Abstract
Background
Studies on microsporidial infection mostly focus on immunodeficiency or immunosuppressive individuals. Therefore, this cross-sectional study describes the prevalence and risk factors of microsporidiosis among asymptomatic individuals in Malaysia.
Methods/Findings
Four hundred and forty seven stool samples were collected and examined for microsporidia after staining with Gram-chromotrope Kinyoun. Demographic, socioeconomic, environmental, and behavioral information were collected by using a pre-tested questionnaire. Overall, 67 (15%) samples were positive for microsporidia. The prevalence of infection was significantly higher among individuals aged more than 15 years compared to those aged <15 years (OR = 1.97, 95% CI = 1.08, 3.62; P = 0.028). Furthermore, logistic regression analysis confirmed that the presence of other family members infected with microsporidia (OR = 8.45; 95% CI = 4.30, 16.62; P<0.001) and being a consumer of raw vegetables (OR = 2.05; 95% CI = 1.15, 3.66; P = 0.016) were the significant risk factors of this infection.
Conclusions
These findings clearly show that exposure to microsporidia is common among Aboriginal population. Further studies using molecular approach on microsporidia isolates from asymptomatic individuals is needed to determine species-specific. The risk factors associated with microsporidiosis will help in identifying more clearly the sources of the infection in the environment that pose a risk for transmission so that preventive strategies can be implemented.
Introduction
Microsporidia have been recognized as opportunistic pathogens of immunocompromised patients, but microsporidiosis is also becoming increasingly common in immunocompetent individuals [1]. Most human microsporidial infections are caused by Enterocytozoon bieneusi or Encephalitozoon intestinalis [2], [3]. E. bieneusi is found mainly in the upper gastrointestinal tract and is associated with chronic diarrhea and loss of weight, although a couple of reports have identified E. bieneusi in respiratory samples [4]. E. intestinalis, the second most frequently identified microsporidian causes disseminated microsporidiosis, affecting the gastrointestinal tract. On the other hand, Encephalitozoon cuniculi is an important parasite of domestic animals including rabbits, dogs, cats, cows, sheep and horses. Symptomatic human infections with this species are rare [5].
Intestinal microsporidia is very small (1 to 2 µm), single celled obligate intracellular parasites characterized by a polar filament that is extruded during the invasion of the host cell [6]. Mature microsporidia spores have thick three-layered walls, can pass through some water treatment filters due to their small size and are resistant to chlorine at concentrations used in treating drinking water. Microsporidia spores have been found in drinking water sources, soil, and domestic and wild animals; suggesting the possibility of water-borne, food-borne, zoonotic, and anthroponotic transmissions [7], [8].
Studies examining the prevalence of human microsporidiosis have been limited to patients who are positive for human immunodeficiency virus (HIV). However, recent molecular epidemiological studies have shown that organ transplant recipients and other immunocompromised patients, as well as immunocompetent individuals are at risk for infections that are mostly asymptomatic [9], [10], [11]. In contrast, only a few reports concerning microsporidial infection of immunocompetent individuals have been published [12], [13], [14], [15]. The most frequent clinical manifestations caused by microsporidia in AIDS patients are diarrhea, nausea, vomiting, malabsorption, and loss of weight. On the other hand, this infection usually causes self-limited diarrhea in immunocompetent individuals [16], [17],[18].
In Malaysia, few reports have been published regarding the prevalence of microsporidiosis among hospitalized patients, HIV infected patients, and Aboriginal communities [19], [20], [21], [22], [23]. However, to our knowledge, reports on the risk factors of microsporidial infection are lacking. Therefore, the present study aimed to determine the prevalence and associated risk factors of microsporidiosis among asymptomatic Aboriginal individuals in rural Malaysia. The establishment of such data will be beneficial for the public health authorities in the planning and implementation of specific prevention and control strategies of this infection in this population.
Materials and Methods
Study area and population surveyed
This cross-sectional study was conducted from June to December, 2011 among 447 Aboriginal participants living in eight villages from three different states (Pahang, Perak, and Negeri Sembilan) in suburban and remote areas of Peninsular Malaysia. The Aborignal is a collective term for a group of indigenous people that usually reside in the interior regions of Peninsular Malaysia. They identify themselves by tribes i.e. Proto-Malay, Negrito, and Senoi. They comprise about 0.6% of the total population in Malaysia. Sample selection was achieved using random selection of villages and random selection of 10 to 15 households per village. Within each village, participants over 2 years of age and those who provided consent to participate were included in this study. Exclusion criteria included children less than 2 years old and refusal to participate.
With regard to the age groups, 194 (43.4%) were less than 15 years old while 253 (56.6%) were 15 years old or more (≥15), with a median age of 20 years [interquartile range (IQR) 9–35]. Participants who participated in this study comprised of 197 (44.1%) males and 250 (55.9%) females.
Sample size
With an expected prevalence of microsporidia among the Aboriginal population in Malaysia at 20% [22], [23], the 95% confidence interval (CI) and an absolute precision of 0.05 [24], the minimum sample size required for the study was estimated to be 246 participants.
Questionnaire
A structured questionnaire was developed in English and then translated to Bahasa Melayu (the national language of Malaysia). The questionnaire was pre-tested among Aboriginal who was admitted to Gombak Hospital, Selangor state. Trained research assistants interviewed participants in person, asking questions for demographic data (i.e. age, gender and education level), socioeconomic background (i.e. occupation, household income, and educational status), behavioral risks (i.e. personal hygiene such as hand washing and food consumption), environmental sanitation and living condition characteristics (i.e. types of water supply, latrine system, sewage disposal system, and presence of domestic animals). Participants were also asked if they had diarrhea and symptoms of gastroenteritis (i.e. fever, vomiting, nausea, abdominal pain, watery stools, and blood or mucus stools). For children, the questionnaire was completed by interviewing their parents or the guardians who had given informed consent.
Stool sample collection
Following the administration of the questionnaire, a wide mouth screw-capped container pre-labeled with the individual's name and code was distributed to each participant for the collection of a stool sample the next day. Their ability to recognize their name was counter-checked. The participant was instructed to scoop a thumb sized stool sample using a provided scoop into the container. Then, the container was placed in a zip-locked plastic bag. Parents and guardians were instructed to monitor their children during the sample collection in order to ensure that they placed their stool samples into the correct container. All participants were asked to provide sufficiently large stool sample (at least 10 grams).
Detection of microsporidia by Gram-chromotrope Kinyoun staining
Thin stool smears were spread on glass slides; air dried, fixed with methanol, and stained with crystal violet for one minute and the excess stain was rinsed off with Gram's iodine. The slides were then stained with Gram's iodine for two minutes. The Gram's iodine solution was removed by gently rinsing with a decolorizer until the flow become colorless. The slides were washed with tap water and stained with chromotrope stained and prepared as described by Moura et al. [25] for eight minutes. The slides were rinsed in 90% acid-alcohol and counterstained with Kinyoun's carbol fuchsin for three minutes. Also, the slides were rinsed in 90% acid-alcohol, 95% alcohol for five minutes, and 100% ethyl alcohol for two minutes [20]. Then mounted with DPX medium (mixture of distyrene, plasticizer, and xylene) and covered with cover slips.
The criterion used to define microsporidia-positive was the presence of one or more pink-blue ovoid structures with a blue spore wall and a belt-like stripe encircling the spore, in at least 100 fields examined under 1000× magnifications, and confirmed by two technologists. The spore seen in the stained samples was graded as follows: 1+ (average number of spores seen was 1–10), 2+ (average number of spores seen was 11–20), and 3+ (average number of spores seen was >21) [19].
Data management and analysis
Data was entered into a Microsoft Access and was cross-checked by the technical staff in order to ensure that data were entered correctly. Statistical analysis was performed using the SPSS version 20 (SPSS, Chicago, IL, USA). Only those participants who had Gram-chromotrope Kinyoun staining result together with complete questionnaire data were included in the final analyses.
For descriptive analysis, rate (percentage) was used to describe the characteristics of the studied population, including the prevalence of microsporidia. A Chi-squares test (χ2) was used to test the associations between the variables. In the univariate analysis, the dependent variable was prevalence of microsporidia, while the independent variables were demographic (gender and age group) and socioeconomic factors, behavioral risks, environmental sanitation, living condition characteristics, and gastrointestinal symptoms. All variables that were significantly associated with the prevalence of microsporidia in the univariate model were included in a logistic regression analysis to identify the risk factors for microsporidiosis with control for the effects of possible confounders. For each statistically significant factor, an odds ratio (OR) and 95% confidence interval (CI) were computed by the univariate and logistic regression analyses. The level of statistical significance was set as P<0.05.
Ethical consideration
Ethical approval was obtained from the Ethics Committee of Universiti Kebangsaan Malaysia Medical Centre (UKMMC) (Reference Number: UKM 1.5.3.5/244/FF-165-2011) and permission for field work was obtained from the Ministry of Rural and Regional Development Malaysia before starting the study. Village meeting were held and village authorities and villagers were handed detailed explanations of the aims, procedures, potential risks, and benefits of the study. During the meeting, they were also informed that their identity and personal information would be kept strictly confidential and they could withdraw from the study at any point of time without citing reasons for doing so. If they agreed to participate, their consent was obtained in written form (signature or thumbprint for those who were illiterate). For children, written informed consent was obtained from their parents. Ethics committee has also approved the consent procedure used in this study.
Results
Characteristics of the study population
Single stool samples were randomly collected from a total of 447 participants. About two thirds (65%) of the parents have low level of education i.e. less than 6 years of formal education. Most of the parents did odd jobs, such as selling forest products without any stable income. Some were daily wage earners working in rubber or palm oil plantations, unskilled laborers in factories or on construction sites. Therefore, 47.8% of the households belonged to people who earned less than RM500 per month (US$162.42). Although 60.9% of the houses have a provision of basic infrastructure such as treated water supply and 66% have a pour flush toilet, at least 39.1% are still using untreated water originating from a nearby river for their domestic needs and 34% still defecate indiscriminately in the river or bush. More than half (55.7%) of the households kept dogs, cats, and poultry as their domestic animals.
Prevalence of microsporidial infection
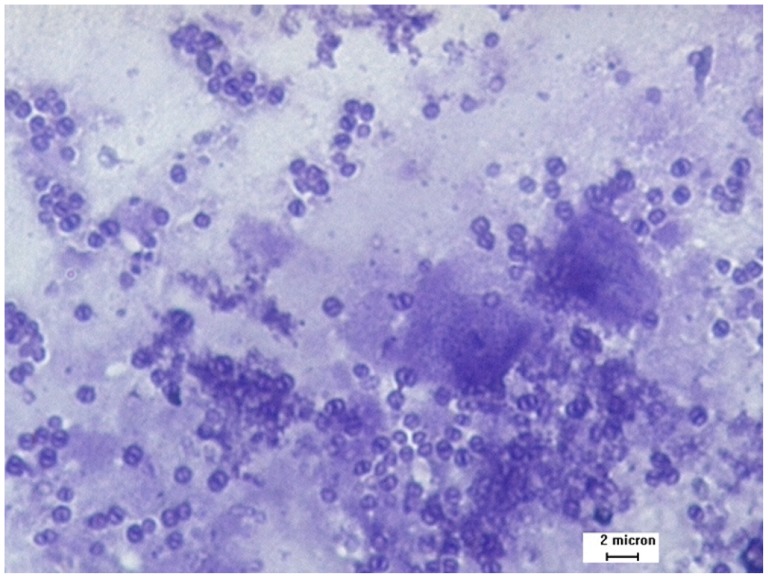
Table 1 shows that 15% (67/447) of the participants were infected with microsporidia. Of the 67 positive smears, 65 (97%) had low spore counts of 1–10 per 100 fields/100×, while only 2 (3%) had moderate spore counts of 11–20 per fields/100× (Figure 1). The prevalence of microsporidial infection was not statistically significant between genders. However, this infection was significantly higher in the age group more than 15 years old.
Table 1. Prevalence of microsporidial infection among asymptomatic Aboriginal according to age groups and gender.
| Orang Asli population | |||
| n positive | n examined | % positive | |
| Age groups (years) | |||
| <15 | 20 | 194 | 10.3 |
| ≥15 | 47 | 253 | 18.6 |
| Gender | |||
| Male | 30 | 197 | 15.2 |
| Female | 37 | 250 | 14.8 |
| Total | 67 | 447 | 15.0 |
n = Number examined.
Figure 1. Microsporidia spores from stool samples stained with Gram-chromotrope Kinyoun under light microscopy (magnification, ×1000).
Clear background and the polar tubes of almost all spores were stained.
Associated risk factors for microsporidiosis
The association of microsporidial infection and sociodemographic characteristics are shown in Table 2. The results of univariate analysis showed that the prevalence of microsporidiosis was significantly higher among individuals aged more than 15 years (18.6%; 95% CI = 1.13, 3.48) when compared with those aged <15 years. Similarly, the prevalence was significantly higher among participants who consume raw vegetables (19.4%; 95% CI = 1.15, 3.36) and those children of mothers with less than 6 years of formal education (16.9%; 95% CI = 1.42, 12.21). Moreover, the presence of other family members infected with microsporidia increased children's odds for infection when compared with their counterparts by 7.4 times (95% CI = 3.89, 14.18)
Table 2. Potential risk factors associated with microsporidial infection among asymptomatic Aboriginal population (univariate analysis, n = 447).
| Variables | No. examined | Infected (%) | OR (95% CI) | P-value |
| Age (years) | ||||
| ≥15 | 253 | 18.6 | 1.99 (1.13,3.48) | 0.015a |
| <15 | 194 | 10.3 | 1 | |
| Gender | ||||
| Male | 197 | 15.2 | 1.03 (0.61,1.74) | 0.900 |
| Female | 250 | 14.8 | 1 | |
| Drinking untreated water | ||||
| Yes | 175 | 16.6 | 1.22 (0.72,2.07) | 0.452 |
| No | 272 | 14.0 | 1 | |
| Bathing and washing in the river | ||||
| Yes | 130 | 16.9 | 1.23 (0.71,2.15) | 0.463 |
| No | 317 | 14.2 | 1 | |
| Not washing hands after playing with soil or gardening | ||||
| Yes | 157 | 13.4 | 0.82 (0.47,1.43) | 0.482 |
| No | 290 | 15.9 | 1 | |
| Presence of domestic animals | ||||
| Yes | 198 | 16.2 | 1.18 (0.70,1.98) | 0.536 |
| No | 249 | 14.1 | 1 | |
| Indiscriminate defecation | ||||
| Yes | 152 | 17.1 | 1.28 (0.75,2.18) | 0.368 |
| No | 295 | 13.9 | 1 | |
| Sewage disposal | ||||
| Outdoor | 197 | 16.8 | 1.28 (0.76,2.15) | 0.354 |
| Common drainage | 250 | 13.6 | 1 | |
| Eating with hands | ||||
| Yes | 288 | 17.0 | 1.61 (0.90,2.87) | 0.106 |
| No | 159 | 11.3 | 1 | |
| Consuming raw vegetables | ||||
| Yes | 217 | 19.4 | 1.97 (1.15,3.36) | 0.012a |
| No | 230 | 10.9 | 1 | |
| Eating fresh fruits | ||||
| Yes | 347 | 15.9 | 1.38 (0.71,2.70) | 0.342 |
| No | 100 | 12.0 | 1 | |
| Father's education | ||||
| Non-educated (<6 yrs) | 180 | 14.4 | 1.60 (0.69,3.71) | 0.266 |
| Educated (≥6 yrs) | 84 | 9.5 | 1 | |
| Mother's education | ||||
| Non-educated (<6 yrs) | 178 | 16.9 | 4.16 (1.42,12.21) | 0.006a |
| Educated (≥6 yrs) | 86 | 4.7 | 1 | |
| Working mothers | ||||
| Yes | 112 | 12.5 | 0.94 (0.45,1.96) | 0.875 |
| No | 152 | 13.2 | 1 | |
| Household members | ||||
| ≥8 (large) | 162 | 19.1 | 1.64 (0.97,2.77) | 0.064 |
| <8 | 285 | 12.6 | 1 | |
| Household monthly income | ||||
| ≤RM500 (low) | 213 | 16.0 | 1.16 (0.69,1.95) | 0.582 |
| >RM500 | 234 | 14.1 | 1 | |
| Other family members infected with microsporidia | ||||
| Yes | 48 | 47.9 | 7.42 (3.89,14.18) | <0.001a |
| No | 399 | 11.0 | 1 |
RM = Malaysian Ringgit; (US$100 = RM307.85) [1st February 2013].
Reference group marked as OR = 1 [OR = Odds Ratio].
CI = Confidence interval.
Significant association (P<0.05).
Multiple logistic regression analysis confirmed that being aged more than 15 years (OR = 1.97, 95% CI = 1.08, 3.62; P = 0.028), consuming raw vegetables (OR = 2.05; 95% CI = 1.15, 3.66; P = 0.016), and the presence of other family members infected with microsporidia (OR = 8.45; 95% CI = 4.30, 16.62; P<0.001) as the significant risk factors for microsporidiosis in the population studied (Table 3).
Table 3. Logistic regression analysis of risk factors associated with microsporidiosis among asymptomatic Aboriginal population.
| Variables | OR | 95% CI | P-value |
| Being aged more than 15 years | 1.97 | 1.08, 3.62 | 0.028 |
| Consuming raw vegetables | 2.05 | 1.15, 3.66 | 0.016 |
| Presence of other family members infected with microsporidia | 8.45 | 4.30, 16.62 | <0.001 |
Co-infection with other intestinal parasites
For all positive stool samples with microsporidia (n = 67), 79.1% showed co-infection with one or more other parasites. Trichuris trichiura was the most common intestinal parasite found in conjunction with microsporidia (58.2%), followed by Entamoeba histolytica/dispar/moshkovskii (25.4%), Entamoeba coli (19.4%), Giardia intestinalis (13.4%), Blastocystis hominis (13.4%), Ascaris lumbricoides (13.4%), hookworm (10.5%), Iodamoeba buetschlii (7.5%), and Entamoeba hartmanni and Chilomastix mesnilii (4.5%).
Discussion
Microsporidiosis in humans occurs worldwide but prevalence vary widely depending on the geographical region, population studied and diagnostic methods used [26], [27]. Prior to the AIDS pandemic, microsporidiosis was rarely identified in humans [28], [29]. Prevalence data for microsporidia in human populations before the era of AIDS relied upon serology based on detecting antibodies to Enterocytozoon bieneusi, Encephalitozoon intestinalis or Encephalitozoon cuniculi, the species that isolated in mammals. Seroprevalence results ranged from 0 to 42%, with the highest rates found in homosexual men in Sweden and in persons with other parasitic infections [30], [31]. A wide range of case reports have identified microsporidia in non-HIV infected individuals but prevalence data based on parasite detection (versus serology) are lacking [32]. The prevalence in non-HIV infected individuals have been reported using microscope studies and polymerase chain reaction (PCR) technique in travelers to endemic areas (3.3 to 10%), children with diarrhea or not (1.7 to 17.4%), and elderly peoples (17.2%) [1], [33], [34].
In this study, the prevalence of microsporidia among the asymptomatic Aboriginal in Malaysia was 15%, which differs from previous local reports [21], [22], [23]. Comparing our findings with studies from other countries showed that the prevalence reported by the present study was similar to those reported in Spain (17%), Uganda (16.8%), Thailand (14.9%), and Czech Republic (15%) [1], [10], [17], [35]. By contrast, considerably lower prevalence rates of 0.7%, 8%, 5.35% and 9.3% were reported in Germany, Peru, Tunisia, and Nigeria among immunocompetent individuals, respectively [36], [37], [38], [39]. The prevalence of microsporidia in the asymptomatic Aboriginal was significantly higher than other groups of Malaysian population and it is in agreement with the earlier assertion that intestinal parasitic infection, in general among the Aboriginal community is higher than in the general healthy population in Malaysia [21], [40]. Tapioca is the main food group for the people in this study and the villagers forage in the jungle for additional sustenance, where there is a risk of acquiring microsporidial infection, owing to the parasite's ubiquity in nature [41]. Moreover, it is possible that low immune status among this people due to the high prevalence of protein-energy malnutrition make them more susceptible to microsporidial infection, as there appears to be a relationship between malnutrition and other enteric parasitic infection in this population [21], [42]. Furthermore, shedding of microsporidia spores is intermittent and it is known that patients diagnosed with microsporidiosis by analysis of biopsy material may have stools devoid of the parasite [43]. Thus, the prevalence of microsporidia among asymptomatic Aboriginal in Malaysia would be expected to be even higher than the analysis of stool indicates.
The present findings showed no significant difference in the prevalence of microsporidia according to gender of the participants, and this is consistent with the results of previous reports [22], [23], [35], [44]. On the other hand, the current study reported significantly higher odds of microsporidial infection among those aged more than 15 years as compared to younger individuals. Likewise, a recent parasitological evaluation of stool samples from Czech Republic citizens (immunocompetent individuals and foreign students of varying age groups) had identified 34 to 56% prevalence rates of shedding E. bieneusi and Encephalitozoon species with the highest prevalence in the group of 50 years and above [35]. Similarly, Norhayati et al. [19] also reported a high prevalence (57.2%) of microsporidiosis among adults aged more than 31 years. The high prevalence rate of the infection among those aged ≥15 years in the present study might be explained by the fact that their behavior is related to more active movement and more independent eating habits compared with children. However, it is also possible that microsporidia persisted but was shed less consistently or at levels below detection in younger age group.
Few studies have been conducted to determine the source and mode of transmission of intestinal microsporidiosis. Information from individual case reports of microsporidiosis in humans and from both natural and experimental infections in animals however has provided insights about the likely modes of transmission of microsporidia to humans [45], [46]. The observations that microsporidia are ubiquitous in nature suggest that several modes of transmission and sources exist for human infections.
The possibility of human-to-human transmission cannot be ruled out. Experimental examples of microsporidia transmission between laboratory animals might suggest that the same infecting species could be transmitted between humans via the same pathways as in animals [34], [47]. On further analysis of risk factor using multiple logistic regression analysis, we found that the presence of other family members infected with microsporidia increases the odds for acquiring microsporidiosis by 8 times. Likewise, a study done by Leelayoova et al. [48] suggested that human-to-human transmission occurred in an orphanage, where a multivariate analysis showed that orphans who were 12–23 months old and living in one particular house were independently associated with E. bieneusi infection and all infected children presented the same genotype in a stool samples. Few studies had also provided evidence to support the role of human-to-human transmission in intestinal microsporidiosis. Individuals with a history of contact with diarrheal patients had two times greater risk of getting the microsporidial infection [49].
Microsporidia are among the parasites of concern for food-borne transmission as a result of globalization of food, faster transport of food, increasing travel of consumers, and changes in food consumption patterns [50], [51]. It is interesting to note that in this study, those who consumed raw vegetables were at 2 times odds of being infected with microsporidia when compared to those who consumed cooked vegetables. Similarly, Calvo et al. [52] identified spores of microsporidia on lettuce, parsley, cilantro, and strawberries in Costa Rica. Furthermore, a recent study conducted by Decraene et al. [53] suggested that cucumber slices in both cheese sandwiches and a salad were the most probable vehicle of transmission for food-borne outbreak associated with E. bieneusi in Sweden. Washing of fresh product such as beans and lettuce remains of utmost important in preventing food-borne illness and should continue to be emphasized. Sometimes, washing may be insufficient to remove all pathogens including microsporidia spores. In this instance, it may be postulated that the level of contamination was quite high that washing procedure was unable to remove enough of the pathogen load, so as to prevent infection. Alternatively, it may be that microsporidia spores are capable of strong adhesion to or internalization in, certain types of product, thereby successfully evading the effects of washing and disinfection. A research conducted in the USA demonstrated that Cryptosporidium oocysts were capable of strongly adhering to spinach plants after contact with contaminated water and were also internalized within the leaves, thus making washing entirely ineffective [54].
Several questions still exist about clinical aspects and consequences of microsporidial infection in humans. In general, the clinical course of microsporidiosis depends on the immune status of the host and site of infection [6]. Immunocompromised hosts infected with microsporidia were most likely to develop disease that often contributed to death [55], [56]. In healthy humans such as travelers, self-limiting diarrhea with duration of about 2–3 weeks has been reported [36], [57]. Furthermore, a study conducted in Czech Republic showed only a minority of diarrheal samples was positive with microsporidia and it was present mainly in formed stool samples [35]. Therefore, it seems important to determine if asymptomatic and persistent microsporidial infection occur in humans, and if so, improved and reliable diagnostic methods are needed for attempting to prevent transmission to others at risk or to reduce the potential for reactivation of infection.
Several limitations apply to our findings. Firstly, identification of microsporidia at species level cannot be achieved by current standard light microscope technique. Transmission electron microscope (TEM) or polymerase chain reaction (PCR) is required to achieve this task. Hence, the findings are presented as microsporidial infection without mention of the species. However, previous study carried out in the same setting reported E. bieneusi and E. intestinalis were the common microsporidia species isolated [58]. Furthermore, the detection limit of light microscope has been determined to be between 104 and 106 microsporidia spores per gram of stool, whereas PCR is able to detect spore concentrations as low as 102 per grams of stool [59], [60]. Thus, the true prevalence of microsporidia has to be determined by highly sensitive techniques such as PCR. Secondly, stool samples may contain spores of non-human microsporidia which acquired from insects, crustacean or fish that can passed through the digestive system. These spores would stain similarly as human microsporidia. Therefore, in order to overcome this limitation, Gram-chromotrope Kinyoun staining was used for identification of microsporidia spores. The staining has higher sensitivity and specificity of 98% and 98.3%, respectively as compared to the reference technique Weber modified trichrome with statistically significant agreement by Kappa statistics (K = 0.941; P<0.001) [20]. Furthermore, morphological characteristics and spore size were used for the identification in the present study. Using the previous staining technique described by Fatmah et al. [20], the wall of each spore was stained blue and the polar tube stained deep blue in color, encircled each spore. The size of each microsporidia spore was approximately 1.5 by 0.9 µm. On the other hand, yeast cells were distinguished by their larger size (3 µm). Bacteria stained faintly with this stain and could not be easily confused with microporida spore. Moreover, Nosema locustae (insects microsporidia) and Pleistophora spp. (arthropods microsporidia) were 2.0 by 3.0 µm and 2.8 by 4 µm in size, respectively [61]. Finally, the unavailability of information on immune status of the participants, therefore, the cause-effect association could not be determined in this study.
In conclusion, the data obtained during the present study showed high prevalence of this infection among the asymptomatic Aboriginal population, raising the question of whether the findings represent true infection resulting in shedding of parasites or ingested parasites that just passed through the gastrointestinal tract without establishing an infection. Since no data exist on the risk factors among this population, our findings on the risk factors associated with microsporidiosis will help in identifying more clearly the sources of the infection in the environment that pose a risk for transmission so that preventive strategies can be implemented. Further studies of people living in different parts of the country also should be performed. Moreover, genotyping of microsporidia isolated from asymptomatic individuals might determine whether there are microsporidia of various levels of virulence.
Funding Statement
This work was supported by UKMMC Fundamental Research Grant (FF-165-2011), Special Research University Grant (UKM-GUP-2011-316) and University of Malaya High Impact Research Grant, UM-MOHE UM.C/625/1/HIR/MOHE/MED/18 from the Ministry of Higher Education Malaysia. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lores B, Lopez-Miragaya I, Arias C, Fenoy S, Torres J, et al. (2002) Intestinal microsporidiosis due to Enterocytozoon bieneusi in elderly human immunodeficiency virus-negative patients from Vigo, Spain. Clin Infect Dis 34: 918–921. [DOI] [PubMed] [Google Scholar]
- 2. Desportes I, Ke Charpentier Y, Galian A, Bernard F, Cochand-Priollet B, et al. (1985) Occurrence of a new microsporidian: Enterocytozoon bieneusi n.g., n.sp., in the enterocytes of a human patient with AIDS. J Protozool 32: 250–254. [DOI] [PubMed] [Google Scholar]
- 3. Cali A, Kotler DP, Orenstein JM (1993) Septata intestinalis N.G., N. Sp., an intestinal microsporidian associated with chronic diarrhea and dissemination in AIDS patients. J Eukaryot Microbiol 40: 101–112. [DOI] [PubMed] [Google Scholar]
- 4. Kotler DP, Orenstein JM (1998) Clinical syndromes associated with microsporidiosis. Adv Parasitol 40: 321–349. [DOI] [PubMed] [Google Scholar]
- 5. Harcourt-Brown FM, Holloway HKR (2003) Encephalitozoon cuniculi in pet rabbits. Vet Rec 152: 427–431. [DOI] [PubMed] [Google Scholar]
- 6. Didier ES (2005) Microsporidiosis: an emerging and opportunistic infection in humans and animals. Acta Trop 94: 61–76. [DOI] [PubMed] [Google Scholar]
- 7. Mathis A, Weber R, Deplozes P (2005) Zoonotic potential of the microsporidia. Clin Microbiol Rev 18: 423–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li N, Xiao L, Wong L, Zhao S, Zhao Y, et al. (2012) Molecular surveillance of Cryptosporidium spp., Giardia duodenalis and Enterocytozoon bieneusi by genotyping and subtyping parasites in wastewater. PLoS Negl Trop Dis 6: e1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coyle CM, Wittner M, Kotler DP, Noyer C, Orenstein JM, et al. (1996) Prevalence of microsporidiosis due to Enterocytozoon bieneusi and Encephalitozoon (Septata) intestinalis among patients with AIDS-related diarrhea: determination by polymerase chain reaction to the microsporidian small-subunit rRNA gene. Clin Infect Dis 23: 1002–1006. [DOI] [PubMed] [Google Scholar]
- 10. Tumwine JK, Kekitiinwa A, Nabukeera N, Akiyoshi DE, Buckholt MA, et al. (2002) Enterocytozoon bieneusi among children with diarrhea attending Mulago Hospital in Uganda. Am J Trop Med Hyg 67: 299–303. [DOI] [PubMed] [Google Scholar]
- 11. Samie A, Obi CL, Tzipori S, Weiss LM, Guerrant RL (2007) Microsporidiosis in South Africa: PCR detection in stool samples of HIV-positive and HIV-negative individuals in school children in Vhembe district, Limpopo Province. Trans R Soc Trop Med Hyg 101: 547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Croppo GP, Gomez-Morales MA, Pozio E (1991) Microsporidia infections inimmunocompetent and immunosuppressed subjects,. Parassitologia 33: 209–218. [PubMed] [Google Scholar]
- 13. Svenungsson BT, Capraru T, Evengard B, Larsson R, Lebbad M (1998) Intestinal microsporidiosis in a HIV-seronegative patient. Scand J infect Dis 30: 314–316. [DOI] [PubMed] [Google Scholar]
- 14. Abreu-Acosta N, Lorenzo-Morales J, Leal-Guio Y, Coronado-Alvarez N, Foronda P, et al. (2005) Enterocytozoon bieneusi (microsporidia) in clinical samples from immunocompetent individuals in Tenerife, Canary Islands, Spain. Trans R Soc Trop Med Hyg 99: 848–855. [DOI] [PubMed] [Google Scholar]
- 15. Sak B, Brady D, Pelikanova M, Kvetonova D, Rost M, et al. (2011) Unapparent microsporidial infection among immunocompetent humans in the Czech Republic. J Clin Microbiol 49: 1064–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sobottka I, Albrecht H, Schottelius J, Schmetz C, Bentfeld M, et al. (1995) Self-limited traveller's diarrhea due to a dual infection with Enterocytozoon bieneusi and Cryptosporidium parvum in immunocompetent HIV-negative child. Eur J Clin Microbiol Infect Dis 14: 919–920. [DOI] [PubMed] [Google Scholar]
- 17. Wanachiwanawin D, Chokephaibulkit K, Lertlaituan P, Ongrotchanakun J, Chinabut P, et al. (2002) Intestinal microsporidiosis in HIV-infected children with diarrhea. Southeast Asian J Trop Public Health 33: 241–245. [PubMed] [Google Scholar]
- 18. Endeshaw T, Kebede A, Verweij JJ, Wolday D, Zewide A, et al. (2006) Intestinal microsporidiosis in diarrheal patients infected with human immunodeficiency virus-1 in Addis Ababa, Ethiopia. Jpn J Infect Dis 59: 306–310. [PubMed] [Google Scholar]
- 19. Norhayati M, Azlin M, Hesham Al-Mekhlafi M, Anisah N, Nor Aini U, et al. (2008) A preliminary study on the prevalence of intestinal microsporidiosis in patients with and without gastrointestinal symptoms in Malaysia. Trans R Soc Trop Med Hyg 102: 1274–1278. [DOI] [PubMed] [Google Scholar]
- 20. Salleh FM, Al-Mekhlafi AM, Nordin A, Yasin AM, Al-Mekhalfi HM, et al. (2011) Evaluation of gram-chromotrope kinyoun staining technique: its effectiveness in detecting microsporidial spores in fecal specimens. Diagn Microbiol Infect Dis 69: 82–85. [DOI] [PubMed] [Google Scholar]
- 21. Lono A, Kumar S, Chye TT (2011) Detection of microsporidia in local HIV-positive population in Malaysia. Trans R Soc Trop Med Hyg 105: 409–413. [DOI] [PubMed] [Google Scholar]
- 22. Norhayati M, Al-Mekhlafi HM, Azlin M, Nor Aini U, Shaik A, et al. (2007) Intestinalmicrosporidial infections among Orang Asli (aborigine) children from Malaysia. Ann Trop Med Parasitol 101: 547–550. [DOI] [PubMed] [Google Scholar]
- 23. Lono A, Kumar GS, Chye TT (2010) Prevalence of microsporidia in an indigenous Orang Asli community in Pahang, Malaysia. Trans R Soc Trop Med Hyg 104: 214–218. [DOI] [PubMed] [Google Scholar]
- 24.Lwanga SK, Lemeshow S (1991) Sample size determination in health studies: A practical manual. Geneva, Switzerland. World Health Organization.
- 25. Moura H, Jorges L, Nunes DAS, Fernando CS, Patricia B, et al. (1996) Gram-chromotrope: a new technique that enhances detection of microsporidial spores in clinical specimens. J Eukaryot Microbiol 43: 94S–95S. [DOI] [PubMed] [Google Scholar]
- 26. Franzen C, Muller A (1999) Molecular techniques for detection, species differentiation and phylogenetic analysis of microsporidia. Clin Microbiol Rev 12: 243–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Didier ES, Weiss LM (2006) Microsporidiosis: Current status. Curr Opin Infect Dis 19: 485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canning EU, Lom J (1986) The Microsporidia of Vertebrates. Academia Press, New York. pp 289.
- 29.Kotler D, Orenstein JM (1999) Clinical syndromes associated withmicrosporidiosis. Washington DC. pp 258–292.
- 30. Hollister WS, Canning EU (1987) An enzyme-linked immunosorbent assay (ELISA) for detection of antibodies to Encephalitozoon cuniculi and its use in determination of infections in man. Parasitology 94: 209–219. [DOI] [PubMed] [Google Scholar]
- 31. Hollister WS, Canning EU, Willcox A (1991) Evidence for widespread occurrence of antibodies to Encephalitozoon cuniculi (Microspora) in man provided by ELISA and other serological tests. Parasitology 102: 33–43. [DOI] [PubMed] [Google Scholar]
- 32.Bryan RT, Schwartz DA (1999) Epidemiology of microsporidiosis. Washington DC. pp 502–516.
- 33. Aoun K, Barbouche R, Bouratbine A, Bejaoui M, Dellagi K, et al. (1997) Microsporidiosis intestinalis chez des enfants attaints de deficits immunitaires primitives. Parasite 4: 386–387. [PubMed] [Google Scholar]
- 34. Didier ES, Stovall ME, Green LC, Brindley PJ, Sestak K, et al. (2004) Epidemiology of microsporidiosis: sources and modes of transmission. Vet Parasitol 126: 145–166. [DOI] [PubMed] [Google Scholar]
- 35. Sak B, Kvac M, Kuceroza Z, Kvetonova D, Sakova K (2011) Latent microsporidial infection in immunocompetent individuals – A longitudinal study. PLoS Negl Trop Dis 5: e1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Muller A, Bialek R, Kamper A, Fatkenheuer G, Salzberger B, et al. (2001) Detection of microsporidia in travelers with diarrhea. J Clin Microbiol 39: 1630–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cama VA, Pearson J, Cabrera L, Pacheco L, Gilman R, et al. (2007) Transmission of Enterocytozoon bieneusi between a child and guinea pigs. J Clin Microbiol 45: 2708–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Anane S, Kaouech E, Belhadj S, Abdelmalek R, Ammari L, et al. (2011) Identification of Enterocytozoon bieneusi by PCR in stools of Tunisian immunocompromised patients. Pathol Biol 59: 234–239. [DOI] [PubMed] [Google Scholar]
- 39. Ayinmode AB, Ojuromi OT, Xiao L (2011) Molecular identification of Enterocytozoon bieneusi isolates from Nigerian children. J Parasitol Res Article ID 129542, 2 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nicholas C, Baer A (2007) Health care for the Orang Asli: consequences of paternalism and colonialism. Kuala Lumpur. pp 119–136.
- 41. Weiss LM, Keohane EM (1998) The uncommon gastrointestinal protozoa: Microsporidia, Blastocystis, Isospora, Dientamoeba and Balantidium. Curr Clin Top Infect Dis 17: 147–187. [PubMed] [Google Scholar]
- 42. Ahmed A, Al-Mekhlafi HM, Al-Adhroey AH, Ithoi I, Abdulsalam AM, et al. (2012) The nutritional impacts of soil-transmitted helminth infections among Orang Asli schoolchildren in rural Malaysia. Parasit Vectors 5: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Clarridge JE, Karkhanis S, Rabeneck L, Marino B, Foote LW (1996) Quantitative light microscopic detection of Enterocytozoon bieneusi in stool specimens: a longitudinal study of human immunodeficiency virus-infected microsporidiosis patients. J Clin Microbiol 34: 520–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nkinin SW, Asonganyi T, Didier ES, Kaneshiro ES (2007) Microsporidian infection is prevalent in healthy people in Cameroon. J Clin Microbiol 45: 2841–2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shadduck JA, Storts R, Adams LG (1996) Selected examples of emerging and re-emerging infectious diseases in animals. Am Soc Microbiol News 62: 586–588. [Google Scholar]
- 46. Deplazes P, Mathis A, Weber R (2000) Epidemiology and zoonotic aspects of microsporidia of mammals and birds. Contrib Microbiol 6: 236–260. [DOI] [PubMed] [Google Scholar]
- 47. Bern C, Kawai V, Vargas D, Rabke-Verani J, Williamson J, et al. (2005) The epidemiology of intestinal microsporidiosis in patients with HIV/AIDS in Lima, Peru. J Infect Dis 191: 1658–1664. [DOI] [PubMed] [Google Scholar]
- 48. Leelayoova S, Subrungruang I, Rangsin R, Chavalitshewinkoon-Petmitr P, Worapong J, et al. (2005) Transmission of Enterocytozoon bieneusi genotype A in a Thai orphanage. Am J Trop Med Hyg 73: 104–107. [PubMed] [Google Scholar]
- 49. Gumbo T, Sarbah S, Gangaidzo IT, Ortega Y, Sterling CR, et al. (1999) Intestinal parasites in patients with diarrhea and human immunodeficiency virus infection in Zimbabwe. AIDS 13: 819–821. [DOI] [PubMed] [Google Scholar]
- 50. Slifko TR, Smith HV, Rose JB (2000) Emerging parasite zoonoses associated with water and food. Int J Parasitol 30: 1379–1393. [DOI] [PubMed] [Google Scholar]
- 51. Orlandi PA, Chu DT, Bier JW, Jackson GJ (2002) Parasites and the food supply. Food Technol 56: 72–81. [Google Scholar]
- 52. Calvo M, Carazo M, Arias ML (2004) Prevalence of Cyclospora sp, Cryptosporidium sp, microsporidia and fecal coliform determination in fresh fruit and vegetables consumed in Costa Rica. Arch Latinoam Nutr 54: 428–432. [PubMed] [Google Scholar]
- 53. Decraene V, Lebbad M, Botero-Kleiven S, Gustavsson AM, Lofdahl M (2012) First reported foodborne outbreak associated with microsporidia, Sweden, October 2009. Epidemiol Infect 140: 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Macarisin D, Bauchan G, Fayer R (2010) Spinacia oleracea L. leaf stomata harboring Cryptosporidium parvum oocysts: a potential threat to food safety. Appl Environ Microbiol 76: 555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Snowden KF, Shadduck JA (1999) Microsporidia of higher vertebrates. In: Wittner M, Weiss LM (eds) The microsporidia and microsporidiosis. American Society of Microbiology Press, Washington DC. pp 393–419.
- 56. Didier ES, Didier PJ, Snowden KF, Shadduck JA (2000) Microsporidiosis in mammals. Microb Infect 2: 709–720. [DOI] [PubMed] [Google Scholar]
- 57. Nageli K (1857) Uber die neue Krankheit die Seindenraupe unde verwandet Organismen. Bot Zeitung 15: 760–761. [Google Scholar]
- 58. Al-Mekhlafi MA, Fatmah MS, Anisah N, Azlin M, Al-Mekhlafi HM, et al. (2011) Species identification of intestinal microsporidia using immunofluorescence antibody assays. Southeast Asian J Trop Med Public Health 42: 19–24. [PubMed] [Google Scholar]
- 59. Muller A, Stellermann K, Hartmann P, Schrappe M, Fatkenheuer G, et al. (1999) A powerful DNA extraction method and PCR for detection of microsporidia in clinical stool specimens. Clin Diagn Lab Immunol 6: 243–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rinder H, Janitschke K, Aspock H, Da Silva AJ, Deplazes P, et al. (1998) Blinded, externally controlled multicenter evaluation of light microscopy and PCR for detection of microsporidia in stool specimens. J Clin Microbiol 36: 1814–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Franzen C, Muller A (2001) Microsporidiosis: human diseases and diagnosis. Microbes Infect 3: 389–400. [DOI] [PubMed] [Google Scholar]