Abstract
Cells use secreted signals (e.g. chemokines and growth factors) and sophisticated vehicles such as argosomes, cytonemes, tunneling nanotubes and exosomes to relay important information to other cells, often over large distances. Exosomes, 30–100-nm intraluminal vesicles of multivesicular bodies (MVB) released upon exocytic fusion of the MVB with the plasma membrane, are increasingly recognized as a novel mode of cell-independent communication. Exosomes have been shown to function in antigen presentation and tumor metastasis, and in transmitting infectious agents. However, little is known about the biogenesis and function of exosomes in polarized cells. In this review, we discuss new evidence suggesting that exosomes participate in the transport of morphogens and RNA, and thus influence cell polarity and developmental patterning of tissues.
Introduction
Eukaryotic cells have evolved elaborate endosomal networks to enable them to communicate with one another, to differentiate into tissues and to adapt to diverse environments [1]. Endosomes are membrane-bound organelles that transport newly synthesized material from the Golgi complex, and endocytosed material from the plasma membrane to various intracellular destinations (Figure 1). Depending on their morphology, their distinct protein and lipid composition, their position within the cell and the cargo that they carry, endosomes are classified as early, late or recycling [1,2]. Within the cell, endosomes are characterized by modular organization, spatial connectivity and functional cooperation, which together helps them to form intricate interconnected networks. In polarized cells, such as epithelial cells and neurons, endosomal networks are even more complex, because these cells interact with different extracellular environments at their apical (axonal) and basolateral (somatodendritic) surfaces (Figure 1b). Endosomes are strategically located at the crossroads between the biosynthetic and endocytic routes in the cell, which enables them to direct both newly synthesized and endocytosed proteins to the appropriate membrane domains or intracellular destinations. Proteins are sorted via their incorporation into different subpopulations of carrier vesicles, a process mediated by sorting signals (e.g. tyrosine and dileucine motifs, glycophosphatidylinositol [GPI] anchors and N-glycans, etc) and signal-decoding machinery (e.g. clathrin adaptors and lipid rafts) [3]. These carrier vesicles are transported to, dock at and fuse with their target plasma membrane domains by the coordinated function of the microtubule and actin cytoskeletons, SNAREs (soluble N-ethylmaleimide-sensitive factor attachment protein receptors), Rabs and other small GTPases and tethering factors, such as the exocyst. Rab GTPases are a family of more than 60 ubiquitously expressed proteins that are indispensable for coordinating various steps of intracellular trafficking, such as vesicle formation, transport and fusion with the target membrane [4]. Within the cell, different Rabs are restricted to specific membrane domains (Figure 1) and help to establish organelle identity. In the endocytic route, internalized proteins and lipids are first incorporated into early endosomes, major sorting platforms where selective remodeling of the lipid bilayer segregates recycling molecules from cargo targeted for degradation[5,6]. Endocytic cargo fated to be degraded is preferentially sorted into 40–100-nm vesicles that bud into the lumen of early endosomes, a process topologically equivalent to outward budding at the plasma membrane [7]. The best-characterized signal to enable sorting into these intralumenal vesicles (ILVs) is ubiquitylation, the covalent addition of ubiquitin subunits to cytoplasmic lysine residues of proteins. Tubular elements of early endosomes undergo fission and subsequent fusion with recycling endosomes, whereas the portion of early endosomes containing ILVs matures or detaches to form multivesicular bodies (MVBs;Box 1). Maturation of early endosomes occurs with the replacement of Rab5 with Rab7, and the accumulation of ILVs containing phosphatidylinositol-3-phosphate (PtdIns(3)P), ubiquitylated cargo (e.g. epidermal growth factor receptor [EGFR]) and other proteins commonly found in ILVs (e.g. tetraspanins) [8]. Upon fusion of the limiting membrane of the MVB with lysosomes (Figure 1), ubiquitylated proteins in ILVs become vulnerable to degradation.
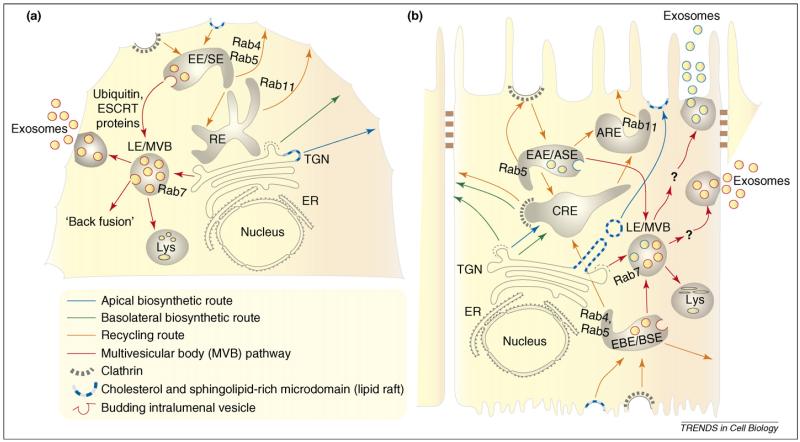
Figure 1.
The endosomal network and the multivesicular bodies (MVBs) pathway. Non-polarized cells (a) have a simpler endosomal system than polarized cells (b). (a) Newly synthesized proteins are transported from the trans-Golgi (TGN) network to the plasma membrane (green and blue arrows). Clustering of proteins into lipid rafts (blue) or interaction of specific motifs in the proteins with clathrin adaptors (green) act as sorting signals. Recycling of endocytosed membrane proteins occurs from early endosomes [also known as sorting endosomes, (EE/SE)] and recycling endosomes (RE, orange arrows). Proteins destined for degradation are sorted into membrane invaginations of the EE/SE. These intralumenal vesicle-containing regions of the EE/SE eventually mature into late endosomes, also known as multivesicular bodies (LE/MVBs). Ubiquitylation of proteins and the ESCRT machinery helps to sort cargo into LE/MVBs and might also participate in their biogenesis [15,85]. Not all ILVs are destined for lysosomal degradation. Fusion of ILVs with the late endosomal limiting membrane, a process called ‘back-fusion’, is essential for the cytoplasmic delivery of certain proteins and viruses [10]. In many cells, the limiting membrane of LE/MVBs fuses with the plasma membrane, releasing ILVs into the extracellular space; these vesicles are now referred to as exosomes. (b). Epithelial cells have distinct apical and basolateral membrane domains separated by tight junctions (brown), and a complex endosomal system that is essential for the establishment and maintenance of polarity [1]. In the biosynthetic route, at the TGN, specific signals help to sort proteins into carrier vesicles that are transported to either the apical or the basolateral membrane. GPI-anchors or association with lipid rafts transport proteins to the apical domain (blue) and proteins containing motifs such as NPXY, YXXΦ, LL and L which interact with clathrin adaptors are transported to the basolateral domain (green) [3]. In the endocytic route, apical and basolateral proteins are first internalized into early apical or basolateral endosomes [also known as sorting endosomes, (EAE/ASE and EBE/BSE)]. Recycling cargo is transported to common recycling endosomes (CREs) and sorted into separate apical and basolateral recycling routes. In epithelial cells, the apical recycling route also includes the Rab11+ apical recycling endosome (ARE) [3]. Little is known about sorting of ubiquitylated cargo, the ESCRT machinery or the biogenesis of LE/MVBs in polarized cells, and phenomena such as back-fusion have yet to be demonstrated. Epithelial cells originating from different organs do release exosomes at both the apical and the basolateral surfaces [11,23,61-64]. Apical and basolateral endocytic pathways have been shown to intersect at the level of late endosomes in epithelial cells [31]; however, because exosomes released apically have a different protein composition from those released at the basolateral surface [11,64], it is also possible that different populations of MVBs generate exosomes destined for apical or basolateral release (see text and Figure 2 for a discussion on polarized sorting into exosomes).
However, not all proteins sorted into the MVB pathway are degraded, and MVBs and/or late endosomes have emerged as important pre-lysosomal sorting stations. There is evidence supporting the existence of different populations of late endosomes [9] and/or different subpopulations of ILVs in a common, late endosomal pool which have different destinations (Figure 1a): ILVs rich in PtdIns(3)P and ubiquitylated proteins head to lysosomes, whereas those enriched in 2,2′-lysobisphosphatidic acid (LBPA, also called bis-monoacyl glycerol phosphate [BMP]) have a non-degradative function [10]. In many cell types, the limiting membrane of the MVB fuses with the plasma membrane, releasing ILVs into the extracellular space. These ILVs are referred to as exosomes (Figure 1 and Box 2).
In this review, we discuss the biogenesis of exosomes from the endosomal system, and we describe how this can differ in polarized and non-polarized cells [11]. A variety of vesicles, tubules and membrane-bound lipid particles have recently been implicated in cell-cell communication: cytonemes, which are actin-based filopodial extensions [12], and argosomes (see below) have been shown to participate in long-range morphogen transport, and tunneling nano-tubes are reported to transport organelles, vesicles and pathogens between cells [13]. To this list, we can now add exosomes. In the latter part of this review, we discuss recent evidence that shows how, through the release of exosomes, the endosomal system participates in intercellular communication, including signaling and the establishment and maintenance of cell polarity and tissue morphogenesis.
Biogenesis of exosomes
Accumulating evidence suggests that some components of the molecular machinery involved in sorting proteins towards the degradative pathway drive membrane invagination in early endosomes, thereby generating MVBs. Central players in this process are the endosomal sorting complexes required for transport, ESCRT-0, ESCRT-I, ESCRT-II and ESCRT-III, which are multimeric protein complexes (reviewed in [14,15]). ESCRT proteins were first identified through yeast genetic screens for vacuolar protein sorting (vps) mutants. vps-mutant yeast cells have an abnormal multi-cisternal compartment (called the class E compartment) in which proteins targeted to the yeast vacuole accumulate. ESCRT complexes deform the endosomal-limiting membrane by specific protein-protein and protein-lipid interactions, thereby orchestrating inward budding of vesicles. ESCRT-0, ESCRT-I and ESCRT-II have ubiquitin-interacting modules that are essential for cargo recognition and sorting. Ubiquitylated cargo first binds to Hrs (hepatocyte growth factor-regulated kinase substrate, a component of ESCRT-0) on flat clathrin coats on the endosomal membrane [15]. Next, ESCRT-I and ESCRT-II are recruited to the membrane, where they cluster the ubiquitylated transmembrane proteins and package them into budding ILVs [16]. ESCRT-III recruits de-ubiquitylating enzymes to remove ubiquitin from cargo proteins before their incorporation into ILVs, and it also recruits Vps4, an ATPase that catalyzes the disassembly of all ESCRTs from membranes. Another class E Vps protein, Alix (apoptosis-linked gene 2 (ALG-2)-interacting protein X), acts as a linker between different ESCRT complexes [17] and might also play a part in intralumenal vesicle generation (Box 3) [18].
The coordinated recruitment and utilization of ESCRT machinery after ubiquitylation is a well-established model of MVB biogenesis and cargo sorting. However, recent work suggests that cargo itself can drive MVB biogenesis [9], that proteins can be sorted into MVB by ubiquitin-independent mechanisms [19,20] and that cargo sorting and MVB biogenesis can occur in a manner independent of both ubiquitin and ESCRTs [21,22]. Thus, in mammalian cells, membrane invagination can be uncoupled from sorting of ubiquitylated cargo, perhaps owing to overlapping functions of ESCRT proteins. It is also likely that the mechanisms underlying MVB biogenesis in higher organisms are more robust in accommodating the increased complexity of endosomal organization.
Proteomic and biochemical analyses of exosomes from dendritic cells, oligodendrocytes and urine show that these exosomes contain class E Vps proteins, including Alix and Tsg101 (tumor susceptibility gene-101), a component of ESCRT-1 [23-25]. However, two recent studies have shown that cells depleted of or expressing dominant negative forms of Hrs, Tsg101, Alix or Vps4 can still secrete exosomes [22,26].
In non-polarized cells, there seem to be two general mechanisms for sorting into ILVs: partitioning into cholesterol and sphingolipid-rich lipid microdomains (the so-called ‘lipid rafts’); and oligomerization (or aggregation) at the plasma membrane (Figure 2a) [26,27]. Many raft-associated proteins such as tetraspanins and GPI-anchored proteins are found in exosomes. Lipid rafts decrease the lateral diffusion of proteins within the plane of the membrane and could inhibit sorting of GPI-anchored proteins and other raft-associated proteins into more fluid membrane domains that undergo fast recycling to the cell surface [5,28]. Prolonged residence on endosomal membranes increases the likelihood that GPI-anchored proteins become sorted into budding ILVs. A similar mechanism of slow recycling to the cell surface could function in sorting cross-linked membrane proteins into exosomes [26,29,30].
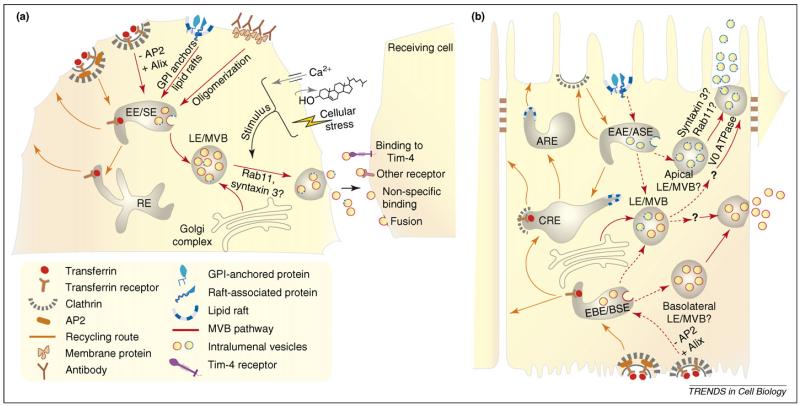
Figure 2.
Sorting and trafficking of exosomes. (a) In non-polarized cells, GPI-anchoring, lipid raft-association or antibody-induced oligomerization sorts proteins into the exosomal pathway [26,27]. Degradation of the clathrin adaptor AP2 reroutes the transferrin receptor away from recycling (orange) and towards the MVB pathway (red) [19]. This is dependent upon binding of the YXXΦ motif of the transferrin receptor to the class E protein Alix. Many studies have shown that increasing intracellular calcium[25,35-39], decreasing membrane cholesterol [36,45,46] or inducing cellular stress [45,49] stimulates the release of exosomes. Once released, exosomes interact with cells by binding to specific receptors such as Tim-4 [57], an endogenous phosphatidylserine receptor [58], or other cell surface molecules [56], or by directly fusing with target cells [55]. (b) Epithelial cells release exosomes at both the apical (intestine, kidney) and basolateral (intestine) surfaces [11,23,61]. Exosomes released apically by intestinal epithelial cells have a different composition than those released basolaterally [11], raising the possibility of differential sorting into apical and basolateral exosomes. However, how this might occur is still largely speculative. Here, we depict two potential sorting mechanisms (red dashed arrows) that might function in polarized exosomal sorting; on the basis of mechanisms operating in non-polarized cells, we hypothesize that GPI-anchoring and association with lipid rafts (which are both apical targeting signals in the biosynthetic route in polarized cells [3]) direct proteins into MVBs destined for fusion with the apical membrane. Sorting into MVBs destined for basolateral release might use interactions between the ESCRT machinery and canonical basolateral sorting signals. In a manner analogous to that seen in non-polarized cells, binding of Alix to the YXXΦ motif of the transferrin receptor might direct it into exosomes destined for basolateral release. It is currently unknown whether apical and basolateral exosomes arise from two separate LE/MVBs or from a common LE/MVB pool [31]. The V0 subunit of the vacuolar ATPase has been implicated in the apical secretion of exosomes [77]; it remains to be investigated if Rab11, which is involved in exosome release in non-polarized cells [50], and syntaxin-3, which is involved in secretory granule exocytosis [52], also function in apical release of exosomes.
Little is known about the MVB pathway and exosome biogenesis in polarized cells. Exosomes released by intestinal epithelial cells at either the apical or the basolateral surface contain proteins normally present on the corresponding plasma membrane [11]. This suggests that epithelial cells have distinct MVB with ILVs into which apical and basolateral proteins are differentially sorted (Figure 2b). Alternatively, since apical and basolateral endocytic routes have been shown to converge at the level of late endosomes in Madin–Darby canine kidney (MDCK) cells [31], exosomes headed to the apical or basolateral membranes might be generated from different intralumenal vesicle populations within a common late endosomal pool. If this is true, then there should be some mechanism of segregating the two intralumenal vesicle populations into secondary organelles that can then be transported to the appropriate membrane (Figure 2b).
We speculate that interactions between membrane proteins and canonical apical or basolateral sorting signals operating in epithelial cells might be used to generate different types of ILVs (Figure 2). For example, GPI-anchors and lipid rafts, known to act as apical sorting mechanisms [3], might drive GPI-anchored proteins and raft-associated proteins into ILVs destined for apical release. Alternatively, interactions between the ESCRT machinery and specific basolateral sorting motifs could direct proteins into ILVs destined for release at the baso-lateral membrane. An example of this is the binding of Alix to the YXXΦ internalization motif of the transferrin receptor in maturing reticulocytes, a process that is necessary for sorting transferrin receptor into exosomes [19]. Degradation of the clathrin adaptor AP2 (adaptor protein 2) is necessary for exosomal sorting of transferrin receptor, suggesting that diverting cargo from endocytic recycling directs them into exosomes. Alix also binds to RME-1, the conserved EH domain-containing protein required for receptor-mediated endocytosis, on recycling endosomes and participates in recycling basolateral cargo, such as major histocompatability (MHC) class I molecules in the Caenor-habditis elegans intestine [32]. These data suggest that, in polarized cells, Alix plays a dual role in basolateral sorting: it cooperates with RME-1 in the recycling route and, when required, it specifically interacts with basolateral proteins to target them into exosomes. This hypothesis is borne out by biochemical and mass spectrometric analysis of apical and basolateral exosomes released by intestinal epithelial cells; apical exosomes contain GPI-anchored proteins (e.g. microsomal dipeptidase) and raft-associated proteins (e.g. syntaxin-3), whereas exosomes released basolaterally are enriched in MHC class I and II molecules [11].
Although this hypothesis is very attractive, it needs to be confirmed in several different epithelial cell types. Additional studies are essential to better understand how polarized sorting into exosomes occurs and whether this involves a single pool of MVBs with different intralumenal vesicle populations or separate apical and basolateral MVBs.
Release and trafficking of exosomes
Exosomes are released both constitutively and in a regulated manner. The molecular machinery involved in the exocytic fusion of MVBs to release exosomes is still under investigation. One hypothesis is that regulated release of exosomes uses similar mechanisms to those involved in the fusion of secretory lysosomes with the plasma membrane [33].
Calcium ionophores, which trigger lysosomal exocytosis [34], stimulate exosome release in many cell types, including epithelial cells and neurons [25,35-39]. Calcium-induced lysosomal exocytosis requires the calcium sensor synaptotagmin VII, which controls the size of the fusion pore, and Rab27a and its effector Munc 13–4, which primes vesicles for fusion at the plasma membrane[34,40]. Recent studies have also implicated the clathrin adaptor AP3 and the v-SNARE TI-VAMP (tetanus neurotoxin-insensitive vesicle-associated membrane protein or VAMP7) in lysosome secretion, but the exact mechanisms by which these proteins participate in exocytosis still need to be elucidated [41-43]. Using total internal reflection fluorescence microscopy (TIRFM) it was observed that lysosomes in close proximity to the plasma membrane were preferentially exocytosed in response to a local spike in intracellular calcium [44]. It remains to be investigated if exocytic fusion of MVBs requires all or some of the machinery used in lysosome fusion and if, as with lysosomes and synaptic vesicles, MVBs can also be primed for exocytosis by virtue of their localization near the plasma membrane.
Decreasing membrane cholesterol or inhibiting cholesterol biosynthesis stimulates the release of exosomes and prostasomes (exosome-like vesicles released by ductal epithelial cells of the prostate gland) [36,45,46]. How might this occur? One possibility is that cholesterol influences the rate of MVB transport to the plasma membrane by controlling the amount of membrane-associated Rab7 and the recruitment of dynein and kinesin, which mediate bi-directional movement of endosomes [47]. Depletion of cell cholesterol increases endosome motility towards the microtubule plus-end by decreasing levels of endosome-associated Rab7 and increasing association with Rab4, which is involved in fast endocytic recycling [48]. However, how cholesterol depletion affects sorting into ILVs has not been studied. Cellular stress (e.g. from exposure to γ-irradiation, DNA damaging agents or heat shock) has also been shown to stimulate exosome secretion [45,49]. DNA damage activates the tumor suppressor p53, which induces the release of exosomes, presumably as a mechanism of tumor surveillance or to affect gene expression patterns in adjacent cells [49].
Overexpression of either Rab11 [50] or citron kinase, a RhoA effector [51], stimulates MVB exocytosis, indicating that these small GTPases are involved in exosome release. Rab11, a marker for recycling endosomes, has been detected in exosomes released by dendritic cells and in prostasomes. [24,46]. Although Rab11 is thought to function predominantly in endocytic recycling, it also participates in regulated exocytosis of secretory vesicles[40,52,53], suggesting that it could act at the intersection of endocytic and secretory pathways in a cell type-specific manner.
In polarized epithelial cells, Rab11a segregates into apical recycling endosomes that are committed to transporting cargo to the apical membrane [1]. Exosomes released at the apical surface of intestinal epithelial cells contain syntaxin-3 [11], which normally localizes to the apical membrane in a microtubule-dependent manner and is necessary for apical exocytosis of post-Golgi vesicles [3]. Both Rab11 and syntaxin-3 participate in the regulated exocytosis of secretory vesicles at the apical membrane of parotid acinar cells [52]. Taken together, these data raise the possibility that syntaxin-3 is also involved in the apical release of exosomes. The involvement of microtubules and the actin cytoskeleton in exosome release is largely unexplored. Their roles might be similar to their involvement in polarized secretion in epithelial cells, wherein microtubules control apical exocytosis, and the actin cytoskeleton controls basolateral exocytosis [3].
Once released, exosomes interact with target cells by fusion, adhesion or direct binding [54-56]. Specific macrophage cell surface receptors for exosomes have recently been identified: two members of the Tim (T-cell immunoglobulin-containing and mucin-domain-containing molecule) family transmembrane proteins Tim1 and Tim4, which bind to phosphatidylserine exposed on the outer leaflet of exosomes [57]. An endogenous phosphatidylserine receptor on monocytes has also been implicated in the uptake of exosomes released by T-cells [58], although it is not known currently whether the Tim or phosphatidylserine receptors are also involved in exosome uptake by other cells.
One impediment to studying the trafficking of exosomes in real time is that their size is below the resolution of the light microscope. Development of better fluorescent probes and sub-diffraction imaging techniques (e.g. stimulated emission depletion [STED] and stochastic optical reconstruction microscopy [STORM]) that can achieve spatial resolutions of 20–40 nm will likely help in visualizing the trafficking of exosomes in living cells [59,60].
Exosomes in polarized cells
Exosomes are released by epithelial cells of the intestine[11,61], kidney [23], salivary gland [62], and ciliary and retinal pigment epithelium [63]. In vitro and in vivo studies have shown that exosomes released at the basolateral surface of intestinal epithelial cells bring antigens from the lumen of the gut into contact with the underlying immune system [61,64]. Apical secretion of exosomes into urine by renal epithelia could be either a conduit for disposing cellular waste or, more plausibly, a way for these cells to regulate their protein repertoire through successive rounds of exosome release and re-uptake by neighboring cells. Proteomic analysis of urinary exosomes also provides a powerful tool to identify disease biomarkers [23].
Although many epithelial cells release exosomes, whether exosomes actually participate in the establishment of apicobasal polarity is an open question (Figure 3). During terminal differentiation of epithelial cells, Rab11-mediated exocytosis and recycling provides additional membrane required for invagination [65] and delivers E-cadherin to the junctional complex [66]. Apical localization of syntaxin-3 is necessary for the formation of a polarized epithelium, because mis-targeting of syntaxin-3 to the basolateral membrane results in the disruption of tight junctions and loss of cell polarity [67]. The presence of both Rab11 and syntaxin-3 in exosomes raises the possibility that heterotypic fusion between MVBs and Rab11 vesicles [40] provides the differentiating cell with a rapidly releasable source of additional membrane and/or proteins.
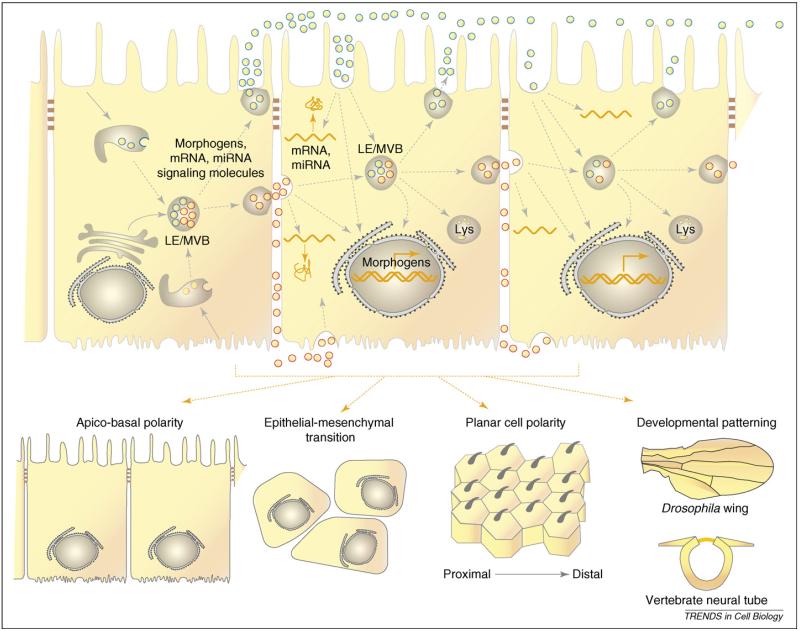
Figure 3.
Potential role of exosomes in cell and tissue polarity. Exosomes released at either the apical or basolateral surfaces of epithelial cells can travel over several cell diameters [74]. Exosomes internalized by dendritic cells traffic through LAMP-1-positive late endsosomes or lysosomes [56]; whether this is true in epithelial cells is currently unknown. Studies have shown that exosomes carry morphogens, mRNA and miRNA, molecules that play important roles in cell and tissue morphogenesis[37,71,77]. Mechanisms involved in sorting morphogens and nucleic acids into exosomes have yet to be elucidated but might involve association with lipid rafts [76] or binding to proteins involved in endosomal sorting [84]. Upon internalization by target cells, exogenous mRNA delivered by exosomes is translated into protein [37], endogenous protein synthesis can be downregulated by exosomal miRNA, and morphogens can influence gene expression. Thus, by modulating the protein repertoire of target cells, it is likely that exosomes could participate in the generation of apicobasal polarity in epithelial cells, planar cell polarity of the epithelial sheet, or the developmental patterning of tissues such as the Drosophila wing and the vertebrate neural tube. Given that tumor-derived exosomes promote tumor invasion, exosomes might also contribute to the epithelial–mesenchymal transition. However, a direct role for exosomes in any of these processes remains to be demonstrated.
Many epithelial tumors secrete exosomes carrying tumor antigens, cytokines, multi-drug transporters, enzymes and death receptors [68]. Tumor-derived exosomes can promote tumor invasion by modulating the immune response and by stimulating angiogenesis and stromal remodeling. However, whether exosomes participate in the epithelial-to-mesenchymal transition, which initiates tumorigenesis, has not yet been investigated (Figure 3).
The presence of exosomes in the nervous system in vivo has yet to be demonstrated, but tissue culture studies show that neurons release exosomes containing glutamate receptor subunits [39], and that oligodendrocytes secrete exosomes containing myelin proteins [22,25]. Thus, exosomes could potentially help regulate neurotransmitter receptor levels at the synapse by targeting certain subunits for degradation, control the production and turnover of myelin membrane proteins, and participate in the progression of neurodegenerative diseases [69].
Exosomes in cell fate and tissue patterning
Exosome-mediated communication is especially useful during the establishment of planar cell polarity and the developmental patterning of tissues (Figure 3). These processes are controlled by morphogens, a small group of evolutionarily conserved, secreted proteins that include members of the Wingless, Hedgehog, fibroblast growth factor (FGF), transforming growth factorβ () and bone morphogenetic protein (BMP) families.
Morphogens are secreted from a localized source and spread across developing tissues, creating concentration gradients that induce distinct patterns of gene expression in target cells. Morphogen gradients are necessary for many processes, including the development of Drosophila wing and leg imaginal discs, and vertebrate neural tube and limb bud [70]. How morphogens travel through tissues to elicit graded responses that integrate positional information with cell fate has been the subject of intense debate. Evidence exists to support mechanisms such as passive diffusion and planar transcytosis (i.e. iterative rounds of endocytosis and recycling to neighboring cells) [70,71]. Given the different biophysical and biochemical properties of different morphogens, morphogen-specific and tissuespecific mechanisms of transport are likely to exist.
Since developing epithelia can be highly convoluted, effective morphogen dispersal requires close association of the morphogen with the plasma membrane and a mechanism of cell-to-cell transfer [72]. Both Wingless and Hedgehog are palmitoylated, and Hedgehog is covalently attached to cholesterol, linking these morphogens to the cell membrane. It was initially reported that Wingless was found in argosomes, vesicles released at the basolateral side of the Drosophila wing imaginal disc epithelium [73]. Within cells, argosomes were detected in endocytic compartments, and argosome-containing organelles moved at speeds consistent with that of molecular motors. In the developing epithelium, the rate of argosome spread was consistent with the rate of Wingless transport. Given that Wingless is present both on the basolateral membrane and in multivesicular endosomes, the authors proposed that argosomes are generated in the same manner as exosomes – through the inward budding of endosomes – and are transported from cell to cell by the exocytic fusion of Wingless-containing MVBs. Only certain regions of the membrane were able to generate argosomes, indicating that, as with exosomes, these vesicles have a non-random protein composition. Although the ultrastructure or size of argosomes was not examined, the fact that they could be visualized by confocal microscopy suggests that they are larger than exosomes.
Subsequent work by these authors showed that argosomes are distinct from stereotypical exosomes, because exogenously expressed CD63 was not found in argosomes, and CD63-containing exosomes did not co-localize with Wingless and Hedgehog [74]. Instead, Wingless and Hedgehog co-purified and co-localized with lipophorin, the Drosophila lipoprotein, and depletion of lipophorin decreased morphogen spread in the wing disc. On the basis of these data, argosomes are now proposed to be lipoprotein particles that associate reversibly with extracellular lipid-linked morphogens and promote their long-range movement. Morphogen transport from cell to cell by lipoproteins is thought to occur passively by successive steps of extraction and re-insertion at the plasma membrane, but the involvement of specific lipoprotein receptors in this process has not been definitively demonstrated [75].
Although exosomes might not be involved in long-range transport of Wingless and Hedgehog, they might participate in morphogen dispersal over short distances, as demonstrated by two observations: lipid-linked morphogens such as Hedgehog and Wingless localize to detergentinsoluble fractions at the plasma membrane [76] and can conceivably be sorted into exosomes directed to the apical membrane; and exosomes produced by wing epithelia have been shown to travel a few cell diameters from the source [74].
Indeed, exciting recent work implicates exosomes in the apical transport of Hedgehog-related proteins essential for the secretion of a normal cuticle in C. elegans [77]. Fusion of MVBs with the apical plasma membrane, and the subsequent release of ILVs required the V0 subunit of the vacuolar ATPase. Fusion events between MVBs and the plasma membrane were blocked in V0 mutants, leading to enlarged, dense MVBs in which Hedgehog-related peptides accumulated. Previous studies have shown that the V0 sector is necessary for homotypic fusion of the vacuole in yeast and for fusion of synaptic vesicles with the plasma membrane in Drosophila [78]; in both these cases, the role of the V0 subunit in fusion was independent of its role in proton translocation. In contrast, vacuole fission in yeast was recently shown to be dependent on the proton pump activity of the V-ATPase [79]. In vacuole fusion, V0 sectors from opposing membranes come together after trans-SNARE pairing, thereby creating a proteolipid channel that spans both membranes, and fusion is triggered upon calcium release from the vacuolar lumen [80]. It is thus likely that the V0 sector functions in the formation and/or stabilization of the fusion pore during exocytic fusion of MVBs with the plasma membrane.
Exosomes can also participate in the Notch signaling pathway, which regulates numerous cell fate decisions during development. Paradoxically, Notch activation in the signal-receiving cell requires endocytosis of its ligand Delta in the signal-sending cell, effectively removing Delta from the cell surface. To resolve this puzzle, a provocative model suggests that, upon ubiquitylation, endocytosed Delta is sorted into intralumenal vesicles of nascent MVBs [71]. Exocytic fusion of MVBs and release of Delta-containing exosomes into the extracellular space will then enable Notch activation in neighboring cells.
Exosomal shuttles for genetic material
Horizontal transfer of genetic material between cells can induce exogenous gene expression and mediate RNA silencing. Recent reports suggest that microvesicles, apoptotic bodies and exosomes are capable of intracellular transport of functional DNA and RNA (Figure 3) [37,81-83].
Exosomes produced by mouse and human mast cells were found to carry ~1300 mRNAs and 121 microRNAs (miRNAs) but were devoid of DNA and rRNA [37]. The relative abundance of mRNAs and miRNAs was different between exosomes and donor cells, and many transcripts found in exosomes were not found in the donors, suggesting that mRNAs are actively sorted into MVBs. Amazingly, incubation of human cells with exosomes from mouse cells induced the production of mouse proteins from mRNAs that were present in exosomes. However, mRNA from mast cell exosomes was transferred to other mast cells but not to CD4+ T-cells, the only other cell type tested. Thus, exosome-mediated horizontal gene transfer is specific, in terms of both the RNAs packaged into exosomes and the cells to which these RNAs are transferred. Transfer of regulatory RNAs between cells by exosomes is a potentially powerful tool for orchestrating gene expression during development. Among the miRNAs found in exosomes were lin-4 and let-7, both of which have known functions in developmental timing, and other miRNAs involved in diverse processes such as angiogenesis and exocytosis [37].
Microvesicles derived from embryonic stem cells carry mRNAs for various transcription factors that are capable of reprogramming hematopoeitic progenitor cells [83]. Endothelial progenitor cells release microvesicles that can initiate angiogenesis in quiescent endothelial cells after mRNA transfer [82]. As with mast cell exosomes, microvesicles also contain a specific subset of RNAs involved in gene transcription, angiogenesis and cell survival. Unlike exosomes, these microvesicles are 100 nm–1 μm in size, and their provenance (i.e. cell surface versus endosomal) is unclear [82,83].
Targeted delivery of exosomes carrying specific functional RNAs raises many questions: what are the mechanisms for active sorting of some, and not other, mRNA species into exosomes, how are these exosomes targeted to recipient cells and, once internalized, how do the RNAs escape degradation? The ESCRT-II complex component Vps36 was recently shown to directly bind to the 3′ untranslated region of bicoid mRNA in the Drosophila oocyte through the GLUE [Gram-like ubiquitin-binding on EAP45 (ELL-associated protein of 45 kDa)] domain of Vsp36 [84]. This interaction, which is essential for localizing the mRNA to the anterior pole, hints at a previously unexplored role for the ESCRT complexes in RNA trafficking, and this role might be independent of their endosomal sorting functions.
Conclusions and perspectives
Exosomes were initially thought to be little more than garbage bags containing proteins and lipids that the cell needed to get rid of. It is now being realized that exosomes are powerful intercellular messengers that can be harnessed for therapeutic purposes. One can imagine exosomes to be itinerant workers who travel away from the source of production and roam from cell to cell to disseminate important information. In recent years, various modes of intercellular communication have been discovered, including cytonemes [12] and argosomes [73], which participate in tissue development. Work summarized in this review points to an exciting role for exosomes in the establishment of cell and tissue polarity. However, this is still an emerging field, and many questions remain unanswered. Are exosomes involved in the establishment of apicobasal polarity or in initiating the epithelial-to-mesenchymal transition? Do apical and basolateral exosomes originate from distinct MVB populations and, as suggested in this review, do exosomal sorting and MVB exocytosis mechanisms parallel those operating in the biosynthetic and/or endocytic routes in epithelial cells? What is the molecular machinery involved in apical and basolateral exosome release? To date, only the V0 subunit of the vacuolar ATPase has been implicated in the apical secretion of exosomes [77]. Are other molecules such as Rab11 and syntaxin-3 also involved? Is the V0 sector also involved in basolateral exosome release? The issue of exosomes in morphogen and RNA transport and in influencing cell fate decisions during development raises even more questions. What determines the cells with which exosomes interact? How does cell-to-cell transport of exosomes occur in developing epithelia and how far do they travel? Do exosomes undergo sequential rounds of endocytosis and secretion (akin to planar transcytosis) or, once internalized, are they lost forever? Answers to these questions will provide a better understanding of these enigmatic vesicles and their roles in cell and tissue morphogenesis.
Box 1. A brief note on terminology.
Multivesicular bodies (MVBs) are sometimes considered to be a subset of late endosomes [86-88]. However, given that all endosomes along the degradative route are multivesicular [85], the converse is also true; late endosomes can be thought of as a type of MVB [8]. To further complicate matters, some mammalian cells have a spherical transport intermediate (~400–500 nm in diameter) that contains ILVs and is distinct from both early and late endosomes. This intermediate, called the endosomal carrier vesicle or multivesicular body, has been shown to fuse with late endosomes [10,89,90]. Because multivesicular bodies that conform to these criteria are not seen in all cell types [91] and given that MVBs from cells such as reticulocytes contain Rab4 and Rab5 [92], which are normally found on early endosomes, there might be cell-specific differences in the generation and usage of transport intermediates between early and late endosomes. To avoid confusion, in this review, we consider late endosomes to be a type of MVB and refer to the trafficking route from early to late endosomes as the MVB pathway.
Box 2. Essentials of exosomes.
Several comprehensive reviews on exosomes have been recently published [33,87,93-96]. Here are the essentials:
Who?
Exosomes were first identified more than 25 years ago, when they were observed as vesicles released by immature red blood cells during differentiation [97]. Exosomes should not be confused with the macromolecular exosome complex, which comprises 3′ → 5′ exonucleases and functions in RNA surveillance [98]. Given that several cell types shed vesicles of various sizes and compositions, a few caveats for using the term ‘exosomes’ have been established [95,96]: vesicles classified as exosomes should be ~30–100 nm in diameter; they should originate as intralumenal vesicles of the MVB pathway released by fusion of the limiting membrane of the MVB with the plasma membrane (as opposed to vesicles shed directly from the cell surface [99]); and, irrespective of the cell type of origin, they should harbor certain proteins and lipids in common (see below).
Where?
Exosomes are released by cells of hematopoietic origin (e.g. B- and T-lymphocytes, platelets, dendritic cells, mast cells, reticulocytes) and non-hematopoietic origin (e.g. neurons, intestinal epithelial cells and tumor cells) [33]. Exosomes are also found in physiological fluids such as plasma, urine [23,45], amniotic fluid [45] and malignant effusions [33].
What?
The protein composition of exosomes reflects the cells from which they are released and their putative functions; exosomes from antigen-presenting cells carry MHC molecules [24,54,100], urinary exosomes have aquaporin-2 and carbonic anhydrase [23], and exosomes released by enterocytes harbor intestinal enzymes [101]. Exosomes produced by different cells contain certain proteins in common, such as those involved in membrane trafficking (e.g. Rabs and annexins) and in MVB biogenesis (e.g. Tsg101 and Alix), and proteins commonly found in ILVs (e.g. tetraspanins CD63 and CD81, etc.), and lack proteins of the MVB limiting membrane (e.g. LAMP-1 and LAMP-2) [23,24,100]. Exosomal membranes are rich in sphingolipids and cholesterol, and they display an increased rate of transbilayer movement of phospholipids, leading to loss of lipid asymmetry and the presence of phosphatidylserine in the outer leaflet[22,87,102,103].
Why?
Many diverse functions have been attributed to exosomes; B-cell exosomes with MHC class I and II, co-stimulatory and adhesion molecules can stimulate antigen-specific T-cell responses and can also participate in integrin-mediated adhesion and signaling. Dendritic cell-derived exosomes loaded with tumor antigens are being investigated for cancer immunotherapy [104]. Exosomal ‘vaccines’ engineered to display viral spike proteins on their membranes can produce neutralizing levels of antibodies when injected into mice [105]. Exosomes in physiological fluids might help in identifying biomarkers for human diseases [23]. Exosomes participate in tumor invasion and transmit viruses, prions and other infectious material [33,106]. Budding of HIV virions occurs in MVB and might use the same molecular machinery involved in exosome biogenesis [26,107]. HIV particles captured by immature dendritic cells escape degradation through exosomes and are capable of trans-infecting T-cells [108].
How?
Filtration and differential centrifugation of cell culture supernatants followed by flotation on sucrose gradients is used to isolate and purify exosomes. Electron microscopy and biochemical analyses are then used to confirm that these vesicles are indeed exosomes. A detailed step-by-step guide on how to isolate and characterize exosomes has recently been published [109]. Proteomic analysis of exosomes is increasingly being performed to identify their protein cargo and to correlate this information with putative functions. Briefly, this involves isolation of exosomes, initial separation of proteins by polyacrylamide gel electrophoresis (PAGE) or high performance liquid chromatography (HPLC), digestion of selected proteins, and, finally, mass spectrometry to identify the proteins [110].
Box 3. A tale of rings, cones and boomerangs.
Intralumenal vesicles are formed when the endosomal membrane bends with a negative curvature away from the cytoplasm (topologically equivalent to outward budding at the plasma membrane), as opposed to the positive curvature of vesicles budding into the cytoplasm during endocytosis. A common theme emerging from studies on ILV biogenesis is that cargo and ultimate fate dictate the mechanisms used to generate negative curvature which involves a fascinating interplay between the intrinsic geometries of proteins and lipids. Ubiquitin, ESCRT proteins and cargo such as EGFR drive the formation of ILVs enriched in Ptd(Ins)3P which head to lysosomes [9]. Until recently, evidence for a direct involvement of ESCRT proteins in ILV biogenesis was lacking. New data point to a role for two isoforms of the ESCRT-III protein CHMP4 (charged multivesicular body protein 4, the human ortholog of yeast snf7 (sucrose non-fermenting 7)) in generating negative membrane curvature. When overexpressed in cells, snf7 assembles into filamentous polymers at the membrane which are arranged into rings and induce the formation of buds and tubules that protrude outward [111]. The late endosomal anionic lipid lysobisphosphatidic acid (LBPA) is a cone-shaped lipid that can generate spontaneous negative curvature as a result of surface area difference between the outer and inner leaflets of the bilayer. LBPA has been shown to induce inward budding in liposomes and Alix (apoptosis-linked gene 2 (ALG-2)-interacting protein X), the mammalian orthologue of yeast Bro1, regulates the fission-fusion balance in LBPA-containing liposomes [112]. The Bro1 domain of Alix is shaped like a boomerang with a convex face composed of basic residues that can bind negatively charged membranes and act as a sensor of negative curvature [18,113,114]. Viruses and bacterial toxins that need to escape from MVBs into the cytosol by back-fusion are sorted into LBPA-rich ILVs, a process that is regulated by Alix [10]. Intriguing new work shows that ceramide, another coneshaped lipid generated by hydrolysis of sphingomyelin by sphingomyelinases, is essential for forming ILVs that are eventually released as exosomes by an oligodendrocyte cell line [22]. Sorting of the raft-associated myelin proteolipid protein into exosomes required the activity of neutral sphingomyelinase 2 but not Alix or ESCRT proteins Hrs, Tsg101 and Vps4. Alix, however, is responsible for sorting transferrin receptor into exosomes in reticulocytes [19], suggesting that there may be cell type- and cargo-specific differences in the requirement for class E Vps proteins. The data on LBPA and ceramide show that endogenously produced cone-shaped lipids can induce the formation of different populations of ILVs, one destined for cytosolic release and the other for release into the extracellular space as exosomes. This is supported by the observation that exosomes are LBPA-poor but rich in ceramide[22,102]. Different mechanisms of generating different ILV populations suggest a hitherto under-appreciated complexity of the MVB pathway; however many outstanding questions remain regarding how ceramide-induced budding is regulated, how various ILV populations are sorted to their final destinations and what the potential implications are for polarized cells.
Acknowledgments
This work was supported by the National Institutes of Health (GM34107 and EY08538), the Research to Prevent Blindness Foundation, the Dyson Foundation and the American Health Assistance Foundation
References
- 1.Perret E, et al. Evolving endosomes: how many varieties and why? Curr. Opin. Cell Biol. 2005;17:423–434. doi: 10.1016/j.ceb.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Gruenberg J. The endocytic pathway: a mosaic of domains. Nat. Rev. Mol. Cell Biol. 2001;2:721–730. doi: 10.1038/35096054. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Boulan E, et al. Organization of vesicular trafficking in epithelia. Nat. Rev. Mol. Cell Biol. 2005;6:233–247. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- 4.Zerial M, McBride H. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 5.Mukherjee S, et al. Endocytic sorting of lipid analogues differing solely in the chemistry of their hydrophobic tails. J. Cell Biol. 1999;144:1271–1284. doi: 10.1083/jcb.144.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunn KW, et al. Iterative fractionation of recycling receptors from lysosomally destined ligands in an early sorting endosome. J. Cell Biol. 1989;109:3303–3314. doi: 10.1083/jcb.109.6.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McMahon HT, Gallop JL. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 2005;438:590–596. doi: 10.1038/nature04396. [DOI] [PubMed] [Google Scholar]
- 8.Russell MR, et al. Molecular mechanisms of late endosome morphology, identity and sorting. Curr. Opin. Cell Biol. 2006;18:422–428. doi: 10.1016/j.ceb.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 9.White IJ, et al. EGF stimulates annexin 1-dependent inward vesiculation in a multivesicular endosome subpopulation. EMBO J. 2006;25:1–12. doi: 10.1038/sj.emboj.7600759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Goot FG, Gruenberg J. Intra-endosomal membrane traffic. Trends Cell Biol. 2006;16:514–521. doi: 10.1016/j.tcb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 11.van Niel G, et al. Intestinal epithelial cells secrete exosomelike vesicles. Gastroenterology. 2001;121:337–349. doi: 10.1053/gast.2001.26263. [DOI] [PubMed] [Google Scholar]
- 12.Hsiung F, et al. Dependence of Drosophila wing imaginal disc cytonemes on Decapentaplegic. Nature. 2005;437:560–563. doi: 10.1038/nature03951. [DOI] [PubMed] [Google Scholar]
- 13.Sowinski S, et al. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat. Cell Biol. 2008;10:211–219. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- 14.Saksena S, et al. ESCRTing proteins in the endocytic pathway. Trends Biochem. Sci. 2007;32:561–573. doi: 10.1016/j.tibs.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Williams RL, Urbe S. The emerging shape of the ESCRT machinery. Nat. Rev. Mol. Cell Biol. 2007;8:355–368. doi: 10.1038/nrm2162. [DOI] [PubMed] [Google Scholar]
- 16.Hurley JH. ESCRT complexes and the biogenesis of multivesicular bodies. Curr. Opin. Cell Biol. 2008;20:4–11. doi: 10.1016/j.ceb.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Babst M. A protein’s final ESCRT. Traffic. 2005;6:2–9. doi: 10.1111/j.1600-0854.2004.00246.x. [DOI] [PubMed] [Google Scholar]
- 18.Odorizzi G. The multiple personalities of Alix. J. Cell Sci. 2006;119:3025–3032. doi: 10.1242/jcs.03072. [DOI] [PubMed] [Google Scholar]
- 19.Geminard C, et al. Degradation of AP2 during reticulocyte maturation enhances binding of hsc70 and Alix to a common site on TFR for sorting into exosomes. Traffic. 2004;5:181–193. doi: 10.1111/j.1600-0854.2004.0167.x. [DOI] [PubMed] [Google Scholar]
- 20.Reggiori F, Pelham HR. Sorting of proteins into multivesicular bodies: ubiquitin-dependent and -independent targeting. EMBO J. 2001;20:5176–5186. doi: 10.1093/emboj/20.18.5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Theos AC, et al. A lumenal domain-dependent pathway for sorting to intralumenal vesicles of multivesicular endosomes involved in organelle morphogenesis. Dev. Cell. 2006;10:343–354. doi: 10.1016/j.devcel.2006.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trajkovic K, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 23.Pisitkun T, et al. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. U. S. A. 2004;101:13368–13373. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thery C, et al. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J. Immunol. 2001;166:7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 25.Kramer-Albers EM, et al. Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: trophic support for axons? Proteomics Clin. Appl. 2007;1:1446–1461. doi: 10.1002/prca.200700522. [DOI] [PubMed] [Google Scholar]
- 26.Fang Y, et al. Higher-order oligomerization targets plasma membrane proteins and HIV gag to exosomes. PLoS Biol. 2007;5:e158. doi: 10.1371/journal.pbio.0050158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Gassart A, et al. Lipid raft-associated protein sorting in exosomes. Blood. 2003;102:4336–4344. doi: 10.1182/blood-2003-03-0871. [DOI] [PubMed] [Google Scholar]
- 28.Chatterjee S, et al. GPI anchoring leads to sphingolipid-dependent retention of endocytosed proteins in the recycling endosomal compartment. EMBO J. 2001;20:1583–1592. doi: 10.1093/emboj/20.7.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muntasell A, et al. T cell-induced secretion of MHC class II-peptide complexes on B cell exosomes. EMBO J. 2007;26:4263–4272. doi: 10.1038/sj.emboj.7601842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vidal M, et al. Aggregation reroutes molecules from a recycling to a vesicle-mediated secretion pathway during reticulocyte maturation. J. Cell Sci. 1997;110:1867–1877. doi: 10.1242/jcs.110.16.1867. [DOI] [PubMed] [Google Scholar]
- 31.Parton RG, et al. Meeting of apical and basolateral endocytic pathways of the madin-darby canine kidney cell in late endosomes. J. Cell Biol. 1989;109:3259–3272. doi: 10.1083/jcb.109.6.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi A, et al. A novel requirement for C. elegans Alix/ALX-1 in RME-1-mediated membrane transport. Curr. Biol. 2007;17:1913–1924. doi: 10.1016/j.cub.2007.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Niel G, et al. Exosomes: a common pathway for a specialized function. J. Biochem. (Tokyo) 2006;140:13–21. doi: 10.1093/jb/mvj128. [DOI] [PubMed] [Google Scholar]
- 34.Andrews NW, Chakrabarti S. There’s more to life than neurotransmission: the regulation of exocytosis by synaptotagmin VII. Trends Cell Biol. 2005;15:626–631. doi: 10.1016/j.tcb.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Savina A, et al. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J. Biol. Chem. 2003;278:20083–20090. doi: 10.1074/jbc.M301642200. [DOI] [PubMed] [Google Scholar]
- 36.Stoeck A, et al. A role for exosomes in the constitutive and stimulus-induced ectodomain cleavage of L1 and CD44. Biochem. J. 2006;393:609–618. doi: 10.1042/BJ20051013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 38.Raposo G, et al. Accumulation of major histocompatibility complex class II molecules in mast cell secretory granules and their release upon degranulation. Mol. Biol. Cell. 1997;8:2631–2645. doi: 10.1091/mbc.8.12.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faure J, et al. Exosomes are released by cultured cortical neurones. Mol. Cell. Neurosci. 2006;31:642–648. doi: 10.1016/j.mcn.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 40.Menager MM, et al. Secretory cytotoxic granule maturation and exocytosis require the effector protein hMunc13-4. Nat. Immunol. 2007;8:257–267. doi: 10.1038/ni1431. [DOI] [PubMed] [Google Scholar]
- 41.Stinchcombe J, et al. Linking albinism and immunity: the secrets of secretory lysosomes. Science. 2004;305:55–59. doi: 10.1126/science.1095291. [DOI] [PubMed] [Google Scholar]
- 42.Charette SJ, Cosson P. Altered composition and secretion of lysosome-derived compartments in dictyostelium AP-3 mutant cells. Traffic. 2008;9:588–596. doi: 10.1111/j.1600-0854.2008.00706.x. [DOI] [PubMed] [Google Scholar]
- 43.Proux-Gillardeaux V, et al. Expression of the Longin domain of TI-VAMP impairs lysosomal secretion and epithelial cell migration. Biol. Cell. 2007;99:261–271. doi: 10.1042/BC20060097. [DOI] [PubMed] [Google Scholar]
- 44.Jaiswal JK, et al. Membrane proximal lysosomes are the major vesicles responsible for calcium-dependent exocytosis in nonsecretory cells. J. Cell Biol. 2002;159:625–635. doi: 10.1083/jcb.200208154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keller S, et al. CD24 is a marker of exosomes secreted into urine and amniotic fluid. Kidney Int. 2007;72:1095–1102. doi: 10.1038/sj.ki.5002486. [DOI] [PubMed] [Google Scholar]
- 46.Llorente A, et al. Cholesterol regulates prostasome release from secretory lysosomes in PC-3 human prostate cancer cells. Eur. J. Cell Biol. 2007;86:405–415. doi: 10.1016/j.ejcb.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 47.Lebrand C, et al. Late endosome motility depends on lipids via the small GTPase Rab7. EMBO J. 2002;21:1289–1300. doi: 10.1093/emboj/21.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen H, et al. Cholesterol level regulates endosome mobility via Rab proteins. Biophys. J. 2007;94:1508–1520. doi: 10.1529/biophysj.106.099366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu X, et al. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 2006;66:4795–4801. doi: 10.1158/0008-5472.CAN-05-4579. [DOI] [PubMed] [Google Scholar]
- 50.Savina A, et al. The exosome pathway in K562 cells is regulated by Rab11. J. Cell Sci. 2002;115:2505–2515. doi: 10.1242/jcs.115.12.2505. [DOI] [PubMed] [Google Scholar]
- 51.Loomis RJ, et al. Citron kinase, a RhoA effector, enhances HIV-1 virion production by modulating exocytosis. Traffic. 2006;7:1643–1653. doi: 10.1111/j.1600-0854.2006.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Castle AM, et al. The minor regulated pathway, a rapid component of salivary secretion, may provide docking/fusion sites for granule exocytosis at the apical surface of acinar cells. J. Cell Sci. 2002;115:2963–2973. doi: 10.1242/jcs.115.14.2963. [DOI] [PubMed] [Google Scholar]
- 53.Khvotchev MV, et al. Divergent functions of neuronal Rab11b in Ca2+-regulated versus constitutive exocytosis. J. Neurosci. 2003;23:10531–10539. doi: 10.1523/JNEUROSCI.23-33-10531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raposo G, et al. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Denzer K, et al. Follicular dendritic cells carry MHC class II-expressing microvesicles at their surface. J. Immunol. 2000;165:1259–1265. doi: 10.4049/jimmunol.165.3.1259. [DOI] [PubMed] [Google Scholar]
- 56.Morelli AE, et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104:3257–3266. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- 57.Miyanishi M, et al. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 58.Zakharova L, et al. T cell exosomes induce cholesterol accumulation in human monocytes via phosphatidylserine receptor. J. Cell. Physiol. 2007;212:174–181. doi: 10.1002/jcp.21013. [DOI] [PubMed] [Google Scholar]
- 59.Willig KI, et al. STED microscopy reveals that synaptotagmin remains clustered after synaptic vesicle exocytosis. Nature. 2006;440:935–939. doi: 10.1038/nature04592. [DOI] [PubMed] [Google Scholar]
- 60.Bates M, et al. Multicolor super-resolution imaging with photo-switchable fluorescent probes. Science. 2007;317:1749–1753. doi: 10.1126/science.1146598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hundorfean G, et al. Luminal antigens access late endosomes of intestinal epithelial cells enriched in MHC I and MHC II molecules: in vivo study in Crohn’s ileitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:G798–G808. doi: 10.1152/ajpgi.00135.2007. [DOI] [PubMed] [Google Scholar]
- 62.Kapsogeorgou EK, et al. Salivary gland epithelial cell exosomes: A source of autoantigenic ribonucleoproteins. Arthritis Rheum. 2005;52:1517–1521. doi: 10.1002/art.21005. [DOI] [PubMed] [Google Scholar]
- 63.McKechnie NM, et al. Hr44 secreted with exosomes: loss from ciliary epithelium in response to inflammation. Invest. Ophthalmol. Vis. Sci. 2003;44:2650–2656. doi: 10.1167/iovs.02-0765. [DOI] [PubMed] [Google Scholar]
- 64.Mallegol J, et al. T84-intestinal epithelial exosomes bear MHC class II/peptide complexes potentiating antigen presentation by dendritic cells. Gastroenterology. 2007;132:1866–1876. doi: 10.1053/j.gastro.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 65.Lecuit T. Junctions and vesicular trafficking during Drosophila cellularization. J. Cell Sci. 2004;117:3427–3433. doi: 10.1242/jcs.01312. [DOI] [PubMed] [Google Scholar]
- 66.Leibfried A, Bellaiche Y. Functions of endosomal trafficking in Drosophila epithelial cells. Curr. Opin. Cell Biol. 2007;19:446–452. doi: 10.1016/j.ceb.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 67.Sharma N, et al. Apical targeting of syntaxin 3 is essential for epithelial cell polarity. J. Cell Biol. 2006;173:937–948. doi: 10.1083/jcb.200603132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iero M, et al. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ. 2008;15:80–88. doi: 10.1038/sj.cdd.4402237. [DOI] [PubMed] [Google Scholar]
- 69.Vella LJ, et al. The role of exosomes in the processing of proteins associated with neurodegenerative diseases. Eur. Biophys. J. 2007;37:323–332. doi: 10.1007/s00249-007-0246-z. [DOI] [PubMed] [Google Scholar]
- 70.Zhu AJ, Scott MP. Incredible journey: how do developmental signals travel through tissue? Genes Dev. 2004;18:2985–2997. doi: 10.1101/gad.1233104. [DOI] [PubMed] [Google Scholar]
- 71.Dudu V, et al. Membrane traffic during embryonic development: epithelial formation, cell fate decisions and differentiation. Curr. Opin. Cell Biol. 2004;16:407–414. doi: 10.1016/j.ceb.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 72.Vincent JP, Dubois L. Morphogen transport along epithelia, an integrated trafficking problem. Dev. Cell. 2002;3:615–623. doi: 10.1016/s1534-5807(02)00323-4. [DOI] [PubMed] [Google Scholar]
- 73.Greco V, et al. Argosomes: a potential vehicle for the spread of morphogens through epithelia. Cell. 2001;106:633–645. doi: 10.1016/s0092-8674(01)00484-6. [DOI] [PubMed] [Google Scholar]
- 74.Panakova D, et al. Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature. 2005;435:58–65. doi: 10.1038/nature03504. [DOI] [PubMed] [Google Scholar]
- 75.Willnow TE, et al. Lipoproteins and their receptors in embryonic development: more than cholesterol clearance. Development. 2007;134:3239–3249. doi: 10.1242/dev.004408. [DOI] [PubMed] [Google Scholar]
- 76.Rietveld A, et al. Association of sterol- and glycosylphosphatidylinositol-linked proteins with Drosophila raft lipid microdomains. J. Biol. Chem. 1999;274:12049–12054. doi: 10.1074/jbc.274.17.12049. [DOI] [PubMed] [Google Scholar]
- 77.Liegeois S, et al. The V0-ATPase mediates apical secretion of exosomes containing Hedgehog-related proteins in Caenorhabditis elegans. J. Cell Biol. 2006;173:949–961. doi: 10.1083/jcb.200511072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Forgac M. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat. Rev. Mol. Cell Biol. 2007;8:917–929. doi: 10.1038/nrm2272. [DOI] [PubMed] [Google Scholar]
- 79.Baars TL, et al. Role of the V-ATPase in regulation of the vacuolar fission-fusion equilibrium. Mol. Biol. Cell. 2007;18:3873–3882. doi: 10.1091/mbc.E07-03-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bayer MJ, et al. Vacuole membrane fusion: V0 functions after trans-SNARE pairing and is coupled to the Ca2+-releasing channel. J. Cell Biol. 2003;162:211–222. doi: 10.1083/jcb.200212004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burghoff S, et al. Horizontal gene transfer from human endothelial cells to rat cardiomyocytes after intracoronary transplantation. Cardiovasc. Res. 2008;77:534–543. doi: 10.1093/cvr/cvm071. [DOI] [PubMed] [Google Scholar]
- 82.Deregibus MC, et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110:2440–2448. doi: 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]
- 83.Ratajczak J, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 84.Irion U, Johnston D. bicoid RNA localization requires specific binding of an endosomal sorting complex. Nature. 2007;445:554–558. doi: 10.1038/nature05503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gruenberg J, Stenmark H. The biogenesis of multivesicular endosomes. Nat. Rev. Mol. Cell Biol. 2004;5:317–323. doi: 10.1038/nrm1360. [DOI] [PubMed] [Google Scholar]
- 86.Piper RC, Katzmann DJ. Biogenesis and function of multivesicular bodies. Annu. Rev. Cell Dev. Biol. 2007;23:519–547. doi: 10.1146/annurev.cellbio.23.090506.123319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fevrier B, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr. Opin. Cell Biol. 2004;16:415–421. doi: 10.1016/j.ceb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 88.Katzmann DJ, et al. Receptor downregulation and multivesicular-body sorting. Nat. Rev. Mol. Cell Biol. 2002;3:893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- 89.Aniento F, et al. Cytoplasmic dynein-dependent vesicular transport from early to late endosomes. J. Cell Biol. 1993;123:1373–1387. doi: 10.1083/jcb.123.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gruenberg J, et al. Characterization of the early endosome and putative endocytic carrier vesicles in vivo and with an assay of vesicle fusion in vitro. J. Cell Biol. 1989;108:1301–1316. doi: 10.1083/jcb.108.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Welsch S, et al. Ultrastructural analysis of ESCRT proteins suggests a role for endosome-associated tubular-vesicular membranes in ESCRT function. Traffic. 2006;7:1551–1566. doi: 10.1111/j.1600-0854.2006.00489.x. [DOI] [PubMed] [Google Scholar]
- 92.Dardalhon V, et al. Fractionation analysis of the endosomal compartment during rat reticulocyte maturation. Cell Biol. Int. 2002;26:669–678. doi: 10.1006/cbir.2002.0917. [DOI] [PubMed] [Google Scholar]
- 93.de Gassart A, et al. Exosome secretion: the art of reutilizing nonrecycled proteins? Traffic. 2004;5:896–903. doi: 10.1111/j.1600-0854.2004.00223.x. [DOI] [PubMed] [Google Scholar]
- 94.Keller S, et al. Exosomes: from biogenesis and secretion to biological function. Immunol. Lett. 2006;107:102–108. doi: 10.1016/j.imlet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 95.Thery C, et al. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 96.Johnstone RM. Exosomes biological significance: A concise review. Blood Cells Mol. Dis. 2006;36:315–321. doi: 10.1016/j.bcmd.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 97.Pan BT, et al. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J. Cell Biol. 1985;101:942–948. doi: 10.1083/jcb.101.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Houseley J, et al. RNA-quality control by the exosome. Nat. Rev. Mol. Cell Biol. 2006;7:529–539. doi: 10.1038/nrm1964. [DOI] [PubMed] [Google Scholar]
- 99.Trams EG, et al. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim. Biophys. Acta. 1981;645:63–70. doi: 10.1016/0005-2736(81)90512-5. [DOI] [PubMed] [Google Scholar]
- 100.Escola JM, et al. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J. Biol. Chem. 1998;273:20121–20127. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- 101.Mallegol J, et al. Phenotypic and functional characterization of intestinal epithelial exosomes. Blood Cells Mol. Dis. 2005;35:11–16. doi: 10.1016/j.bcmd.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 102.Laulagnier K, et al. Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem. J. 2004;380:161–171. doi: 10.1042/BJ20031594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wubbolts R, et al. Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J. Biol. Chem. 2003;278:10963–10972. doi: 10.1074/jbc.M207550200. [DOI] [PubMed] [Google Scholar]
- 104.Chaput N, et al. Dendritic cell derived-exosomes: biology and clinical implementations. J. Leukoc. Biol. 2006;80:471–478. doi: 10.1189/jlb.0206094. [DOI] [PubMed] [Google Scholar]
- 105.Kuate S, et al. Exosomal vaccines containing the S protein of the SARS coronavirus induce high levels of neutralizing antibodies. Virology. 2007;362:26–37. doi: 10.1016/j.virol.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fevrier B, et al. Exosomes: a bubble ride for prions? Traffic. 2005;6:10–17. doi: 10.1111/j.1600-0854.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- 107.Pelchen-Matthews A, et al. Endosomes, exosomes and Trojan viruses. Trends Microbiol. 2004;12:310–316. doi: 10.1016/j.tim.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 108.Wiley RD, Gummuluru S. Immature dendritic cell-derived exosomes can mediate HIV-1 trans infection. Proc. Natl. Acad. Sci. U. S. A. 2006;103:738–743. doi: 10.1073/pnas.0507995103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thery C, et al. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006 doi: 10.1002/0471143030.cb0322s30. Chapter 3, Unit 3.22. [DOI] [PubMed] [Google Scholar]
- 110.Olver C, Vidal M. Proteomic analysis of secreted exosomes. Subcell. Biochem. 2007;43:99–131. doi: 10.1007/978-1-4020-5943-8_7. [DOI] [PubMed] [Google Scholar]
- 111.Hanson PI, et al. Plasma membrane deformation by circular arrays of ESCRT-III protein filaments. J. Cell Biol. 2008;180:389–402. doi: 10.1083/jcb.200707031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Matsuo H, et al. Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science. 2004;303:531–534. doi: 10.1126/science.1092425. [DOI] [PubMed] [Google Scholar]
- 113.Fujii K, et al. Beyond Tsg101: the role of Alix in ‘ESCRTing’ HIV-1. Nat. Rev. Microbiol. 2007;5:912–916. doi: 10.1038/nrmicro1790. [DOI] [PubMed] [Google Scholar]
- 114.Kim J, et al. Structural basis for endosomal targeting by the Bro1 domain. Dev. Cell. 2005;8:937–947. doi: 10.1016/j.devcel.2005.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]