), the average blood vessel diameter (E) and the percentage of blood vessels with the indicated diameter range (F) determined from analysis of 7 control and 9 MMP-1-deficient primary tumors. ***, P<0.001; two-tailed Student’s t-test.
HEp3-derived MMP-1 regulates the microarchitecture of intratumoral blood vessels
To investigate whether the effects of MMP-1 on tumor cell invasion and intravasation in vivo may be indirect and independent from the putative collagenolytic activity of MMP-1 on matrix degradation, we examined the involvement of MMP-1 in the induction of tumor angiogenesis, an MMP-dependent process that ultimately provides aggressive cancer cells with conduits for vascular spreading. To address this possibility, we evaluated the extent of angiogenic network development within intramesodermal microtumors originating from HEp3-hi/diss cells treated with either control or MMP-1 siRNA constructs. Both tumor types exhibited comparable numbers of CAM blood vessels coalescing towards the border of intramesodermal microtumors (Fig. 4B). However, intratumoral angiogenic vessels networks appear distinctly different between control and MMP-1-silenced microtumors. Thus, more than 70% of control microtumors contained well-developed, perfusable intratumoral angiogenic blood vessels. In contrast, almost 90% of MMP-1-deficient HEp3-hi/diss microtumors failed to develop robust intratumoral angiogenic networks and therefore these intramesodermal microtumors presented with rather thin, poorly perfused blood capillaries (Fig. 4C and 4D). Thus, the average diameter of five most prominent intratumoral blood vessels was 25 μm within individual control microtumors, but reached only 12 μm in microtumors developing from MMP-1-silenced cells (Fig. 4E). More detailed distribution analysis indicated that almost 60% of blood vessels in control HEp3-hi/diss microtumors were of ≥20 μm in diameter, whereas an 80% majority of vessels in MMP-1-deficient microtumors had a lumen diameter less than 15 μm and only 7% of blood vessels were ≥20 μm in diameter (Fig. 4F). Together these findings suggested that under in vivo conditions, the MMP-1 produced by aggressive HEp3 cells is involved in the regulation of intratumoral angiogenesis and the overall structure of the tumor neovasculature.
MMP-1 regulates development of intratumoral angiogenic blood vessels that sustain HEp3-hi/diss cell intravasation
The above notion was further investigated in a modification of the spontaneous metastasis assay, which involves the establishment of topical CAM microtumors in ex ovo-incubated embryos by grafting 5-6 collagen droplets each containing 1×105 cells (Fig. 5A). Importantly, this newly-established assay fully reproduced dissemination differentials, manifested by control and MMP-1-deficient HEp3-hi/diss cells in the standard in ovo model, and demonstrated >90% reduction in intravasation and liver metastasis by silencing of MMP-1 (Fig. 5B). Notably, this inhibition of tumor dissemination occurred in the absence of significant cell invasion into the surrounding stroma (Fig. 5C), further suggesting the involvement of mechanisms other than MMP-1-mediated collagen-remodeling. To probe for this notion, we analyzed possible correlations between the levels of MMP-1-mediated tumor cell dissemination and the structure of intratumoral vessels visualized by epifluorescence microscopy of topical CAM tumors. Both control and MMP-1-silenced microtumors triggered robust vascular responses, attracting large and medium size CAM blood vessels, which under low magnification appear to converge onto topically developing microtumors (Fig. 5C). However, quantitative analysis of images acquired at higher magnification indicated that almost 90% of control microtumors had well-developed intratumoral angiogenic networks as opposed to less than 40% of siMMP-1 microtumors. Western blot analysis of CAM microtumors excised from individual embryos confirmed that MMP-1 siRNA silencing in HEp3-hi/diss cells was still sustained in microtumor tissue at the end of the 6 day-long experiment (Fig. 5D). This differential in MMP-1 expression closely correlated with the pattern and extent of intratumoral vasculature development manifested in a higher density of well-defined, lumen-containing angiogenic vessels within control microtumors (Fig. 5E, top panels). In contrast, MMP-1-deficient microtumors exhibited a substantially reduced density of lumen-containing vessels, most of which appeared poorly perfused and less interconnected (Fig. 5E, bottom panels). Furthermore, the angiogenic vessels within the two types of microtumors differed significantly in their mean lumen diameter (12.1±0.9 μm in control tumors versus 6.9±0.3 μm in MMP-1-deficient tumors; P<0.001) (Fig. 5F, bars on the left). In addition, diameter distribution analysis indicated that approximately 30% of blood vessels in control HEp3-hi/diss microtumors contained lumens with diameter of more than 15 μm, whereas microtumors developing from siMMP-1-treated cells were almost completely deprived of this size of blood vessels (28.3±4.2% versus 0.8±0.8%, P<0.0001) (Fig. 5F, bars on the right). Importantly, this significant differential in the size and overall quality of the intratumoral angiogenesis is highly comparable with the intravasation and metastasis differentials displayed by the two cell types as measured in the same model system (Fig. 5B).
Figure 5. Functional contribution of MMP-1 produced by HEp3-hi/diss cells to the development and microarchitecture of intratumoral vasculature.
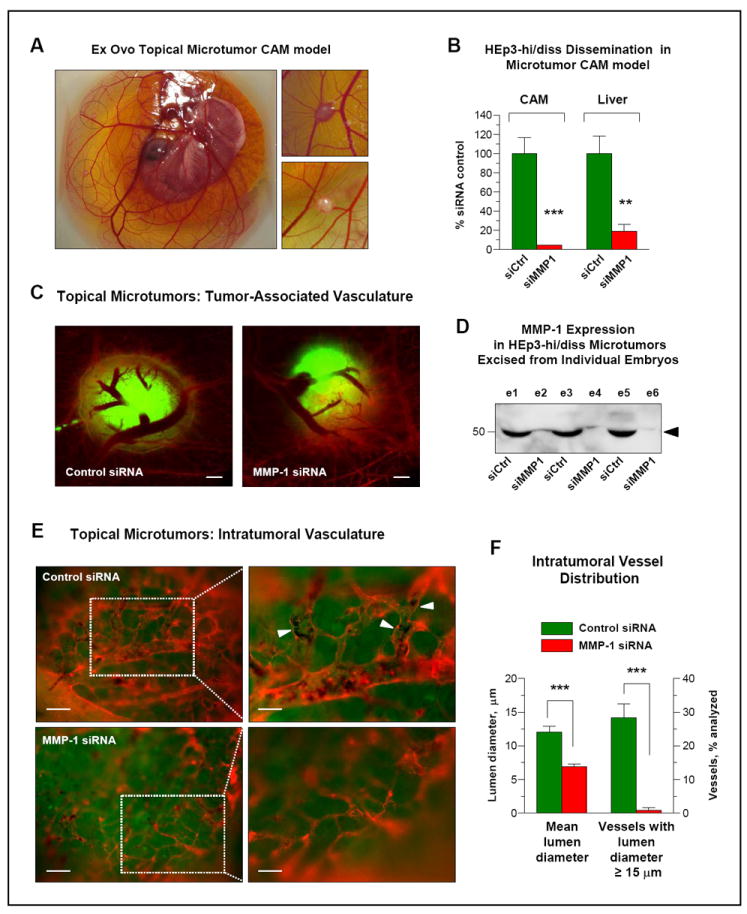
(A) Topical microtumor CAM model. HEp3-hi/diss cells were grafted on the top of intact CAM of day 10 embryos incubated ex ovo. Topical CAM tumors developed 6 days after cells grafting are circled with dotted line. Enlarged images of two microtumors are presented on the right.
(B) MMP-1 expression is critical for HEp3 intravasation and spontaneous metastasis. HEp3-hi/diss cells transfected with control (siCtrl) or MMP-1-specific (siMMP1) siRNAs were grafted on the CAM of the ex ovo embryos to generate topical microtumors. After 6 days of incubation, the levels of intravasation to the CAM and metastasis to the liver were quantified by Alu-qPCR. ** and ***, P<0.01 and <0.0001, respectively; two-tailed Student’s t-test.
(C) Silencing MMP-1 does not affect the number of CAM blood vessels coalescing towards HEp3-hi/diss topical microtumors. HEp3-hi/diss cells were treated with control and MMP-1 siRNAs, labeled with CellTracker Green, and grafted on the CAM. Six days after cell grafting, the chick embryo vasculature was highlighted in red with LCA and the CAM with topical microtumors visualized in a fluorescent microscope. There is no major difference in the vessels coming towards microtumors regardless of their MMP-1 expression status. Bar, 500 μm.
(D) Western blot analysis of microtumors for MMP-1 expression. Topical microtumors originating from HEp3-hi/diss cells treated with control siRNA (siCtrl; e1, e3, e5) and MMP-1 siRNA (siMMP1; e2, e4, e6) were excised from individual embryos, lysed and probed by Western blotting under reducing conditions for MMP-1 expression. The position of the 52-kDa MMP-1 band is indicated on the right.
(E) Downregulation of MMP-1 modifies microarchitecture of angiogenic blood vessel network within HEp3-hi/diss microtumors. Fluorescently labeled vasculature (red) was examined within topical microtumors (green) at an original magnification of 100x (left; bar, 25 μm) or 200x (right; bar, 50 μm). Note well-developed intratumoral vessels in control microtumors (top) as compared to underdeveloped, collapsed vessels in MMP-1-silenced tumors (bottom). Arrowheads point to blood vessels containing intravascular tumor cells surrounded by erythrocytes in control siRNA microtumors.
(F) Quantitative analysis of intratumoral blood vessels. Digital images of 15 control and 8 MMP-1-deficient microtumors were analyzed for the mean diameter of intratumoral blood vessels (left Y-axis) and percentage of intratumoral blood vessels with a lumen diameter of ≥15 μm (right Y-axis). Data are means ± SEM from one representative experiment employing 4 embryos bearing control tumors and 3 embryos bearing MMP-1-deficient tumors. ***, P<0.0001; two-tailed Student’s t-test.
Together, our angiogenesis analyses performed on live microtumors indicate that MMP-1 may facilitate tumor cell intravasation by inducing development of a dilated intratumoral angiogenic vasculature capable of sustaining tumor cell intravasation, whereas MMP1 silencing appears to severely compromise vascular microarchitecture by narrowing vessel diameters, which in turn, would prevent tumor cell intravasation.
MMP-1 regulates endothelial permeability and HEp3-hi/diss transendothelial migration
Tumor cell penetration into vessels via an MMP-1-mediated disruption of the endothelial barrier can constitute an additional mechanism whereby carcinoma cell-produced MMP-1 would facilitate intravasation independent of the collagenolytic activity of MMP-1 and its putative effects on stromal invasion. Since MMP-1-silencing did not affect the overall adhesive, migratory and invasive capacities of HEp3-hi/diss cells (Suppl. Fig. S3), we further explored the possibility that the ability of tumor cells to cross over an endothelial barrier may not depend on collagenolytic activity of MMP-1. This was investigated in a Transwell assay, which combines measurement of endothelium permeability by Evans blue exudation with coordinated quantification of tumor cell transendothelial migration in an environment free of interstitial collagen (Fig. 6). Control, MMP-1 competent HEp3-hi/diss cells, but not the MMP-1-silenced cells (Fig. 6A), were able to induce substantial Evans blue diffusion (Fig. 6D), strongly implicating carcinoma cell-produced MMP-1 in the disruption of endothelium integrity. This notion was further affirmed when plasmin-activated recombinant MMP-1 (Fig. 6B) was able to rescue dampened levels of endothelial permeability induced in the presence of MMP-1-silenced HEp3-hi/diss cells (Fig. 6D, left). Furthermore, control HEp3-hi/diss cells exhibited approximately 3-4-fold higher rates of transendothelial migration as compared with their MMP-1-silenced counterparts, thereby establishing a strong positive link between MMP-1 production by carcinoma cells and their ability to cross endothelial barriers. This link is further re-enforced by more than 2-fold increase of transendothelial migration of MMP-1-silenced cells by addition of activated exogenous MMP-1 (Fig. 6D, right).
Figure 6. Tumor MMP-1 functionally regulates the permeability of endothelial cell barriers and contributes to HEp3-hi/diss transendothelial migration.
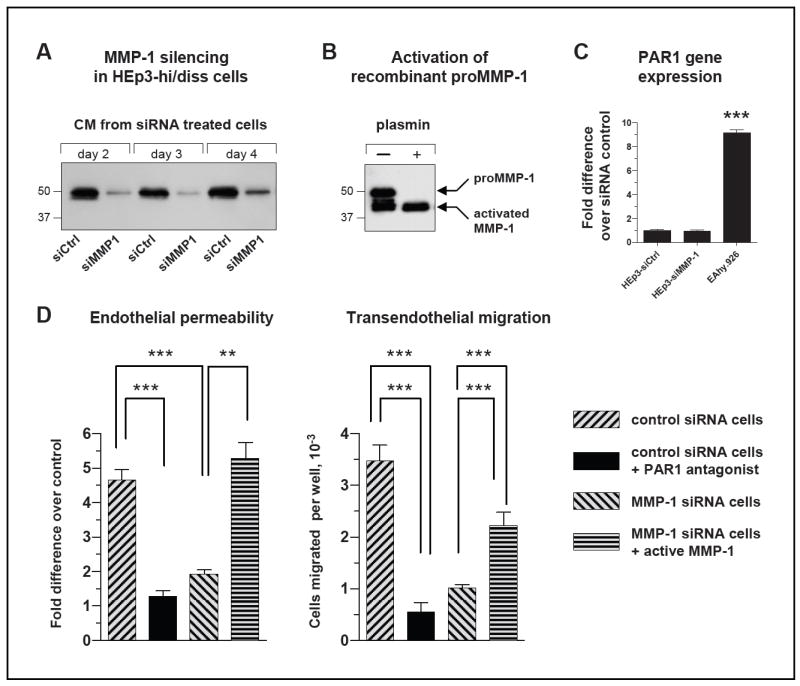
(A) Analysis of MMP-1 production by HEp3-hi/diss cells. Western blot analysis of MMP-1 was performed on samples of serum-free conditioned medium collected at the indicated time points from HEp3-hi/diss cells transfected with control siRNA or MMP-1 siRNA. Position of mol. wt. markers is indicated on the left in kDa. One day after treatment with control siRNA and MMP-1 siRNA constructs, HEp3-hi/diss cells were pre-labeled with CellTracker Green and plated into Transwells containing endothelial cell layers resistant to Evans blue diffusion for experiments described in (D).
(B) Activation status of recombinant MMP-1 used in rescue experiments. Recombinant MMP-1, represented by both the proenzyme and enzyme species, was further activated by 50 nM plasmin for 2 hr at 37°C and analyzed by SDS-PAGE under reducing conditions. Note the full conversion of the ~52-kDa proenzyme into ~44-46-kDa enzyme species. Position of mol. wt. markers is indicated on the left in kDa. Position of proMMP-1 and activated MMP-1 is indicated on the right.
(C) Gene expression analysis of PAR1 in HEp3-hi/diss carcinoma cells and endothelial cells. PAR1 expression was analyzed by RT-qPCR using human specific PAR1 primers and cDNA generated from the control siRNA-treated (siCtrl) and MMP-1 siRNA-treated (siMMP-1) HEp3-hi/diss cells and EA.hy926 cells. The relative levels of PAR1 gene expression were normalized to the GAPDH levels (ΔCt) and then ΔΔCT values for each cell line and condition were calculated according to the 2-(ΔΔCT) formula. The data are expressed as fold difference over control siRNA HEp3-hi/diss cells. Bars are means±SEM; ***, P< 0.001.
(D) Left, MMP-1 produced by HEp3-hi/diss cells functionally regulates endothelial layer permeability. The inserts, containing the endothelial layers resistant to dye penetration, were overlaid with HEp3-hi/diss cells treated with control or MMP-1 siRNA as illustrated in (A) and labeled with CellTracker Green. Recombinant MMP-1 was activated as illustrated in (B) and added at 3 nM to some of the Transwells containing MMP1-siRNA-treated HEp3-hi/diss cells. After 24-hr incubation, the PAR1 antagonist SCH79797 was added at 5 μM to Transwells seeded with the control siRNA-treated HEp3-hi/diss cells, followed by the addition of Evans blue at 48 hr to measure endothelial permeability. Pooled data are from 3 independent experiments employing from 3 to 6 Transwells for each experimental condition. The data are presented as fold difference in fluorescence intensity compared to no treatment control (1.0) and are means±SEM. Right, Tumor MMP-1 and endothelial PAR1 functionally contribute to HEp3-hi/diss transendothelial migration. After Evans blue permeability test, tumor cells were collected from the bottom chamber of Transwells to determine the efficiency of transendothelial migration. Relative numbers of the HEp3-hi/diss cells that had transmigrated into the lower chambers were determined by flow cytometry analysis of green fluorescent cells. Numbers of transmigrated cells per well are presented as means±SEM. Statistical significance was evaluated using two-tailed unpaired Student’s t-test. *, ** and ***, P<0.05, <0.005 and <0.0001, respectively.
To verify whether carcinoma-produced MMP-1 disrupted endothelium integrity through processes involving PAR1 activation (29), we used a specific PAR1 antagonist, SCH79797, which was added at 5 μM into Transwells. The PAR1 antagonist significantly and concomitantly diminished the ability of MMP-1-competent HEp3-hi/diss cells to induce endothelial permeability and cross over the transendothelial barrier (Fig. 6D, black bars). Correspondingly, these two diminished capacities of the MMP-1-deficient cells were rescued by the addition of exogenous active MMP-1 (Fig. 6D, horizontally-striped bars). Since gene expression of PAR1 in the targeted endothelial cells is 10-fold higher than in HEp3-hi/diss cells and has not been affected by siRNA downregulation of MMP-1 (Fig. 6C), these in vitro findings suggest that a tumor MMP-1/endothelial PAR1 axis is likely involved in altering vascular integrity and rendering epidermoid carcinoma cells with the ability to efficiently penetrate through endothelial barriers.
Tumor MMP-1 and endothelial PAR1 functionally regulate the permeability of intratumoral vasculature in vivo
To validate that MMP-1 functionally contributes to the development of an intratumoral vasculature manifesting high levels of endothelial permeability, we compared in vivo the exudation levels of permeable dextran within the topical HEp3-hi/diss microtumors. Thus, tumor-bearing embryos were first, inoculated with TRITC-labeled dextran (155 kDa) and then, after one hour incubation, perfused with non-permeable FITC-conjugated dextran (2,000 kDa). Microtumors were evaluated in a fluorescent microscope for the levels of low mol. wt. dextran exudation (red fluorescence) and perfusion of intratumoral vasculature (green fluorescence). The leakage of permeable dextran and the volume of perfusable vasculature in individual microtumors were then quantified fluorometrically.
No exudation of low mol. wt. dextran was observed in the CAM tissue distal to the tumors (Fig. 7A, left). However, the permeable dextran was detected at significant levels within control MMP-1-competent microtumors as evidenced by diffuse red fluorescence (Fig. 7A, middle). In contrast, the intensity of red fluorescence was significantly reduced by MMP-1 silencing, (Fig. 7A, right), which could be appreciated more readily in a monochromatic tone (Fig. 7A, lower panels). Permeable dextran exudation was quantified in the lysates of individual microtumors either relative to the volume of perfusable intratumoral vasculature or as an independent parameter. This analyses confirmed that specific vascular permeability in MMP-1-deficient tumors was diminished 2.3-fold (P<0.05) compared to control microtumors (Fig. 7B, top). Furthermore, the comparison of absolute levels of dextran exudation independent of the volume of perfusable vasculature in the individual tumors indicated approximately a 10-fold differential in the overall vascular permeability between control and MMP-1-silenced microtumors (Fig. 7B, bottom).
Figure 7. Tumor MMP-1 functionally regulates the permeability of intratumoral vasculature via PAR1-mediated signaling.
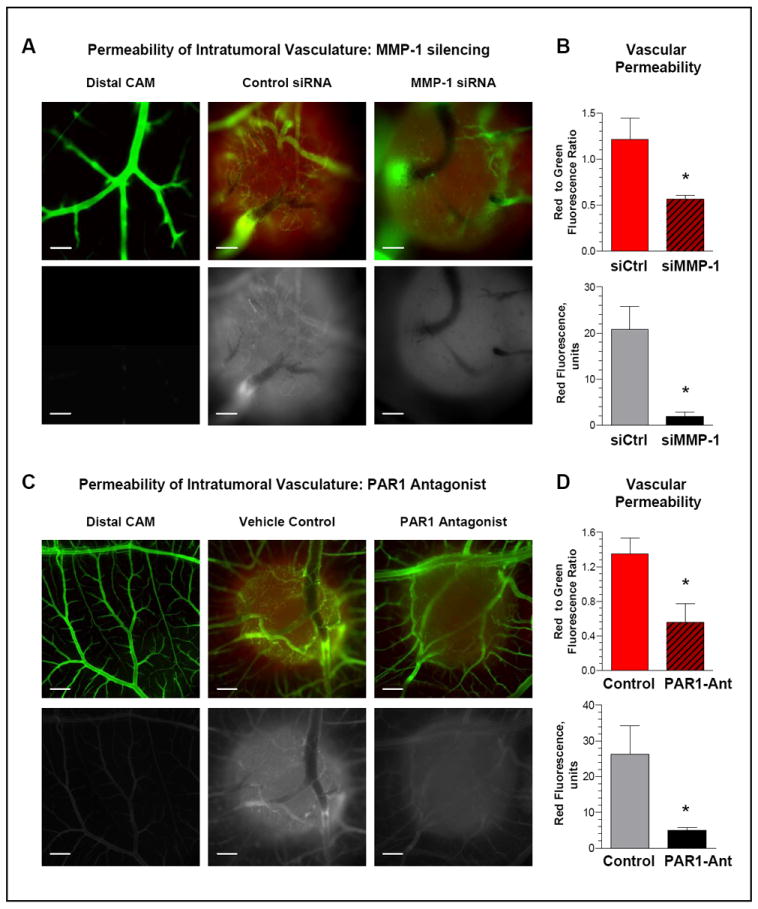
(A) Permeability of intratumoral vasculature is regulated by tumor-produced MMP1. Topical CAM microtumors were initiated from non-labeled HEp3-hi/diss cells. Six days after cell grafting, the embryos were injected i.v. with the permeable low mol. wt. TRITC-dextran. After 1-hr incubation, the embryos were inoculated with the non-permeable high mol. wt. FITC-dextran. The portions of the CAM with and without microtumors were visualized in fluorescent microscope and images were acquired at an original 10x magnification. Top panels, green and red fluorescence signals are merged. Bottom panels, red fluorescence is depicted monochromatically in white in order to highlight differential exudation of low mol. wt. dextran. Bar, 200 μm.
(B) Quantitative analysis of intratumoral permeability. Following imaging, individual microtumors were lysed and levels of red and green fluorescence were measured at 557 and 492 nm, respectively. Top graph, Permeability of intratumoral vasculature in individual tumors is presented as ratio of dextran exudation (red fluorescence) to total volume of perfusable vasculature (green fluorescence). Bottom graph, levels of dextran exudation presented as red fluorescence units. Data are means±SEM; *, P<0.05.
(C) Permeability of intratumoral vasculature is regulated by PAR1-mediated signaling. Developing HEp3-hi/diss microtumors were treated on day 2 and 4 with the PAR1 antagonist SC79797 (PAR1-Ant) or vehicle control. Dextran permeability in the distal CAM and microtumors was analyzed as described in (A). Original magnification, 4x. Bar, 500 μm.
(D) Quantitative analysis of intratumoral permeability. CAM microtumors treated with PAR1 antagonist SC79797 (PAR1-Ant) or vehicle (Control) were dissected and lysed. Vascular permeability was measured as described in (B). Data are means±SEM; *, P<0.05.
The PAR1 antagonists, SCH79797 and RWJ56110, previously shown to target endothelial PAR1 in chick embryos (30), were employed to substantiate in vivo the link between tumor-produced MMP-1 and PAR1-mediated signaling within intratumoral vasculature. The HEp3-hi/diss cells were grafted on the CAM of the ex vivo chick embryos and the developing microtumors were treated with either of PAR1 antagonists or vehicle. Similar to MMP-1 silencing, SC79797 caused a significant reduction in the levels of permeable dextran exudation compared to the vehicle-control tumors as depicted visually or quantified fluorometrically (Fig. 7C and 7D). When related to perfusable vascular volume, specific vascular permeability in SC79797 -treated tumors was diminished 2.4-fold (P<0.05), whereas the overall levels of dextran exudation indicated a more that 5-fold decrease caused by this specific inhibitor of PAR1 signaling (P<0.005). Comparable results were obtained for the PAR1 antagonist RWJ56110 (Suppl. Fig. S4), further strengthening the observed inhibitory effects of SCH79797. In addition to significant diminishment of vascular permeability, the treatment with SCH79797 also reduced the levels of HEp3-hi/diss CAM intravasation (Suppl. Fig. S5). Together, these findings are consistent with the notion that tumor-produced MMP-1 functionally regulates endothelial integrity in the intratumoral vasculature via a PAR1 signaling axis.
To corroborate our findings generated with HEp3-hi/diss cells, we have screened a number of human head and neck carcinomas, including FaDu, Detroit 562, SCC-9, SCC-15, SCC-25 and A-253, obtained from the ATCC. From six tested cell lines, we chose Detroit 562 carcinoma since these cells produce MMP-1, form primary CAM tumors, and intravasate into the CAM vasculature. To examine the effects of MMP-1 on vascular integrity and cell intravasation of Detroit 562 cells, we downregulated MMP-1 by RNA interference and employed the control and MMP-1-depleted cells in our CAM microtumor assay (Suppl. Fig. S6). Complementing our major findings in HEp3-hi/diss cells, downregulation of MMP-1 by siRNA in Detroit 562 cells also resulted in a concomitant decrease of vascular permeability and intravasation, thereby corroborating our notion that carcinoma-produced MMP-1 is involved in the regulation of vascular integrity and tumor cell dissemination.
Discussion
MMP-1 is one of the most up-regulated proteinases in a variety of carcinomas (31-33), but the precise role of this interstitial collagenase in carcinoma progression and metastasis remains unclear. Thus, although MMP-1 efficiently cleaves native triple helical collagen in vitro, its contribution to matrix degradation and stromal invasion in vivo has been overshadowed by the well documented role of another collagenase, namely MT1-MMP (MMP-14) (34, 35). In the present study we have demonstrated that MMP-1 expression correlated positively with the overall metastatic ability of HEp3-hi/diss cells, a highly metastatic variant of the human head and neck SCC, HEp3. In our search for MMPs that could in part be responsible for the substantial dissemination differential between HEp3 variants, we profiled the expression of several MMP genes and identified Mmp1 as the most differentially overexpressed in HEp3-hi/diss cells compared to HEp3-lo/diss cells. While expression of the Mmp1 gene has been found to be the third best oral SCC tumor predictor (5), we have demonstrated for the first time the direct involvement of MMP-1 enzyme in SCC metastasis. Thus, the ability of HEp3-hi/diss cells to complete intravasation and metastatic dissemination from the primary tumor to secondary organs depended on MMP-1 expression, both at gene and protein levels, strongly suggesting that MMP-1 could be a critical contributor to vascular intravasation of carcinoma cells. Since the intravasation step is the least studied step in the entire metastatic cascade, the identification of a contributing molecule in a live animal model is a significant finding.
The functional contribution of the MMP-1 protein to spontaneous dissemination of HEp3-hi/diss cells was confirmed by downregulation of MMP-1 by RNA interference. Importantly, the inhibitory effects of MMP-1 deficiency on metastatic dissemination of MMP-1-silenced cells were reversed by recombinant MMP-1 exogenously supplemented to developing HEp3-hi/diss primary tumors. Furthermore, exogenously-supplemented purified MMP-1 substantially increased the intrinsically low disseminating potential of HEp3-lo/diss cells. Together, our findings validate MMP-1 as a critical proteinase that functionally regulates in vivo the intravasation process of epidermoid carcinoma cells.
Despite the fact that specific silencing of MMP-1 in HEp3-hi/diss cells by two RNAi approaches substantially, up to 97% reduced intravasation and metastatic dissemination, stromal invasion in vivo was reduced only partially. Moreover, when the MMP-1-silenced HEp3-hi/diss cells and their control counterparts were examined for ability to invade across native collagen and basement membrane barriers, no significant reduction in invasion was observed for the MMP-1-silenced cells. These results are in apparent discrepancy with recently published data on the proteolytic role of murine MMP-1a (homologue of human MMP-1) in collagen and Matrigel invasion of murine lung cancer and melanoma cells (36), but are in agreement with the lack of MMP-1-mediated effects on matrix invasion of human tumor cells demonstrated previously by the Weiss laboratory (34, 35, 37). Our findings suggested that other functional catalytic properties of MMP-1, aside from the putative collagen degradation and extracellular matrix remodeling normally associated with an interstitial collagenase, might be involved in the indicated contribution of MMP-1 to vascular intravasation.
Immunohistochemical analysis of primary tumors showed a substantial diminishment in the number of intravascular HEp3-hi/diss cells within tumor-associated blood vessels when carcinoma cell MMP-1 was specifically down-regulated, suggesting that MMP-1 activity might be involved directly in the actual blood vessel entry process. This notion, along with the lack of substantial effects of MMP-1 silencing on tumor growth and stromal invasion in the in vivo settings, prompted us to examine the mechanistic involvement of MMP-1 in modulating interactions between tumor cells and intratumoral vasculature. By utilizing our new intramesodermal microtumor model, we found that MMP-1-silenced HEp3-hi/diss microtumors had a thin and poorly perfused blood vessel network. More detailed evaluation by epifluorescent microscopy demonstrated a dramatic difference in the microarchitecture of intratumoral vessels between control, MMP-1-competent microtumors and MMP-1-silenced HEp3-hi/diss microtumors. Thus, MMP-1 downregulation resulted in development of weak and immature tumor vascular networks represented mainly by vessels of 7-12 μm in diameter, i.e. with a lumen size that apparently would not be amenable for efficient intravasation of the larger carcinoma cells. Importantly, the presence of an altered vascular structure in MMP-1-deficient tumors closely coordinated with the reduced rates and numbers of intravascular tumor cells and the substantially diminished levels of tumor cell intravasation and metastasis. These results further substantiate the functional significance of tumor-derived MMP-1 in regulation of intratumoral angiogenesis, vascular structure and integrity, and ultimately, the tumor cell capacity for vascular penetration. Overall, our findings demonstrate that development of an intravasation-supporting intratumoral vasculature depends on specific mechanisms involving tumor-produced MMP-1, likely independent of its collagenolytic activity. That MMP-1 can be a critical contributing factor to the ability of carcinoma cells to regulate the development of tumor vasculature sustaining intravasation and metastasis, was further corroborated by the inhibitory effects of MMP-1 downregulation in another human SCC, Detroit 562.
Having observed MMP-1-dependent changes in vascular structure in our in vivo metastatic models, we investigated the effects of MMP-1-modulation on endothelial barrier integrity, namely on endothelial permeability during tumor cell transendothelial migration. Control MMP-1-competent and MMP-1-silenced HEp3-hi/diss cells were compared in our dual Transwell assay that uniquely combines quantification of both tumor cell-induced permeability of endothelial barriers and tumor cell transendothelial migration. We have demonstrated for the first time that tumor-produced MMP-1 can regulate the permeability of the endothelial barrier and make it more penetrable for transmigrating tumor cells. It has previously been shown in a non-tumor setting that exogenously-added recombinant MMP-1 enzyme can induce vascular permeability and endothelial barrier disruption in mice undergoing endotoxin-induced sepsis (29). By using the EA.hy926 endothelial cell line in vitro, the authors delineated a putative MMP-1-involving mechanism and demonstrated that endotoxin-mediated endothelial barrier disruption was mediated by overall PAR1 activation via an induction of Rho-GTP activity (29). A study by Goerge et al. also reported that the PAR1 pathway can be activated by conditioned medium containing MMP-1 secreted by tumor cells (38). Furthermore, in a number of recent clinical studies MMP-1 and PAR1 co-expression or co-localization in tumor tissues positively correlated with poor prognosis or unfavorable outcome for three different types of human carcinomas (39-41).
The findings of our study unify MMP-1-induced PAR1 activation demonstrated selectively in either tumor cells (42-44) or endothelial cells (29, 45) and also complements PAR1 activation induced in immortalized endothelial cells by conditioned medium harvested from MMP-1-producing tumor cells (38). By direct introduction of MMP-1-producing, but, importantly, PAR1-deficient HEp3-hi/diss cells, to PAR1-positive endothelial cells and measuring endothelial barrier permeability in the presence or absence of a specific PAR1 antagonist, our study for the first time provides direct evidence that the MMP-1/PAR1 axis indeed plays a functional role during tumor cell-endothelial cell interactions in vitro. More importantly, our in vivo studies also indicate that HEp3-hi/diss-produced MMP-1 might mediate the complex effects on intratumoral vascular microarchitecture and permeability via endothelial PAR1.
There are several suggested mechanisms by which PAR1-activation might be involved in increasing vascular permeability. Activation of PAR1 on vascular endothelial cells induces their activation and promotes exocytosis of Weibel-Palade bodies containing the chemokine IL-8 (38). In turn, the released IL-8, via binding to the receptor CXCR2 expressed on tumor cells, can contribute to the disruption of the endothelial barrier by enhancing tumor cell force generation and cytoskeletal remodeling dynamics in tumor cells directly confronting the endothelium (46). Additionally, cleaved and activated PAR1 is a strong activator of G12/13–driven Rho-GTPases, which, in turn, induce actin-dependent contraction of endothelial cells, resulting in a persistently increased endothelial permeability (47, 48). Since platelet MMP-1 can cleave PAR1 at a distinct site that strongly activates Rho-GTP pathways (49), it is plausible that MMP-1 produced by tumor cells can activate PAR1 on confronting vascular endothelial cells, leading to the development of an intravasation-sustaining vasculature that facilitates active entry of the aggressive carcinoma cells that produced the MMP-1.
It remains unknown whether or not the overall process of cancer cell intravasation is directly linked to PAR1-modulated endothelial permeability by tumor-produced MMP-1, but our study suggests that a tumor MMP-1/endothelial PAR1 signaling axis could be a valid molecular target to functionally compromise the intratumoral vasculature during anti-metastatic therapy of patients with aggressive epidermoid carcinomas. This contention could be especially important in view that it might not be the surrounding vasculature adjacent to the primary tumor, but rather the intratumoral vasculature that provides major conduits for primary tumor dissemination.
Supplementary Material
Acknowledgments
The authors wish to thank Chenxing Li for her excellent technical assistance.
Funding
This work was supported by postdoctoral fellowships from the Lundbeck and Willum Kann Rasmussen Foundations (A.J-J.); NIH grants R01CA105412 and R01CA129484 (J.P.Q.); a postdoctoral research fellowship from Nachwuchsförderungskredit/Stiefel-Zangger Foundation, University of Zurich (I.R.), and Human Diversity and Re-Entry Award from NIH/NCI (E.Z.).
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–74. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 2.Fingleton B. Matrix metalloproteinases: roles in cancer and metastasis. Front Biosci. 2006;11:479–91. doi: 10.2741/1811. [DOI] [PubMed] [Google Scholar]
- 3.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 4.Dasgupta S, Dash R, Das SK, Sarkar D, Fisher PB. Emerging strategies for the early detection and prevention of head and neck squamous cell cancer. J Cell Physiol. 2012;227:467–73. doi: 10.1002/jcp.22767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ziober AF, Patel KR, Alawi F, Gimotty P, Weber RS, Feldman MM, et al. Identification of a gene signature for rapid screening of oral squamous cell carcinoma. Clin Cancer Res. 2006;12:5960–71. doi: 10.1158/1078-0432.CCR-06-0535. [DOI] [PubMed] [Google Scholar]
- 6.Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–24. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minn AJ, Kang Y, Serganova I, Gupta GP, Giri DD, Doubrovin M, et al. Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors. J Clin Invest. 2005;115:44–55. doi: 10.1172/JCI22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta GP, Nguyen DX, Chiang AC, Bos PD, Kim JY, Nadal C, et al. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature. 2007;446:765–70. doi: 10.1038/nature05760. [DOI] [PubMed] [Google Scholar]
- 9.Harima Y, Ikeda K, Utsunomiya K, Shiga T, Komemushi A, Kojima H, et al. Identification of genes associated with progression and metastasis of advanced cervical cancers after radiotherapy by cDNA microarray analysis. Int J Radiat Oncol Biol Phys. 2009;75:1232–9. doi: 10.1016/j.ijrobp.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen-Preiss SM, Quigley JP. Detection and characterization of low abundance cellular proteins that specifically increase upon loss of the metastatic phenotype. J Cell Biochem. 1993;51:219–35. doi: 10.1002/jcb.240510214. [DOI] [PubMed] [Google Scholar]
- 11.Zijlstra A, Mellor R, Panzarella G, Aimes RT, Hooper JD, Marchenko ND, et al. A quantitative analysis of rate-limiting steps in the metastatic cascade using human-specific real-time polymerase chain reaction. Cancer Res. 2002;62:7083–92. [PubMed] [Google Scholar]
- 12.Toolan HW. Transplantable human neoplasms maintained in cortisone-treated laboratory animals: H.S. No. 1; H.Ep. No. 1; H.Ep. No. 2; H.Ep. No. 3; and H.Emb.Rh. No. 1. Cancer Res. 1954;14:660–6. [PubMed] [Google Scholar]
- 13.Ossowski L, Reich E. Experimental model for quantitative study of metastasis. Cancer Res. 1980;40:2300–9. [PubMed] [Google Scholar]
- 14.Ossowski L, Reich E. Changes in malignant phenotype of a human carcinoma conditioned by growth environment. Cell. 1983;33:323–33. doi: 10.1016/0092-8674(83)90414-2. [DOI] [PubMed] [Google Scholar]
- 15.Gordon JR, Quigley JP. Early spontaneous metastasis in the human epidermoid carcinoma HEp3/chick embryo model: contribution of incidental colonization. Int J Cancer. 1986;38:437–44. doi: 10.1002/ijc.2910380321. [DOI] [PubMed] [Google Scholar]
- 16.Brooks PC, Lin JM, French DL, Quigley JP. Subtractive immunization yields monoclonal antibodies that specifically inhibit metastasis. J Cell Biol. 1993;122:1351–9. doi: 10.1083/jcb.122.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Testa JE, Brooks PC, Lin JM, Quigley JP. Eukaryotic expression cloning with an antimetastatic monoclonal antibody identifies a tetraspanin (PETA-3/CD151) as an effector of human tumor cell migration and metastasis. Cancer Res. 1999;59:3812–20. [PubMed] [Google Scholar]
- 18.Hooper JD, Zijlstra A, Aimes RT, Liang H, Claassen GF, Tarin D, et al. Subtractive immunization using highly metastatic human tumor cells identifies SIMA135/CDCP1, a 135 kDa cell surface phosphorylated glycoprotein antigen. Oncogene. 2003;22:1783–94. doi: 10.1038/sj.onc.1206220. [DOI] [PubMed] [Google Scholar]
- 19.Zijlstra A, Lewis J, Degryse B, Stuhlmann H, Quigley JP. The inhibition of tumor cell intravasation and subsequent metastasis via regulation of in vivo tumor cell motility by the tetraspanin CD151. Cancer Cell. 2008;13:221–34. doi: 10.1016/j.ccr.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deryugina EI, Zijlstra A, Partridge JJ, Kupriyanova TA, Madsen MA, Papagiannakopoulos T, et al. Unexpected effect of matrix metalloproteinase down-regulation on vascular intravasation and metastasis of human fibrosarcoma cells selected in vivo for high rates of dissemination. Cancer Res. 2005;65:10959–69. doi: 10.1158/0008-5472.CAN-05-2228. [DOI] [PubMed] [Google Scholar]
- 21.Partridge JJ, Madsen MA, Ardi VC, Papagiannakopoulos T, Kupriyanova TA, Quigley JP, et al. Functional analysis of matrix metalloproteinases and tissue inhibitors of metalloproteinases differentially expressed by variants of human HT-1080 fibrosarcoma exhibiting high and low levels of intravasation and metastasis. J Biol Chem. 2007;282:35964–77. doi: 10.1074/jbc.M705993200. [DOI] [PubMed] [Google Scholar]
- 22.Bekes EM, Deryugina EI, Kupriyanova TA, Zajac E, Botkjaer KA, Andreasen PA, et al. Activation of pro-uPA is critical for initial escape from the primary tumor and hematogenous dissemination of human carcinoma cells. Neoplasia. 2011;13:806–21. doi: 10.1593/neo.11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conn EM, Botkjaer KA, Kupriyanova TA, Andreasen PA, Deryugina EI, Quigley JP. Comparative Analysis of Metastasis Variants Derived from Human Prostate Carcinoma Cells. Roles in Intravasation of VEGF-Mediated Angiogenesis and uPA-Mediated Invasion. Am J Pathol. 2009;175:1638–52. doi: 10.2353/ajpath.2009.090384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agarwal D, Goodison S, Nicholson B, Tarin D, Urquidi V. Expression of matrix metalloproteinase 8 (MMP-8) and tyrosinase-related protein-1 (TYRP-1) correlates with the absence of metastasis in an isogenic human breast cancer model. Differentiation. 2003;71:114–25. doi: 10.1046/j.1432-0436.2003.710202.x. [DOI] [PubMed] [Google Scholar]
- 25.Montel V, Kleeman J, Agarwal D, Spinella D, Kawai K, Tarin D. Altered metastatic behavior of human breast cancer cells after experimental manipulation of matrix metalloproteinase 8 gene expression. Cancer Res. 2004;64:1687–94. doi: 10.1158/0008-5472.can-03-2047. [DOI] [PubMed] [Google Scholar]
- 26.Gutierrez-Fernandez A, Fueyo A, Folgueras AR, Garabaya C, Pennington CJ, Pilgrim S, et al. Matrix metalloproteinase-8 functions as a metastasis suppressor through modulation of tumor cell adhesion and invasion. Cancer Res. 2008;68:2755–63. doi: 10.1158/0008-5472.CAN-07-5154. [DOI] [PubMed] [Google Scholar]
- 27.Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, et al. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61–5. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- 28.Itoh Y, Seiki M. MT1-MMP: a potent modifier of pericellular microenvironment. J Cell Physiol. 2006;206:1–8. doi: 10.1002/jcp.20431. [DOI] [PubMed] [Google Scholar]
- 29.Tressel SL, Kaneider NC, Kasuda S, Foley C, Koukos G, Austin K, et al. A matrix metalloprotease-PAR1 system regulates vascular integrity, systemic inflammation and death in sepsis. EMBO Mol Med. 2011;3:370–84. doi: 10.1002/emmm.201100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zania P, Kritikou S, Flordellis CS, Maragoudakis ME, Tsopanoglou NE. Blockade of angiogenesis by small molecule antagonists to protease-activated receptor-1: association with endothelial cell growth suppression and induction of apoptosis. J Pharmacol Exp Ther. 2006;318:246–54. doi: 10.1124/jpet.105.099069. [DOI] [PubMed] [Google Scholar]
- 31.Brinckerhoff CE, Rutter JL, Benbow U. Interstitial collagenases as markers of tumor progression. Clin Cancer Res. 2000;6:4823–30. [PubMed] [Google Scholar]
- 32.Ala-aho R, Kahari VM. Collagenases in cancer. Biochimie. 2005;87:273–86. doi: 10.1016/j.biochi.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 33.Szarvas T, vom Dorp F, Ergun S, Rubben H. Matrix metalloproteinases and their clinical relevance in urinary bladder cancer. Nat Rev Urol. 2011;8:241–54. doi: 10.1038/nrurol.2011.44. [DOI] [PubMed] [Google Scholar]
- 34.Hotary KB, Allen ED, Brooks PC, Datta NS, Long MW, Weiss SJ. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell. 2003;114:33–45. doi: 10.1016/s0092-8674(03)00513-0. [DOI] [PubMed] [Google Scholar]
- 35.Rowe RG, Weiss SJ. Breaching the basement membrane: who, when and how? Trends Cell Biol. 2008;18:560–74. doi: 10.1016/j.tcb.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Foley CJ, Luo C, O’Callaghan K, Hinds PW, Covic L, Kuliopulos A. Matrix metalloprotease-1a promotes tumorigenesis and metastasis. J Biol Chem. 2012;287:24330–8. doi: 10.1074/jbc.M112.356303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ota I, Li XY, Hu Y, Weiss SJ. Induction of a MT1-MMP and MT2-MMP-dependent basement membrane transmigration program in cancer cells by Snail1. Proc Natl Acad Sci U S A. 2009;106:20318–23. doi: 10.1073/pnas.0910962106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goerge T, Barg A, Schnaeker EM, Poppelmann B, Shpacovitch V, Rattenholl A, et al. Tumor-derived matrix metalloproteinase-1 targets endothelial proteinase-activated receptor 1 promoting endothelial cell activation. Cancer Res. 2006;66:7766–74. doi: 10.1158/0008-5472.CAN-05-3897. [DOI] [PubMed] [Google Scholar]
- 39.Fujimoto D, Hirono Y, Goi T, Katayama K, Yamaguchi A. Prognostic value of protease-activated receptor-1 (PAR-1) and matrix metalloproteinase-1 (MMP-1) in gastric cancer. Anticancer Res. 2008;28:847–54. [PubMed] [Google Scholar]
- 40.Du X, Wang S, Lu J, Cao Y, Song N, Yang T, et al. Correlation between MMP1-PAR1 axis and clinical outcome of primary gallbladder carcinoma. Jpn J Clin Oncol. 2011;41:1086–93. doi: 10.1093/jjco/hyr108. [DOI] [PubMed] [Google Scholar]
- 41.Peng HH, Zhang X, Cao PG. MMP-1/PAR-1 signal transduction axis and its prognostic impact in esophageal squamous cell carcinoma. Braz J Med Biol Res. 2012;45:86–92. doi: 10.1590/S0100-879X2011007500152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005;120:303–13. doi: 10.1016/j.cell.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 43.Yang E, Boire A, Agarwal A, Nguyen N, O’Callaghan K, Tu P, et al. Blockade of PAR1 signaling with cell-penetrating pepducins inhibits Akt survival pathways in breast cancer cells and suppresses tumor survival and metastasis. Cancer Res. 2009;69:6223–31. doi: 10.1158/0008-5472.CAN-09-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blackburn JS, Liu I, Coon CI, Brinckerhoff CE. A matrix metalloproteinase-1/protease activated receptor-1 signaling axis promotes melanoma invasion and metastasis. Oncogene. 2009;28:4237–48. doi: 10.1038/onc.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blackburn JS, Brinckerhoff CE. Matrix metalloproteinase-1 and thrombin differentially activate gene expression in endothelial cells via PAR-1 and promote angiogenesis. Am J Pathol. 2008;173:1736–46. doi: 10.2353/ajpath.2008.080512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mierke CT, Zitterbart DP, Kollmannsberger P, Raupach C, Schlotzer-Schrehardt U, Goecke TW, et al. Breakdown of the endothelial barrier function in tumor cell transmigration. Biophys J. 2008;94:2832–46. doi: 10.1529/biophysj.107.113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beckers CM, van Hinsbergh VW, van Nieuw Amerongen GP. Driving Rho GTPase activity in endothelial cells regulates barrier integrity. Thromb Haemost. 2010;103:40–55. doi: 10.1160/TH09-06-0403. [DOI] [PubMed] [Google Scholar]
- 48.Thennes T, Mehta D. Heterotrimeric G proteins, focal adhesion kinase, and endothelial barrier function. Microvasc Res. 2012;83:31–44. doi: 10.1016/j.mvr.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trivedi V, Boire A, Tchernychev B, Kaneider NC, Leger AJ, O’Callaghan K, et al. Platelet matrix metalloprotease-1 mediates thrombogenesis by activating PAR1 at a cryptic ligand site. Cell. 2009;137:332–43. doi: 10.1016/j.cell.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


