Abstract
Respiratory syncytial virus (RSV) is a major cause of pneumonia and wheezing in infants and the elderly, but to date there is no licensed vaccine. We developed a gold nanorod construct that displayed the major protective antigen of the virus, the fusion protein (F). Nanorods conjugated to RSV F were formulated as a candidate vaccine preparation by covalent attachment of viral protein using a layer-by-layer approach. In vitro studies using ELISA, confocal microscopy and circular dichroism revealed that conformation-dependent epitopes were maintained during conjugation, and transmission electron microscopy studies showed that a dispersed population of particles could be achieved. Human dendritic cells treated with the vaccine-induced immune responses in primary human T cells. These results suggest that this vaccine approach may be a potent method for immunizing against viruses such as RSV with surface glycoproteins that are targets for the human immune response.
1. Background
Respiratory syncytial virus (RSV) is a ubiquitous human respiratory pathogen, causing severe disease primarily in children and the elderly. The majority of infants are infected within their first year of life. Worldwide, RSV is the leading viral cause of lower respiratory tract infections, accounting for an estimated 65 million infections and 1 million deaths annually. In the U.S., RSV infection causes over 100,000 hospitalizations per year [1, 2]. RSV infection is also a significant cause of severe respiratory distress among immune compromised and elderly populations [1, 3, 4]. Despite the significant health burden produced by RSV, there is currently no licensed vaccine.
Increasingly, there is a realization that the commonality in sizes and general structural features between nanoparticles and their biological counterparts could translate into significant applications of nanoclusters to a wide variety of fields in biology and medicine. As a vaccine delivery vehicle, gold nanoclusters are very attractive candidates. A gold nanorod of approximately 21 by 57 nm in size is stabilized by protein ligands (2 × 10−8 M on average) on its surface, creating a potential for a high level of presentation of antigen. Naturally-occurring viral particles often are in this size regime, and many viruses such as RSV exhibit a roughly filamentous shape [5–8]. Under appropriate conditions, surface-stabilizing ligands on gold nanoparticles may be exchanged [9, 10], resulting in the potential for formulating a vaccine candidate incorporating both B cell and T helper cell epitopes into a single construct. Finally, colloidal gold is phagocytosed by antigen presenting cells such as macrophages and dendritic cells (DCs) [11–13], which are key players in the induction of protective immune responses. We sought to test whether the major protective antigen for RSV that is a surface protein, the fusion (F) protein, could be displayed on the surface of a gold nanorod to yield a vaccine candidate that induces superior protective efficacy relative to other available delivery systems. To test this hypothesis, we generated an experimental gold nanorod RSV F vaccine candidate and investigated its ability to induce an immune response in primary cultures of human DCs and T lymphocytes. We found that the construct was taken up effectively by DCs, and this inoculation resulted in the robust induction of a human T cell response in cells exposed to vaccine-treated DCs.
The major protective antigen in the RSV virion particle is the fusion (F) protein [14], which mediates fusion of virus and host cell lipid bilayer membranes during viral entry into cells [15]. Antibodies to the head domain of RSV F protein neutralize the virus [16–19], and the protein also induces T cells that are an important component of immunity to the virus [20, 21]. Therefore, we designed the vaccine construct to incorporate a recombinant version of the F protein made as a soluble protein that maintains the trimeric state of the protein as in the virion particle. The soluble protein was expressed in, and secreted by, mammalian cells in culture, facilitating native glycosylation and folding. The secreted protein was purified to a high degree by chromatography.
2. Materials and Methods
2.1 Chemicals
Chloroauric acid (HAuCl4 • 3H2O), sodium borohydride (NaBH4), silver nitrate, ascorbic acid, poly (acrylic acid, sodium salt), (PAA, MW ~ 15,000 g/mol), poly (allylamine hydrochloride), (PAH, MW ~ 15,000 g/mol), and sodium chloride, ≥ 99 %, were obtained from Aldrich and used as is. Hexadecyltrimethylammonium bromide (CTAB), Sigma Ultra, 99%, and 2-(N-Morpholino)ethanesulfonic acid (MES for buffer) were obtained from Sigma chemicals and used without further purification. 1-Ethyl-3-[3- dimethylaminopropyl]carbodiimide Hydrochloride (EDC) was obtained from Pierce chemicals. Deionized (DI) water (resistivity ~ 18.2 MΩ) was used for all experiments and dilutions unless otherwise noted.
2.2 Gold Nanorod Preparation
Gold nanorods were prepared as follows. A volume 0.6 mL of ice cold NaBH4 was added to a solution containing 0.1 M CTAB and 2.5 × 10 −4 M HAuCl4 under vigorous stirring for 10 min. The resulting solution contained approximately 4 nm gold spheres that were used as a “seed” solution in the preparation of nanorods. To a 250 mL flask the following reagents were added in order: 95 mL CTAB, 800 μL AgNO3, 5 mL HAuCl4, 550 μL ascorbic acid, and 120 μL seed solution. The final solution was mixed gently and allowed to sit overnight ≥ 12 hours, followed by purification via two rounds of centrifugation (to remove excess CTAB and metallic ions) at 9,384 x g for 20 min and resuspended in distilled water at 25% the original volume.
2.3 PAA Coating
A volume of 200 μL of a PAA solution (10 mg/mL) was added to two 1 mL aliquots of gold nanorods simultaneously with 100 μL of a 10 mM NaCl solution. The resulting solution was allowed to mix for 30 min, followed by one round of centrifugation (17,000 x g for 8–10 min) and resuspended in 2 × 1 mL distilled water.
PAH Coating
PAA-coated rods were coated further with a layer of PAH by the addition of 200 μL of PAH (10 mg/mL) added simultaneously with 100 μL of a 10 mM NaCl solution. The resulting solution was mixed for 30 min followed by purification via centrifugation (17,000 x g for 8–10 min) and resuspended in 2 × 1 mL MES buffer pH 5.5. Samples were stored at room temperature for further surface modification.
2.4 EDC-Mediated Covalent RSV Fusion Protein Attachment
To each of the 1 mL PAH coated rods, 54 μg, (3 × 10−7 M), of recombinant RSV fusion protein was added followed by 15 min of mixing. Then 1.3 mg of EDC was added to each tube, mixed for several minutes, and stored overnight at 4 °C. Samples were purified via two rounds of centrifugation (17,000 x g for 10 min) and combined into 1 mL total volume in sterile water.
2.5 Recombinant RSV Fusion (F) Protein
The RSV F ectodomain construct (pcDNA3.1-FECTO-myc/His), was obtained by PCR amplification of the gene encoding the F protein ectodomain, removing the cDNAs encoding the transmembrane domain and cytoplasmic tail regions, from a full-length RSV F protein sequence optimized cDNA. The PCR product was cloned directionally into the pcDNA3.1/myc-His B plasmid (Invitrogen) using restriction sites 5′-BamHI-EcoRI - 3′. Ligated product was transformed into competent E. coli strain DH5α cells, and plasmids purified with the QIAprep Miniprep Kit (Qiagen). All plasmid constructs were sequenced to confirm in-frame cloning with the C-terminal c-myc epitope and polyhistidine (6x His) tag of the expression vector. The pcDNA3.1-FECTO-myc/His recombinant plasmid was transfected into suspension 293-F cells, as recommended by the manufacturer (Freestyle 293 Expression System, Invitrogen). Transfected cells were incubated for 4 days, then the cells were centrifuged for 10 minutes at 100 x g at 4 °C and supernatant filtered through 0.2 μm filters prior to purification and stored at 4 °C. Purification of 6xHis-tagged RSV FECTO protein was performed by immobilized metal ion affinity chromatography using pre-packed 5 mL HisTrap HP Ni-Sepharose columns (GE Healthcare Bio-Sciences AB, Uppsala, Sweden), according to the manufacturer’s recommended protocol. Purified protein was concentrated and dialyzed against phosphate buffered saline through Amicon Ultra centrifugal filters with a 30 kD molecular weight cutoff (Millipore, Billerica, MA). Purified preparations were identified by denaturing, non-reducing SDS-PAGE analysis as a single, silver-stained band at the expected 60 kD migration. Concentration was determined by the Bradford dye assay. Purified RSV F protein was stored at 4 °C until analyzed.
2.6 Particle Stability Measurements
UV-vis spectroscopy was performed on an Agilent model 8453, 845 X UV-vis spectrophotometer and used to assess particle stability in 1X PBS and 1% serum containing medium before and after protein coatings.
2.7 Transmission Electron Microscopy (TEM)
TEM measurements were performed on a Philips TEM instrument operating at an accelerating voltage of 160 kV. For TEM, grids were prepared by placing a carbon coated copper grid on top of the samples for 2 hours, and then drying them.
2.8 Enzyme Linked Immunosorbent Assay (ELISA)
Purified RSV F protein was concentrated to 1 mg/mL as indicated by A280 and used for both nanorod conjugations and standard curves. The monoclonal antibody palivizumab (Medimmune) was used as capture antibody; a volume of 100 μL at 10 μg/mL was added to 22 wells of a 96-well plate and allowed to incubate for 1 hour. After 3 washes in 300 μL of PBS, 300 μL of a 2 % by weight BSA solution was added to all wells and allowed to incubate for ≥ 1 hour. The BSA solution was removed and 100 μL of serially diluted RSV F protein (beginning at 1:1,000 dilution and continuing by half until a total of 7 wells was occupied), PBS, water, nanorod-fusion protein conjugate (1:10 dilution), supernatant of nanorod-RSV F protein reaction after the first centrifugation (1:100 dilution), and supernatant of nanorod-RSV F reaction after the second centrifugation (1:10 dilution), and any additional supernatants from washing steps were each added in triplicate to the blocked wells of the 96-well plate and incubated for 1 hour. Following antigen binding, all wells were rinsed 3 times with PBS followed by the addition to all antigen bound wells of 100 μL of a mixture of three purified murine monoclonal antibodies that bind to RSV F protein. After a one-hour incubation, the wells were rinsed 3 times with PBS followed by the addition of 100 μL secondary antibody (goat anti-human HRP conjugate) at a 1:5,000 dilution. After 1 hour, the wells were rinsed 5 times with PBS and 100 μL of tetramethylbenzidine substrate was added for a period of 10 mins. The reaction was quenched with 2M H2SO4, and the absorbance measured at 450 nm. Plate reader experiments were performed on a Bio-Tex, Synergy HT spectrofluorimeter instrument with an absorption wavelength of 450 nm at maximum sensitivity. Fourier Transform Infrared Spectroscopy (FTIR) measurements were carried out on a Burker Tensor 27 FTIR microscope in the reflection mode. 360 scans of each sample were recorded at an amplifier resolution of 0.05 V. Samples were drop cast on silica substrates.
2.9 Dendritic Cell (DC) Maturation and T cell Activation
Human peripheral blood was collected with the approval of the Vanderbilt University Institutional Review Board. Sixty mL of peripheral blood was collected from healthy adult donors. Peripheral blood mononuclear cells (PBMCs) were isolated using density gradient centrifugation using Ficoll (Sigma Histopaque 1077). Monocytes were isolated using CD14+ magnetic beads (Miltenyi, cat. 130-050-201), as directed by the manufacturer. Dendritic cells (DCs) were derived from monocytes in the presence of recombinant human GM-CSF (800 IU/mL, PeproTech Inc.) and recombinant human IL-4 (500 IU/mL, PeproTech Inc) in Aim V medium (Invitrogen) supplemented with 10% human AB serum (Sigma) at a concentration of 1 × 106 cells/mL for 6 days. Concurrently, T cells were isolated from the sample donor PBMCs using CD3+ magnetic beads (Miltenyi, cat. 130-050-101), as directed by the manufacturer. T cells were cultured in AimV medium supplemented with 10% human AB serum, 1% non-essential amino acids (Invitrogen), 2 mM L-glutamine (Mediatech), penicillin-streptomycin (Mediatech), 0.1% β-mercaptoethanol (Sigma), and recombinant human IL-2 (100 IU/mL, PeproTech Inc.) at a concentration of 1 × 106 cells/mL.
Monocyte-derived DCs (MDDCs) (2 × 104 cells) were pulsed with PBS, purified RSV fusion (F) protein in PBS, RSV F protein coated-nanorods in PBS, or PAH-coated nanorods in PBS for two hours at 37° C. DCs were pelleted, washed two times with 1X PBS, resuspended in AimV complete DC medium as described above, and cultured overnight at 37° C in 5% CO2. The next day, 2 × 105 T cells were stained with CFSE (Invitrogen) as directed by the manufacturer and were overlaid onto donor-matched nanorod-activated MDDCs. Cells were co-cultured for 4 days. T cell proliferation was analyzed using a custom five-laser LSRII cytometer (BD Biosciences). Flow cytometry data sets were analyzed using FlowJo software v7.5 (TreeStar).
2.10 Circular Dichroism (CD) Measurements
CD measurements were collected on an AVIV spectrometer using a 10 mm low volume cuvette. Spectra were collected from 260-185 nm at a bandwidth of 2 nm and an averaging time of 2 seconds at a protein concentration of 8 × 10−8 M. Molecular grade water was run under the same experimental conditions and the signal from that solution subtracted from all samples. The signal from PAH-coated rods also was subtracted from F protein-coated rods in order to eliminate any contribution from gold nanorods on the observed spectra. Secondary structure analysis was performed using Borland Delphi CD Spectra Deconvolution software Version 2.1, using advanced spectra deconvolution analysis.
3. Results
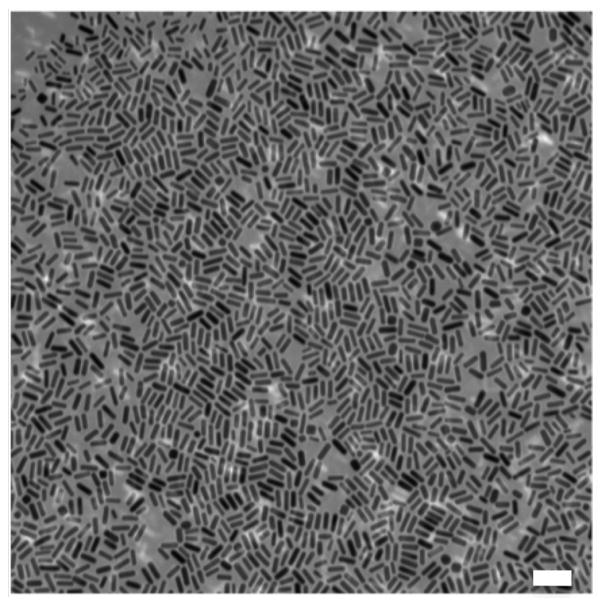
Gold nanorods were synthesized using a seed-mediated approach, as previously described [22,23]. The synthesized gold nanorods maintain a cetyltrimethylammonium bromide (CTAB) bilayer on their surface, which provides water solubility and results in a net positive surface charge. Figure 1 shows a representative transmission electron micrograph (TEM) image of particles used in this study, which revealed a monodispersed solution of particles having a rod-like morphology with an average width and length of 21 nm ± 2.0 nm and 57 nm ± 3.4 nm, respectively.
Figure 1.
TEM image of gold nanorods used in this study. Scale bar = 100 nm in length.
The conjugation approach we used is outlined in Figure 2. Gold nanorods were prepared for covalent attachment of viral protein using a layer-by-layer approach [24, 25], with the charged polyelectrolytes poly(acrylic acid) (PAA) and polyallyamine hydrogen chloride (PAH), respectively, resulting in exposed primary amine functionality on the particle surface. RSV fusion protein was added to the nanorod solution in excess (3 × 10−7 M), followed by the subsequent addition of the carbodiimide EDC for 14–16 hours in order to induce amide bond formation between nanorods and proteins. Total protein concentrations of the conjugated preparations were determined by enzyme linked immunosorbent assay (ELISA) in comparison to known standards and found to be 2 × 10−8 M, on average. Supernatants from both rounds of centrifugation also were assayed for completeness, indicating an approximate 70% total protein recovery rate with ~ 8% bound to the nanorod surface. Fourier transform infrared spectroscopy (FTIR) measurements (see Supplemental Figure 1) were used to confirm the presence of amide I (~ 1630–1695 cm−1) and amide II (~1500–1650 cm−1) bands of protein-conjugated nanorods, which was consistent with the IR trace for protein alone.
Figure 2.
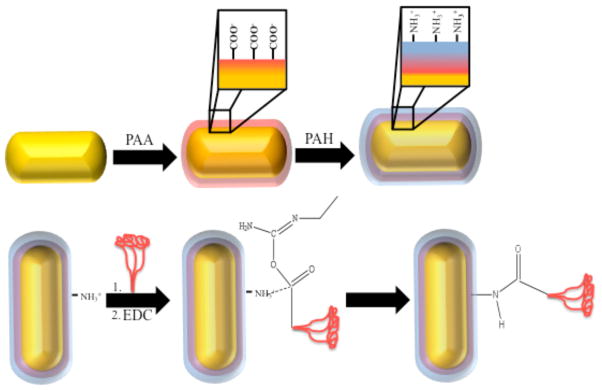
Schematic showing the method of conjugation of F protein (represented by red structure) to the gold nanorod surface.
Figure 3 shows a micrograph of nanorods obtained by TEM before (a) or after (b) protein conjugation. The micrographs confirmed particle integrity and stability following F protein conjugation. Furthermore, stability tests were performed by treating as-prepared nanorods, PAH-coated nanorods, or F protein-coated nanorods with 1X PBS (pH 7.4) in order to determine particle integrity under physiological pH and osmolarity. Particles were tested using ultra-violet visible (UV-vis) absorption after 1X PBS addition at 5-min intervals, up to 20 mins. Traces from each sample were overlaid and compared by monitoring red shifts and broadening in plasmon bands, characteristics consistent with particle aggregation (Figure 4A–B). While a slight broadening and red shift was observed, RSV F protein-coated particles exhibited substantially better stability when compared to plain PAH-coated particles, providing additional evidence that the substantial coverage of particles with protein had been achieved. Additionally, RSV F protein-coated particles were assayed in phenol-red-free cell medium (OPTI-MEM® I) containing 1 % fetal bovine serum and found to further maintain stability (see Supplemental Figure 2).
Figure 3.
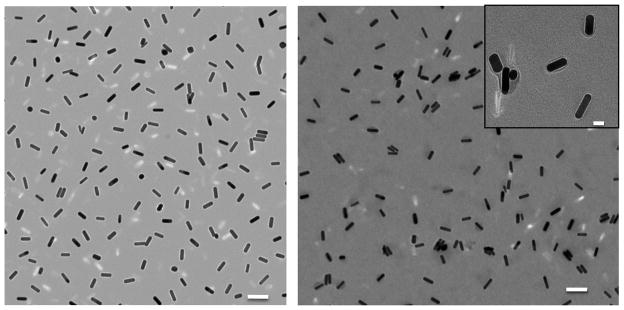
Transmission electron micrograph of PAH coated gold nanorods (left) and fusion protein coated gold nanorods (right). Scale bar = 100 nm. Inset scale bar = 20 nm.
Figure 4.
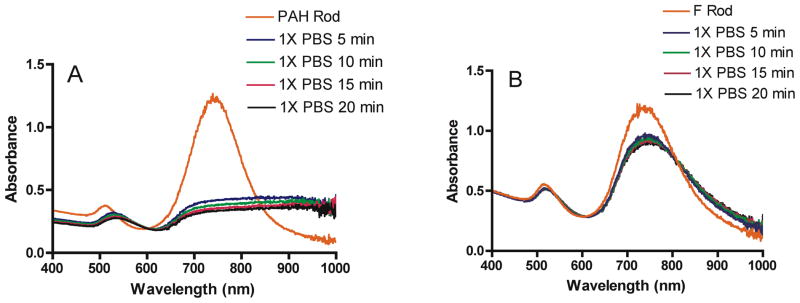
Absorbance in the UV-visible spectrum of A) PAH-coated rods or B) RSV F protein-coated rods after resuspension in 1X PBS. Spectra were collected every 5 min up to 20 min.
The RSV F protein contains significant proportions of beta sheet, alpha helical, and random coil structures in the predicted structure of the trimeric F protein [26]. We tested the vaccine construct using circular dichroism (CD), to test whether the RSV F protein-conjugated to nanorods maintained these three secondary motifs. For circular dichroism (CD) measurements, F protein was conjugated at a concentration of 8 × 10−8 M in order to improve CD signal. Furthermore, as a result of the higher protein concentrations, samples used for CD analysis were subjected to two additional rounds of centrifugation at 17,000 x g for 10 min. CD measurements were collected and monitored from 260-185 nm using protein alone, nanorods, PAH-coated nanorods, or RSV F protein-coated nanorods. Protein-coated nanorods maintained a secondary structure similar to that of free protein, suggesting retention of optimal protein folding after rod conjugation (Figure 5). PAH and F protein were run together in the absence of rods indicating no significant change in protein secondary structure (data not shown). While these data confirmed an intact secondary structure for F protein on the nanorod surface, there was a significant increase (22% → 35%) in helicity post nanoparticle conjugation.
Figure 5.
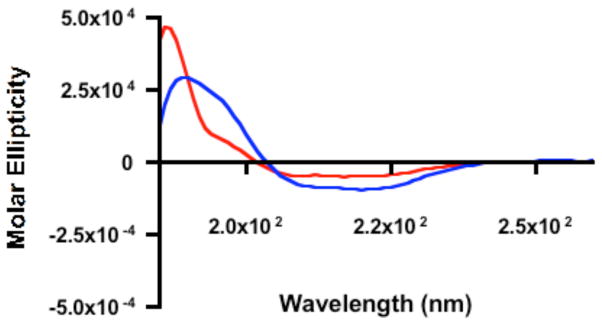
Circular dichroism of RSV F protein (red trace) or RSV F protein-nanorod conjugates (blue trace) suggests an intact secondary structure after F protein-nanorod conjugation. Specifically, F protein: helix: 22%, beta sheet: 42% random coil: 36%; F protein-nanorod conjugates: helix: 35%, beta sheet: 31% random coil: 34%.
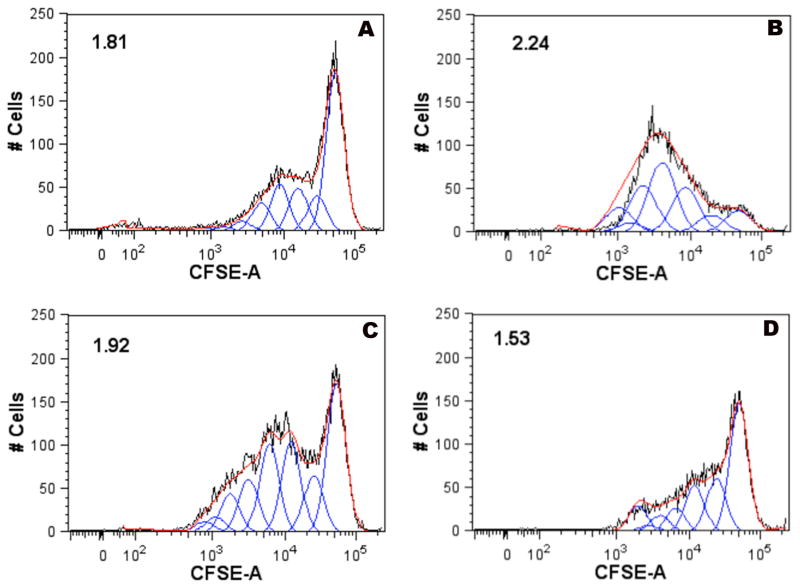
The purpose of developing the vaccine construct was to test whether gold nanorods could deliver protective viral antigens effectively to human antigen presenting cells in order to initiate the induction of a protective immune response. Therefore, we next tested the ability of the F protein-conjugated nanorods to be taken up by human primary DCs and induce active immune responses in primary human T cells. This co-culture system has been shown previously to mimic the interactions that occur in human lymph nodes following infection or vaccination. Prior to mixing with immunized DCs, the T cells were labeled in order to monitor the subsequent degree of T cell proliferation induced by immunized DCs via flow cytometry using a nuclear intercalating dye (CFSE 5-[and-6]-carboxyfluorescein diacetate, succinimidyl ester, mixed isomers). Figure 6 shows a representative flow cytometric plot that shows the dilution of the nuclear dye in discrete intervals (indicated by peaks of increasing lower mean fluorescence as the dye becomes diluted by cell division). The results showed that DCs that had been exposed to RSV F protein-coated nanorods induced significantly more proliferation of stimulated T cells (Proliferation Index =1.92) compared with that induced by DCs treated with PAH-coated rods (Proliferation Index =1.53) or PBS alone (Proliferation Index =1.81), and slightly less than that of pure fusion protein at similar total protein concentrations. Similar results were observed for a second donor. The substantial T cell proliferation observed clearly suggests that the nanorod-F protein conjugates are not significantly cytotoxic.
Figure 6.
Flow cytometric analysis of proliferation of human T cells exposed to autologous DCs treated with solutions as follows (a) PBS, (b) RSV F protein alone, (c) RSV F protein-coated nanorods, or (d) PAH-coated rods. The X-axis shows the fluorescence intensity of cells labeled with the nuclear stain CFSE on a log10 scale. The Y-axis shows the # of cells at the indicated fluorescence. A shift of the curves from the right of the plots to the left indicates a dilution of the dye on a per-cell basis, consistent with proliferation of cells. Values shown in the upper left hand corners for each plot are the corresponding proliferation indexes, where proliferation index: is the total number of divisions divided by the number of cells that went into division. The proliferation index only takes into account the cells that underwent at least one division, i.e., only responding cells are reflected in the proliferation index. This value is useful to compare from sample to sample, as it considers only the fraction of responding cells.
4. Discussion and Conclusion
A covalent attachment of viral protein to nanorod is preferred over an electrostatic interaction for attachment, due to the need for high stability of the complex in a physiological environment after inoculation in vivo. This conjugation approach provides superior particle stability at physiological pH and osmolarity likely due the buffering protein layer. The increased helicity of nanorod-F protein composites may be explained by the conjugation approach that results in covalent amide bond formation between a previously PAH-coated nanorod and the protein allowing for the observed change in secondary structure. This finding is in contrast to other reports that rely on physical absorption of peptides or proteins to nanoparticle surfaces using electrostatic attachment and/or hydrophobic interactions, often resulting in loss of helical content [27, 28]. Furthermore, the use of colloidal gold nanoparticles as a template for vaccine development is limited, and to our knowledge this report is the first example using gold nanoparticles with a rod-like morphology as a template for full-length antigenic protein conjugation for vaccine delivery.
These results show that conjugation of a protective glycoprotein antigen from the major human viral pathogen RSV stabilizes an optimal protein conformation, facilitates interaction with human antigen presenting cells, and allows efficient induction of human immune responses to the viral protein. From a practical perspective, the protein-encapsulated nanorods are processed easily. The combination of the chemical and physical properties of protein-conjugated gold nanorods offers a robust platform for the development of a potential new vaccine candidate. Such a combination of properties stem, in large part, from the unique nanoscale properties of these materials. Consequently, monolayer protected clusters present an effective modular scaffold to bridge the gap between traditional particulate formulation and chimeric biological formulations.
These data provide biologic evidence of proof-of-concept that viral proteins covalently attached to gold nanoparticles can induce human T cell proliferation, evidence of immune stimulation. More extensive quantitative studies are needed to determine the extent to which gold nanorods may augment an RSV F response.
Supplementary Material
Acknowledgments
Support: The work was supported by grants from the March of Dimes, NIH grants R21 AG30321 (NIA) and R01 EB04537 (NIBIB), and a Clinical Scientist Award in Translational Research from the Burroughs Wellcome Foundation (to JEC). JWS was supported by NIH training grant T90 DA22873. NJT was supported by NIH training grant T32 HL069765 and F32 AI080117. Vanderbilt University supported the work through an Innovation and Discovery in Engineering and Science (IDEAS) award. Flow cytometry experiments were performed in the VMC Flow Cytometry Shared Resource of Vanderbilt University Medical Center’s Digestive Disease Research Center (supported by NIH grant P30 DK058404) and the Vanderbilt Ingram Cancer Center (supported by NIH grant P30 CA68485).
We thank Dr. Jonas Perez for helpful discussions during the course of this work, Dr. Anh Hoang for technical support in IR spectroscopy experiments, and Frances House for excellent technical support in virology.
Footnotes
Supporting Information Available. Detailed descriptions of methods and materials are provided. Results from experiments referenced above showing data from Fourier transform spectroscopy (FTIR) measurements are included, as well as particle stability in serum-containing medium, as measured using UV-vis spectroscopy.
References
- 1.Thompson WW, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 2.Shay DK, et al. Bronchiolitis-associated mortality and estimates of respiratory syncytial virus-associated deaths among US children, 1979–1997. J Infect Dis. 2001;183(1):16–22. doi: 10.1086/317655. [DOI] [PubMed] [Google Scholar]
- 3.Falsey AR, et al. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352(17):1749–59. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 4.Walsh EE, Peterson DR, Falsey AR. Risk factors for severe respiratory syncytial virus infection in elderly persons. J Infect Dis. 2004;189(2):233–8. doi: 10.1086/380907. [DOI] [PubMed] [Google Scholar]
- 5.Gower TL, et al. RhoA signaling is required for respiratory syncytial virus-induced syncytium formation and filamentous virion morphology. J Virol. 2005;79(9):5326–36. doi: 10.1128/JVI.79.9.5326-5336.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCurdy LH, Graham BS. Role of plasma membrane lipid microdomains in respiratory syncytial virus filament formation. J Virol. 2003;77(3):1747–56. doi: 10.1128/JVI.77.3.1747-1756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Utley TJ, et al. Respiratory syncytial virus uses a Vps4-independent budding mechanism controlled by Rab11-FIP2. Proc Natl Acad Sci U S A. 2008;105(29):10209–14. doi: 10.1073/pnas.0712144105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santangelo PJ, Bao G. Dynamics of filamentous viral RNPs prior to egress. Nucleic Acids Res. 2007;35(11):3602–11. doi: 10.1093/nar/gkm246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kassam A, et al. Place exchange reactions of alkyl thiols on gold nanoparticles. J Am Chem Soc. 2006;128(11):3476–7. doi: 10.1021/ja057091q. [DOI] [PubMed] [Google Scholar]
- 10.Hostetler MJ, Templeton AC, Murray RW. Dynamics of place-exchange reactions on monolayer-protected gold cluster molecules. Langmuir. 1999;15(11):3782–3789. [Google Scholar]
- 11.Villiers CL, et al. Analysis of the toxicity of gold nano particles on the immune system: effect on dendritic cell functions. J Nanopart Res. 2010;12(1):55–60. doi: 10.1007/s11051-009-9692-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shukla R, et al. Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: a microscopic overview. Langmuir. 2005;21(23):10644–54. doi: 10.1021/la0513712. [DOI] [PubMed] [Google Scholar]
- 13.Connor EE, et al. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1(3):325–7. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- 14.Connors M, et al. Respiratory syncytial virus (RSV) F, G, M2 (22K), and N proteins each induce resistance to RSV challenge, but resistance induced by M2 and N proteins is relatively short-lived. J Virol. 1991;65(3):1634–7. doi: 10.1128/jvi.65.3.1634-1637.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brock SC, et al. The transmembrane domain of the respiratory syncytial virus F protein is an orientation-independent apical plasma membrane sorting sequence. J Virol. 2005;79(19):12528–35. doi: 10.1128/JVI.79.19.12528-12535.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beeler JA, van Wyke Coelingh K. Neutralization epitopes of the F glycoprotein of respiratory syncytial virus: effect of mutation upon fusion function. J Virol. 1989;63(7):2941–50. doi: 10.1128/jvi.63.7.2941-2950.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbas CF, 3rd, et al. Human monoclonal Fab fragments derived from a combinatorial library bind to respiratory syncytial virus F glycoprotein and neutralize infectivity. Proc Natl Acad Sci U S A. 1992;89(21):10164–8. doi: 10.1073/pnas.89.21.10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crowe JE, Jr, et al. Isolation of a second recombinant human respiratory syncytial virus monoclonal antibody fragment (Fab RSVF2-5) that exhibits therapeutic efficacy in vivo. J Infect Dis. 1998;177(4):1073–6. doi: 10.1086/517397. [DOI] [PubMed] [Google Scholar]
- 19.Crowe JE, et al. Monoclonal antibody-resistant mutants selected with a respiratory syncytial virus-neutralizing human antibody fab fragment (Fab 19) define a unique epitope on the fusion (F) glycoprotein. Virology. 1998;252(2):373–5. doi: 10.1006/viro.1998.9462. [DOI] [PubMed] [Google Scholar]
- 20.Rock MT, Crowe JE., Jr Identification of a novel human leucocyte antigen-A*01-restricted cytotoxic T-lymphocyte epitope in the respiratory syncytial virus fusion protein. Immunology. 2003;108(4):474–80. doi: 10.1046/j.1365-2567.2003.01619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venter M, et al. Respiratory syncytial virus nucleoprotein-specific cytotoxic T-cell epitopes in a South African population of diverse HLA types are conserved in circulating field strains. J Virol. 2003;77(13):7319–29. doi: 10.1128/JVI.77.13.7319-7329.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy CJ, et al. Anisotropic metal nanoparticles: Synthesis, assembly, and optical applications. J Phys Chem B. 2005;109(29):13857–70. doi: 10.1021/jp0516846. [DOI] [PubMed] [Google Scholar]
- 23.Murphy CJ, et al. One-dimensional colloidal gold and silver nanostructures. Inorg Chem. 2006;45(19):7544–54. doi: 10.1021/ic0519382. [DOI] [PubMed] [Google Scholar]
- 24.Gole A, Murphy CJ. Polyelectrolyte-coated gold nanorods: Synthesis, characterization and immobilization. Chemistry of Materials. 2005;17(6):1325–1330. [Google Scholar]
- 25.Norman RS, et al. Targeted photothermal lysis of the pathogenic bacteria, Pseudomonas aeruginosa, with gold nanorods. Nano Lett. 2008;8(1):302–6. doi: 10.1021/nl0727056. [DOI] [PubMed] [Google Scholar]
- 26.Zhao X, et al. Structural characterization of the human respiratory syncytial virus fusion protein core. Proc Natl Acad Sci U S A. 2000;97(26):14172–7. doi: 10.1073/pnas.260499197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Treuel L, et al. The influence of surface composition of nanoparticles on their interactions with serum albumin. Chemphyschem. 2010;11(14):3093–9. doi: 10.1002/cphc.201000174. [DOI] [PubMed] [Google Scholar]
- 28.Vallee A, Humblot V, Pradier CM. Peptide interactions with metal and oxide surfaces. Acc Chem Res. 2010;43(10):1297–306. doi: 10.1021/ar100017n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.