Abstract
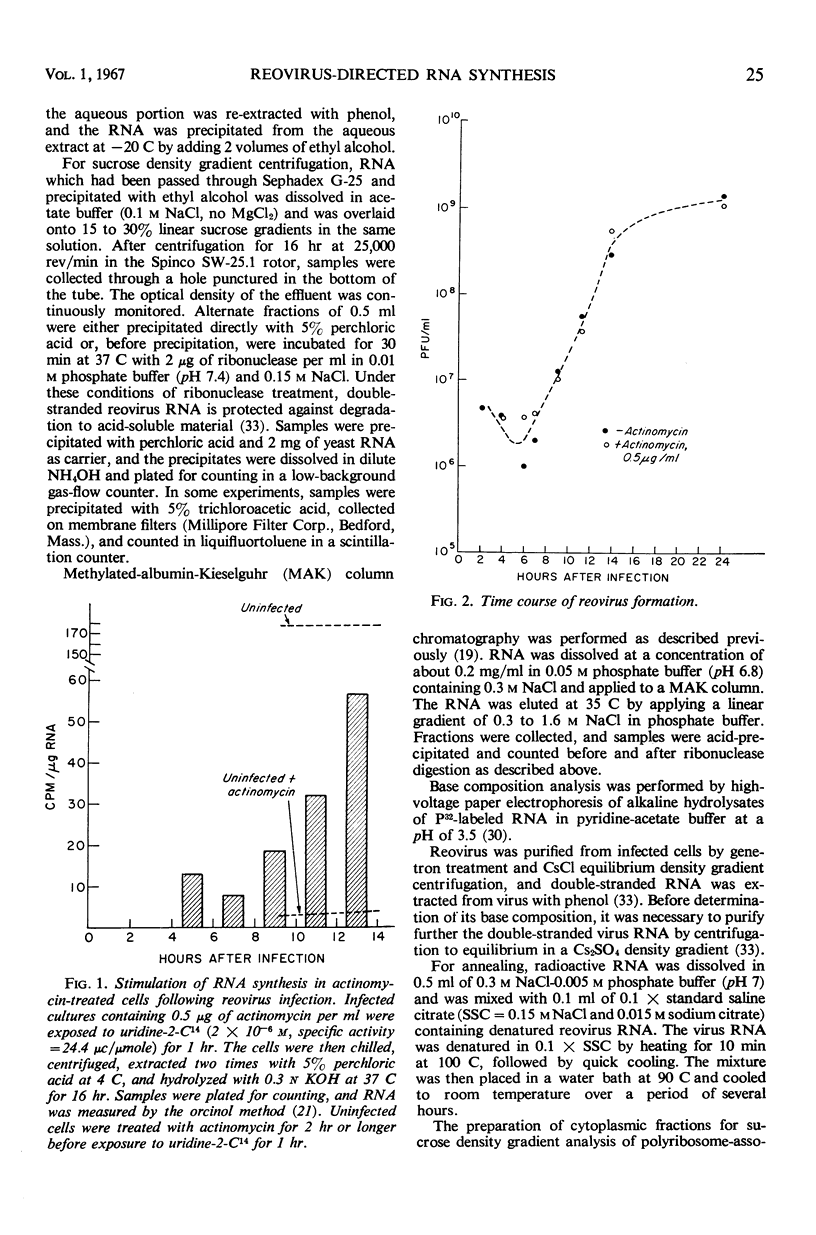
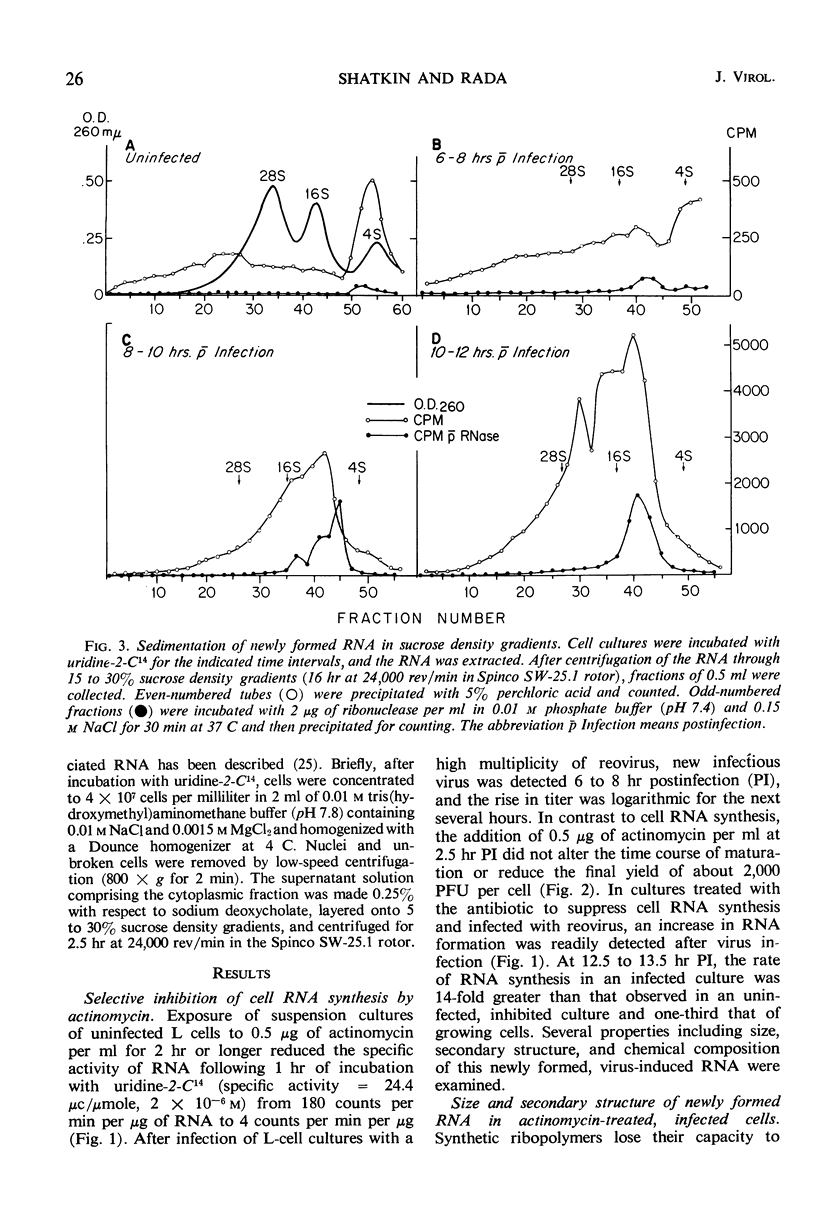
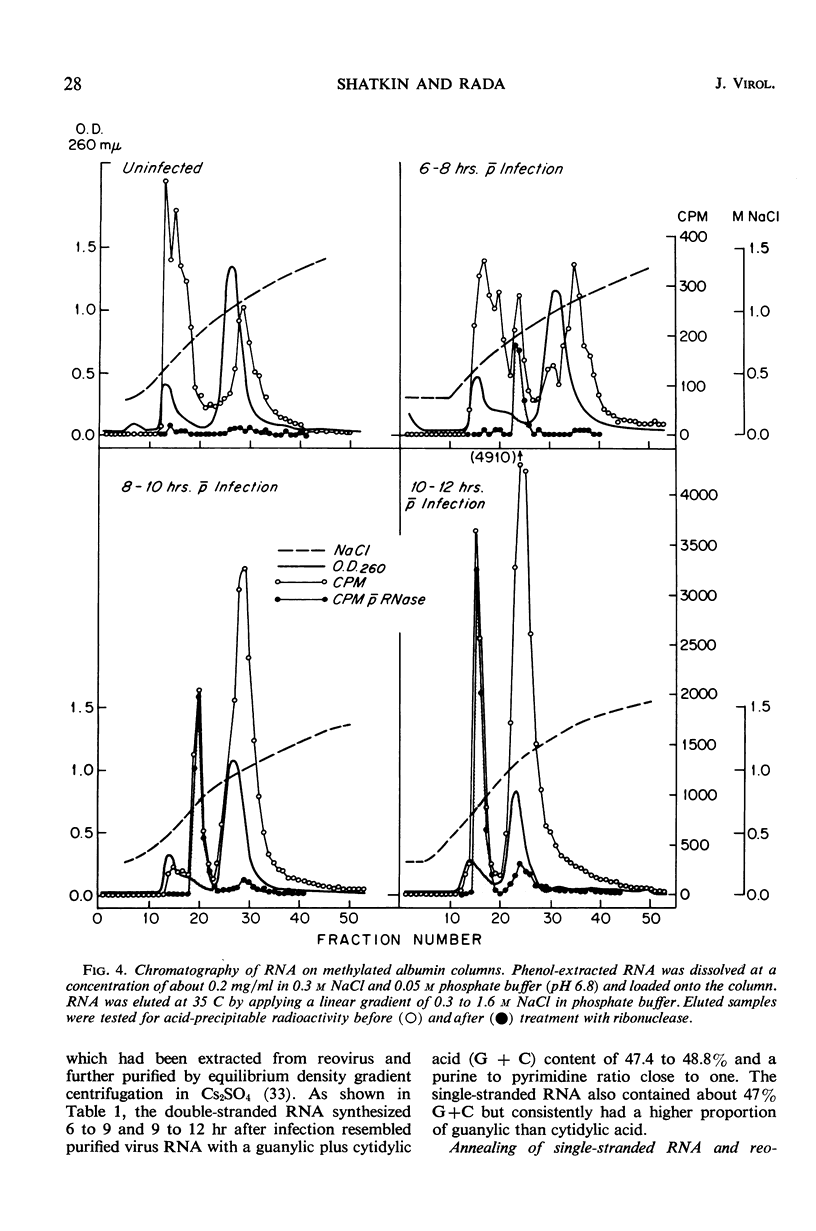
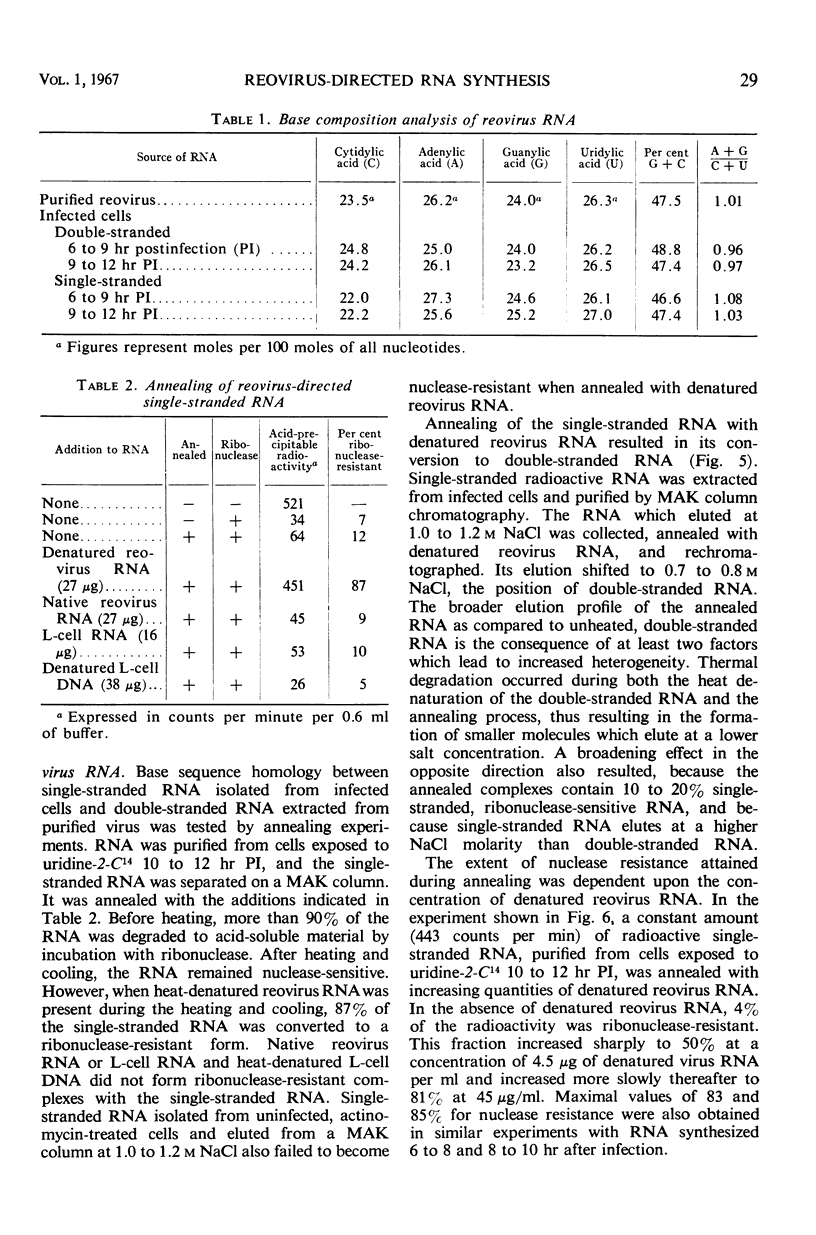
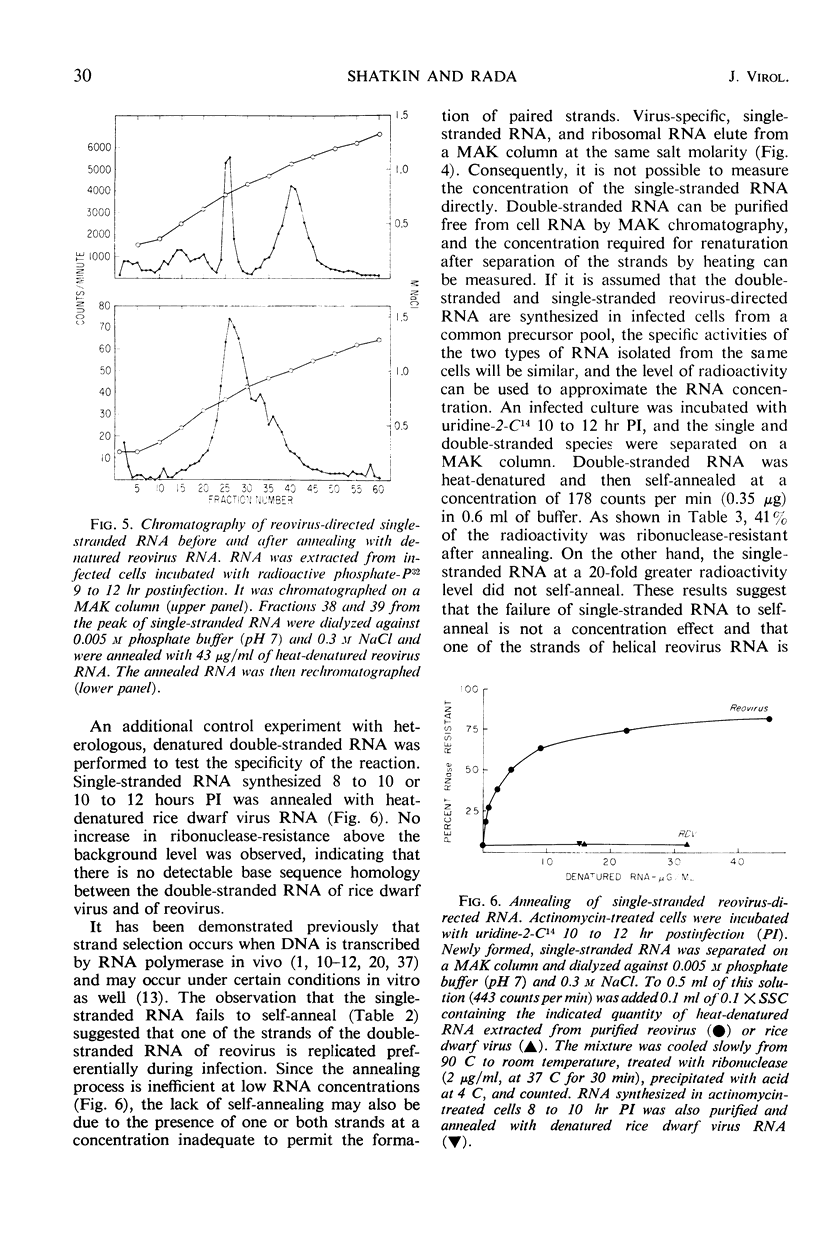
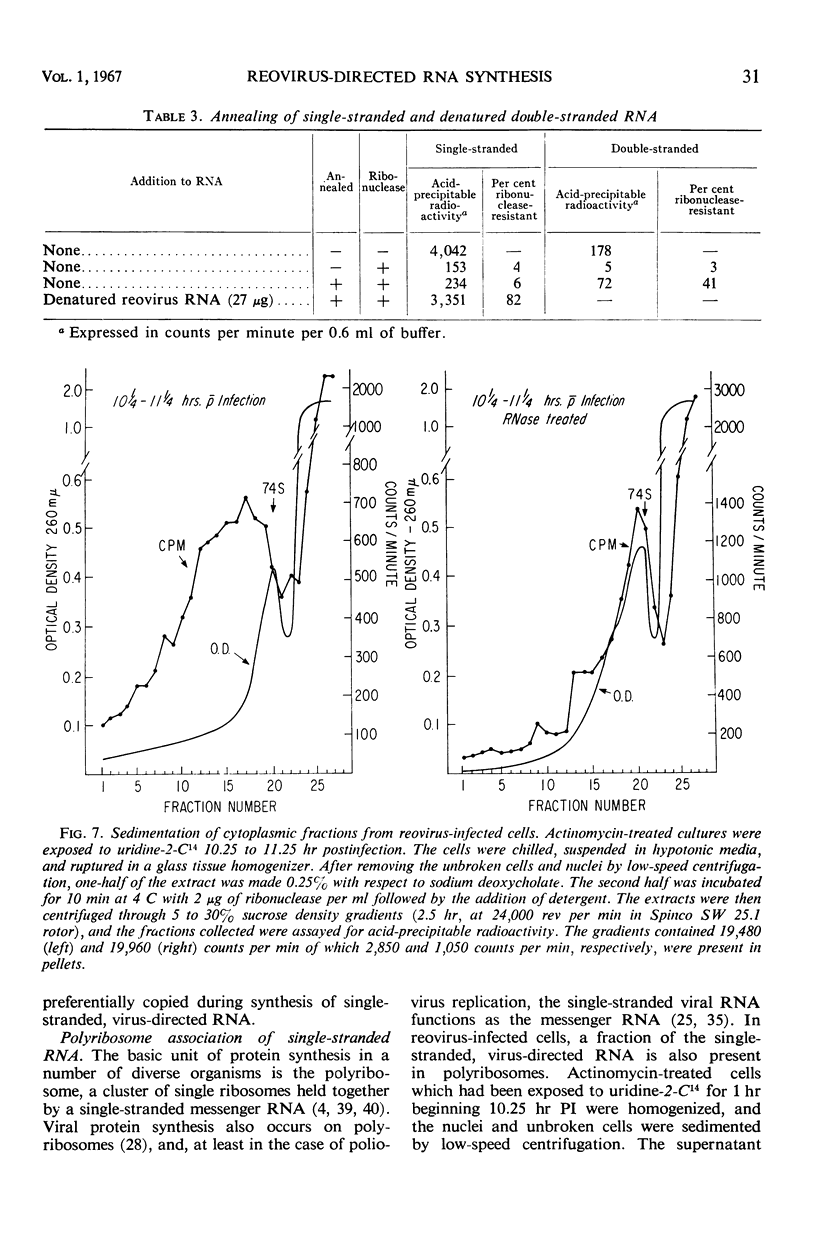
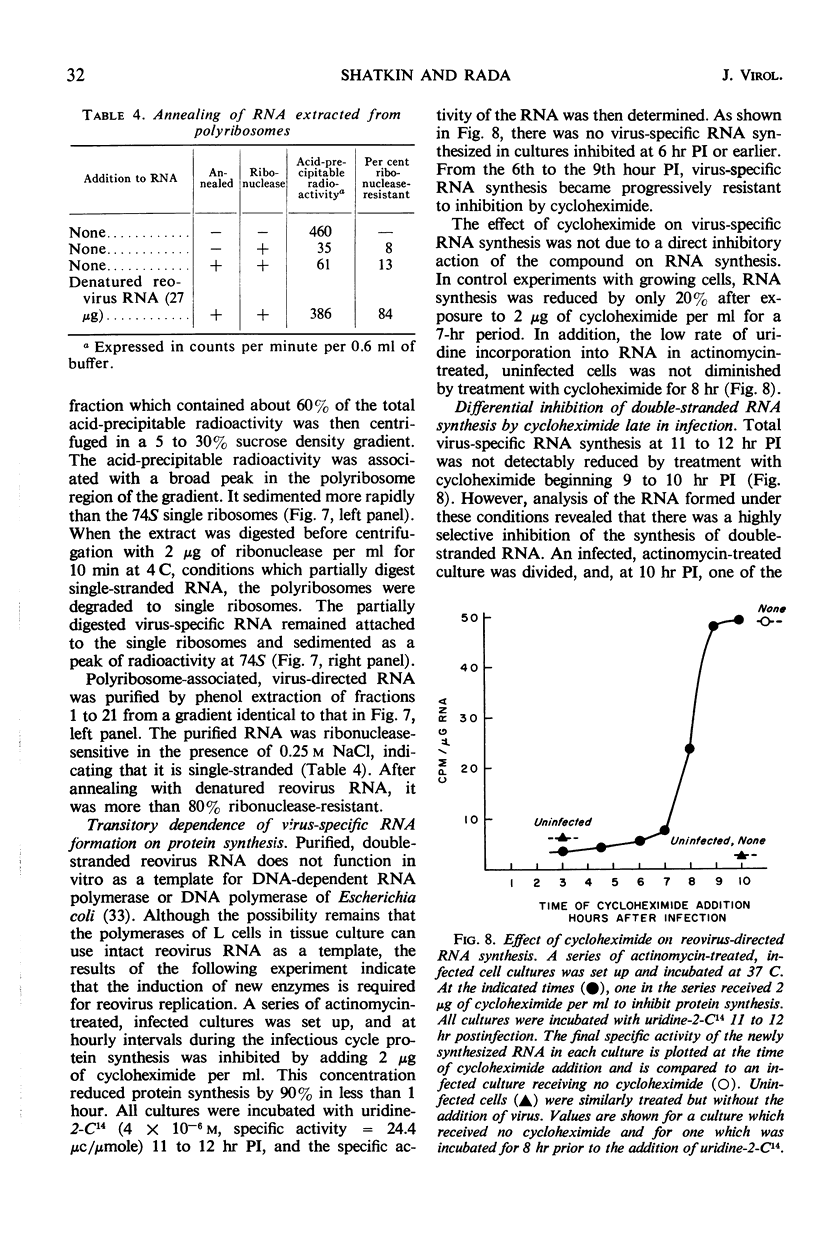
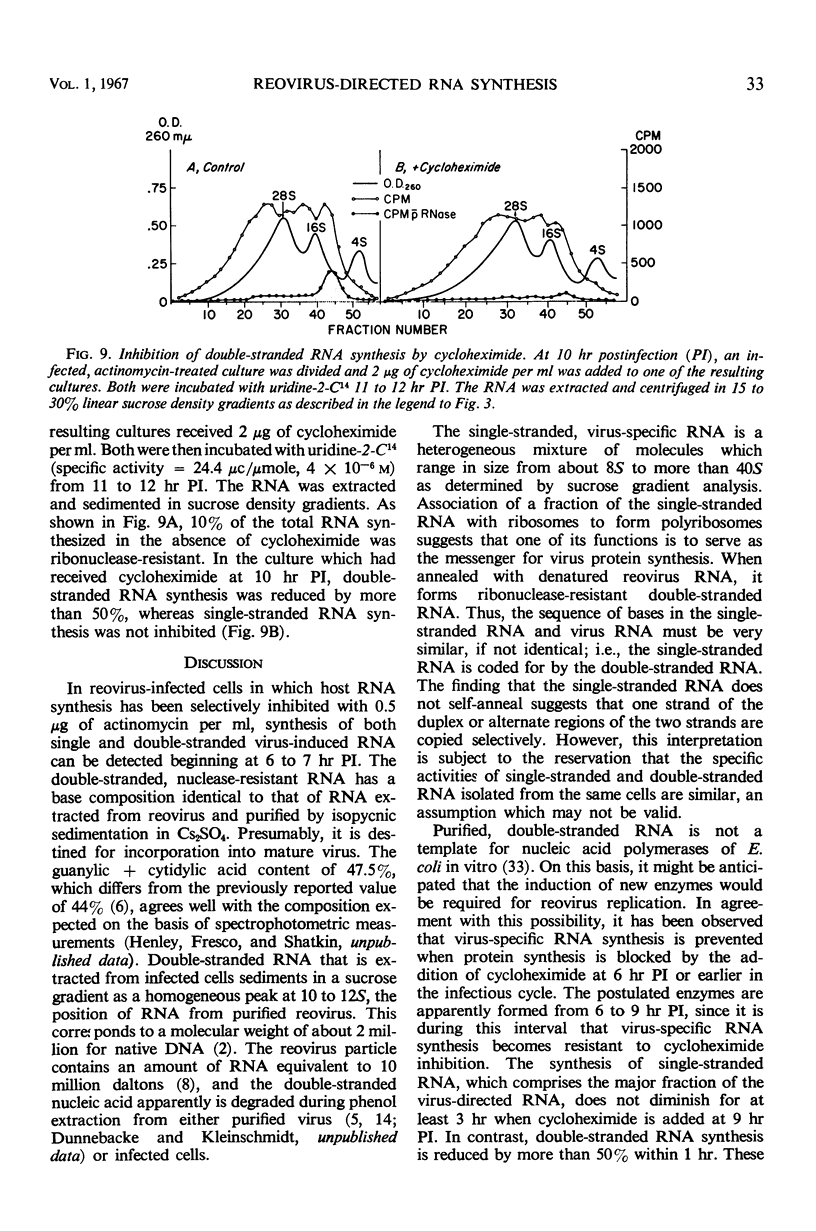
Reovirus replication in L-929 mouse fibroblasts was unaffected by 0.5 μg of actinomycin per ml, a concentration which inhibited cell ribonucleic acid (RNA) synthesis by more than 90%. Under these conditions of selective inhibition, the formation of both single-stranded and double-stranded virus-specific RNA was detected beginning at 6 hr after infection. The purified double-stranded RNA was similar in size and base composition to virus RNA and presumably was incorporated into mature virus. The single-stranded RNA formed ribonuclease-resistant duplexes when annealed with denatured virus RNA but did not self-anneal, thus indicating that it includes copies of only one strand of the duplex. The single-stranded RNA was polyribosome-associated and may function as the virus messenger RNA. Production of both types of virus-induced RNA required protein synthesis 6 to 9 hr after infection. At later times in the infectious cycle, only double-stranded RNA synthesis was dependent on continued protein formation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- GIERER A. Function of aggregated reticulocyte ribosomes in protein synthesis. J Mol Biol. 1963 Feb;6:148–157. doi: 10.1016/s0022-2836(63)80131-x. [DOI] [PubMed] [Google Scholar]
- GOMATOS P. J., STOECKENIUS W. ELECTRON MICROSCOPE STUDIES ON REOVIRUS RNA. Proc Natl Acad Sci U S A. 1964 Dec;52:1449–1455. doi: 10.1073/pnas.52.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOMATOS P. J., TAMM I. ANIMAL AND PLANT VIRUSES WITH DOUBLE-HELICAL RNA. Proc Natl Acad Sci U S A. 1963 Nov;50:878–885. doi: 10.1073/pnas.50.5.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOMATOS P. J., TAMM I., DALES S., FRANKLIN R. M. Reovirus type 3: physical characteristics and interaction with L cells. Virology. 1962 Jul;17:441–454. doi: 10.1016/0042-6822(62)90139-3. [DOI] [PubMed] [Google Scholar]
- GOMATOS P. J., TAMM I. MACROMOLECULAR SYNTHESIS IN REOVIRUS-INFECTED L CELLS. Biochim Biophys Acta. 1963 Aug 20;72:651–653. [PubMed] [Google Scholar]
- GUILD W. R., ROBINSON M. Evidence for message reading from a unique strand of pneumococcal DNA. Proc Natl Acad Sci U S A. 1963 Jul;50:106–112. doi: 10.1073/pnas.50.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomatos P. J., Tamm I. THE SECONDARY STRUCTURE OF REOVIRUS RNA. Proc Natl Acad Sci U S A. 1963 May;49(5):707–714. doi: 10.1073/pnas.49.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEINSCHMIDT A. K., DUNNEBACKE T. H., SPENDLOVE R. S., SCHAFFER F. L., WHITCOMB R. F. ELECTRON MICROSCOPY OF RNA FROM REOVIRUS AND WOUND TUMOR VIRUS. J Mol Biol. 1964 Nov;10:282–288. doi: 10.1016/s0022-2836(64)80046-2. [DOI] [PubMed] [Google Scholar]
- Kubinski H., Koch G. Regulation of the synthesis of various ribonucleic acids in animal cells. Biochem Biophys Res Commun. 1966 Feb 3;22(3):346–351. doi: 10.1016/0006-291x(66)90489-x. [DOI] [PubMed] [Google Scholar]
- Kudo H., Graham A. F. Selective inhibition of reovirus induced RNA in L cells. Biochem Biophys Res Commun. 1966 Jul 20;24(2):150–155. doi: 10.1016/0006-291x(66)90711-x. [DOI] [PubMed] [Google Scholar]
- Kudo H., Graham A. F. Synthesis of reovirus ribonucleic acid in L cells. J Bacteriol. 1965 Oct;90(4):936–945. doi: 10.1128/jb.90.4.936-945.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANGRIDGE R., GOMATOS P. J. The structure of RNA. Reovirus RNA and transfer RNA have similar three-dimensional structures, which differ from DNA. Science. 1963 Aug 23;141(3582):694–698. doi: 10.1126/science.141.3582.694. [DOI] [PubMed] [Google Scholar]
- MANDELL J. D., HERSHEY A. D. A fractionating column for analysis of nucleic acids. Anal Biochem. 1960 Jun;1:66–77. doi: 10.1016/0003-2697(60)90020-8. [DOI] [PubMed] [Google Scholar]
- MARMUR J., GREENSPAN C. M. TRANSCRIPTION IN VIVO OF DNA FROM BACTERIOPHAGE SP8. Science. 1963 Oct 18;142(3590):387–389. doi: 10.1126/science.142.3590.387. [DOI] [PubMed] [Google Scholar]
- Miura K. I., Kimura I., Suzuki N. Double-stranded ribonucleic acid from rice dwarf virus. Virology. 1966 Apr;28(4):571–579. doi: 10.1016/0042-6822(66)90242-x. [DOI] [PubMed] [Google Scholar]
- Miura K. I., Muto A. Lack of messenger RNA activity of a double-stranded RNA. Biochim Biophys Acta. 1965 Dec 9;108(4):707–709. doi: 10.1016/0005-2787(65)90068-7. [DOI] [PubMed] [Google Scholar]
- NIRENBERG M. W., MATTHAEI J. H. The dependence of cell-free protein synthesis in E. coli upon naturally occurring or synthetic polyribonucleotides. Proc Natl Acad Sci U S A. 1961 Oct 15;47:1588–1602. doi: 10.1073/pnas.47.10.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penman S., Scherrer K., Becker Y., Darnell J. E. POLYRIBOSOMES IN NORMAL AND POLIOVIRUS-INFECTED HELA CELLS AND THEIR RELATIONSHIP TO MESSENGER-RNA. Proc Natl Acad Sci U S A. 1963 May;49(5):654–662. doi: 10.1073/pnas.49.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REICH E., FRANKLIN R. M., SHATKIN A. J., TATUMEL Action of actinomycin D on animal cells and viruses. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1238–1245. doi: 10.1073/pnas.48.7.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALZMAN N. P., SHATKIN A. J., SEBRING E. D. THE SYNTHESIS OF A DNA-LIKE RNA IN THE CYTOPLASM OF HELA CELLS INFECTED WITH VACCINIA VIRUS. J Mol Biol. 1964 Mar;8:405–416. doi: 10.1016/s0022-2836(64)80204-7. [DOI] [PubMed] [Google Scholar]
- SCHARFF M. D., SHATKIN A. J., LEVINTOW L. ASSOCIATION OF NEWLY FORMED VIRAL PROTEIN WITH SPECIFIC POLYRIBOSOMES. Proc Natl Acad Sci U S A. 1963 Oct;50:686–694. doi: 10.1073/pnas.50.4.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHERRER K., DARNELL J. E. Sedimentation characteristics of rapidly labelled RNA from HeLa cells. Biochem Biophys Res Commun. 1962 Jun 4;7:486–490. doi: 10.1016/0006-291x(62)90341-8. [DOI] [PubMed] [Google Scholar]
- SEBRING E. D., SALZMAN N. P. AN IMPROVED PROCEDURE FOR MEASURING THE DISTRIBUTION OF P32O4--AMONG THE NUCLEOTIDES OF RIBONUCLEIC ACID. Anal Biochem. 1964 May;8:126–129. doi: 10.1016/0003-2697(64)90177-0. [DOI] [PubMed] [Google Scholar]
- SHATKIN A. J. ACTINOMYCIN AND THE DIFFERENTIAL SYNTHESIS OF REOVIRUS AND L CELL RNA. Biochem Biophys Res Commun. 1965 May 3;19:506–510. doi: 10.1016/0006-291x(65)90154-3. [DOI] [PubMed] [Google Scholar]
- SHATKIN A. J. Actinomycin inhibition of ribonucleic acid synthesis and poliovirus infection of HeLa cells. Biochim Biophys Acta. 1962 Aug 20;61:310–313. doi: 10.1016/0926-6550(62)90095-6. [DOI] [PubMed] [Google Scholar]
- SINGER M. F., JONES O. W., NIRENBERG M. W. The effect of secondary structure of the template activity of polyribonucleotides. Proc Natl Acad Sci U S A. 1963 Mar 15;49:392–399. doi: 10.1073/pnas.49.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A. J. Inactivity of purified reovirus RNA as a template for E. coli polymerases in vitro. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1721–1728. doi: 10.1073/pnas.54.6.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D. F., Levintow L. Constitution and function of polyribosomes of poliovirus-infected HeLa cells. Virology. 1965 Sep;27(1):44–53. doi: 10.1016/0042-6822(65)90142-x. [DOI] [PubMed] [Google Scholar]
- TOCCHINI-VALENTINI G. P., STODOLSKY M., AURISICCHIO A., SARNAT M., GRAZIOSI F., WEISS S. B., GEIDUSCHEK E. P. ON THE ASYMMETRY OF RNA SYNTHESIS IN VIVO. Proc Natl Acad Sci U S A. 1963 Nov;50:935–942. doi: 10.1073/pnas.50.5.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMITA K. I., RICH A. X-RAY DIFFRACTION INVESTIGATIONS OF COMPLEMENTARY RNA. Nature. 1964 Mar 21;201:1160–1163. doi: 10.1038/2011160a0. [DOI] [PubMed] [Google Scholar]
- WARNER J. R., KNOPF P. M., RICH A. A multiple ribosomal structure in protein synthesis. Proc Natl Acad Sci U S A. 1963 Jan 15;49:122–129. doi: 10.1073/pnas.49.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WETTSTEIN F. O., STAEHELIN T., NOLL H. Ribosomal aggregate engaged in protein synthesis: characterization of the ergosome. Nature. 1963 Feb 2;197:430–435. doi: 10.1038/197430a0. [DOI] [PubMed] [Google Scholar]



